Abstract
Tetrahydrobiopterin (BH4) is an essential cofactor required for enzymatic activity of endothelial nitric oxide synthase (eNOS). Recently, it has been shown that vascular protective effects of erythropoietin (EPO) are dependent on activation of eNOS. Therefore, our objective was to characterize the effect of EPO on biosynthesis of BH4 in vascular wall. Incubation of isolated C57BL/6J mouse aortas for 18 hours with recombinant human EPO (1–50 U/ml) caused concentration-dependent increase in intracellular BH4 levels and activity of GTP-cyclohydrolase I. Maximal biosynthesis of BH4 was detected at therapeutic concentrations of 5 U/mL. Removal of the endothelium abolished EPO-induced biosynthesis of BH4 demonstrating that the vascular endothelium is a major source of BH4. Treatment with a selective phosphatidylinositol 3-kinase inhibitor wortmannin significantly reduced BH4 biosynthesis stimulated by EPO. The stimulatory effect of EPO on vascular GTP-cyclohydrolase I activity, BH4 production, and phosphorylation of eNOS was also detected in-vivo in mice treated with recombinant human EPO. These effects of EPO were abolished in Akt1-deficient mice. In addition, EPO significantly increased systolic blood pressure and number of circulating platelets in protein kinase Bα/Akt1-deficient mice. Our results demonstrate that EPO stimulates biosynthesis of BH4 in vascular endothelium and that the increase in BH4 levels is caused by de-novo biosynthesis of BH4 via phosphatidylinositol 3-kinase/Akt1 pathway. This effect is most likely designed to provide optimal intracellular concentration of cofactor necessary for EPO-induced elevation of eNOS activity.
Keywords: Tetrahydrobiopterin, endothelium, erythropoietin, GTP-cyclohydrolase I, protein kinase B, vasculature, mice
Introduction
Nitric oxide (NO) generated by endothelial nitric oxide synthase (eNOS) in vascular endothelium is a potent vasodilator and regulator of systemic blood pressure, vascular remodeling, and angiogenesis.1 Tetrahydrobiopterin (BH4) is an essential cofactor required for enzymatic activity of eNOS.1,2 The biosynthesis of BH4 is dependent on activity of the rate-limiting enzyme GTP-cyclohydrolase I (GTPCH I).3 During activation of eNOS isoform, BH4 is needed for allosteric and redox activation of its enzymatic activity.4 Indeed, inhibition of GTPCH I with 2,4-diamino-6-hydroxy-pyrimidine, results in almost complete depletion of intracellular BH4 and abolishes formation of NO in response to activation of eNOS.5
Erythropoietin (EPO) is a hypoxia-induced hormone that is essential for erythropoiesis in bone marrow mediated via activation of EPO receptors (EPOR).6 Recently, it has been demonstrated that classic homodimeric EPOR is also expressed in non-hematopoietic tissues, including endothelial cells7 indicating that EPO has direct vascular effects including NO production, inhibition of apoptosis, and stimulation of angiogenesis.8–11 In endothelial and other cells EPOR is coupled to activation of phosphatidylinositol-3 kinase (PI3-kinase)/protein kinase B (PKB or Akt) signaling pathway.12,13 PI3-kinase/Akt is a critical pathway modulating cell survival but is also a well documented activator of eNOS.14 There are three different Akt isoforms. Akt1 or PKBα is the predominant isoform in endothelial cells.15 Indeed, genetic inactivation of Akt1 (but not Akt2) impairs revascularization after ischemia.15,16 Akt2/PKBβ-deficient mice display insulin resistance and exhibit a diabetic phenotype.17 Expression of Akt3/PKBγ is undetectable in vascular tissue.16 The exact molecular mechanisms underlying beneficial vascular effects of EPO in-vivo are not well defined. Therefore, in the present study we hypothesized that EPO stimulates vascular biosynthesis of BH4 by activation of PI3-kinase/Akt1 signal transduction pathway.
Methods
Experimental Animals
Male C57BL/6J (wild-type) mice, heterozygous Akt1 (Akt1+/−) mice, homozygous Akt1 (Akt1−/−) mice (C57BL/6J-Akt1tm1Mbb), and homozygous eNOS (eNOS−/−) mice (C57BL/6J-Nos3tm1Unc) were obtained from Jackson Laboratory (Bar Harbor, ME). EPO-transgenic mice were provided by Dr. M. Gassmann (Vetsuisse Faculty, Zürich, Switzerland). Mice were maintained on standard chow with free access to drinking water. Housing facilities and all experimental protocols were approved by the Institutional Animal Care and Use Committee of the Mayo Clinic and comply with the National Institute of Health Guide for the Care and Use of Laboratory Animals. Mice were randomly distributed to a control group (PBS) and an EPO group (recombinant human EPO alpha, 1000 U/kg body weight, biweekly, s.c.; Amgen, Thousand Oaks, CA).11 After 14 days of treatment systolic blood pressure (SBP) was recorded11 and the animals were euthanized (pentobarbital, 60 mg/kg body weight, i.p.) and aortas and lungs were harvested.
