Abstract
Background/Aims
Liver transplantation usually cures hepatocellular carcinoma when the Milan selection criteria are applied, whereas there is substantial risk of posttransplant recurrence with tumors beyond these criteria. This study uses molecular data to identify a subgroup of patients who, despite having hepatocellular carcinoma beyond Milan criteria, have favorable outcomes.
Methods
Allelic imbalance of 18 microsatellites was analyzed in 70 consecutive patients (35 within Milan, 35 beyond Milan criteria) transplanted for hepatocellular carcinoma of whom 24 recurred and 46 survived at least 5 years recurrence-free. Fractional allelic imbalance (the fraction of significant microsatellites that demonstrated allelic imbalance) and relevant clinical/pathological variables were tested for correlation with time to recurrence.
Results
Allelic imbalance in 9/18 microsatellites correlated with recurrence. Fractional allelic imbalance >0.27 and macrovascular invasion were independent predictors of recurrence in patients with tumors beyond Milan criteria; the probability of recurrence at 5 years was 85% with fractional allelic imbalance ≥0.27 vs 10% when <0.27 (p=0.0002). An algorithm including Milan criteria and fractional allelic imbalance status is 89% accurate in predicting tumor recurrence after transplantation.
Conclusion
Analysis of allelic imbalance of 9 microsatellites identifies a subgroup of patients who, despite having hepatocellular carcinoma beyond Milan criteria, have a low risk of posttransplant recurrence.
Keywords: Hepatocellular carcinoma, allelic imbalance, liver transplantation extended indications, biomarkers
Introduction
Hepatocellular carcinoma (HCC) is a major health problem worldwide (1), and liver transplantation (LT) has come to play an important role in its management. Risk estimation of postsurgical tumor recurrence is an essential element in selecting patients with HCC for LT. In current clinical practice, this estimation is based on the size and number of tumor nodules and the presence of macroscopic vascular invasion (VI) as defined on preoperative imaging studies. The empirical rule proposed by the Milan group for selection of patients -one nodule ≤ 5cm or 2–3 nodules all ≤ 3cm, and without macroscopic VI-has been shown to provide survival rates above 70% at 5 years with ~10% likelihood of recurrence (2). These so-called Milan criteria have been validated by several groups and are widely employed for candidate selection in the US and Europe alike (3, 4). Despite their proven utility, however, it is well-recognized that some patients with tumors that exceed these criteria are also potentially curable by LT (5).
Efforts to refine recurrence prediction based on preoperatively available clinical and radiological variables (6) have led to the current realization that tumor size and number are imperfect surrogates for predicting HCC metastatic potential. Increasingly, attention is being turned to molecular data for insights into tumor behavior. Gene expression studies using microarray analysis to define global expression patterns are beginning to yield potentially useful information, but remain preliminary (7–10).
Losses and /or gains of chromosomal DNA at tumor suppressor and/or oncogene loci have long been recognized as an important element in tumorigenesis and cancer progression (11), and analysis of allelic imbalance (AI) of microsatellites (MS) situated near these loci is a method to detect such copy number change. This study, based on the detection of AI of a set of previously defined MS(12), defines molecular markers that can predict tumor recurrence in patients with HCC beyond Milan criteria, thus providing a rationale for expanding the conventional indications published in HCC management guidelines(13).
MATERIALS AND METHODS
Between September 1990 and December 1997, 160 patients with HCC underwent LT at Mount Sinai. Patients who died in <5 years without evidence of HCC recurrence (n=45) were excluded. Among the 115 patients remaining, 70 were included in the present study (35 within Milan, and 35 beyond Milan criteria), while the remainder were excluded for technical reasons including lack of sufficient pathological material or adequate tumor DNA suitable for analysis due to pre-LT chemoembolization.
The primary aim of the study was to identify molecular markers of HCC recurrence after LT among the 18 MS chosen as informative markers in our previous analyses (12). The 18 MS analyzed represent loci from 9 chromosomes within 17 distinct cytogenetic bands.
This study was approved by the Institutional Review Boards at The Mount Sinai Hospital and the University of Pittsburgh.
Clinical data
Patient characteristics are summarized in Table 1. Age, sex, underlying liver disease, tumor size and number, within vs. beyond Milan criteria (based on pathology; many cases predated the availability of helical computerized tomography), alpha-fetoprotein (AFP), VI (none vs. micro-vs. macroscopic), and histologic grade (well vs. moderately vs. poorly-differentiated; data unavailable in two cases), as well as date of LT, date of recurrence, and date of death, were recorded for all patients.
Table 1.
