Abstract
Aims
One of the main causes of cardiovascular complications in diabetes is the hyperglycemia-induced cell injury, and mitochondrial fission has been implicated in the apoptotic process. We investigated the role of mitochondrial fission in high glucoseinduced cardiovascular cell injury.
Methods
We used several types of cultured mouse, rat, and bovine cells from the cardiovascular system, and evaluated mitochondrial morphology, reactive oxygen species (ROS) levels and apoptotic parameters in sustained high glucose incubation. Adenoviral infection was used for the inhibition of the fission protein DLP1.
Results
We found that mitochondria were short and fragmented in cells incubated in sustained high glucose conditions. Under the same conditions, cellular ROS levels were high and cell death was increased. We demonstrated that the increased level of ROS causes mitochondrial permeability transition (MPT), phosphatidylserine exposure, cytochrome c release, and caspase activation in prolonged high glucose conditions. Importantly, maintaining tubular mitochondria by inhibiting mitochondrial fission in sustained high glucose conditions normalized cellular ROS levels and prevented the MPT and subsequent cell death. These results demonstrate that mitochondrial fragmentation is an upstream factor for ROS overproduction and cell death in prolonged high glucose conditions.
Conclusion
These findings indicate that the fission-mediated fragmentation of mitochondrial tubules is causally associated with enhanced production of mitochondrial ROS and cardiovascular cell injury in hyperglycemic conditions.
Introduction
Hyperglycemia induces cell injury in most cell types studied and is directly linked to the cardiovascular complications in diabetes. Mitochondria are an integral part of apoptosis, as they retain multiple apoptosis-activating factors1–3. Mitochondria in mammalian cells form reticular networks composed of filamentous tubules which constantly move and change shape through fission and fusion4. The most studied and characterized pathway for mitochondrial fission in mammalian cells involves the dynamin-like protein DLP1/Drp1 and its putative receptor hFis15–9. DLP1 is a dynamin-related large GTPase that binds to the mitochondrial surface for the fission reaction through the function of hFis16,9,10. Two dynamin-related proteins, mitofusin (Mfn) and OPA1, have been found to mediate fusion of outer and inner membranes, respectively11–15.
Mitochondrial fission and fusion have become a focus of attention recently due to their involvement in apoptosis16–19. Mitochondrial fragmentation is often associated with apoptosis, and mitochondrial fission proteins mediate the apoptosis-associated fragmentation of mitochondria20,21. Although it is debatable, inhibition of mitochondrial fragmentation has been shown to delay or prevent the release of mitochondrial apoptotic factors, suggesting that fission may participate in mitochondrial membrane permeabilization during the early stage of apoptosis20,21. Opening of the mitochondrial permeability transition (MPT) pore is one mechanism to induce the mitochondrial outer membrane permeabilization and apoptotic factor release during apoptosis. Increased levels of reactive oxygen species (ROS) are also known to induce the MPT and to cause apoptotic cell death22,23.
One of the main causes of hyperglycemic complications is the increased level of ROS. The electron transport chain (ETC) of mitochondria has been shown to be the site of ROS overproduction in hyperglycemia24–26. High glucose concentrations result in increased metabolic input into mitochondria, which overwhelms the ETC causing mitochondrial hyperpolarization, leading to electron backup within the ETC and ROS overproduction24–26. Studies indicate that increased levels of ROS can directly cause cell and tissue injury by apoptosis and oxidative stress in hyperglycemia27–29.
Although the dynamic nature of mitochondrial tubules has been recognized for some time, functional significance of mitochondrial dynamics is not well understood. In our previous study, we demonstrated that a rapid increase of the ROS level within minutes of hyperglycemic exposure and subsequent ROS fluctuation require mitochondrial fission, suggesting an active physiological role of mitochondrial fission in regulating mitochondrial activity30. However, the involvement of mitochondrial fission in apoptosis caused by hyperglycemic insult has not been tested. Cardiovascular cell injury is one of the most common complications in diabetes. In the present study, to gain mechanistic insight of whether and how mitochondrial fission participates in hyperglycemia-induced death of cells in the cardiovascular system, we examined correlations among mitochondrial fission, ROS, MPT, and apoptosis in prolonged hyperglycemic conditions. Our data demonstrated that sustained high glucose conditions cause the MPT and cell death through increased ROS production. More importantly, we found for the first time that inhibition of mitochondrial fission ameliorated high glucose-mediated cell death by normalizing the ROS level. These findings indicate that mitochondrial fission is an early component that regulates mitochondrial ROS production during hyperglycemia-induced cell death.
Materials and Methods
Cell isolation and culture conditions
Neonatal rat ventricular myocytes were prepared as described previously31. The cells were collected in medium consisting of DMEM supplemented with 50 units/ml penicillin/streptomycin, 10% calf serum, and 10% horse serum and preplated in 10-cm tissue culture dishes. After 2–3 hours, unattached cells were plated in 35-mm wells (~1 × 104 cells/well). The cultures were exposed to 3500 rads of γ-irradiation from a cesium source at 24-hour post culturing to limit proliferation of nonmyocardial cells. Cells were washed every second day and used for experiments after 4–14 days in culture. The cell line H9c2 (ATCC CRL-1446) was maintained in DMEM supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100µg/ml streptomycin. Bovine aortic endothelial cells (BAEC) were a gift from Dr. Zheng-Gen Jin (University of Rochester Medical Center) and were maintained in medium 199 supplemented with 10% FBS as described32. Cells in passages 5–8 were used for the experiments. Mouse aortic smooth muscle cells (SMC) were isolated and cultured as described33. Thoracic aortas were removed from 8-week-old C57BL/6 mice (Jackson Laboratory). Adventitia and endothelium were removed after digestion of the aortic segments with collagenase and further digested with elastase / collagenase cocktail. Cells were grown in DMEM supplemented with 10% FBS, 100U/ml penicillin, and 100µg/ml streptomycin. SMC lineage was confirmed by immunostaining with anti-α-actin antibody, and cells in passages 4–6 were used for experiments. For overexpression of the dominant-negative mutant DLP1-K38A, an adenovirus carrying DLP1-K38A (Ad-DLP1-K38A) was used. Ad-DLP1-K38A was constructed and amplified per manufacturer’s instruction (AdEasy system, Stratagene, Inc). Cells were incubated overnight in the normal glucose medium containing Ad-DLP1-K38A. After rinsing cells, the high glucose medium was added and further incubated for 30 and 48 hours. Handling of rats and mice conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
ROS and cell death assessments
The level of reactive oxygen species was detected using the fluorescent probe dihydroethidium (DHE) (Molecular Probes) and carboxy-dichlorodihydrofluorescein (H2DCFDA) (Molecular Probes) per manufacturer’s instructions. Cells were loaded with 25µM carboxy-H2DCFDA in PBS or 5µM DHE in HEPES buffer at 37°C for 30 minutes. Images were acquired at room temperature and fluorescence intensity was measured using IPLab imaging software (Scanalytics, Inc.). Cell death was measured by trypan blue exclusion assay, and apoptotic cell death was evaluated by FITC-Annexin V (BioVision Inc.) staining per manufacturer’s instruction. Caspase activity was measured by Rhodamine 110 bis-L-aspartic acid amide (Molecular Probes) as described previously34. Briefly, cells were loaded with 50µM rhodamine 110 bis-L-aspartic acid amide for 30 minutes at room temperature. Fluorescence images were acquired at room temperature and quantified for individual cells as the indicator of the presence of activated caspases.