In-Vitro Studies
Isolated aortic rings of wild-type mice were incubated with EPO at various concentrations in minimal essential medium for 18 hours at 37°C as described.5
Measurements of BH4, 7,8-dihydrobiopterin, and GTPCH I Enzyme Activity
Biopterin levels and GTPCH I activity were determined in fresh aortas using reverse-phase HPLC method asdescribed previously.18
Western Blot Analysis
Primary antibodies against eNOS, Ser1177-phosphorylated eNOS (Transduction Labs), Akt1, Akt2, Ser473-phosphorylated Akt1 (Upstate), GTPCH-I18, EPO-R (Santa Cruz), JAK2, Tyr1007/1008-phosphorylated JAK2 (Cell Signaling), and anti-β-actin (Sigma) were used.19
Calculations and Statistical Analysis
All results are expressed as means ± SEM and “n” indicates the number of animals from which tissues were harvested. Single values were compared by one-way ANOVA with Bonferroni’s correction for multiple comparisons. For simple comparisons between two groups, an unpaired Student’s t-test was used where appropriate. A value of P<0.05 was considered significant.
For an expanded Methods section, please see http://hyper.ahajournals.org.
Results
Stimulatory Effects of EPO on Endothelial BH4 Synthesis
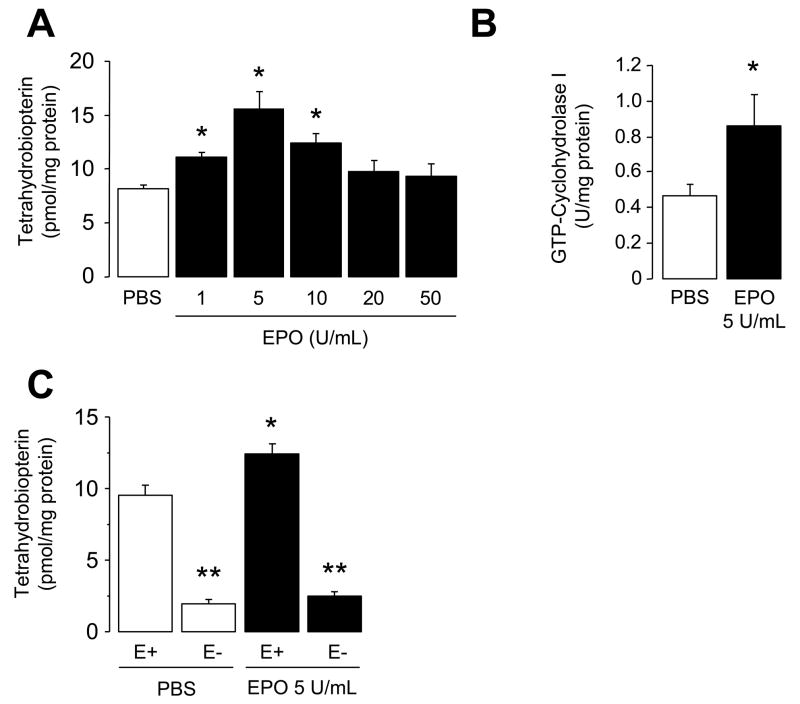
Incubation of isolated wild-type mouse aorta for 18 hours in MEM supplemented with EPO (1–50 U/ml) causes concentration-dependent increase in intracellular levels of BH4 levels with maximum biosynthesis occurring at concentrations of 5 U/mL (P<0.05; Figure 1A). Oxidative products of BH4, 7,8-dihydrobiopterin (7,8-BH2) levels, were unaffected by EPO indicating that EPO does not increase oxidation of BH4 (data not shown). In addition, GTPCH I activity is augmented by EPO (P<0.05; Figure 1B). Inhibition of GTPCH I with 2,4-diamino-6-hydroxypyrimidine (10 mmol/L) abolished the increase in BH4 levels induced by EPO (data not shown; n=3). Furthermore, removal of the endothelium prevented the stimulatory effect of EPO on BH4 biosynthesis (P<0.05; Figure 1C).
Figure 1.
Effects of in-vitro EPO treatment on enzymatic activity of GTPCH I and BH4 biosynthesis in wild-type mouse aorta. A, Bar graphs showing tetrahydrobiopterin (BH4) levels in aortas of wild-type mice after incubation with various concentrations (1–50 U/mL) of EPO for 18 hours (n=6–8). Maximal BH4 biosynthesis occurred at 5 U/mL EPO. B, GTP-cyclohydrolase I (GTPCH I) enzymatic activity in isolated aorta after 18 hours exposure to 5 U/mL EPO (n=8). C, Effect of endothelial removal on BH4 levels in the aortas of wild-type mice. Please note that removal of the endothelium (E-) eliminated stimulatory effects of EPO on BH4 biosynthesis in the aorta of wild-type (n=5). * P<0.05 vs. PBS treated aortas; ** P<0.05 vs. with endothelium (ANOVA with Bonferroni’s). E+ indicates aorta with endothelium.