Patient and tumor characteristics (n=70)
| Age | 55.3 ± 10.4 | |
| Sex | ||
| Male | 54 | |
| Female | 16 | |
| Underlying disease: | ||
| Hepatitis C | 37 | |
| Alcohol | 9 | |
| Cryptogenic/NASH | 9 | |
| Hepatitis B | 8 | |
| Other | 7 | |
| Number of Tumor Nodules | ||
| 1 | 31 | |
| 2 | 15 | |
| 3 | 6 | |
| >3 | 18 | |
| Vascular Invasion | ||
| None | 26 | |
| Microscopic | 32 | |
| Macroscopic | 12 | |
| Largest Tumor Diameter | ||
| ≤5cm | 54 | |
| > 5cm | 16 | |
| mean: 4.0 ± 2.8 cm | ||
| Histologic Differentiation Grade * | ||
| Well | 37 | |
| Moderate | 23 | |
| Poor | 8 | |
| Milan Criteria | ||
| Within Criteria | 35 | |
| Beyond Criteria | 35 | |
2 cases with missing histologic differentiation data
DNA analysis
Microdissection and AI experiments were done according to previously published methods (12, 14). Briefly, paired samples of tumor and non-tumor liver were prepared from each patient. Genomic DNA was isolated from tissue microdissected manually under stereomicroscopic observation and amplified via PCR with fluorescent-labeled oligonucleotides that flank the MS. PCR products were separated by capillary electrophoresis (ABI 310: Applied Biosystems) and the ratio of the two alleles for each MS was determined using GeneScan software (Applied Biosystems, Foster City, CA). Manual microdissection was employed in preference to the more cumbersome laser-capture technology based on side-by-side comparison in our laboratory that failed to demonstrate any advantage to LCM in this setting. In addition to analysis of HCC(12, 14, 15), the techniques employed in the current work have been previously validated in a variety of other tumors including lung (16), ovary (17), bone (18), hemangioblastoma (19), thyroid (20), pancreas(21), meningioma (22), melanoma (23), neuroendocrine (24), and esophagus (25).
In each patient, MS found to be homozygous in non-tumor liver were designated as noninformative for that patient. MS demonstrating heterozygosity in non-tumor liver were scored as showing AI in the tumor when the ratio of the individual allele peaks in the tumor fell outside the range of 0.66 to 1.50. Replicate analysis was performed in every case, with concordance of 85–100% and a standard deviation varying among patients from 0.06 to 0.2.
Statistical analysis
The primary endpoint was time to recurrence. Baseline characteristics of patients are expressed as mean ± SD. Length of follow-up and time to recurrence are expressed as median (range). Follow-up was computed from the date of LT until recurrence or the last visit before December 31st, 2007.
Each MS was analyzed individually for association of AI with time to recurrence using the log-rank test. Multivariate analysis of AI data is complicated by the fact that each MS is noninformative in some cases, and thus conventional Cox proportional hazard analysis cannot be applied. In order to overcome this issue and to provide an index of the overall level of genomic instability, fractional AI (FAI) was calculated for each patient based on the results of those MS that demonstrated significant correlation with time to recurrence. FAI was defined as the ratio of the number of MS demonstrating AI to the total number of informative MS. A receiver operating characteristic (ROC) curve was constructed to determine the optimal FAI cut-off value (discriminatory value: 0.27).
Univariate analysis of the associations of clinical/pathological variables including Milan criteria (within vs. beyond), AFP (< 1000 vs. ≥ 1000 ng/ml, cut-off determined by ROC curve); grade (well/moderately vs. poorly differentiated), VI (none vs. microscopic vs. macroscopic), and FAI (≥0.27 vs. <0.27) with time to recurrence was carried out by comparing Kaplan-Meier survival curves using the log-rank test. Variables associated with recurrence (p < 0.05) were included in a multivariate Cox proportional hazard analysis. Similar multivariate analysis was carried out on that subset of cases with tumors beyond Milan criteria (n=35).
Sensitivity, specificity, positive and negative predictive values, and likelihood ratio were calculated for FAI as a predictor of HCC recurrence. Finally, the accuracy of a strategy to predict recurrence that incorporates the Milan criteria and the molecular data was assessed.
RESULTS
Among the 70 patients included in the study, 24 developed HCC recurrence at a median 12.5 months (5–53 months). Recurrence rates at 1 and 5 years were 13.5% and 34.8%. Initial and ultimate sites of HCC recurrence are detailed in Table 2. Median follow-up was 78.8 months (5–148 months). Among the 18 MS studied, the mean noninformative rate was 25.7%, with a range from 17.1% to 38.6%. The mean number of noninformative MS per patient was 4.6, with a range from 1–8.
Table 2.