Cobalt quenching calcein assay
The MPT was assayed by measuring calcein fluorescence quenched by cobalt ion in mitochondria as described previously9,35. The cells were loaded with 1.0µM calcein-acetomethoxy ester (Molecular Probes) and 1.0mM CoCl2 in Hank's balanced salt solution (Invitrogen) for 20 minutes at 37°C. At the end of the incubation, mitochondria were labeled with 25nM MitoTracker Red CMXRos (Molecular Probes) for 10 minutes. Fluorescence images were acquired for both calcein and MitoTracker, and the extent of the MPT was assessed by the decrease of the calcein fluorescence intensity. Of note, MitoTracker Red CMXRos was not sensitive to ROS levels and mitochondrial membrane potential in our experimental conditions.
Computer-assisted analyses of mitochondrial morphology
Quantitative analyses of mitochondrial morphology were performed using methods described previously30,36–38. Digital images were processed through a convolve filter using the NIH-developed ImageJ software (Wayne Rasband, NIH) to obtain isolated and equalized fluorescent pixels. After converting to masks, individual mitochondria (particles) were subjected to particle analyses to acquire values for circularity (4π·Area/perimeter2) and lengths of major and minor axes as well as the number of particles. From these values, form factor (FF: the reciprocal of circularity value) and aspect ratio (AR: major axis/minor axis) were calculated. Both parameters have a minimal value of 1 when it is a small perfect circle and the values increase as mitochondria elongate. High values for AR represent elongated tubular mitochondria, and increase of FF indicates increase of mitochondrial complexity (length and branching)36.
Indirect immunofluorescence
Indirect immunofluorescence was performed as described previously9. The cells were fixed in 4% paraformaldehyde and permeabilized, and then incubated in blocking buffer containing 5% horse serum for 1 hour at 37°C. The mouse anti-DLP1 (BD Transduction Laboratory) or mouse monoclonal anti-cytochrome c (BD Bioscience Pharmingen) antibodies were used as primary antibodies. For secondary antibodies, Alexa 488 or 594-conjugated antibodies (Molecular Probes) were used. After appropriate rinsing, coverslips were mounted in ProLong antifade reagent (Molecular Probes) on glass slides and cells were viewed with an Olympus IX71 epifluorescence microscope. Fluorescence images were acquired with an Evolution QEi camera (Mediacybernetics, Inc.) driven by IPLab imaging software (Scanalytics, Inc.). Acquired images were adjusted using Adobe Photoshop (Adobe Systems Inc.) software.
Results
Sustained exposure to high glucose conditions increases ROS production and cell death
Because cardiovascular injury is one of the most common diabetic complications linked to elevated ROS levels, we examined the cellular ROS levels in cultured primary rat neonatal cardiomyocytes incubated in sustained high glucose conditions. Cells were initially maintained in media containing 1g/L (5.56mM) glucose for the normal glucose concentration. For high glucose incubation, cells were transferred to media with either moderate (20mM) or severe (35mM) hyperglycemic condition for 30 and 48 hours. ROS levels were measured by increased ethidium fluorescence resulting from oxidation of DHE. In both 20 and 35mM concentrations, the high glucose incubation significantly increased ethidium fluorescence. An approximately two-fold increase of ROS was observed in cells incubated with 35mM glucose compared with normal conditions (Fig. 1A). The increase of ROS in 20mM glucose was less than that in 35mM glucose. Next we tested whether cell death occurs under the same high glucose incubation conditions. Proportions of trypan blue positive cells were 3–4 fold higher in both 20 and 35mM glucose concentrations, indicating an increase of cell death (Fig. 1A). Experiments using aortic endothelial and smooth muscle cells produced the similar results (supplementary figure 1). Annexin V cell surface labeling of cells incubated in high glucose conditions showed an increased labeling (Fig. 1A), suggesting that the high glucose-mediated cell death includes apoptosis.
Figure 1.