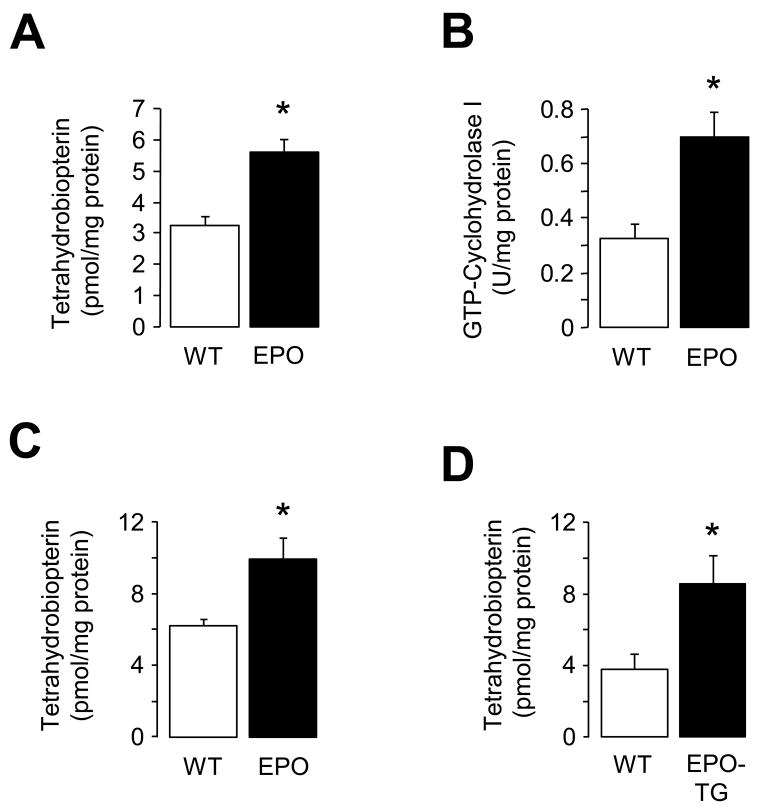
We next investigated whether EPO increases vascular BH4 production under in vivo conditions. Treatment with EPO for 3 days increased GTPCH I enzymatic activity and biosynthesis of BH4 in the aorta of wild-type mice (P<0.05; Figure 2A and 2B). Levels of 7,8-BH2 were low in the aorta of wild-type mice (0.3±0.2 pmol/mg; n=5) and they were unaffected by EPO. Similar stimulatory effects of EPO on BH4 biosynthesis were observed in wild-type mice treated with EPO for 14 days (P<0.05; Figure 2C) or in EPO-transgenic mice (P<0.05; Figure 2D).
Figure 2.
Effects of in-vivo EPO treatment on enzymatic activity of GTPCH I and BH4 biosynthesis in wild-type mouse aorta. A, Tetrahydrobiopterin (BH4) levels after EPO treatment for 3 days (n=5–7). B, Enzymatic activity of GTPCH I after EPO treatment for 3 days (n=4–6). C, BH4 levels after EPO treatment for 2 weeks (n=7). D, BH4 levels in EPO-transgenic (EPO-TG) mice (n=8–9). Results are mean ± SEM. * P<0.05 vs. wild-type mice (unpaired t-test). WT indicates wild-type.
Mechanisms of EPO-Induced BH4 Synthesis
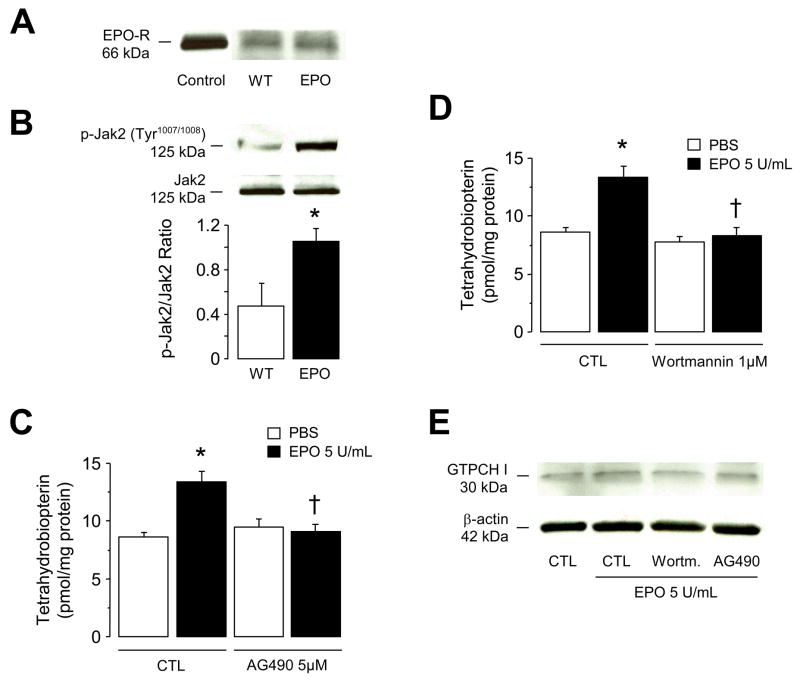
We investigated signal transduction pathways coupling endothelial EPOR to BH4 metabolism. We here provide the first evidence showing that EPOR is expressed in mouse aorta and that expression of EPOR was not affected by EPO treatment for 2 weeks (Figure 3A). Further analysis demonstrated that, EPO increased phosphorylation of Janus kinase 2 (JAK2) at Tyr1007/1008, the key kinase in the signal transduction pathways activated by EPOR20 (P<0.05; Figure 3B). Indeed, in-vitro treatment of intact isolated mouse aortas with a JAK2 inhibitor AG490 (5 μmol/L) inhibited stimulatory effect of EPO on BH4 (P<0.05; Figure 3C) indicating that in vascular endothelium, coupling between EPOR and JAK2 is an important step in control of BH4 metabolism. We next analyzed downstream signaling pathways such as PI3-kinase/Akt, protein kinase C, as well as signal transducer and activator of transcription factors (STAT). In isolated mouse aortas, inhibition of PI3-kinase by wortmannin (1 μmol/L) significantly diminished EPO-induced BH4 biosynthesis (P<0.05; Figure 3D). Wortmannin alone had no effect on BH4 metabolism (Figure 3D), suggesting that stimulatory effect of EPO is dependent on activation of PI3-kinase. Furthermore, GTPCH I protein expression was unchanged in mouse aorta after in-vitro incubation with EPO and in the presence of wortmannin or AG490 (Figure 3E). On the other hand, incubation of aortas with parthenolide (5 μmol/L), a specific inhibitor of STAT, with general protein kinase inhibitor chelerythrine (3 μmol/L), or with casein kinase 2 (CK2) inhibitor TBB (5 μmol/L) did not abolish EPO-induced BH4 biosynthesis (data not shown; n=5–9).
