Abstract
Relapsing-remitting experimental autoimmune encephalomyelitis (EAE), a multiple sclerosis model, is induced in mice by injection of myelin proteolipid protein (PLP) encephalitogenic peptide, PLP139–151, in adjuvant. In this study, prior to EAE induction, mice were vaccinated with a bacterial plasmid encoding a PLP-ubiquitin fusion (pCMVUPLP). During the relapse phase of EAE, clinical signs, histopathologic changes, in vitro lymphoproliferation to PLP139–151 and interferon-γ levels were reduced in pCMVUPLP-vaccinated mice, compared to mock-vaccinated mice (controls). Lymphocytes from pCMVUPLP-vaccinated mice produced interleukin-4, a cytokine lacking in controls. Thus, pCMVUPLP-vaccination can modulate the relapse after EAE induction.
Keywords: CNS demyelinating disease, Experimental autoimmune encephalomyelitis, DNA vaccines, Multiple sclerosis, Ubiquitin degradation pathway
1. Introduction
Relapsing-remitting experimental autoimmune encephalomyelitis (RR-EAE) is a CD4+ T helper (Th) type 1-mediated disease of the central nervous system (CNS) (Pender, 1995). RR-EAE mimics many of the clinical and pathological features of the human demyelinating disease multiple sclerosis (MS). RR-EAE induced in SJL/J mice using an encephalitogenic peptide of myelin proteolipid protein (PLP139–151) emulsified in complete Freund’s adjuvant (CFA) is characterized by an initial acute attack that lasts an average of 7 days which is then followed by an irregular RR phase (McRae et al., 1995; Tsunoda and Fujinami, 1996; Whitham et al., 1991).
While the pathogenesis of MS is unknown, several features, including genetics, age and environmental factors, contribute to the disease. Epidemiologic studies have demonstrated that the prevalence of MS is low around the equatorial belt of the world and as one progresses north and south from this region, the incidence of MS increases [(Kurtzke, 1993), reviewed in (Libbey and Fujinami, 2002; Libbey and Fujinami, 2003)]. Migration studies have shown that individuals moving after the age of 15 from an area of high-risk for MS to an area of low incidence of MS retain their high-risk for MS phenotype. Conversely, individuals migrating before the age of 15 acquire the low-risk phenotype of the area to which they have moved. These data have been interpreted as indicating that infections early in life in high-risk or high endemic MS areas contribute to or imprint the body’s immune system to the high-risk MS phenotype. The converse of this is that infections early in life in the low-risk MS regions may actually protect individuals from developing MS (Granieri et al., 2001; Granieri and Casetta, 1997; Kurtzke, 1993). In addition, there are several reports indicating that exacerbations of MS occur in close association with microbial infections (Álvarez-Lafuente et al., 2006; Andersen et al., 1993; Berti et al., 2002; Buljevac et al., 2002; Christensen, 2007; Correale et al., 2006; De Keyser et al., 1998; Edwards et al., 1998; Gilden, 2002; Granieri et al., 2001; Kriesel et al., 2004; Kriesel and Sibley, 2005; Marrie et al., 2000; Metz et al., 1998; Panitch, 1994; Rapp et al., 1995; Sibley et al., 1985; Sospedra and Martin, 2006; Wandinger et al., 2000). Therefore from an epidemiologic standpoint, infections, whether viral, bacterial, or parasitic, probably contribute to the pathogenesis of MS.
Molecular mimicry is a sharing of immunologic determinants between a microbe, virus or bacteria, and the host. We have previously demonstrated that recombinant viruses encoding self-proteins could either prime for autoimmune CNS disease or protect from autoimmune CNS disease (Barnett et al., 1993; Barnett et al., 1996; Wang et al., 1999; Wang and Fujinami, 1997). We have also reported that vaccination with naked DNA (Tsunoda et al., 1999) and DNA encoding self-CNS antigens (whole PLP, PLP139–151, PLP178–191) (Tsunoda et al., 1998) enhanced the acute EAE and led to an increase in relapses. DNA immunization using naked DNA or DNA encoding a self-CNS antigen (PLP) resulted in increased proliferative responses to the sensitizing antigen. In addition, the immune response was of an enhanced Th1 type compared to mock-vaccinated RR-EAE mice (Tsunoda et al., 1999). These constructs mirror organisms which have cross-reacting epitopes with self-CNS proteins. In these instances the naked DNA (containing CpG motifs) and the DNA encoding self-antigens appear to prime mice for the development of RR-EAE.
In the present study, we tested whether ubiquitination of a self-protein, PLP, could modulate the type of priming that we had previously observed. It is known that ubiquitination improves entry into and presentation by the class I major histocompatibility complex (MHC) pathway (Rodriguez et al., 1997; Rodriguez et al., 1998) and leads to an enhanced CD8+ cytotoxic T lymphocyte (CTL) response. Therefore, we constructed a bacterial plasmid encoding a modified ubiquitin gene in frame with the PLP gene (pCMVUPLP). We then examined whether targeting the encoded PLP antigen, provided by the DNA vaccination, to the intracellular degradation pathway could modulate RR-EAE. We found that the ubiquitinated PLP DNA vaccine could downregulate the immune response observed during the relapse after EAE induction. Mice vaccinated with pCMVUPLP (the ubiquitinated form of PLP) had a milder clinical disease with fewer lesions in the brain and spinal cord during the relapse. Lymphoproliferative responses to PLP139–151 in pCMVUPLP-vaccinated mice were also reduced, compared to mock-vaccinated mice. This was accompanied by a decrease in interferon (IFN)-γ production and an increase in interleukin (IL)-4 production. In addition, data from CTL experiments suggest that CD8+ T cells are activated and expanded after EAE induction in pCMVUPLP-vaccinated mice. These CD8+ T cells could contribute to the modulation of disease by regulating the CD4+ Th1 encephalitogenic T cells.
2. Materials and Methods
2.1. Plasmid construction
Constructs used for intramuscular (i.m.) injection were based on pCMV, a plasmid that contains the widely expressed immediate-early promoter from cytomegalovirus. The PLP gene was fused to a modified ubiquitin gene [ubiquitin-A76, (Rodriguez et al., 1997)] and subcloned into the NotI restriction enzyme site of plasmid pCMV, which was derived by excision of the β-galactosidase gene from pCMV-β (Clontech, Palo Alto, CA). The resultant expression vector was named pCMVUPLP (Theil et al., 2001). Plasmid DNA was purified from transformed DH5α Escherichia coli with the EndoFree Plasmid Maxi Kit (Qiagen, Chatsworth, CA).
2.2. DNA immunization and EAE induction
Groups of 3–5 SJL/J mice (3–4-weeks-old, female, Jackson Laboratory, Bar Harbor, ME) were vaccinated i.m. a total of 3 times at 1-week intervals with 100 µg pCMVUPLP plasmid DNA, or were mock-vaccinated, with phosphate-buffered saline (PBS) or empty vector, pCMV. Plasmid DNA was dissolved in 1 N saline and 50 µg were injected into each anterior tibial muscle with a 28-gauge needle. Mice were observed and weighed every other day.