Sustained incubation of neonatal cardiomyocytes or H9c2 cells in high glucose conditions increases ROS production and cell death. (A) Neonatal cardiomyocytes were maintained in a normal glucose concentration (5.56mM). ROS levels and cell death were assessed at 30 and 48 hours after the cells were transferred to media containing 20 and 35mM glucose. ROS levels were measured by DHE oxidation (A, left panel). Quantification of fluorescence intensity showed approximately a two-fold increase of ROS in 35mM glucose compared to normal conditions at both 30- and 48-hour incubations. The increase of ROS in 20mM glucose was less than that in 35mM glucose. Trypan blue staining showed a 3–4 fold increase of cell death in 20 and 35mM glucose concentrations (A, middle panel). Cell surface labeling with annexin V was used to assess whether the cell death in high glucose incubation is possibly apoptotic (A, right panel). Cells incubated in 20 and 35mM glucose showed increased annexin V labeling. Error bars represent SEM. * and ** indicate statistically significant differences compared with the Normal Glc at the corresponding time points at p< 0.05 and p<0.01, respectively. (B) The cardiac myoblast cell line H9c2 shows increased ROS levels. H9c2 cells showing increased ethidium (upper panels) and DCF (lower panels) fluorescence in prolonged high glucose conditions (35mM Glc). Cells in normal glucose (5.56mM) had low ROS levels (left panels). Fluorescence quantification shows an approximately two-fold increase of the ROS level after 30- and 48-hour incubations in 35mM glucose (right panels). Scale bar: 20µm. (C–E) Prolonged high glucose incubation of H9c2 cells increases cell death. Proportions of trypan blue-positive cells (C) and annexin V-positive cells (D) were increased by approximately 5 and 10 folds at 30 and 48 hours, respectively, in 35mM glucose incubation. A significant increase of fluorescence resulting from caspase-mediated cleavage of the substrate rhodamine-110 bis-L-aspartic acid amide was observed in cells after a 48-hour incubation in 35mM glucose (E). Addition of 30mM mannitol instead of glucose increased neither cell death nor caspase activity. Error bars represent SEM.
Mitochondrial tubules are short and fragmented in sustained high glucose conditions
We next examined whether the mitochondrial morphology is altered in hyperglycemia-induced cell death. Because mitochondrial morphology in primary neonatal heart cells was difficult to discern due to varying shapes and cell clumping, we used the rat heart myoblast cell line H9c2 for these experiments. We first tested whether H9c2 cells respond to high glucose conditions similarly to primary neonatal cardiomyocytes. A two-fold increase of the ethidium fluorescence intensity was observed after 30- and 48-hour incubations in 35mM glucose concentration (Fig. 1B upper panels). Because fluorescent ethidium produced from DHE oxidation binds to DNA in the nucleus, cells with increased ROS showed pronounced nuclear labeling. Another ROS probe, carboxy-H2DCFDA, gave results similar to the ones obtained with DHE (Fig 1B lower panels). The proportion of trypan blue positive cells was approximately 5 fold higher at 30 hours and increased by10 fold at 48 hours in high glucose concentration (Fig. 1C). In addition, an increased cell surface annexin V labeling was also observed after prolonged incubation in high glucose (Fig. 1D and supplementary figure 2). The caspase activity assay using the caspase substrate Rhodamine-110 bis-L-aspartic acid amide also showed a significant increase of fluorescence resulting from caspase-mediated cleavage of the substrate in the sustained high glucose incubation (Fig. 1E). Adding 30mM L-glucose (results not shown) or mannitol instead of D-glucose did not increase cell death and the apoptotic parameters, indicating that the cell injury observed in high glucose incubation resulted from glucose metabolism and not from the osmotic stress of the high sugar concentration. These results indicate that sustained high glucose incubation causes cell death in H9c2 cells.
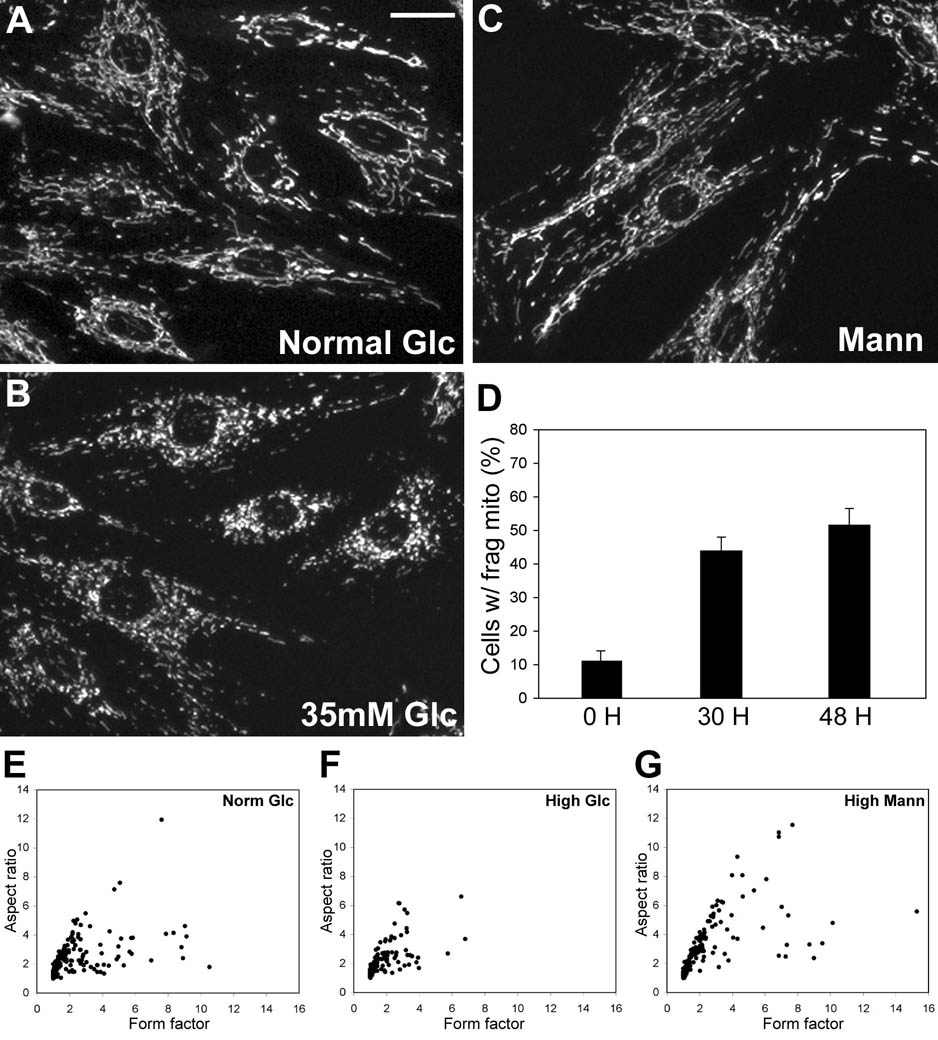
MitoTracker staining showed that mitochondrial morphology of H9c2 cells in the normal glucose concentration was mostly tubular (Fig. 2A). After incubating cells in the sustained high glucose concentration, much shorter and smaller mitochondria were prevalent in these cells, indicating mitochondrial fragmentation (Fig. 2B). Approximately 50% of cells incubated in the high glucose concentration for 30 and 48 hours contained clearly fragmented mitochondria (Fig. 2D). Although mitochondrial fragmentation in the remainder of the cells was less obvious, mitochondrial tubules were generally shorter in these cells. The same concentration of mannitol did not cause a detectable change in mitochondrial morphology (Fig. 2C). For objective quantification of mitochondrial morphology, mitochondrial shapes were analyzed by measuring the form factor and aspect ratio. Representative data show that the majority of mitochondria in cells from high glucose incubation are short and small (Fig. 2F), compared to those from normal glucose (Fig. 2E) or high mannitol conditions (Fig. 2G). We also observed that the number of mitochondria increases in high glucose incubation. Although the number of mitochondria varied greatly from cell to cell, numbers in 5 individual cells from normal and high glucose conditions ranged 107–225 and 262–442, respectively.