Figure 3.
Mechanisms underlying increased BH4 biosynthesis induced by EPO. A, Representative Western blot analysis of EPO receptor (EPOR) in mouse aorta. Please note that EPOR is present in the aorta and was unaffected after 2 weeks treatment with EPO. Lysate derived from mouse spleen was loaded as control. B, Two weeks treatment with EPO increased protein expression of Tyr1007/1008-phosphorylated JAK2 in wild-type mouse lung. The bar graph indicates the results of the relative densitometry compared with JAK2 protein (n=4 independent experiments). C through E, isolated wild-type mouse aortas were pretreated in-vitro for 1 hour with 5 μmol/L of JAK2-inhibitor AG490, 1 μmol/L of PI3-K inhibitor wortmannin, or control vehicle (CTL; 0.05% DMSO). Following treatments 5 U/mL of EPO was added and incubated for additional 18 hours. C, Tetrahydrobiopterin (BH4) levels after treatment with AG490 (n=6–7). D, BH4 levels after treatment with wortmannin (n=6–7). E, Representative Western blot analysis of GTPCH I protein expressions in mouse aortas (n=3 independent experiments). Results are mean ± SEM. * P<0.05 vs. control wild-type mice; † P<0.05 vs. EPO-treated mouse aortas (ANOVA with Bonferroni’s).
Characteristics of Akt1-Deficient Mice
Number of red blood cell along with hematocrit and hemoglobin were increased following the administration of EPO for 14 days in wild-type, Akt1+/−, and Akt1−/− mice (P<0.05; Table 1) but there was no difference between EPO treated wild-type, Akt1+/−, and Akt1−/− mice. EPO treatment for 14 days did not increase number of circulating white blood cells (Table 1). Number of platelets was significantly increased in EPO-treated Akt1−/− mice (P<0.05; Table 1). Furthermore, EPO significantly increases SBP in Akt1−/−mice compared to wild-type and Akt1+/− mice after 14 days of treatment (P<0.05; Table 1).
Table 1.
Effect of 2 weeks treatment with EPO on blood cells profile and systolic blood pressure in wild-type and Akt1-deficient mice.
| Parameters | C57BL/6J | C57BL/6J + EPO | Akt1+/− + EPO | Akt1−/− + EPO |
|---|---|---|---|---|
| White blood cells (103/mm3) | 10.4±0.6 | 11.8±0.8 | 9.3±0.2 | 10.4±0.5 |
| Lymphocytes (103/mm3) | 9.6±0.5 | 10.9±0.7 | 8.4±0.2 | 9.5±0.4 |
| Monocytes (103/mm3) | 0.4±0.1 | 0.7±0.2 | 0.1±0.1 | 0.3±0.1 |
| Granulocytes (103/mm3) | 0.5±0.1 | 0.5±0.1 | 0.8±0.1 | 0.7±0.2 |
| Red blood cells (106/mm3) | 9.9±0.3 | 12.8±0.4 * | 13.1±0.4 * | 13.6±0.5 * |
| Hematocrit (%) | 42.1±0.8 | 54.8±1.1 * | 57.1±1.6 * | 52.8±1.6 * |
| Hemoglobin (g/dL) | 15.1±0.2 | 18.9±0.3 * | 21.1±0.5 * | 17.0±0.5 * |
| Platelets (103/mm3) | 754±39 | 766±32 | 768±79 | 1049±81 *† |
| SBP (mmHg) | 115±4 | 123±2 | 119±2 | 125±2 * |
C57BL/6J indicates wild-type mice; EPO, erythropoietin; SBP, systolic blood pressure. Data are means ± SEM (n=5–8).
P<0.05 vs. C57BL/6J mice;
P<0.05 vs. EPO-treated C57BL/6J and Akt1+/− mice (ANOVA + Bonferroni’s).