Initial and ultimate sites of HCC recurrence (n=24)
| Liver | Extrahepatic* | Both | |
|---|---|---|---|
| At time of recurrence | 3 | 17 | 4 |
| At time of death | 2 | 13 | 9 |
Extrahepatic sites:
Lung 12
Bone 9
Adrenal 4
Lymph nodes 3
Brain 2
Peritoneum 1
Chest wall 1
Predictors of recurrence after LT
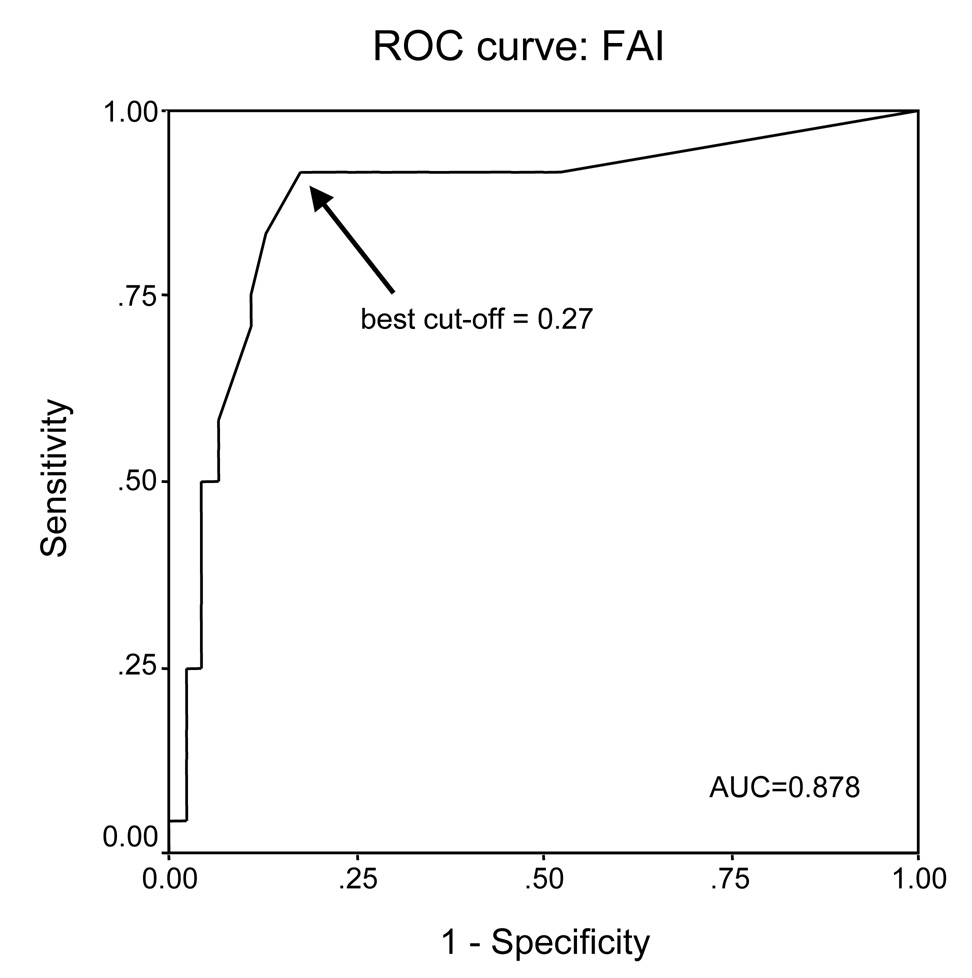
AI was significantly associated with reduced time to recurrence for 9 of the 18 MS studied (Table 3). The ROC curve used to determine the optimal cut-off for FAI to be 0.27 is shown in Figure 2; the area under the curve is 0.878.
Table 3.
Gene loci and related MS. Nine of the 18 MS tested were significantly associated with HCC recurrence.
| Locus | Gene | Microsatellite | GenBank | % non-informative | % AI overall | % AI Milan=no | %AI Milan=yes | %AI recur=yes | %AI recur=no | p value |
|---|---|---|---|---|---|---|---|---|---|---|
| 1p34.2 | L-MYC | MYCL 5NT | M19720 | 24.3% | 34.0% | 57.7% | 11.1% | 75.0% | 9.1% | <0.0001 |
| 1p36.21 | ** | D1S407 | L18040 | 28.6% | 36.0% | 53.6% | 13.6% | 60.0% | 20.0% | 0.0016 |
| 3p24.3 | OGG! | D3S2303 | L17972 | 28.6% | 34.0% | 44.0% | 24.0% | 60.0% | 22.9% | 0.0085 |
| 3p26.3 | OGG1 | D3S1539 | L16393 | 21.4% | 29.1% | 36.7% | 20.0% | 52.4% | 14.7% | 0.0026 |
| 5q21 | MCC | MCC E10 | M62397 | 37.1% | 11.4% | 14.3% | 8.7% | 21.4% | 6.7% | 0.2 |
| 5q23.1 | APC | D5S592 | L16423 | 24.3% | 34.0% | 40.0% | 28.5% | 38.9% | 31.4% | 0.57 |
| 5q23.2 | APC | D5S615 | L18737 | 24.3% | 24.5% | 31.0% | 16.7% | 36.8% | 17.7% | 0.09 |
| 7q22.3 | MET | D7S1530 | L30387 | 22.9% | 22.2% | 16.0% | 27.6% | 16.7% | 25.0% | 0.46 |
| 8q24.3 | C-MYC | D8S373 | L16320 | 25.7% | 23.1% | 33.3% | 14.3% | 41.2% | 14.3% | 0.024 |
| 9p21.1 | CDKN2A | D9S251 | L18726 | 25.7% | 17.3% | 24.0% | 11.1% | 25.0% | 13.9% | 0.29 |
| 9p23 | CDKN2A | D9S254 | L18040 | 17.1% | 31.0% | 43.3% | 17.9% | 42.9% | 24.3% | 0.12 |
| 10q23.33 | PTEN | D10S520 | L16357 | 28.6% | 20.0% | 20.8% | 19.2% | 35.7% | 13.9% | 0.06 |
| 10q23.32 | PTEN | D10S1173 | L30341 | 25.7% | 30.8% | 30.8% | 30.7% | 42.1% | 24.2% | 0.14 |
| 17p12 | TP53 | D17S1289 | G09615 | 17.1% | 10.3% | 15.2% | 4.0% | 22.7% | 2.8% | 0.0031 |
| 17p12 | TP53 | D17S974 | G07961 | 22.9% | 20.4% | 33.3% | 7.4% | 40.0% | 8.8% | 0.0006 |
| 17p13.1 | TP53 | TP53 L1 | M13121 | 38.6% | 11.6% | 11.1% | 12.0% | 11.1% | 11.8% | 0.98 |
| 17q25.1 | ** | D17S1163 | L30445 | 18.6% | 14.0% | 24.1% | 3.6% | 31.6% | 5.3% | 0.0022 |
| 18q21.33 | DCC | D18S814 | L17776 | 34.3% | 47.8% | 72.7% | 25.0% | 80.0% | 32.3% | 0.0022 |
no known tumor-related gene at these loci
Figure 2.
Receiver operating characteristic (ROC) curves, indicating the best cut-off for fractional allelic imbalance (FAI) in the cohort of 70 patients to identify recurrence. The 9 MS significantly related with time to recurrence were used to calculate this ratio, defined as the number of MS showing AI/ total number of informative MS. A cut-point ≥0.27 (arrow), has a sensitivity of 90%, specificity of 54%, and correctly classified 72% of the cases.