One week after the last vaccination, mice received subcutaneously (at the base of the tail) 100 nM PLP139–151, emulsified in CFA. After EAE induction, mice were observed and weighed daily. Mice were followed for clinical signs of disease according to the following scale (Tsunoda et al., 1998): 0 - no clinical disease; 0.5 - loss of tonicity of the distal half of the tail; 1 - complete loss of tail tonicity; 2 - mild hind leg paresis; 3 - moderate hind leg paralysis; 4 - complete paraplegia; and 5 - quadriplegia, moribund state or death. Mice were euthanized with isoflurane. All experiments were performed twice.
Previously, we have shown that pCMVPLP (lacking ubiquitin) vaccination before EAE induction resulted in the most severe EAE (Tsunoda et al., 1998), while pCMV injection before EAE induction also resulted in more severe EAE than EAE induced with PLP139–151/CFA alone (Tsunoda et al., 1999). Therefore, in this study, we used pCMV-vaccinated mice followed with PLP139–151/CFA-EAE induction as a control group, unless otherwise noted.
2.3. Histology
Mice sacrificed on day 26 post-EAE induction were perfused with PBS followed by 4% paraformaldehyde. Brains were embedded as 5 coronal slabs in paraffin. Spinal cords were divided into 10 to 12 transverse slabs and were embedded in paraffin by standard methods (Tsunoda and Fujinami, 1996). Sections were stained with hematoxylin and eosin (H&E) and Luxol-fast blue.
Mononuclear cell (MNC) infiltration of the meninges and parenchyma was assessed according to the following scoring system: 0 - no infiltration; 1 - meninges were slightly infiltrated; 2 - perivascular cuffing in the meninges and lateral ventricle (brain), and perivascular cuffing in the meninges comprised of up to two cell rows (spinal cord); 3 - perivascular cuffing in meninges, all ventricles, cerebellum and brain stem (brain), and severe perivascular cuffing in the meninges and ventral funiculus (spinal cord); and 4 - entire meninges and all funiculi were severely infiltrated (spinal cord). Demyelination in the spinal cord was assessed according to the following scoring system: 0 - no demyelination; 1 - white matter adjacent to the meninges was replaced with gliotic cells; 2 - less than half of the white matter was replaced with infiltrating cells and gliotic cells; 3 - more than half of the white matter was replaced with infiltrating cells and gliotic cells.
Immunohistochemistry was performed using anti-CD3 antibody (DAKO Corporation, Carpenteria, CA) and the avidin-biotin peroxidase complex (ABC) technique with 3,3′-diaminobenzidine tetrahydrochloride (Sigma, St. Louis, MO) in 0.01% hydrogen peroxide (H2O2) in PBS. Sections were analyzed by Image-Pro Plus version 4.5.1 (Media Cybernetics, Inc., Silver Spring, MD).
2.4. Proliferation assay
The inguinal lymph nodes and spleens from mice in each experimental group were removed at days 23 and 28 post-EAE induction and pooled. Lymphocytes were isolated using Histopaque-1083 (Sigma). A volume of 0.2 ml containing 2 × 105 cells in RPMI 1640 media (Mediatech, Herndon, VA), supplemented with 1% glutamine (Mediatech), 1% penicillin/streptomycin (Mediatech), 5 × 10−5 M 2-mercaptoethanol (Sigma), and 10% fetal bovine serum (Invitrogen, San Diego, CA), was added to each well and PLP139–151, PLP178–191, myelin basic protein (MBP)84–104 (Vanderlugt et al., 2000) and myelin oligodendrocyte glycoprotein (MOG)92–106 (Amor et al., 1994) peptides, which are encephalitogenic in SJL/J mice, were added at 50 µg/ml. Lymphocytes prepared as above from mice at day 4 post-EAE induction were incubated with irradiated antigen presenting cells (APCs), irradiated APCs transfected with either pCMVPLP (lacks ubiquitin) or pCMVUPLP, irradiated APCs infected with a recombinant vaccinia virus (VV) that expresses PLP (VVplp) or PLP139–151 peptide. PPD (tuberculin purified protein derivative) was used as a positive control. The cells were cultured for 72 hrs and each well was pulsed with 1 µCi of 3H-thymidine (PerkinElmer, Waltham, MA). Cells were cultured for another 24 hrs. Cultures were harvested onto filters using a multiwell cell harvester (Flow Laboratories, McLean, VA) and counted using standard liquid scintillation techniques. All cultures were performed in triplicate. Results are expressed as stimulation index [experimental counts per minute (cpm)/control cpm].
2.5. Cytokine assays
Lymphocytes from the same animals used in the proliferation assay were cultured at 2 × 106 cells/ml in 6-well plates and stimulated with 50 µg/ml PLP139–151. Culture supernatants were harvested 24 and 48 hrs after stimulation. IL-4, IL-10 and IFN-γ were measured using the enzyme-linked immunosorbent assay (ELISA) system, OptEIA™ (BD Pharmingen, San Jose, CA), according to the manufacturer’s instructions. IL-17 was measured using mouse IL-17 ELISA MAX™ Set Deluxe (BioLegend, San Diego, CA).
2.6. Cytotoxicity assays
2.6.1. Preparation of target cells
A simian virus 40-transformed murine fibroblast cell line, PSJLSV (PSJL) (H-2s), kindly provided by Barbara Knowles (Jackson Laboratory, Bar Harbor, ME), was used as a target in the CTL assays. On the day of assay, PSJL cells were labeled with Na251CrO4 (PerkinElmer) for 1 hr, and, at the same time, these cells were infected with VVplp at a multiplicity of infection (MOI) of 10 (Barnett et al., 1993). The same 51Cr loading procedure was applied to control targets: uninfected PSJL cells, PSJL cells infected with VVsc11 (recombinant VV expressing β-galatosidase), and human Jurkat cells (American Type Culture Collection, Manassas, VA).
2.6.2. Preparation of effector cells
In EAE, encephalitogenic T cells have been thought to engage in bystander killing, using the Fas-FasL interaction without MHC-T cell receptor (TCR) interaction (Thilenius et al., 1999). Available syngeneic target cells, PSJL cells, are potentially susceptible to Fas-FasL mediated killing (Tsunoda et al., 2002). Therefore, it would be difficult to analyze CTL responses if we harvest the cells from mice after induction of encephalitogenic T cells, which are expected to be induced by day 6. Thus, in this study, we chose day 4 to examine the induction of CTL by vaccination with pCMVUPLP.