Effects of EPO on BH4 Synthesis in Akt1-Deficient Mice
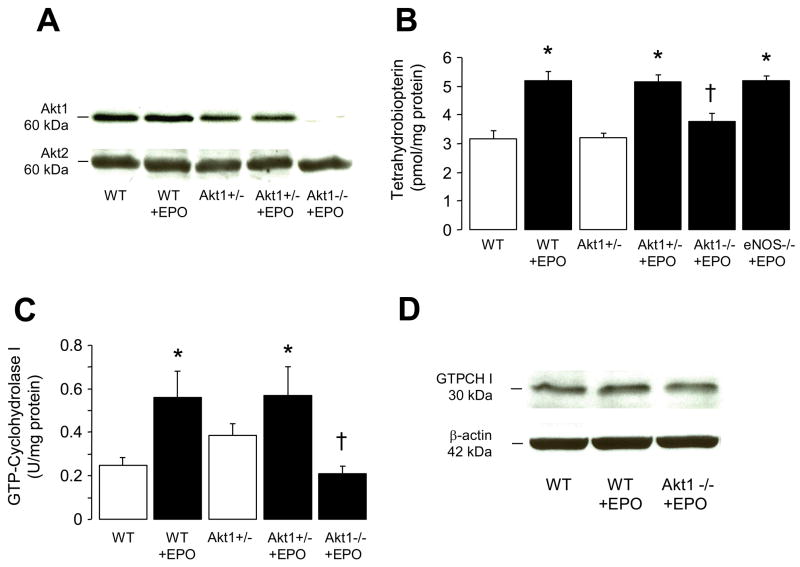
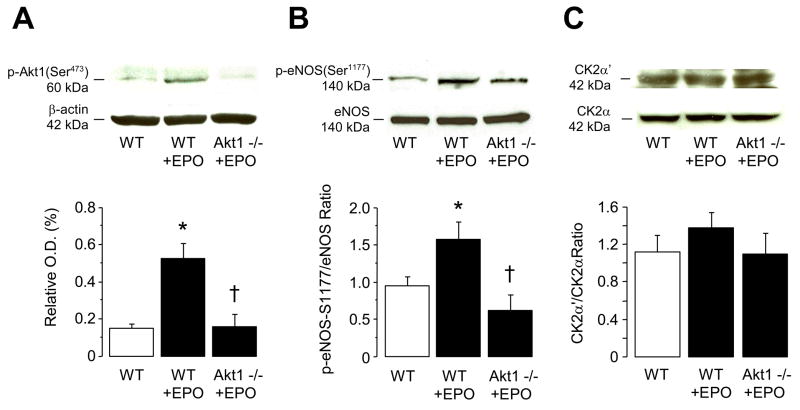
To establish whether stimulatory effect of EPO is dependent on activation of PI3-K dependent Akt phosphorylation, we performed studies on Akt1-deficient mice. Western blot analysis confirmed that Akt1 protein expression was reduced in Akt1+/− mice and was absent in Akt1−/− mice (Figure 4A), consistent with previous findings obtained on other tissues of Akt1-deficient mice.16,21 Akt2 protein expression was unchanged in Akt1−/− mice and those treated with EPO (Figure 4A). Stimulatory effect of EPO on BH4 synthesis was abolished in Akt1−/− mice but not in Akt1+/− or eNOS−/− mice aortas (P<0.05; Figure 4B). In addition, increased enzymatic activity of GTP-cyclohydrolase I by EPO treatment for 14 days was also abolished in aortas of Akt1−/− mice compared to wild-type and Akt1+/− mice (P<0.05; Figure 4C). GTPCH I protein expression was unaltered in EPO treated wild-type and Akt1−/− mice as compared with untreated wild-type mice (Figure 4D).
Figure 4.
Role of Akt1 in BH4 biosynthesis after 2 weeks treatment with EPO. A, Western blot analyses of Akt1 and Akt2 protein expressions in lung from wild-type, Akt+/−, and Akt1−/− mice (n=3 independent experiments). B, Tetrahydrobiopterin (BH4) levels in aortas of wild-type, Akt+/−, Akt1−/−, and eNOS−/− mice (n=5–7). C, GTP-cyclohydrolase I (GTPCH I) enzymatic activity in aortas of wild-type, Akt+/−, and Akt1−/− mice (n=4–6). D, Representative Western blot analysis of GTPCH I protein expression in lung from wild-type and Akt1−/− mice (n=3 independent experiments). WT indicates wild-type (C57BL/6J). Results are mean ± SEM. * P<0.05 vs. wild-type mice; † P<0.05 vs. EPO-treated mice (ANOVA with Bonferroni’s).
Two weeks treatment with EPO also increased protein expressions of phosphorlyated Akt1 at Ser473 and eNOS at Ser1177 in wild-type mice (P<0.05; Figure 5A and 5b, respectively). Maintained serine phosphorylation and activation of eNOS was prevented in Akt1−/− mice (P<0.05; Figure 5B). In contrast, EPO did not affect protein expressions of α′ and α subunits of protein kinase CK2 in wild-type and Akt1−/− mice (Figure 5C).
Figure 5.
Effect of 2 weeks treatment with EPO on A, protein expression of Ser473-phosphorylated Akt1, B, protein expression of Ser1177-phosphorylated eNOS, and C, protein expression of α′ and α subunits of casein kinase 2 (CK2) in lung of wild-type and Akt−/− mice. The bar graphs indicate the results of the relative densitometry. Data are shown as means ± SEM (n=3–5 independent experiments). * P<0.05 vs. wild-type mice; † P<0.05 vs. EPO-treated wild-type mice (ANOVA with Bonferroni’s).
Discussion
In the present study, we report several novel findings. First, therapeutic concentrations of EPO activate vascular GTPCH I (rate-limiting enzyme in biosynthesis of BH4) thereby increasing intracellular concentration of BH4. Second, EPO stimulates production of BH4 exclusively in endothelial cells. Third, the effect of EPO is independent of shear stress imposed on vascular endothelium by elevated number of circulating red blood cells. Fourth, pharmacological and molecular genetic analysis established that activation of EPOR/PI3-kinase/Akt1 signal transduction pathway is responsible for the stimulatory effect of EPO on production of BH4. Fifth, genetic inactivation of Akt1 signaling favors prohypertensive effect of EPO. In aggregate, these findings provide new insights into the molecular mechanisms underlying non-hematopoietic vascular effects of EPO.