Figure 2.
Mitochondrial tubules are short and fragmented in sustained high glucose conditions. MitoTracker staining shows the tubular mitochondrial morphology in H9c2 cells in normal glucose conditions (A). Small and fragmented mitochondria were evident in cells incubated in 35mM glucose for 48 hours (B), whereas 30mM mannitol did not affect mitochondrial morphology (C). Scale bar: 20µm. Approximately half of the cell population contained fragmented mitochondria after high glucose incubation (D). Error bars represent SEM. (E–G) Computer-assisted morphology analyses of mitochondria. Individual mitochondria from cells incubated in the high glucose concentration for 48 hours have lower values for both form factor and aspect ratio (F: short and fragmented mitochondria) whereas many of those from normal glucose and high mannitol conditions show increased values (E and G: tubular mitochondria).
Mitochondrial fission mediated by DLP1 is necessary for mitochondrial fragmentation in sustained high glucose conditions
The main cellular processes determining mitochondrial morphology are fission and fusion of mitochondrial tubules39. The dynamin-like GTPase DLP1/Drp1 mediates mitochondrial fission. To test whether mitochondrial fission is necessary for the mitochondrial fragmentation that we observed in sustained high glucose conditions, we examined mitochondrial morphology in cells infected with the adenovirus carrying a dominant-negative mutant form of DLP1, DLP1-K38A (Ad-DLP1-K38A). An adenovirus carrying green fluorescent protein (GFP) was used as a control in which cell and mitochondrial morphologies were unaffected upon infection (Supplementary figure 3). Immunoblotting showed a significantly increased DLP1-K38A expression in cell lysate from virus-infected cells (Fig. 3A). Because a larger spliced variant of DLP1 was used to create the DLP1-K38A virus, overexpressed DLP1-K38A ran slower than the endogenous DLP1 in gel electrophoresis. DLP1 immunofluorescence showed the presence of bright punctate aggregates, characteristic of the DLP1-K38A expression5,40, in all cells in culture infected with Ad-DLP1-K38A (Fig. 3B). In addition, cells infected with Ad-DLP1-K38A contained elongated and entangled mitochondria, indicating that mitochondrial fission was inhibited in these cells (Fig. 3B). After a prolonged incubation in high glucose concentrations, Ad-DLP1-K38A infection abolished the mitochondrial fragmentation, showing elongated tubular mitochondria (Fig. 3E) whereas fragmented mitochondria were prevalent in control cells (Fig. 3D). Less than 5% of Ad-DLP1-K38A-infected cells showed fragmented mitochondria after 30- and 48-hour incubations in the high glucose concentration (Fig. 3F). Computer-assisted analyses of mitochondrial morphology also showed abolition of high glucose-mediated mitochondrial fragmentation by DLP1-K38A (Fig. 3G–I). These results demonstrate that mitochondrial fragmentation in sustained hyperglycemic conditions requires the mitochondrial fission protein DLP1.
Figure 3.
The mitochondrial fission protein DLP1 is necessary for mitochondrial fragmentation in sustained high glucose conditions. (A) Immunoblotting of H9c2 cell lysates from uninfected (Un), control virus-infected (Ad-GFP), and DLP1-K38A virus-infected (Ad-DLP1-K38A) cells. While endogenous DLP1 was detected as a faint band in all three lysates, a dense band of the slightly larger DLP1 (asterisk) was found in Ad-DLP1-K38A-infected cells, indicating an overexpression of DLP1-K38A. (B) DLP1 immunofluorescence showed the presence of characteristic bright punctate aggregates, indicative of the DLP1-K38A expression (left panel). Ad-DLP1-K38A-infected cells show the elongated mitochondrial morphology due to inhibition of mitochondrial fission (right panel). Scale bar: 20µm. (C–F) MitoTracker staining of control-infected cells shows the tubular morphology in normal glucose conditions (C), but a fragmented phenotype after the 35mM glucose incubation for 48 hours (D). Cells infected with Ade-DLP1-K38A contained elongated mitochondria and no fragmented phenotype in high glucose incubation (E). Scale bar: 10µm. Less than 5% of Ad-DLP1-K38A-infected cells contained fragmented mitochondria after 30- and 48-hour incubations in high glucose conditions (F) Error bars represent SEM. (G–I) Computer-assisted quantitative analyses of mitochondrial morphology in cells from normal glucose (G), high glucose (H), and DLP1-K38A/high glucose (I) conditions. Mitochondria in cells expressing DLP1-K38A have higher values for both form factor and aspect ratio in the high glucose conditions, indicating the abolition of high glucose-induced mitochondrial fragmentation.