Effector cells were prepared by pooling splenocytes and inguinal lymphocytes, harvested 4 days after EAE induction with PLP139–151/CFA, from 2 mice vaccinated with pCMVUPLP. These cells were treated with an NH4Cl red blood cell-lysing solution and were used in primary and secondary CTL assays. For the secondary CTL assay, the effector cells were stimulated in vitro for 6 days with naïve SJL/J mouse splenocytes that had been infected with VVplp at a MOI of 1, cultured overnight and irradiated at 2000 rad (137Cs irradiator). Effector cells were washed 3 times prior to use.
2.6.3. 51Cr release assay
Target cell lysis was assessed by a 5-hr short-term 51Cr release assay. The target cells (104) were placed in wells of 96-well, round-bottomed microtiter plates. Effector cells were used at a concentration of 106/100 µl, and threefold serial dilutions were made to provide effector-to-target cell (E/T) ratios of 100:1, 33:1, 11:1 and 3:1. After a 5-hr incubation, the radioactivity released from the cells was measured, as counts per minute (cpm), by means of a model 20/20 gamma counter (Iso-Data, Inc., Palatine, IL). The percentage of target cell lysis was calculated using the following equation: % lysis = [(experimental release cpm – spontaneous release cpm)/(maximal release cpm – spontaneous release cpm)] × 100.
2.7. Statistical analyses
All statistical analyses were performed using the StatView program (SAS Institute Inc., Cary, NC). Either the unpaired t-test (Student’s t-test) or analysis of variance (ANOVA), followed when indicated by the Fisher’s Protected Least Significant Difference (Fisher’s PLSD) post hoc test, were used to determine group differences for continuous data.
3. Results
3.1. Clinical disease
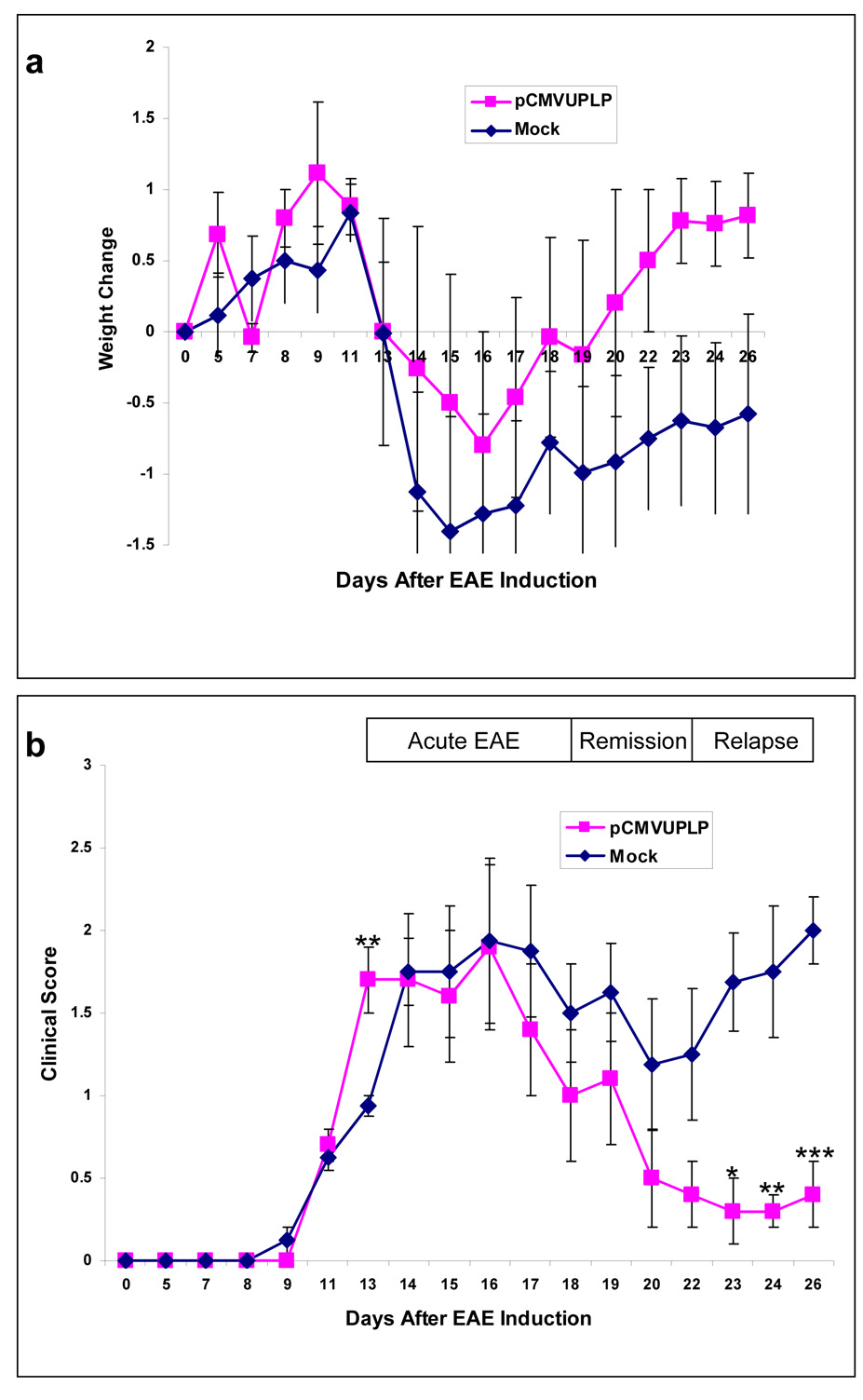
In PLP139–151-induced EAE, most mice develop the first attack (onset) at around day 13, the first remission at around day 18, and the second attack (the first relapse) at around day 22 post-EAE induction (Tsunoda et al., 2007). After the first relapse, the disease courses between individual mice are not synchronized (Tsunoda et al., 2007). Thus, in this study, we decided to observed mice during the first 1 month post-EAE induction. Acute clinical signs of RR-EAE were present in both pCMVUPLP-vaccinated and mock-vaccinated mice beginning on days 13–14 post-EAE induction with PLP139–151/CFA. Clinical signs in RR-EAE were characterized by tail and hindlimb paresis that progressed to flaccid paralysis, accompanied by urinary incontinence. The average clinical score and variation in weight of pCMVUPLP-vaccinated and mock-vaccinated mice are shown in Fig. 1 for days 0 through 26. There was a trend for the pCMVUPLP-vaccinated mice to lose less weight, compared to mock-vaccinated mice, though the difference never reached significance (Fig. 1a). The clinical scores were significantly lower for the pCMVUPLP-vaccinated mice, compare to mock-vaccinated mice, for the days 23, 24 and 26 post-EAE induction (p < 0.05, p < 0.01 and p = 0.0001, respectively, t-test); however, the clinical score for the pCMVUPLP-vaccinated mice was significantly higher, compared to mock-vaccinated mice, for day 13 post-EAE induction (p < 0.01, t-test) (Fig. 1b). Both pCMVUPLP-vaccinated and mock-vaccinated mice recovered partially from the initial acute attack. Mock-vaccinated mice had their first relapse around day 22 after sensitization. Mice vaccinated with pCMVUPLP without PLP139–151/CFA challenge or challenged with PBS/CFA had no disease (data not shown). Therefore, pCMVUPLP injections per se did not induce CNS disease. However, pCMVUPLP injections did downregulate the clinical disease induced with PLP139–151/CFA during the relapses, while not preventing the relapses altogether.