Prior studies from several groups including ours demonstrated that vascular protective effect of EPO is mediated by phosphorylation of Akt, eNOS, and subsequent increase in production of NO.9,11,13 Since enzymatic activity of eNOS is critically dependent on availability of BH4 it was of major interest to determine the effect of EPO on metabolism of BH4. Our results demonstrate that EPO causes concentration-dependent increase in intracellular levels of BH4 with the maximal BH4 biosynthesis detected at therapeutic concentration of 5 U/ml. Pharmacological inhibition of GTPCH I abolished stimulatory effect of EPO on BH4 thereby reinforcing conclusion regarding the importance of GTPCH I activation as a major molecular mechanism responsible for the EPO effect. We and others have demonstrated that endothelium is a major source of BH4 in intact mouse aorta.18,22 Consistent with these observations, mechanical removal of endothelial cells abolished stimulatory effect of EPO on BH4 biosynthesis thus demonstrating that activation of endothelial cells by EPO results in increased availability of BH4. Detected increase in BH4 levels in arteries treated with EPO is quantitatively similar to the levels reported in genetically modified mice over-expressing GTPCH I specifically in endothelial cells.23,24 Indeed, a number of prior studies demonstrated that elevation of BH4 concentration in vascular endothelium detected in our experiments is vascular protective, and can prevent endothelial dysfunction induced by hypercholesterolemia, diabetes, and hypertension.22–24
Recently, our group reported that in vivo increase in blood flow causes up-regulation of GTPCH I activity in arterial wall.25 This finding was corroborated by in vitro studies demonstrating stimulatory effect of shear stress on BH4 production in cultured endothelial cells.26 Since EPO increases number of circulating red blood cells thereby causing elevation of shear stress imposed by the circulating blood on the endothelial layer of blood vessel27, it was important to rule out the possibility that the effect of EPO is mediated indirectly by changes in shear stress rather than direct activation of EPOR. Therefore, we performed experiments on mice treated with EPO only for three days. Short term exposure to EPO does not affect production of red blood cells11 thus excluding increased shear stress as an explanation for the observed effects of EPO. Indeed, elevation of BH4 observed three days after treatment with EPO was not significantly different from the elevation detected in aorta of mice treated with EPO for two weeks. Based on these observations, we concluded that EPO has direct stimulatory effect on GTPCH I activity and BH4 biosynthesis in vascular endothelium, and that this effect is independent of hematopoietic effects of EPO. This conclusion is further reinforced by the fact that the promoter region of the mouse GTPCH I28 does not have shear stress response elements.29 In addition, in our previous study we demonstrated that EPO treatment for 3 or 14 days stimulated phosphorylation of eNOS in the arterial wall to a similar degree11 thus demonstrating that high number of red blood cells and subsequent elevation of shear stress are not responsible for the effect of EPO.
Expression and activation of EPOR on endothelium is well established.7,30 Furthermore, it has been shown that EPO induces homodimerization of cell surface EPOR with subsequent autophosphorylation and activation of the receptor associated JAK2 tyrosine kinase leading to activation of several downstream cellular pathways including PI3-kinase/Akt, STAT, and protein kinase C.6,20 Of interest, we detected higher expression of phosphorylated JAK2 in EPO treated mice suggesting that increased phosphorylation and activation of JAK2 is the molecular mechanisms underlying observed effect of EPO on BH4 synthesis. Indeed, pharmacological inhibition of JAK2 with AG490 abolished EPO-induced BH4 biosynthesis. Existing literature indicates that in endothelial and other cells EPOR/JAK2 is coupled to activation of PI3-kinase/Akt signaling pathway.12,13,31 Our study showed that PI3-kinase activity was an upstream activator of Akt1 because pharmacological and genetic inactivation of PI3-kinase/Akt1 abolished the stimulatory effects of EPO on GTPCH I activity and biosynthesis of BH4 in mouse aorta. These observations provide new insights into the molecular mechanisms responsible for EPO-induced elevation of vascular BH4. Inspection of mouse GTPCH I protein sequence (NCBI accession number NP032128) revealed that in contrast to eNOS, GTPCH I does not contain the consensus sequence for phosphorylation by Akt1 (RXRXXS/T).32 Most recently, it has been reported that GTPCH I possesses 6 consensus sequences for phosphorylation by CK2.26 Furthermore, it has been shown that shear stress-induced activation of GTPCH I is mediated through activation of catalytic subunits of CK2α′ in endothelial cells.26 However, in our study we could not detect any effect of EPO on protein expressions ofα′ and α subunits of CK2, whereas inhibition of CK2 failed to prevent EPO-induced BH4 synthesis. Thus, the exact molecular mechanisms responsible for the Akt1-mediated activation of GTPCH I remain to be determined.
We also wish to point out that treatment with EPO caused significant increase in arterial blood pressure and number of circulating platelets in Akt1-deficient mice. This observation may help to explain adverse cardiovascular effects of EPO including hypertension and thrombosis.11,33 Although the analysis of the exact mechanism of EPO-induced hypertension is beyond the scope of our study, we wish to point out that a number of prior studies demonstrated an important role of BH4 in pathogenesis of hypertension and attendant dysfunction of endothelium.22,24,34–36 The clinical importance of impaired EPOR/Akt1 signal transduction pathway in development of adverse cardiovascular effects of EPO requires further studies.