On univariate analysis, high FAI, macroscopic VI, tumor beyond Milan criteria, AFP ≥ 1000 ng/ml, and poor histologic grade were all significantly associated with recurrence (Table 4). The actuarial probability of recurrence at 5 years was significantly higher in patients with FAI ≥ 0.27 (n=30) than in patients with FAI < 0.27 (n=40) (75% vs. 5%, respectively; p<0.0001 (Figure 3). Multivariate analysis revealed FAI (p<.001; hazard ratio = 39.7, 95% C.I. = 5.3–299.5) and macroscopic VI (p=.004; hazard ratio = 3.6, 95% C.I = 1.5–8.6) to be independent predictors of recurrence. Overall, FAI predicted 60/70 cases correctly (86%), compared to 53/70 (76%) correctly predicted by Milan criteria; however, only 3/35 patients within Milan criteria developed recurrence.
Table 4.
Univariate analyses of predictors of recurrence in 70 patients.
| Recurrence (n=24) |
No recurrence (n=46) |
p(log-rank) | |
|---|---|---|---|
| Univariate | |||
| Histologic differentiation* | |||
| Well/moderate | 18 | 42 | .03 |
| Poor | 5 | 3 | |
| Vascular invasion | |||
| None | 1 | 23 | |
| Microscopic | 13 | 20 | <.0001** |
| Macroscopic | 10 | 3 | |
| Alpha-fetoprotein | |||
| < 1000 ng/ml | 15 | 42 | .0009 |
| ≥ 1000 ng/ml | 9 | 4 | |
| Milan Criteria | |||
| Within Criteria | 3 | 32 | <.0001 |
| Beyond Criteria | 21 | 14 | |
| FAI | |||
| < .27 | 2 | 38 | <.0001 |
| ≥ .27 | 22 | 8 | |
| Multivariate analysis | Hazard ratio (95% CI) | P | |
| FAI | 39.7 (5.3–299.5) | <0.001 | |
| Macroscopic VI | 3.6 (1.5–8.6) | 0.004 | |
2 cases with missing histologic differentiation data
None/Microscopic vs Macroscopic
Figure 3.
Actuarial probability of recurrence in 70 cases, dividing the patients according to FAI. FAI ≥ 0.27: 30 cases, 5-year recurrence rate: 75%; FAI <0.27: 40 cases, 5-year recurrence rate: 5%
Patients with HCC beyond Milan criteria were then analyzed separately; 21/35 patients in this group developed recurrence. On univariate analysis, FAI and macroscopic VI were significantly associated with recurrence (Table 5). Recurrence was noted in 20/24 patients with FAI ≥ 0.27 vs. 1/11 patients with FAI < 0.27. The actuarial probability of recurrence at 5 years was significantly higher in patients with FAI ≥ 0.27 than in patients with FAI < 0.27 (85% vs 10% respectively, p=0.0002; Figure 4). FAI predicted recurrence of HCC beyond Milan criteria with a sensitivity of 83%, specificity of 91%, PPV of 96%, NPV of 71%, overall accuracy of 86%, and a likelihood ratio of 3.3. On multivariate analysis, both FAI (p=.006; hazard ratio = 17.3, 95%C.I.= 2.3–130.5) and macroscopic VI (p=.018; hazard ratio = 3.0, 95%C.I. = 1.2–7.3) were independent predictors of recurrence.