Fig. 1.
Weight change (a) and clinical score (b) after experimental autoimmune encephalomyelitis (EAE) induction with the myelin proteolipid protein (PLP) peptide, PLP139–151, emulsified in complete Freund’s adjuvant (CFA). Mice were either vaccinated (pCMVUPLP), prior to EAE induction, with the bacterial plasmid, pCMVUPLP, which encodes the ubiquitinated form of PLP (5 mice), or mock-vaccinated controls (Mock) with phosphate-buffered saline (3 mice) or empty vector, pCMV (5 mice). No significant differences were found for weight change. pCMVUPLP mice were found to have a significantly higher clinical score at day 13 post-EAE induction (**, p < 0.01, t-test); however the control mice had significantly higher clinical scores at days 23, 24 and 26 post-EAE induction (*, p < 0.05; **, p < 0.01 and ***, p = 0.0001, respectively, t-test). Values are the means ± standard error of the mean (SEM).
3.2. Histological findings
Brains and spinal cords were examined histopathologically 26 days post-EAE induction. The histopathology of RR-EAE in the brain and spinal cord of mock-vaccinated mice was characterized by abundant MNC infiltration around vessels (perivascular cuffing) in the meninges and white matter (Fig. 2a). Lesions were prominent in the periventricular zone around lateral ventricles, the white matter of the cerebellar folia and the thoracolumbar segment of the spinal cord. Often present around the inflammatory cell cuffs, an abundant accumulation of macrophages and microglia was present with or without rings of demyelination. Classical plaque-like demyelination as seen in MS was not common.
Fig. 2.
Spinal cord histopathology 26 days post-EAE induction with PLP that followed either mock vaccination with empty vector, pCMV (a,b), or vaccination with pCMVUPLP (c,d), which encodes the ubiquitinated form of PLP. (a) Mock-vaccinated mice had severe meningitis (arrow) with subpial demyelination (arrowhead) in the ventral root exit zone of the spinal cord white matter. (c) pCMVUPLP-vaccinated mice had mild meningitis with demyelination in regions of the spinal cord similar to mock-vaccinated mice. (b,d) In consecutive sections, both groups of mice had CD3+ T cells in the meninges and the parenchyma of the spinal cord white matter. (a,c) Luxol fast blue stain. (b,d) Immunohistochemistry against CD3. Magnification: a-d, × 100; inset, × 360.
The histopathology, measured as MNC infiltration in the brain, MNC infiltration in the spinal cord and demyelination, was significantly less severe (p < 0.05, p < 0.01 and p < 0.0001, respectively, t-test) in the mice vaccinated with pCMVUPLP and challenged with PLP139–151/CFA than in the mock-vaccinated mice (Fig. 3). In general in both groups, demyelination was present in the spinal cord but was absent in the brain. In the brains of pCMVUPLP-vaccinated mice, MNC infiltration was seen around the lateral ventricles and, in only one mouse (1/5), MNC infiltration was present in the cerebellum. In contrast, the cerebellum was always affected in the mock-vaccinated mice. In the spinal cord the same anatomical region was affected, in pCMVUPLP-vaccinated and mock-vaccinated mice (Fig. 2c).
Fig. 3.
Mononuclear cell (MNC) infiltration of the brain and spinal cord and demyelination were scored as described in the Methods section for pCMVUPLP-vaccinated mice (5 mice, pCMVUPLP) and mock-vaccinated mice (5 mice, Mock) for day 26 post-EAE induction. MNC infiltration in the brain and spinal cord and demyelination were significantly less severe (*, p < 0.05; **, p < 0.01 and ***, p < 0.0001, respectively, t-test) in the pCMVUPLP mice than in the mock-vaccinated mice. Values are the means ± SEM.
To determine the extent of T cell infiltration, immunohistochemistry on serial sections was performed. In both groups of mice, perivascular cuffing was positive for CD3+ T cells (Fig. 2b,d); more than half of the cells comprising a cuff stained positive using CD3 antibodies.
3.3 Proliferation assay
In general, CD8+ T cells recognize endogenous (intracellular) protein epitopes presented by MHC class I, and CD4+ T cells recognize exogenous (extracellular) protein epitopes presented by MHC class II (Leifert et al., 2004). We measured CD4+ T cell proliferative responses of inguinal lymph nodes and spleens, using exogenously added PPD and different CD4-specific encephalitogenic epitopes reported in SJL/J mice.
Lymphocytes, harvested on day 23 post-EAE induction, from mice vaccinated with pCMVUPLP and challenged with PLP139–151/CFA, when stimulated in vitro with PLP139–151, had a lower proliferative response than lymphocytes from mice that were mock-vaccinated (p < 0.01, t-test) (Fig. 4a). No significant proliferation to other known encephalitogenic peptides was detected at this time point.
Fig. 4.
Lymphoproliferative responses. (a) Lymphocytes from mice vaccinated with pCMVUPLP and challenged with PLP139–151/CFA (2 mice, pCMVUPLP) or mock-vaccinated (2 mice, Mock) were harvested on day 23 post-EAE induction and stimulated in vitro with PLP139–151, PLP178–191, myelin oligodendrocyte glycoprotein (MOG)92–106, or myelin basic protein (MBP)84–104. Lymphocyte proliferation was significantly less in response to PLP139–151 stimulation in the pCMVUPLP lymphocytes than the lymphocytes from mock-vaccinated mice (**, p < 0.01, t-test). (b) Lymphocytes from mice vaccinated with pCMVUPLP and challenged with PLP139–151/CFA (2 mice each) were harvested on days 23 and 28 post-EAE induction and stimulated in vitro with PLP139–151, PLP178–191, MOG92–106, MBP84–104, or PPD (tuberculin purified protein derivative). Lymphocyte proliferation was significantly less in response to PLP139–151 stimulation on day 23 than on day 28 (**, p < 0.01, t-test). Some proliferation to PLP178–191 could be seen during the relapse phase, possibly indicating epitope spreading. (c) Lymphocytes from mice vaccinated with pCMVUPLP and challenged with PLP139–151/CFA (2 mice) were harvested on day 4 post-EAE induction and stimulated in vitro with irradiated antigen presenting cells (APC) (CONTROL), APC transfected with pCMVUPLP (pCMVUPLP) and pCMVPLP (pCMVPLP), APC infected with a recombinant vaccinia virus that expresses PLP (VVplp), PLP139–151 peptide (PLP139), or PPD. Lymphocytes proliferated in response to VVplp, PLP139 and pCMVUPLP, but not to CONTROL or pCMVPLP. Lymphocyte proliferation was significantly greater in response to pCMVUPLP compared to CONTROL or pCMVPLP (§, p < 0.001, ANOVA). All assays were performed in triplicate. Values are the means ± SEM.