Perspectives
Akt1 is an important protein responsible for eNOS activation in response to stimulation of several signal transduction pathways. Akt1 phosphorylates and activates eNOS whereas impaired Akt1 kinase activity results in endothelial dysfunction. Furthermore, it is well established that BH4 is critical co-factor for enzymatic activity of eNOS by which NO is generated in endothelium, and that the vascular protective effects of EPO are dependent on Akt1-induced phosphorylation and activation of eNOS. The results of the present study provide the first direct evidence that EPO stimulates BH4 synthesis through the de novo synthetic pathway involving activation of GTPCH I via PI3-kinase/Akt1 signaling pathway. The ability of EPO to upregulate GTPCH I activity and phosphorylation of eNOS in coordinated fashion is most likely designed to optimize the production of NO in vascular endothelium. Our results also suggest that impaired Akt1 signaling is an important mechanism underlying pro-hypertensive effect of EPO.
Acknowledgments
The authors would like to thank Suzanne M. Greiner for assistance with blood cell counts.
Source of Funding
This work was supported by National Institutes of Health grant HL-53524, by Roche Foundation for Anemia Research, and by the Mayo Foundation. Dr. d’Uscio is the recipient of Scientist Development Grant from the American Heart Association (07-30133N).
Footnotes
Conflict on Interest Disclosures
None
References
- 1.Pollock JS, Förstermann U, Mitchell JA, Warner TD, Schmidt HH, Nakane M, Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991;88:10480–10484. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt K, Werner ER, Mayer B, Wachter H, Kukovetz WR. Tetrahydrobiopterin-dependent formation of endothelium-derived relaxing factor (nitric oxide) in aortic endothelial cells. Biochem J. 1992;281:297–300. doi: 10.1042/bj2810297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nichol CA, Smith GK, Duch DS. Biosynthesis and metabolism of tetrahydrobiopterin and molybdopterin. Annu Rev Biochem. 1985;54:729–764. doi: 10.1146/annurev.bi.54.070185.003501. [DOI] [PubMed] [Google Scholar]
- 4.Raman CS, Li H, Martasek P, Kral V, Masters BS, Poulos TL. Crystal structure of constitutive endothelial nitric oxide synthase: a paradigm for pterin function involving a novel metal center. Cell. 1998;95:939–950. doi: 10.1016/s0092-8674(00)81718-3. [DOI] [PubMed] [Google Scholar]
- 5.Kinoshita H, Milstien S, Wambi C, Katusic ZS. Inhibition of tetrahydrobiopterin biosynthesis impairs endothelium-dependent relaxations in canine basilar artery. Am J Physiol. 1997;273:H718–H724. doi: 10.1152/ajpheart.1997.273.2.H718. [DOI] [PubMed] [Google Scholar]
- 6.Richmond TD, Chohan M, Barber DL. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol. 2005;15:146–155. doi: 10.1016/j.tcb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Anagnostou A, Liu Z, Steiner M, Chin K, Lee ES, Kessimian N, Noguchi CT. Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci U S A. 1994;91:3974–3978. doi: 10.1073/pnas.91.9.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher JW. Erythropoietin: physiology and pharmacology update. Exp Biol Med (Maywood) 2003;228:1–14. doi: 10.1177/153537020322800101. [DOI] [PubMed] [Google Scholar]
- 9.Santhanam AV, Smith LA, Akiyama M, Rosales AG, Bailey KR, Katusic ZS. Role of endothelial NO synthase phosphorylation in cerebrovascular protective effect of recombinant erythropoietin during subarachnoid hemorrhage-induced cerebral vasospasm. Stroke. 2005;36:2731–2737. doi: 10.1161/01.STR.0000190021.85035.5b. [DOI] [PubMed] [Google Scholar]
- 10.Urao N, Okigaki M, Yamada H, Aadachi Y, Matsuno K, Matsui A, Matsunaga S, Tateishi K, Nomura T, Takahashi T, Tatsumi T, Matsubara H. Erythropoietin-mobilized endothelial progenitors enhance reendothelialization via Akt-endothelial nitric oxide synthase activation and prevent neointimal hyperplasia. Circ Res. 2006;98:1405–1413. doi: 10.1161/01.RES.0000224117.59417.f3. [DOI] [PubMed] [Google Scholar]
- 11.d’Uscio LV, Smith LA, Santhanam AV, Richardson D, Nath KA, Katusic ZS. Essential role of endothelial nitric oxide synthase in vascular effects of erythropoietin. Hypertension. 2007;49:1142–1148. doi: 10.1161/HYPERTENSIONAHA.106.085704. [DOI] [PubMed] [Google Scholar]
- 12.Miura O, Nakamura N, Ihle JN, Aoki N. Erythropoietin-dependent association of phosphatidylinositol 3-kinase with tyrosine-phosphorylated erythropoietin receptor. J Biol Chem. 1994;269:614–620. [PubMed] [Google Scholar]