Table 5.
Univariate and multivariate analyses of predictors of recurrence in 35 patients with tumors beyond Milan Criteria.
| Recurrence (n=21) |
No recurrence (n=14) |
p(log-rank) | |
|---|---|---|---|
| Univariate | |||
| Histologic differentiation* | |||
| Well/moderate | 15 | 13 | .08 |
| Poor | 5 | 1 | |
| Vascular invasion | |||
| None | 1 | 4 | |
| Microscopic | 10 | 8 | .007** |
| Macroscopic | 10 | 2 | |
| Alpha-fetoprotein | |||
| < 1000 ng/ml | 12 | 10 | .31 |
| ≥ 1000 ng/ml | 9 | 4 | |
| FAI | |||
| < .27 | 1 | 10 | .0002 |
| ≥ .27 | 20 | 4 | |
| Multivariate analysis | Hazard ratio (95% CI) | P | |
| FAI | 17.3 (2.3–130.5) | 0.006 | |
| Macroscopic VI | 3.0 (1.2–7.3) | 0.018 | |
1 case with missing histologic differentiation data
None/Microscopic vs Macroscopic
Figure 4.
Actuarial probability of recurrence in 35 cases exceeding Milan criteria, dividing the patients according to FAI. FAI ≥ 0.27: 24 cases, 5-year recurrence rate: 85%; FAI <0.27: 11 cases, 5-year recurrence rate: 10%.
The relationship between vascular invasion and FAI with regard to HCC recurrence is illustrated in cross-tabular form in Table 6. One of 14 patients with microscopic VI and FAI < .27 developed recurrence vs. 12/18 with FAI ≥ 0.27; 0/3 patients with macroscopic VI and FAI < .27 recurred vs. 10/10 with FAI ≥0.27.
Table 6.
Cross-tabulation of VI and FAI vs. recurrence in patients with HCC within and beyond Milan Criteria
| WITHIN MILAN CRITERIA (n=35) | ||
|---|---|---|
| Recurrence | ||
| No | Yes | |
| FAI < 0.27 (n=29) | ||
| No VI | 18 | 0 |
| Micro VI | 9 | 1 |
| Macro VI | 1 | 0 |
| FAI 0.27 ≥ (n=6) | ||
| No VI | 1 | 0 |
| Micro VI | 3 | 2 |
| Macro VI | 0 | 0 |
| BEYOND MILAN CRITERIA (n=35) | ||
| Recurrence | ||
| No | Yes | |
| FAI < 0.27 (n=11) | ||
| No VI | 3 | 1 |
| Micro VI | 5 | 0 |
| Macro VI | 2 | 0 |
| FAI ≥ 0.27 (n=24) | ||
| No VI | 1 | 0 |
| Micro VI | 3 | 10 |
| Macro VI | 0 | 10 |
Description and accuracy of the final predictive model
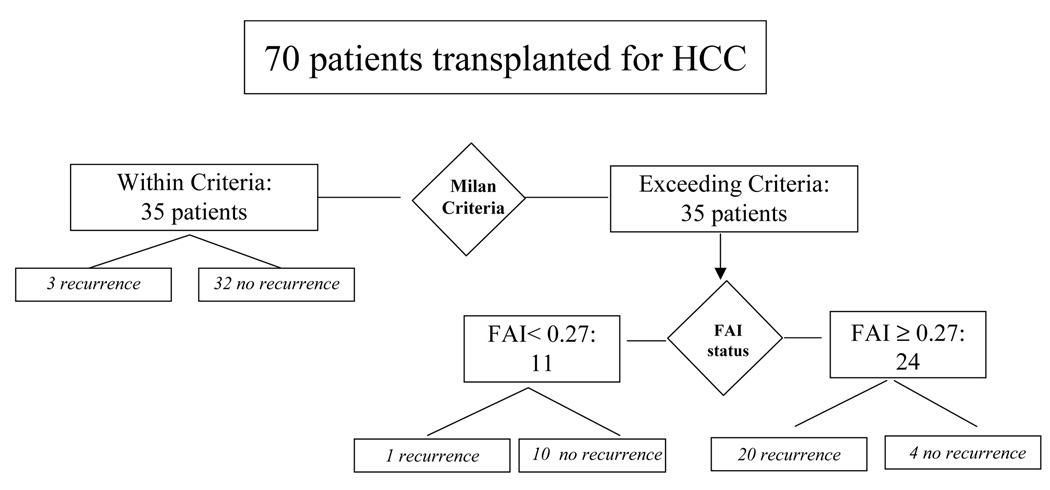
A predictive model for recurrence was created, assuming that HCC within Milan criteria will not recur, and predicting recurrence in patients with HCC beyond Milan criteria based on FAI ≥ 0.27. The model yielded a sensitivity of 83%, specificity of 91%, PPV of 83%, and NPV of 91%, an overall accuracy of 89%, and a likelihood ratio of 7.7 (Figure 5).