The lymphoproliferative response, to the PLP139–151 peptide, of lymphocytes obtained from the pCMVUPLP-vaccinated mice was higher on day 28 post-EAE induction than on day 23 post-EAE induction (p < 0.01, t-test) (Fig. 4b). Some proliferation to another PLP epitope (PLP178–191) could be seen during the relapse. This may reflect epitope spreading (Fig. 4b).
To see potential MHC class I restricted CD8+ T cell responses, we used APCs (spleen cells) transfected with pCMVPLP (which lacks ubiqitin), pCMVUPLP, or VVplp-infected APCs as stimulator in lymphoproliferative assays. Lymphocytes, isolated at day 4 post-EAE induction, from mice vaccinated with pCMVUPLP proliferated when incubated with irradiated APCs transfected with pCMVUPLP, but not to APCs transfected with pCMVPLP (p < 0.001, ANOVA, Fisher’s PLSD), or to control irradiated APCs (p < 0.001, ANOVA, Fisher’s PLSD) (Fig. 4c). This suggests that enhanced degradation and processing of PLP occurs in the APCs with the ubiquitinated vaccine. Furthermore, this finding suggests that the proliferative response might be due to an MHC class I restricted interaction, although no PLP-specific CD8+ T cell responses have been discovered in SJL/J mice.
3.4. Cytokines
Lymphocytes from pCMVUPLP-vaccinated mice or mock-vaccinated mice were harvested 23 days after EAE induction and were stimulated with PLP139–151, as described. Then, IFN-γ and IL-4 were measured in culture fluids. Four times higher amounts of IFN-γ were detected in the mock-vaccinated mice compared to pCMVUPLP-vaccinated mice at the 48 hr time point (Fig. 5a). IL-4 was detectable only in the pCMVUPLP-vaccinated group and not in the mock-vaccinated group (Fig. 5b). IL-10 was below the detection limit (31.3 pg/ml) in all samples. These findings suggest a dampening of the Th1 response. IL-4 production contributes to the lessened Th1 response. Since IL-17 has been associated with exacerbation in some EAE models (McClain et al., 2007), we also measured IL-17 in the supernatant. We detected no or a low level of IL-17 in the pCMVUPLP-vaccinated group (exp. 1, not detectable; exp. 2, 76 pg/ml), while IL-17 was not detectable in the mock-vaccinated group (detection limit, 7.8 pg/ml). Therefore, IL-17 production was not associated with disease severity in our current EAE model.
Fig. 5.
Cytokine production. Levels of interferon (IFN)-γ (a) and interleukin (IL)-4 (b) in the supernatants of lymphocytes, harvested from vaccinated mice (2 mice, pCMVUPLP), and mock-vaccinated mice (2 mice, Mock) on day 23 post-EAE induction, were measured by enzyme-linked immunosorbent assay (ELISA) at 24 and 48 hours after in vitro stimulation with PLP139–151. (a) IFN-γ levels were 4 times higher in the mock-vaccinated mice than the pCMVUPLP mice at 48 hr. (b) IL-4 was detectable only in the pCMVUPLP mice. All assays were performed in duplicate. Values are the means ± SEM.
3.5. CTL assay
We tested whether pCMVUPLP vaccination could induce PLP-specific CTL responses. In CTL assays, we used lymphocytes harvested on day 4 post-EAE induction from pCMVUPLP-vaccinated mice as effector cells; syngeneic PSJL cells infected with VVplp, control VVsc11, or uninfected PSJL cells were used as target cells. The lymphocytes showed substantial cytotoxicity against both VVplp and VVsc11 infected cell, but not uninfected cells (% lysis at E/T ratio = 100: VVplp infected, 42%; VVsc11 infected, 32%; uninfected, 0%; % lysis at E/T ratio = 33: VVplp infected, 28%; VVsc11 infected, 31%; uninfected, 0%). In vitro secondary stimulation with VVplp-infected APCs did not enhance the CTL responses (% lysis at E/T ratios = 100 and 33: VVplp infected, 12 and 9%, respectively). Although we did not see killing of uninfected syngeneic cells, the lymphocytes killed human Jurkat cells (% lysis at E/T ratios = 100, 33, and 11: 27%, 15%, and 12%, respectively). Blocking experiments suggested that the cyototoxicity against Jurkat cells was mediated by the Fas-FasL pathway (data not shown).
4. Discussion
The current study demonstrates that DNA vaccination with a cDNA encoding an ubiquitinated autologous protein reduces clinical disease. Studies done by Rodriguez et al. (1997) and the present work show that 1) ubiquitinated vaccines efficiently induce a CD8+ CTL response and 2) these types of T cells are involved in disease attenuation.
CD8+ T cells are present in the lesions in EAE and MS [reviewed in (Johnson et al., 2007)]. CD8+ suppressor or regulatory T cells have been described which are reactive to encephalitogenic T cells or to myelin protein (Kumar and Sercarz, 1996; Sun et al., 1988). Furthermore, a key feature of effective DNA vaccination is the efficient generation of long-lasting CD8+ T cell immunity (Riedl et al., 2006). By targeting the antigens to the proteasome pathway, we are inducing immune cells specific for epitopes presented by MHC class I, and these cells could elicit a suppressive function upon disease induction. Several mechanisms have been proposed for how CD8+ T cells could downregulate a destructive CD4+ T cell response. Sun and Klinert (1989) demonstrated that CD8+ T cells could modulate EAE by eliminating the CD4+ effector T cells through direct cytotoxicity. Kumar and Sercarz (1996) suggested that CD8+ T cells could recognize distinct TCR determinants in a MHC class I context displayed on the surface of effector CD4+ T cells. However, both authors were exploring the role of CD8+ T cells in EAE models that do not develop spontaneous relapses. In the RR-EAE model, we found that CD8+ T cells, induced by DNA vaccination, reduced clinical disease during the relapse after EAE induction. In CTL assays, we found that pCMVUPLP vaccination induced cytotoxic responses against virus infected syngeneic cells (PSJL) as well as xenogeneic cells (Jurkat). Since Jurkat is Fas positive and Fas blocking experiments inhibited the killing (unpublished data), this xenogeneic killing could be mediated by the Fas-FasL pathway (Thilenius et al., 1999). Since CD8+ T cells, in theory, suppress CD4+ T cells by the Fas-FasL pathway, we are investigating the phenotype of the killer cells and involvement of the Fas-FasL pathway in the killing. We are also investigating whether similar cytotoxicity can be detected during the relapse phase of EAE, and if so, whether the killing is correlated with the clinical severity of EAE.