- 13.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106:2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- 14.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Somanath PR, Razorenova O, Chen WS, Hay N, Bornstein P, Byzova TV. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188–1196. doi: 10.1038/nm1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 18.d’Uscio LV, Katusic ZS. Increased vascular biosynthesis of tetrahydrobiopterin in apolipoprotein E-deficient mice. Am J Physiol Heart Circ Physiol. 2006;290:H2466–H2471. doi: 10.1152/ajpheart.00366.2005. [DOI] [PubMed] [Google Scholar]
- 19.d’Uscio LV, Milstien S, Richardson D, Smith L, Katusic ZS. Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circ Res. 2003;92:88–95. doi: 10.1161/01.res.0000049166.33035.62. [DOI] [PubMed] [Google Scholar]
- 20.Witthuhn BA, Quelle FW, Silvennoinen O, Yi T, Tang B, Miura O, Ihle JN. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993;74:227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- 21.Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, Kadowaki T, Hay N. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alp NJ, Mussa S, Khoo J, Cai S, Guzik T, Jefferson A, Goh N, Rockett KA, Channon KM. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I overexpression. J Clin Invest. 2003;112:725–735. doi: 10.1172/JCI17786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du YH, Guan YY, Alp NJ, Channon KM, Chen AF. Endothelium-specific GTP cyclohydrolase I overexpression attenuates blood pressure progression in salt-sensitive low-renin hypertension. Circulation. 2008;117:1045–1054. doi: 10.1161/CIRCULATIONAHA.107.748236. [DOI] [PubMed] [Google Scholar]
- 25.Lam CF, Peterson TE, Richardson DM, Croatt AJ, d’Uscio LV, Nath KA, Katusic ZS. Increased blood flow causes coordinated upregulation of arterial eNOS and biosynthesis of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol. 2006;290:H786–793. doi: 10.1152/ajpheart.00759.2005. [DOI] [PubMed] [Google Scholar]
- 26.Widder JD, Chen W, Li L, Dikalov S, Thony B, Hatakeyama K, Harrison DG. Regulation of tetrahydrobiopterin biosynthesis by shear stress. Circ Res. 2007;101:830–838. doi: 10.1161/CIRCRESAHA.107.153809. [DOI] [PubMed] [Google Scholar]
- 27.Boulanger CM, Amabile N, Guerin AP, Pannier B, Leroyer AS, Mallat CN, Tedgui A, London GM. In vivo shear stress determines circulating levels of endothelial microparticles in end-stage renal disease. Hypertension. 2007;49:902–908. doi: 10.1161/01.HYP.0000259667.22309.df. [DOI] [PubMed] [Google Scholar]
- 28.Ichinose H, Ohye T, Matsuda Y, Hori T, Blau N, Burlina A, Rouse B, Matalon R, Fujita K, Nagatsu T. Characterization of mouse and human GTP cyclohydrolase I genes. Mutations in patients with GTP cyclohydrolase I deficiency. J Biol Chem. 1995;270:10062–10071. doi: 10.1074/jbc.270.17.10062. [DOI] [PubMed] [Google Scholar]
- 29.Resnick N, Gimbrone MA., Jr Hemodynamic forces are complex regulators of endothelial gene expression. Faseb J. 1995;9:874–882. doi: 10.1096/fasebj.9.10.7615157. [DOI] [PubMed] [Google Scholar]
- 30.Beleslin-Cokic BB, Cokic VP, Yu X, Weksler BB, Schechter AN, Noguchi CT. Erythropoietin and hypoxia stimulate erythropoietin receptor and nitric oxide production by endothelial cells. Blood. 2004;104:2073–2080. doi: 10.1182/blood-2004-02-0744. [DOI] [PubMed] [Google Scholar]
- 31.Ruscher K, Freyer D, Karsch M, Isaev N, Megow D, Sawitzki B, Priller J, Dirnagl U, Meisel A. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci. 2002;22:10291–10301. doi: 10.1523/JNEUROSCI.22-23-10291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Obata T, Yaffe MB, Leparc GG, Piro ET, Maegawa H, Kashiwagi A, Kikkawa R, Cantley LC. Peptide and protein library screening defines optimal substrate motifs for AKT/PKB. J Biol Chem. 2000;275:36108–36115. doi: 10.1074/jbc.M005497200. [DOI] [PubMed] [Google Scholar]
- 33.Lindenblatt N, Menger MD, Klar E, Vollmar B. Darbepoetin-alpha does not promote microvascular thrombus formation in mice: role of eNOS-dependent protection through platelet and endothelial cell deactivation. Arterioscler Thromb Vasc Biol. 2007;27:1191–1198. doi: 10.1161/ATVBAHA.107.141580. [DOI] [PubMed] [Google Scholar]
- 34.Hong HJ, Hsiao G, Cheng TH, Yen MH. Supplemention with tetrahydrobiopterin suppresses the development of hypertension in spontaneously hypertensive rats. Hypertension. 2001;38:1044–1048. doi: 10.1161/hy1101.095331. [DOI] [PubMed] [Google Scholar]
- 35.Cosentino F, Patton S, d’Uscio LV, Werner ER, Werner-Felmayer G, Moreau P, Malinski T, Luscher TF. Tetrahydrobiopterin alters superoxide and nitric oxide release in prehypertensive rats. J Clin Invest. 1998;101:1530–1537. doi: 10.1172/JCI650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porkert M, Sher S, Reddy U, Cheema F, Niessner C, Kolm P, Jones DP, Hooper C, Taylor WR, Harrison D, Quyyumi AA. Tetrahydrobiopterin: a novel antihypertensive therapy. J Hum Hypertens. 2008 doi: 10.1038/sj.jhh.1002329. In press. [DOI] [PubMed] [Google Scholar]