Figure 5.
Algorithm proposed for selection of HCC candidates for liver transplantation integrating the Milan criteria and the molecular markers. All patients with HCC meeting Milan are eligible; 3/35 developed recurrence. Patients with HCC beyond Milan are eligible if FAI <0.27; 1/11 developed recurrence. Conversely, patients with HCC beyond Milan and FAI ≥ 0.27 show an unacceptable risk of recurrence (20/24, 5-year recurrence arte of 85%).
DISCUSSION
Our findings suggest that AI analysis improves the ability to predict post-LT HCC recurrence and can be used as a tool to identify patients who, despite having HCC beyond Milan criteria, have an acceptable outcome profile. An underlying hypothesis of this work is that alterations in chromosomal DNA in regions containing tumor-associated genes will result in altered gene expression leading to altered tumor biology. Either loss of an allele of a tumor suppressor gene or increased copy number of an oncogene can result in a tumorigenic phenotype (the methodology for analysis of AI employed in this study does not distinguish loss from gain). In addition to identifying specific regions of gains and/or losses, genome-wide AI analysis can also be used as an index to assess overall genomic stability (26).
The 18 MS analyzed in this report have been previously tested in a number of cancer types including lung (16), ovarian (17), bone (18), thyroid (20), pancreatobiliary (21), brain (22), and melanoma (23). Their selection was largely based on empiric observation of an association between AI at these loci and cancer. The 9 MS for which AI correlated with HCC recurrence comprise the panel used to calculate FAI; attempts to reduce the number of MS used to calculate FAI while preserving its predictive ability were unsuccessful due to the high incidence of noninformative MS.
One of the 9 significant MS, MYCL 5NT, is located within the known oncogene l-myc on chromosome 1p34, and among all the MS tested this one had the strongest correlation with HCC recurrence (p<0.0001). The remaining MS are situated at distances ranging from 3–12 megabase pairs from known tumor suppressor genes or oncogenes; while suggestive, AI at these MS does not constitute direct evidence of copy number change of these known genes.
Among all factors examined, FAI correlated most strongly with post-LT HCC recurrence with 86% of cases correctly predicted, better than any clinical variable or combination thereof. Nevertheless, among the 35 patients with HCC within Milan criteria, 4 of 6 who were predicted to recur by FAI did not. Application of Milan criteria has been repeatedly shown to result in a low incidence of post-LT HCC recurrence (3). Our data suggest that, when discovered at an early clinical stage, even tumors with relatively high malignant potential are potentially curable with LT.
It is important to recognize that the data analyzed in this study are based on samples taken at pathological examination of whole livers; incorporation of FAI into the LT candidate selection algorithm presumes that FAI as determined on pretransplant biopsy will correlate well with findings at pathology. It is by no means clear that this is the case, and lack of validation on this point remains an important hurdle. A recent retrospective study comparing tumor grade on preoperative needle core biopsy to grade on final transplant or resection pathology in 93 patients (27) demonstrated the sensitivity of biopsy in identifying poorly-differentiated HCC to be only 35%. In this study biopsy was performed for diagnosis, for which a single core from a single nodule is adequate, and median tumor diameter was 7cm (range 2–23). The optimal parameters for performing biopsy for prognosis have not been defined. A prospective study evaluating the accuracy of laparoscopic biopsy in identifying poorly-differentiated HCC incorporating multiple samples per nodule with the number a function of tumor diameter, sampling of all nodules, and use of fine needle aspiration cytology as well as core biopsy, is currently underway at the University of Padua (Cillo U, personal communication), where tumor grade has been integrated into the LT candidate selection algorithm (28); the Padova study will provide a more realistic assessment of the ability to identify poorly-differentiated HCC on pre-LT biopsy. While it has been our aim to develop a tool to improve candidate selection, AI studies would also have relevance even if only performed after transplant to identify patients at high risk for recurrence, potentially influencing the choice of immunosuppression and the intensity of follow-up.
The data on tumor size and number used to determine whether HCC was within or beyond Milan criteria in this study were based on findings at pathology rather than on pre-LT imaging studies because many of the study patients were transplanted before the introduction at our center of arterial phase imaging via helical CT or MRI. With improved imaging technology the discrepancy between imaging and pathology findings is greatly diminished (29). Nevertheless, it remains a widely-accepted fact that current pre-LT imaging understages disease in 15–25% of cases, primarily related to the presence of additional small (<2cm) nodules (30). The clinical significance of this discrepancy is debatable in view of the excellent results repeatedly achieved when patients with HCC are transplanted based on their tumors meeting Milan criteria on pre-LT imaging. The essential conclusion of our study, that post-LT recurrence of HCC exceeding Milan criteria can be predicted using AI analysis, should apply just as well if based on radiologic data; any bias thus introduced would tend to include fewer patients in the beyond Milan criteria group, and their tumors would tend to be more advanced, than when patients are classified according to pathology results.