Increased ROS levels in sustained high glucose conditions cause MPT and cell death
We observed that the number of cells showing Annexin V-positive labeling increased in sustained high glucose incubation, indicative of potential apoptotic cell death (Fig. 1). Because high levels of ROS can cause the MPT that leads to apoptosis23, we examined whether the MPT occurs in high glucose incubation by cobalt quenching calcein (Co-Q) assay9,35. In this assay, cobalt ions move into the mitochondria upon the MPT and quench mitochondrial calcein fluorescence. Decreases of calcein fluorescence in mitochondria indicate the MPT. Incubating H9c2 cells in high glucose concentrations for 48 hrs resulted in loss or significant decrease of mitochondrial calcein fluorescence (Fig. 4B’). We randomly selected 40 cells each in normal and high glucose conditions and measured calcein fluorescence intensity. Although levels of calcein staining in mitochondria varied from cell to cell, the average value of calcein fluorescence was markedly reduced in cells incubated in the high glucose concentration for 48 hours (Fig. 4C). Adding the MPT inhibitor bongkrekic acid (BA) to cells incubated in high glucose conditions prevented the decrease of the mitochondrial calcein fluorescence, confirming that prolonged high glucose conditions cause MPT.
Figure 4.
Increased ROS levels in sustained high glucose conditions cause the MPT. (A, A’, B, B’) H9c2 cells were incubated with calcein-AM and CoCl2 to assess the MPT in normal and high glucose conditions. Mitochondria were labeled with MitoTracker (A and B). Mitochondria in a majority of the cells in normal glucose conditions were calcein-positive (A’), whereas the mitochondrial calcein fluorescence was lost or decreased upon the MPT, which is more prevalent in cells incubated in 35mM glucose for 48 hours (B’). Scale bar: 20µm. (C) Quantification of calcein fluorescence in 40 randomly selected cells. High glucose incubation (HG) decreased calcein fluorescence. Bongkrekic acid (BA, 5µM), FCCP (100nM), or MnTMPyP (25µM) prevented the decrease of the mitochondrial calcein fluorescence in high glucose conditions. (D–F) Blocking the MPT or the ROS increase in high glucose incubation decreases apoptotic cell death. Trypan blue staining shows an approximately three-fold decrease of cell death in cells treated with BA, FCCP, or MnTMPyP after 48 hours in 35mM glucose (D). Annexin V cell surface labeling (E) and caspase activity (F) were also reduced by treating cells with BA, FCCP or MnTMPyP in high glucose condition. Error bars represent SEM.
We next tested whether the increased ROS is the cause of the MPT in high glucose conditions. We have shown that mild uncoupling of mitochondria by a low concentration of FCCP (p-trifluoromethoxy carbonyl cyanide phenyl hydrazone, 100nM) abolished the ROS overproduction in high glucose conditions30. Cell viability and mitochondrial morphology was unaffected in the presence of this concentration of FCCP30. In cells treated with FCCP, a normal level of calcein fluorescence was found after a 48-hour incubation in high glucose conditions (Fig. 4C), indicating that the MPT was prevented. We also used a cell permeable manganese superoxide dismutase (MnSOD) mimetic, Mn(III)tetrakis(1-methyl-4-pyridyl)porphyrin (MnTMPyP) which prevents mitochondrial ROS increase41,42. Consistent with data from FCCP-treated cells, cells incubated with MnTMPyP in high glucose conditions maintained mitochondrial calcein staining (Fig. 4C). These results demonstrate that the elevated ROS level is the cause of the MPT in sustained hyperglycemic conditions.
We also tested whether the increased ROS is the cause of cell death in sustained high glucose conditions. The number of trypan blue positive cells decreased by three folds in cells treated with BA, FCCP, or MnTMPyP after 48 hours in the 35mM glucose concentration (Fig. 4D). Likewise, apoptotic indicators including annexin V cell surface labeling and caspase activity also showed decreases in cells treated with these agents in the high glucose incubation (Fig. 4E, F). These results demonstrate that an increased ROS level is the causal factor that induces MPT and cell death in sustained high glucose conditions.
Inhibition of mitochondrial fission prevents high glucose-induced cell death
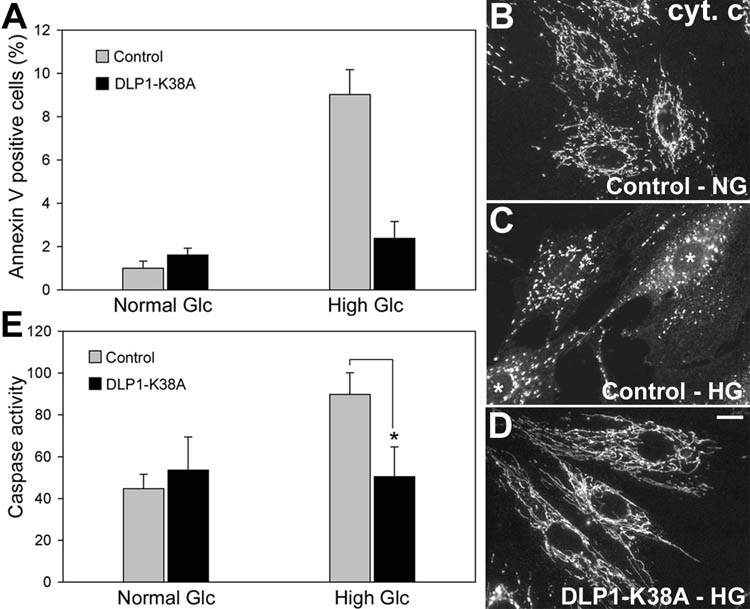
We examined the role of mitochondrial fission in hyperglycemia-induced cell death. When mitochondrial fission was blocked by Ad-DLP1-K38A, we observed a drastic decrease in annexin V-positive cells incubated in high glucose conditions for 48 hours (Fig. 5A). In immunofluorescence with anti-cytochrome c antibodies, a diffuse cytosolic distribution of cytochrome c was observed in addition to fragmented mitochondria after a 48-hour high glucose incubation in control cells (Fig. 5C), indicating cytochrome c release from the mitochondria. However, cells infected with Ad-DLP1-K38A exhibited no cytosolic cytochrome c in high glucose conditions. Clear retention of cytochrome c within the mitochondria was evident in cells with elongated mitochondria caused by the DLP1-K38A-induced fission block (Fig. 5D). Furthermore, the Ad-DLP1-K38A infection also normalized caspase activities in high glucose incubation (Fig. 5E). These data demonstrate that mitochondrial fragmentation is a necessary factor for apoptosis in sustained high glucose conditions.