The cytokine profile of the relapsing phase in RR-EAE is of a Th1 type [IFN-γ and tumor necrosis factor (TNF)-α], and the cytokines are produce by CD4+ effector T cells. In the remission phase, cytokines of a Th2 type (IL-4) have been detected (Begolka et al., 1998). The source of the Th2 type cytokines is not exclusively CD4+ T cells; other cell types involved in an inflammatory process have been found to produce transforming growth factor (TGF)-β (Miller et al., 1992). Miller et al. (1992) demonstrated the protective role of TGF-β produced by CD8+ T cells in oral tolerization to MBP in monophasic EAE (Lewis rat injected in footpad with MBP/CFA). In the mice vaccinated with pCMVUPLP and challenged with PLP139–151/CFA, compared to the mock-vaccinated mice, IL-4 was detectable only in the pCMVUPLP-vaccinated group. We hypothesize that the cytokine profile observed in our experiments reflects the prolonged remission phase mice were in at the time of sacrifice and not a Th2 response induced directly by the pCMVUPLP vaccine. This aspect is still under investigation, since IL-4 has been shown to play a decisive role in leading the immune response towards a Th2 type response, which is known to be protective in many EAE models (Falcone and Bloom, 1997; Karpus et al., 1992; Racke et al., 1994). IL-4 has also been shown to downregulate macrophage activity by inhibiting protein kinase C epsilon activity and reducing nitric oxide (NO) expression (Sands et al., 1994). It is still possible that suppressor cells induced by the pCMVUPLP vaccine diminish clinical and pathological disease by downregulating macrophages and glial cell activity in the brain and spinal cord.
Vaccination with naked DNA has previously been explored in EAE and has been found to be effective in some cases and not in others. DNA vaccines, with specificity for the TCR V genes commonly found on pathogenic T cells that cause EAE, can protect animals from developing EAE, as demonstrated in DA rats, PL/J mice and SJL/J mice sensitized with MBP (Buch and Waisman, 2006; Kumar et al., 2001; Matsumoto, 2005; Miyakoshi et al., 2003; Waisman et al., 1996). This protection has been found to be due to the induction of Th2 type immunity, by inducing a shift in the phenotype of the T cells responding to the antigen and utilizing the relevant V gene segment, ultimately resulting in a cytokine shift from Th1 to Th2 and thus protection from EAE (Buch and Waisman, 2006; Kumar et al., 2001; Waisman et al., 1996). This method, however, requires knowledge of the exact TCR repertoire relevant for a particular disease and individual (Waisman et al., 1996).
DNA vaccines have also been constructed which encode receptors for various chemokines known to be active in the process of EAE relapse (Matsumoto, 2005). Administration of these decoy chemokine receptor DNA vaccines encoding the binding sites of CXC chemokine receptor 3 (CXCR3) and CC chemokine receptor 2 (CCR2), receptors for IFN-γ-inducible protein-10 (IP-10) and monocyte chemoattractant protein-1 (MCP-1), respectively, was found to suppress relapse, but not the acute attack, in EAE induced in DA rats by means of immunization with purified myelin (Matsumoto, 2005).
Most DNA vaccination studies have demonstrated that DNA injection can modulate diseases/infections, if DNA is given before disease induction. Vaccination with a DNA construct encoding the entire PLP gene was shown to either suppress or exacerbate EAE in SJL/J mice depending on when the vaccine was given (Selmaj et al., 2000). Exacerbation occurred if the interval between DNA vaccination and sensitization with PLP139–151 was less than 10 weeks, and suppression occurred if the interval was greater than 10 weeks (Selmaj et al., 2000). In the current study, we injected pCMVUPLP before EAE induction and found that it suppressed EAE. It would be worth testing in future experiments whether pCMVUPLP vaccination can suppress ongoing EAE when it is given after EAE induction or disease onset.
DNA vaccines with a myelin epitope alone (PLP139–151) resulted in reduction of the incidence and severity of the acute phase of PLP139–151-induced EAE, probably due to T cell anergy (Ruiz et al., 1999). Alternately, DNA covaccination, with a PLP139–151 vaccine and an IL-4 vaccine, drives the response to a Th2 type response rather than anergy (Garren et al., 2001). It is thought that both the IL-4 and myelin peptide vaccines are taken up by APCs which present the expressed myelin epitopes on MHC class II, thus recruiting antigen-specific T cells. The APCs also secrete IL-4 which influences the Th2 type phenotype of the antigen-specific T cells and thus protects from EAE (Garren et al., 2001).
Still other covaccination methods have been tested which incorporate a mixture of myelin epitopes from multiple myelin proteins and IL-4 [(Ho et al., 2005; Robinson et al., 2003), reviewed in (Fontoura et al., 2005)]. These studies demonstrated a significant reduction in relapse rate in the PLP139–151-induced EAE model in SJL/J mice [(Ho et al., 2005; Robinson et al., 2003), reviewed in (Fontoura et al., 2005)].
Previously in our laboratory, vaccination with cDNAs encoding PLP (whole PLP, PLP139–151, and PLP178–191 in pCMV) was shown to enhance RR-EAE (Tsunoda et al., 1998). Vaccination with the constructs alone induced a PLP-specific lymphoproliferative response of a CD4+ T cell mediated nature, but not CNS disease; whereas vaccination and subsequent peptide challenge resulted in a clinically and histologically more severe chronic EAE with enhanced lymphoproliferative responses to PLP (Tsunoda et al., 1998). The differences between the Tsunoda et al. (1998) study (enhancement of EAE) and the Ruiz et al. (1999) study (amelioration of EAE) (above) include examination of difference phases of the disease (chronic and acute, respectively), the use of older mice by Ruiz et al. (1999), the use of different expression vectors, the use of different plasmid preparation kits, the use of different immunization protocols, and the use of different EAE induction methods (Ruiz et al., 1999; Tsunoda et al., 1998).
In the current study, in which vaccination was with a bacterial plasmid, pCMVUPLP, encoding the ubiquitinated form of PLP, amelioration of EAE was observed (reduced MNC infiltration and demyelination, less proliferation to the encephalitogenic peptide PLP139–151 and dampening of the Th1 response). Thus, targeting the PLP antigen to the intracellular degradation pathway by ubiquitination could modulate RR-EAE in a suppressive manner.
Ubiquitin is a 76 amino-acid protein that binds covalently to target proteins, shepherding them to the proteasome for degradation (Hung et al., 2006; Pickart, 2004). Targeting proteins encoded by DNA vaccines to the proteasome has been studied as a way of increasing the immunogenicity of DNA vaccines. The fusion of antigen to ubiquitin significantly enhances the presentation of the antigen by MHC class I and, thus, the antigen-specific CD8+ T cell immune response to the antigen (Andersson and Barry, 2004; Hung et al., 2006). In this system, target epitope recognition by CTL can be blocked by a proteasome inhibitor, as we have previously demonstrated (Rodriguez et al., 1997). Interestingly, however, different proteasome inhibitors can not only impair but also enhance the presentation of MHC class I restricted immunodominant and subdominant epitopes in some cases (Gavioli et al., 2002). Therefore, in order to understanding the mechanism of PLP antigen presentation in greater detail, further experiments, such as analyzing the effect of different proteasome inhibitors on the generation of possible subdominant and immunodominant PLP epitopes in vitro, will be required.