Vascular invasion has long been recognized as an important risk factor for HCC recurrence after resection or LT. In this study, both VI and FAI correlated with HCC recurrence, though FAI was the more significant. As can be seen in Table 5, even among patients with VI, FAI remained a strong predictor of recurrence: 1/18 patients with VI (micro- or macroscopic) who had FAI < .27 recurred, vs. 22 / 28 patients with VI and FAI ≥ .27.
In conclusion, AI of 9 individual MS was significantly associated with post-LT recurrence of HCC. FAI, with a cut-off as determined by ROC curve analysis of 0.27, was a powerful independent predictor of recurrence of HCC beyond Milan criteria, with a sensitivity of 83% and a specificity of 91%. An algorithm assuming that HCC meeting Milan criteria will not recur, and using FAI to predict the remainder, yielded 89% accuracy (62/70 patients predicted correctly). Molecular analysis based on AI at specific loci appears to reflect the inherent metastatic potential of HCC, and adds significantly the ability to predict post-LT recurrence compared to models based solely on clinical data. Prospective validation of the utility of this algorithm in a series of consecutive cases based on pre-LT imaging and biopsy is required.
Figure 1.
Overall probability of recurrence in the 70 patients with HCC treated by liver transplantation from 1990 to 1997. Twenty–four patients presented recurrence.
ACKNOWLEDGMENTS
Myron Schwartz is supported by NIH grant K24 DK 60498-01.
Josep M. Llovet is supported by a grant from Instituto de Salud Carlos III (PI02/0596, Fondo de Investigaciones Sanitarias 2002–2005); Professor of Research-Institut Catala de Recerca Avancada (ICREA).
Abbreviations
- HCC
hepatocellular carcinoma
- LT
liver transplant
- VI
vascular invasion
- MS
microsatellite
- AI
allelic imbalance
- FAI
fractional allelic imbalance
- AFP
alphafetoprotein
- ROC
receiver operating characteristic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 2.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz M. Liver transplantation for hepatocellular carcinoma. Gastroenterology. 2004;127:S268–S276. doi: 10.1053/j.gastro.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 5.Roayaie S, Frischer JS, Emre SH, Fishbein TM, Sheiner PA, Sung M, et al. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg. 2002;235:533–539. doi: 10.1097/00000658-200204000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao FY, Ferrell L, Bass NM, Bacchetti P, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: Comparison of the proposed UCSF criteria with the milan criteria and the pittsburgh modified TNM criteria. Liver Transpl. 2002;8:765–774. doi: 10.1053/jlts.2002.34892. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Wurmbach E. Gene expression profiles in hepatocellular carcinoma: Not yet there. J Hepatol. 2004;41:336–339. doi: 10.1016/j.jhep.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Lee JS, Chu IS, Heo J, Calvisi DF, Sun Z, Roskams T, et al. Classification and prediction of survival in hepatocellular carcinoma by gene expression profiling. Hepatology. 2004;40:667–676. doi: 10.1002/hep.20375. [DOI] [PubMed] [Google Scholar]
- 9.Iizuka N, Hamamoto Y, Oka M. Predicting individual outcomes in hepatocellular carcinoma. Lancet. 2004;364:1837–1839. doi: 10.1016/S0140-6736(04)17455-2. [DOI] [PubMed] [Google Scholar]
- 10.Kurokawa Y, Matoba R, Takemasa I, Nagano H, Dono K, Nakamori S, et al. Molecular-based prediction of early recurrence in hepatocellular carcinoma. J Hepatol. 2004;41:284–291. doi: 10.1016/j.jhep.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 11.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 12.Marsh JW, Finkelstein SD, Demetris AJ, Swalsky PA, Sasatomi E, Bandos A, et al. Genotyping of hepatocellular carcinoma in liver transplant recipients adds predictive power for determining recurrence-free survival. Liver Transpl. 2003;9:664–671. doi: 10.1053/jlts.2003.50144. [DOI] [PubMed] [Google Scholar]
- 13.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 14.Finkelstein SD, Marsh W, Demetris AJ, Swalsky PA, Sasatomi E, Bonham A, et al. Microdissection-based allelotyping discriminates de novo tumor from intrahepatic spread in hepatocellular carcinoma. Hepatology. 2003;37:871–879. doi: 10.1053/jhep.2003.50134. [DOI] [PubMed] [Google Scholar]