Figure 5.
Inhibiting mitochondrial fission prevents high glucose-induced apoptosis. H9c2 cells were infected with Ad-DLP1-K38A and assessed for apoptosis after a 48-hour incubation in 35mM glucose. The number of annexin V-positive cells in the Ad-DLP1-K38A-infected culture in high glucose conditions was as low as that in normal glucose conditions (A). Cytochrome c immunofluorescence indicates cytochrome c in tubular mitochondria in normal glucose conditions (B), whereas it shows an increased cytosolic staining (cells marked by asterisks) along with fragmented mitochondria in cells incubated in the high glucose concentration (C). Cells infected with Ad-DLP1-K38A in high glucose conditions still show cytochrome c retained in elongated tubular mitochondria (D). Scale bar: 10µm. The Ad-DLP1-K38A infection normalized caspase activities in high glucose incubation (E). Error bars represent SEM. * indicates a statistically significant difference compared with the Control at p< 0.05.
Inhibiting mitochondrial fission in sustained high glucose incubation prevents ROS overproduction and MPT
Our data indicate that both mitochondrial fragmentation and increased ROS levels are upstream events in high glucose-induced apoptotic cell death. To test a correlation between mitochondrial fragmentation and high ROS levels in high glucose-mediated cell death, we assessed ROS levels of cells in which mitochondrial fission was blocked by DLP1-K38A. In cells infected with control virus, the ROS level increased by approximately two fold after a 48-hour incubation in the high glucose concentration (Fig. 6B, D). Remarkably, cells infected with Ad-DLP1-K38A showed a low level of ROS in high glucose conditions, similar to that of cells in the normal glucose concentration (Fig. 6C, D). This result indicates that mitochondrial fragmentation is causal to increasing the ROS level in sustained high glucose incubation. Because increased levels of ROS cause the MPT in high glucose conditions (Fig. 4), we also tested whether inhibiting mitochondrial fission prevents the MPT in sustained high glucose incubation. We found that cells infected with Ad-DLP1-K38A in high glucose conditions exhibited calcein levels similar to those of cells in normal glucose conditions (Fig. 6E), indicating that the mitochondrial fission process is necessary for the high glucose-induced MPT that leads to apoptosis.
Figure 6.
Inhibiting mitochondrial fission in sustained high glucose incubation prevents ROS overproduction and MPT. (A–D) Control cells show low levels of ROS in normal glucose conditions (A), and elevated ROS in the 48-hour high glucose incubation (B). ROS levels did not increase in H9c2 cells infected with Ad-DLP1-K38A in the 48-hour incubation in high glucose conditions (C). Scale bar: 20µm. Quantification of ethidium fluorescence indicates that ROS levels of Ad-DLP1-K38A-infected cells in high glucose conditions were similar to those in normal glucose incubation (D). Error bars represent SEM. ** indicates a statistically significant difference compared with the Control at p<0.01. (E) Co-Q assays for the MPT show that H9c2 cells infected with Ad-DLP1-K38A in high glucose conditions exhibited calcein levels similar to those of cells in normal glucose conditions. (F, G) Inhibition of mitochondrial fission in primary cells from cardiovascular system prevented the ROS increase in sustained high glucose conditions. Ad-DLP1-K38A infection decreased ROS levels in BAEC (F) and SMC (G) incubated for 48 hours in both 20 and 35mM glucose. Error bars represent SEM.
Our data with H9c2 cells demonstrate that mitochondrial fission plays an important role in ROS overproduction and subsequent ROS-induced cell death in prolonged incubation in high glucose conditions. Because the cardiovascular system is a main target of hyperglycemic ROS damage, we tested the role of mitochondrial fission in ROS overproduction in primary cultures of aortic endothelial and smooth muscle cells. These cells exhibited increased levels of ROS and increased cell death in sustained high glucose conditions (supplementary figure 4). Consistent with the observations made with H9c2 cells, both aortic endothelial and smooth muscle cells expressing DLP1-K38A showed an ROS level similar to that in cells of normal glucose conditions (Fig. 6F and G). Taken together, our experimental data demonstrate that mitochondrial fragmentation occurring in sustained high glucose conditions is an upstream component that is necessary for ROS generation, MPT, and cell death.
Discussion
One of the initial consequences of hyperglycemia is an increased generation of ROS which is a main factor contributing to the development and progression of diabetic complications24–26,43,44. Our results showed that, while increased cell death was unequivocal in sustained high glucose incubation, the proportion of dying cells in the whole cell population was small (10–15%), consistent with other reports26–29,45,46. The majority of cells in hyperglycemic incubation exhibited increased ROS levels but approximately a tenth of those showed cell death traits, indicating that increased ROS was not directly translated into cell death. ROS is an important component of cellular signaling pathways at stimulative concentrations47,48. On the other hand, when the ROS level is above a certain threshold, it induces the MPT and also perturbs the cellular redox balance and shift cells into a state of oxidative stress, leading to cell death22,44,49. Therefore, we speculate that although ROS levels increase in most cells incubated in high glucose concentrations, individual cells respond differently depending on the level of ROS and the efficiency of ROS detoxification in given cells, resulting in cell stimulation or death.
The involvement of mitochondrial fission in apoptosis has been demonstrated in cell death induced by different apoptotic stimuli16,20. We found that mitochondria are fragmented in sustained high glucose conditions through the mitochondrial fission process. Inhibition of mitochondrial fission blocked the MPT and cytochrome c release in hyperglycemic incubation, suggesting the involvement of mitochondrial fission in the early apoptotic stage. These results allow us to add the hyperglycemic insult to the panel of stimuli with which mitochondrial fission participates in apoptotic cell death.