DNA vaccines encoding an antigen fused to ubiquitin have been studied with respect to several viral, bacterial and parasitic infections, including papillomavirus (cancer), Plasmodium yoelii (malaria), pseudorabies virus, Mycobacterium tuberculosis, hepatitis C virus, lymphocytic choriomeningitis virus, HIV, and influenza virus (Andersson and Barry, 2004; Brandsma et al., 2007; Brulet et al., 2007; Delogu et al., 2000; Delogu et al., 2002; Fu et al., 1998; Gravier et al., 2007; Leachman et al., 2002; Liu et al., 2002; Rodriguez et al., 1997; Tobery and Siliciano, 1997; Vidalin et al., 1999; Wang et al., 2004). The approach also has been exploited in studies of antitumor therapy and allergies (Bauer et al., 2006; Duan et al., 2006; Zhang et al., 2005).
Interestingly, a regulatory role of CD8+ T cells has been reported in the disease caused by the Theiler’s murine encephalomyelitis virus (TMEV), a viral model for MS. The DA virus, a strain of TMEV, causes at first a subclinical or asymptomatic disease in the CNS, which is then followed by a chronic destructive CD4+ T cell response (Tsunoda and Fujinami, 1996). At first the immune system seems to cope with the infection. The first asymptomatic phase is believed to be the result of a prompt immune response; the induction of regulatory CD8+ T cells keeps CD4+ effector T cells in check (Nicholson et al., 1996; Rodriguez et al., 1991). Similar protective immune machinery might be functioning in EAE mice vaccinated with pCMVUPLP. To clarify the potential regulatory role of CD8+ T cells, we are planning experiments, such as in vivo adoptive transfer of CD8+ T cells (protected versus non-protected mice) to EAE mice, in vivo depletion of CD8+ T cells in vaccinated mice, and in vitro addition or depletion of CD8+ T cells in CTL as well as lymphoproliferative assays.
Relapsing in the pCMVUPLP-vaccinated mice could be the result of clonal CD8+ T cell exhaustion, or the result of continuous accumulation of Th1 cytokines or proinflammatory cytokine [IFN-γ, IL-12, tumor necrosis factor (TNF)-α]. The source of these cytokines is probably macrophages and activated glial cells in the brain, or newly attracted effector CD4+ T cells.
In this study we underscore that different methods of DNA vaccination can modulate autoimmunity. Ubiquitinated DNA vaccination represents a powerful tool in studying the pathogenesis of autoimmunity. CD8+ T cells may play a decisive role in regulating RR-EAE. We demonstrated that DNA vaccination modulated the onset, remission and the first relapse of RR-EAE. We are planning to determine: 1) whether DNA vaccination can decrease the number of relapses during the late chronic stage and, 2) whether DNA injection after disease onset or during the chronic stage can modulate the clinical course of EAE. These future directions will be important for potential therapeutic applications of DNA vaccination in MS.
Acknowledgements
We are grateful to Ms. Kathleen Borick for preparation of the manuscript. We thank Nikki Kirkman, BS, for technical assistance. This work was supported by NIH AI581501.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Álvarez-Lafuente R, Garcia-Montojo M, de Las Hervas V, Bartolome M, Arroyo R. Clinical parameters and HHV-6 active replication in relapsing-remitting multiple sclerosis patients. J. Clin. Virol. 2006;37 Suppl 1:S24–S26. doi: 10.1016/S1386-6532(06)70007-5. [DOI] [PubMed] [Google Scholar]
- Amor S, Groome N, Linington C, Morris MM, Dornmair K, Gardinier MV, Matthieu J-M, Baker D. Identification of epitopes of myelin oligodendrocyte glycoprotein for the induction of experimental allergic encephalomyelitis in SJL and Biozzi AB/H mice. J. Immunol. 1994;153:4349–4356. [PubMed] [Google Scholar]
- Andersen O, Lygner PE, Bergstrom T, Andersson M, Vahlne A. Viral infections trigger multiple sclerosis relapses: A prospective seroepidemiological study. J. Neurol. 1993;240:417–422. doi: 10.1007/BF00867354. [DOI] [PubMed] [Google Scholar]
- Andersson HA, Barry MA. Maximizing antigen targeting to the proteasome for gene-based vaccines. Mol. Ther. 2004;10:432–446. doi: 10.1016/j.ymthe.2004.05.035. [DOI] [PubMed] [Google Scholar]
- Barnett LA, Whitton JL, Wada Y, Fujinami RS. Enhancement of autoimmune disease using recombinant vaccinia virus encoding myelin proteolipid protein. J. Neuroimmunol. 1993;44:15–25. doi: 10.1016/0165-5728(93)90263-x. [published erratum appears in J. Neuroimmunol. 48:120, 1993] [DOI] [PubMed] [Google Scholar]
- Barnett LA, Whitton JL, Wang LY, Fujinami RS. Virus encoding an encephalitogenic peptide protects mice from experimental allergic encephalomyelitis. J. Neuroimmunol. 1996;64:163–173. doi: 10.1016/0165-5728(95)00165-4. [DOI] [PubMed] [Google Scholar]
- Bauer R, Scheiblhofer S, Kern K, Gruber C, Stepanoska T, Thalhamer T, Hauser-Kronberger C, Alinger B, Zoegg T, Gabler M, Ferreira F, Hartl A, Thalhamer J, Weiss R. Generation of hypoallergenic DNA vaccines by forced ubiquitination: preventive and therapeutic effects in a mouse model of allergy. J. Allergy Clin. Immunol. 2006;118:269–276. doi: 10.1016/j.jaci.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Begolka WS, Vanderlugt CL, Rahbe SM, Miller SD. Differential expression of inflammatory cytokines parallels progression of central nervous system pathology in two clinically distinct models of multiple sclerosis. J. Immunol. 1998;161:4437–4446. [PubMed] [Google Scholar]
- Berti R, Brennan MB, Soldan SS, Ohayon JM, Casareto L, McFarland HF, Jacobson S. Increased detection of serum HHV-6 DNA sequences during multiple sclerosis (MS) exacerbations and correlation with parameters of MS disease progression. J. NeuroVirol. 2002;8:250–256. doi: 10.1080/13550280290049615-1. [DOI] [PubMed] [Google Scholar]