- 15.Kirimlioglu H, Dvorchick I, Ruppert K, Finkelstein S, Marsh JW, Iwatsuki S, et al. Hepatocellular carcinomas in native livers from patients treated with orthotopic liver transplantation: Biologic and therapeutic implications. Hepatology. 2001;34:502–510. doi: 10.1053/jhep.2001.26633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dacic S, Ionescu DN, Finkelstein S, Yousem SA. Patterns of allelic loss of synchronous adenocarcinomas of the lung. Am J Surg Pathol. 2005;29:897–902. doi: 10.1097/01.pas.0000164367.96379.66. [DOI] [PubMed] [Google Scholar]
- 17.Krishnamurti U, Sasatomi E, Swalsky PA, Jones MW, Finkelstein SD. Microdissection-based mutational genotyping of serous borderline tumors of the ovary. Int J Gynecol Pathol. 2005;24:56–61. [PubMed] [Google Scholar]
- 18.Rao UN, Goodman M, Chung WW, Swalski P, Pal R, Finkelstein S. Molecular analysis of primary and recurrent giant cell tumors of bone. Cancer Genet Cytogenet. 2005;158:126–136. doi: 10.1016/j.cancergencyto.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Beckner ME, Sasatomi E, Swalsky PA, Hamilton RL, Pollack IF, Finkelstein SD. Loss of heterozygosity reveals non-VHL allelic loss in hemangioblastomas at 22q13. Hum Pathol. 2004;35:1105–1111. doi: 10.1016/j.humpath.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Hunt JL, Fowler M, Lomago D, Niehouse L, Sasatomi E, Swalsky P, et al. Tumor suppressor gene allelic loss profiles of the variants of papillary thyroid carcinoma. Diagn Mol Pathol. 2004;13:41–46. doi: 10.1097/00019606-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Khalid A, Pal R, Sasatomi E, Swalsky P, Slivka A, Whitcomb D, et al. Use of microsatellite marker loss of heterozygosity in accurate diagnosis of pancreaticobiliary malignancy from brush cytology samples. Gut. 2004;53:1860–1865. doi: 10.1136/gut.2004.039784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JY, Finkelstein S, Hamilton RL, Rekha R, King JT, Jr, Omalu B. Loss of heterozygosity analysis of benign, atypical, and anaplastic meningiomas. Neurosurgery. 2004;55:1163–1173. doi: 10.1227/01.neu.0000141081.07086.a0. [DOI] [PubMed] [Google Scholar]
- 23.Ariyanayagam-Baksh SM, Baksh FK, Swalsky PA, Finkelstein SD. Loss of heterozygosity in the MXI1 gene is a frequent occurrence in melanoma. Mod Pathol. 2003;16:992–995. doi: 10.1097/01.MP.0000087421.44975.1C. [DOI] [PubMed] [Google Scholar]
- 24.Dacic S, Finkelstein SD, Baksh FK, Swalsky PA, Barnes LE, Yousem SA. Small-cell neuroendocrine carcinoma displays unique profiles of tumor-suppressor gene loss in relationship to the primary site of formation. Hum Pathol. 2002;33:927–932. doi: 10.1053/hupa.2002.126875. [DOI] [PubMed] [Google Scholar]
- 25.Raja S, Finkelstein SD, Baksh FK, Gooding WE, Swalsky PA, Godfrey TE, et al. Correlation between dysplasia and mutations of six tumor suppressor genes in barrett's esophagus. Ann Thorac Surg. 2001;72:1130–1135. doi: 10.1016/s0003-4975(01)03005-3. [DOI] [PubMed] [Google Scholar]
- 26.Iacobuzio-Donahue CA, van der Heijden MS, Baumgartner MR, Troup WJ, Romm JM, Doheny K, et al. Large-scale allelotype of pancreaticobiliary carcinoma provides quantitative estimates of genome-wide allelic loss. Cancer Res. 2004;64:871–875. doi: 10.1158/0008-5472.can-03-2756. [DOI] [PubMed] [Google Scholar]
- 27.Pawlik TM, Gleisner AL, Anders RA, Assumpcao L, Maley W, Choti MA. Preoperative assessment of hepatocellular carcinoma tumor grade using needle biopsy: Implications for transplant eligibility. Ann Surg. 2007;245:435–442. doi: 10.1097/01.sla.0000250420.73854.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cillo U, Vitale A, Bassanello M, Boccagni P, Brolese A, Zanus G, et al. Liver transplantation for the treatment of moderately or well-differentiated hepatocellular carcinoma. Ann Surg. 2004;239:150–159. doi: 10.1097/01.sla.0000109146.72827.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burrel M, Llovet JM, Ayuso C, Iglesias C, Sala M, Miquel R, et al. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: An explant correlation. Hepatology. 2003;38:1034–1042. doi: 10.1053/jhep.2003.50409. [DOI] [PubMed] [Google Scholar]
- 30.Ravaioli M, Ercolani G, Cescon M, Vetrone G, Voci C, Grigioni WF, et al. Liver transplantation for hepatocellular carcinoma: Further considerations on selection criteria. Liver Transpl. 2004;10:1195–1202. doi: 10.1002/lt.20239. [DOI] [PubMed] [Google Scholar]