Our study demonstrated that increased ROS levels in sustained hyperglycemic conditions cause the MPT and lead to cell death presumably via apoptosis. The adenine nucleotide translocase inhibitor BA decreased cell death by preventing the MPT in hyperglycemic incubation (Fig. 4). Although cyclosporine A (CsA) is the more commonly used inhibitor of the MPT, we found that mitochondria became fragmented upon treating cells with CsA alone. On the other hand, the BA treatment itself did not affect mitochondrial morphology. How CsA causes mitochondrial fragmentation is unclear. It is possible that the calcineurin-inhibiting activity of CsA may play a role in a potential signaling for controlling mitochondrial morphology.
Our data also show that the increased ROS level is the direct cause of the MPT because decreasing the ROS levels either by FCCP or MnTMPyP in high glucose incubation prevents the MPT (Fig. 4). However, for the FCCP-mediated effect, it is possible that FCCP decreases the mitochondrial Ca2+ influx to prevent the MPT although the involvement of Ca2+ in this condition is not clear. The same treatments also significantly decreased cell death in the high glucose conditions (Fig. 4). These data further support the notion that decreasing the cellular ROS level in hyperglycemic conditions would prevent cell and tissue injury and alleviate hyperglycemic complications. By blocking mitochondrial fragmentation in high glucose incubation, we prevented the ROS increase and the subsequent cell death (Fig. 5, 6), indicating that mitochondrial fragmentation in sustained hyperglycemia is an active component in regulating mitochondrial ROS generation and cell death.
The impending question is how the morphological change of mitochondria can alter ROS levels in hyperglycemic conditions. Our data as well as data from others indicate that the ROS increase in high glucose conditions is mostly of mitochondrial origin26,30,50. In mitochondria, the majority of ROS is produced in the ETC. Electrons escaped from the ETC react with oxygen to produce superoxide radicals that can be further converted to various ROS. The ETC resides within the elaborately folded inner membrane, and individual components of the ETC are presumed to be arranged in a specific manner for efficient transfer of electrons. It is possible that a large-scale change of mitochondrial membrane by fragmentation of membrane tubules in hyperglycemic conditions may change structural organization and arrangement of ETC components within the membrane. This organizational derangement of the ETC may lead to perturbation of ETC activity, causing ROS overproduction. Further biochemical and structural studies will be necessary to determine the mechanisms of ROS overproduction from fragmented mitochondria in hyperglycemic conditions.
In this study, we observed mitochondrial fragmentation in cells incubated in prolonged hyperglycemic conditions and investigated its relation to ROS generation and cell injury. We found that the mitochondrial fission process mediated by DLP1 is necessary for mitochondrial fragmentation that leads to the increase of ROS levels and cell death in hyperglycemic conditions. Based on our data that mitochondrial fragmentation is the upstream factor for the hyperglycemia-induced ROS overproduction and cell injury, mitochondrial fission machinery can be a novel target to ameliorate ROS-mediated cardiovascular complications in hyperglycemia.
Supplementary Material
Acknowledgments
We are grateful to Li Wang for helping in aortic smooth muscle cell isolation. We also thank Madhavika Serasinghe for critical reading and Randy Fox for technical assistance.
Funding: National Institute of Health (DK073858 and DK078618 to YY; HL033333 to S-SS)
Footnotes
Conflict of Interest: none declared
References
- 1.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 2.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 3.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- 4.Bereiter-Hahn J. Behavior of mitochondria in the living cell. Int Rev Cytol. 1990;122:1–63. doi: 10.1016/s0074-7696(08)61205-x. [DOI] [PubMed] [Google Scholar]
- 5.Pitts KR, Yoon Y, Krueger EW, McNiven MA. The dynamin-like protein DLP1 is essential for normal distribution and morphology of the endoplasmic reticulum and mitochondria in mammalian cells. Mol Biol Cell. 1999;10:4403–4417. doi: 10.1091/mbc.10.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stojanovski D, Koutsopoulos OS, Okamoto K, Ryan MT. Levels of human Fis1 at the mitochondrial outer membrane regulate mitochondrial morphology. J Cell Sci. 2004;117:1201–1210. doi: 10.1242/jcs.01058. [DOI] [PubMed] [Google Scholar]
- 8.Yoon Y, Krueger EW, Oswald BJ, McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol. 2003;23:5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu T, Fox RJ, Burwell LS, Yoon Y. Regulation of mitochondrial fission and apoptosis by the mitochondrial outer membrane protein hFis1. J Cell Sci. 2005;118:4141–4151. doi: 10.1242/jcs.02537. [DOI] [PubMed] [Google Scholar]
- 10.Yoon Y, Pitts KR, Dahan S, McNiven MA. A novel dynamin-like protein associates with cytoplasmic vesicles and tubules of the endoplasmic reticulum in mammalian cells. J Cell Biol. 1998;140:779–793. doi: 10.1083/jcb.140.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci U S A. 2004;101:15927–15932. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santel A, Fuller MT. Control of mitochondrial morphology by a human mitofusin. J Cell Sci. 2001;114:867–874. doi: 10.1242/jcs.114.5.867. [DOI] [PubMed] [Google Scholar]
- 14.Ishihara N, Jofuku A, Eura Y, Mihara K. Regulation of mitochondrial morphology by membrane potential, and DRP1-dependent division and FZO1-dependent fusion reaction in mammalian cells. Biochem Biophys Res Commun. 2003;301:891–898. doi: 10.1016/s0006-291x(03)00050-0. [DOI] [PubMed] [Google Scholar]
- 15.Pfanner N, Wiedemann N, Meisinger C. Double membrane fusion. Science. 2004;305:1723–1724. doi: 10.1126/science.1104244. [DOI] [PubMed] [Google Scholar]
- 16.Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol. 2005;6:657–663. doi: 10.1038/nrm1697. [DOI] [PubMed] [Google Scholar]
- 17.Karbowski M, Youle RJ. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ. 2003;10:870–880. doi: 10.1038/sj.cdd.4401260. [DOI] [PubMed] [Google Scholar]
- 18.Perfettini JL, Roumier T, Kroemer G. Mitochondrial fusion and fission in the control of apoptosis. Trends Cell Biol. 2005;15:179–183. doi: 10.1016/j.tcb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Sugioka R, Shimizu S, Tsujimoto Y. Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J Biol Chem. 2004;279:52726–52734. doi: 10.1074/jbc.M408910200. [DOI] [PubMed] [Google Scholar]
- 20.Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 21.Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell. 2004;15:5001–5011. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, et al. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta. 1998;1366:177–196. doi: 10.1016/s0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- 23.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 25.Green K, Brand MD, Murphy MP. Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes. 2004;53:S110–S118. doi: 10.2337/diabetes.53.2007.s110. [DOI] [PubMed] [Google Scholar]
- 26.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 27.Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome c-mediated caspase-3 activation pathway. Diabetes. 2002;51:1938–1948. doi: 10.2337/diabetes.51.6.1938. [DOI] [PubMed] [Google Scholar]
- 28.Russell JW, Golovoy D, Vincent AM, Mahendru P, Olzmann JA, Mentzer A, et al. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J. 2002;16:1738–1748. doi: 10.1096/fj.01-1027com. [DOI] [PubMed] [Google Scholar]
- 29.Detaille D, Guigas B, Chauvin C, Batandier C, Fontaine E, Wiernsperger N, et al. Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes. 2005;54:2179–2187. doi: 10.2337/diabetes.54.7.2179. [DOI] [PubMed] [Google Scholar]
- 30.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colecraft HM, Egamino JP, Sharma VK, Sheu SS. Signaling mechanisms underlying muscarinic receptor-mediated increase in contraction rate in cultured heart cells. J Biol Chem. 1998;273:32158–32166. doi: 10.1074/jbc.273.48.32158. [DOI] [PubMed] [Google Scholar]
- 32.Wong C, Jin ZG. Protein kinase C-dependent protein kinase D activation modulates ERK signaling pathway and endothelial cell proliferation by vascular endothelial growth factor. J Biol Chem. 2005;280:33262–33269. doi: 10.1074/jbc.M503198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kodali RB, Kim WJ, Galaria II, Miller C, Schecter AD, Lira SA, et al. CCL11 (Eotaxin) induces CCR3-dependent smooth muscle cell migration. Arterioscler Thromb Vasc Biol. 2004;24:1211–1216. doi: 10.1161/01.ATV.0000131654.90788.f5. [DOI] [PubMed] [Google Scholar]
- 34.Huang P, Yu T, Yoon Y. Mitochondrial clustering induced by overexpression of the mitochondrial fusion protein Mfn2 causes mitochondrial dysfunction and cell death. Eur J Cell Biol. 2007;86:289–302. doi: 10.1016/j.ejcb.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Petronilli V, Di Lisa F, Bernardi P. Commitment to apoptosis by GD3 ganglioside depends on opening of the mitochondrial permeability transition pore. J Biol Chem. 1999;274:22581–22585. doi: 10.1074/jbc.274.32.22581. [DOI] [PubMed] [Google Scholar]
- 36.Koopman WJ, Verkaart S, Visch HJ, van der Westhuizen FH, Murphy MP, van den Heuvel LW, et al. Inhibition of complex I of the electron transport chain causes O2−. - mediated mitochondrial outgrowth. Am J Physiol Cell Physiol. 2005;288:C1440–C1450. doi: 10.1152/ajpcell.00607.2004. [DOI] [PubMed] [Google Scholar]
- 37.De Vos KJ, Allan VJ, Grierson AJ, Sheetz MP. Mitochondrial function and actin regulate dynamin-related protein 1-dependent mitochondrial fission. Curr Biol. 2005;15:678–683. doi: 10.1016/j.cub.2005.02.064. [DOI] [PubMed] [Google Scholar]
- 38.Hom JR, Gewandter JS, Michael L, Sheu SS, Yoon Y. Thapsigargin induces biphasic fragmentation of mitochondria through calcium-mediated mitochondrial fission and apoptosis. J Cell Physiol. 2007;212:498–508. doi: 10.1002/jcp.21051. [DOI] [PubMed] [Google Scholar]
- 39.Chan DC. Mitochondrial fusion and fission in mammals. Ann Rev Cell Dev Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- 40.Yoon Y, Pitts KR, McNiven MA. Mammalian dynamin-like protein DLP1 tubulates membranes. Mol Biol Cell. 2001;12:2894–2905. doi: 10.1091/mbc.12.9.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Remondino A, Kwon SH, Communal C, Pimentel DR, Sawyer DB, Singh K, et al. Beta-adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of the mitochondrial pathway. Circ Res. 2003;92:136–138. doi: 10.1161/01.res.0000054624.03539.b4. [DOI] [PubMed] [Google Scholar]
- 42.Cai L, Wang J, Li Y, Sun X, Wang L, Zhou Z, et al. Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes. 2005;54:1829–1837. doi: 10.2337/diabetes.54.6.1829. [DOI] [PubMed] [Google Scholar]
- 43.Nishikawa T, Edelstein D, Brownlee M. The missing link: a single unifying mechanism for diabetic complications. Kidney Int Suppl. 2000;77:S26–S30. doi: 10.1046/j.1523-1755.2000.07705.x. [DOI] [PubMed] [Google Scholar]
- 44.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 45.Verzola D, Bertolotto MB, Villaggio B, Ottonello L, Dallegri F, Salvatore F, et al. Oxidative stress mediates apoptotic changes induced by hyperglycemia in human tubular kidney cells. J Am Soc Nephrol. 2004;15:S85–S87. doi: 10.1097/01.asn.0000093370.20008.bc. [DOI] [PubMed] [Google Scholar]
- 46.Allen DA, Harwood S, Varagunam M, Raftery MJ, Yaqoob MM. High glucose-induced oxidative stress causes apoptosis in proximal tubular epithelial cells and is mediated by multiple caspases. FASEb J. 2003;17:908–910. doi: 10.1096/fj.02-0130fje. [DOI] [PubMed] [Google Scholar]
- 47.Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;28:1456–1462. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 48.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 49.Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res. 2003;93:292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- 50.Lin Y, Berg AH, Iyengar P, Lam TK, Giacca A, Combs TP, et al. The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species. J Biol Chem. 2005;280:4617–4626. doi: 10.1074/jbc.M411863200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.