- Brandsma JL, Shlyankevich M, Zelterman D, Su Y. Therapeutic vaccination of rabbits with a ubiquitin-fused papillomavirus E1, E2, E6 and E7 DNA vaccine. Vaccine. 2007;25:6158–6163. doi: 10.1016/j.vaccine.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brulet J-M, Maudoux F, Thomas S, Thielemans K, Burny A, Leo O, Bex F, Hallez S. DNA vaccine encoding endosome-targeted human papillomavirus type 16 E7 protein generates CD4+ T cell-dependent protection. Eur. J. Immunol. 2007;37:376–384. doi: 10.1002/eji.200636233. [DOI] [PubMed] [Google Scholar]
- Buch T, Waisman A. Protection from autoimmunity by DNA vaccination against T-cell receptor. Methods Mol. Med. 2006;127:269–280. doi: 10.1385/1-59745-168-1:269. [DOI] [PubMed] [Google Scholar]
- Buljevac D, Flach HZ, Hop WCJ, Hijdra D, Laman JD, Savelkoul HFJ, van der Meché FGA, Van Doorn PA, Hintzen RQ. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain. 2002;125:952–960. doi: 10.1093/brain/awf098. [DOI] [PubMed] [Google Scholar]
- Christensen T. Human herpesviruses in MS. Int. MS J. 2007;14:41–47. [PubMed] [Google Scholar]
- Correale J, Fiol M, Gilmore W. The risk of relapses in multiple sclerosis during systemic infections. Neurology. 2006;67:652–659. doi: 10.1212/01.wnl.0000233834.09743.3b. [DOI] [PubMed] [Google Scholar]
- De Keyser J, Zwanikken C, Boon M. Effects of influenza vaccination and influenza illness on exacerbations in multiple sclerosis. J. Neurol. Sci. 1998;159:51–53. doi: 10.1016/s0022-510x(98)00139-7. [DOI] [PubMed] [Google Scholar]
- Delogu G, Howard A, Collins FM, Morris SL. DNA vaccination against tuberculosis: expression of a ubiquitin-conjugated tuberculosis protein enhances antimycobacterial immunity. Infect. Immun. 2000;68:3097–3102. doi: 10.1128/iai.68.6.3097-3102.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delogu G, Li A, Repique C, Collins F, Morris SL. DNA vaccine combinations expressing either tissue plasminogen activator signal sequence fusion proteins or ubiquitin-conjugated antigens induce sustained protective immunity in a mouse model of pulmonary tuberculosis. Infect. Immun. 2002;70:292–302. doi: 10.1128/IAI.70.1.292-302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Hisaeda H, Shen J, Tu L, Imai T, Chou B, Murata S, Chiba T, Tanaka K, Fehling HJ, Koga T, Sueishi K, Himeno K. The ubiquitin-proteasome system plays essential roles in presenting an 8-mer CTL epitope expressed in APC to corresponding CD8+ T cells. Int. Immunol. 2006;18:679–687. doi: 10.1093/intimm/dxl005. [DOI] [PubMed] [Google Scholar]
- Edwards S, Zvartau M, Clarke H, Irving W, Blumhardt LD. Clinical relapses and disease activity on magnetic resonance imaging associated with viral upper respiratory tract infections in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 1998;64:736–741. doi: 10.1136/jnnp.64.6.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone M, Bloom BR. A T helper cell 2 (Th2) immune response against non-self antigens modifies the cytokine profile of autoimmune T cells and protects against experimental allergic encephalomyelitis. J. Exp. Med. 1997;185:901–907. doi: 10.1084/jem.185.5.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontoura P, Garren H, Steinman L. Antigen-specific therapies in multiple sclerosis: going beyond proteins and peptides. Int. Rev. Immunol. 2005;24:415–446. doi: 10.1080/08830180500379655. [DOI] [PubMed] [Google Scholar]
- Fu T-M, Guan L, Friedman A, Ulmer JB, Liu MA, Donnelly JJ. Induction of MHC class I-restricted CTL response by DNA immunization with ubiquitin-influenza virus nucleoprotein fusion antigens. Vaccine. 1998;16:1711–1717. doi: 10.1016/s0264-410x(98)00134-0. [DOI] [PubMed] [Google Scholar]
- Garren H, Ruiz PJ, Watkins TA, Fontoura P, Nguyen L-VT, Estline ER, Hirschberg DL, Steinman L. Combination of gene delivery and DNA vaccination to protect from and reverse Th1 autoimmune disease via deviation to the Th2 pathway. Immunity. 2001;15:15–22. doi: 10.1016/s1074-7613(01)00171-6. [DOI] [PubMed] [Google Scholar]
- Gavioli R, Vertuani S, Masucci MG. Proteasome inhibitors reconstitute the presentation of cytotoxic T-cell epitopes in Epstein-Barr virus-associated tumors. Int. J. Cancer. 2002;101:532–538. doi: 10.1002/ijc.10653. [DOI] [PubMed] [Google Scholar]
- Gilden DH. Multiple sclerosis exacerbations and infection. Lancet Neurol. 2002;1:145. doi: 10.1016/s1474-4422(02)00066-2. [DOI] [PubMed] [Google Scholar]
- Granieri E, Casetta I. Part III: Selected reviews common childhood and adolescent infections and multiple sclerosis. Neurology. 1997;49:S42–S54. doi: 10.1212/wnl.49.2_suppl_2.s42. [DOI] [PubMed] [Google Scholar]
- Granieri E, Casetta I, Tola MR, Ferrante P. Multiple sclerosis: infectious hypothesis. Neurol. Sci. 2001;22:179–185. doi: 10.1007/s100720170021. [DOI] [PubMed] [Google Scholar]
- Gravier R, Dory D, Rodriguez F, Bougeard S, Beven V, Cariolet R, Jestin A. Immune and protective abilities of ubiquitinated and non-ubiquitinated pseudorabies virus glycoproteins. Acta Virol. 2007;51:35–45. [PubMed] [Google Scholar]
- Ho PP, Fontoura P, Platten M, Sobel RA, DeVoss JJ, Lee LY, Kidd BA, Tomooka BH, Capers J, Agrawal A, Gupta R, Zernik J, Yee MK, Lee BJ, Garren H, Robinson WH, Steinman L. A suppressive oligodeoxynucleotide enhances the efficacy of myelin cocktail/IL-4-tolerizing DNA vaccination and treats autoimmune disease. J. Immunol. 2005;175:6226–6234. doi: 10.4049/jimmunol.175.9.6226. [DOI] [PubMed] [Google Scholar]
- Hung C-F, Yang M, Wu TC. Modifying professional antigen-presenting cells to enhance DNA vaccine potency. Methods Mol. Med. 2006;127:199–220. doi: 10.1385/1-59745-168-1:199. [DOI] [PubMed] [Google Scholar]
- Johnson AJ, Suidan GL, McDole J, Pirko I. The CD8 T cell in multiple sclerosis: suppressor cell or mediator of neuropathology? Int. Rev. Neurobiol. 2007;79:73–97. doi: 10.1016/S0074-7742(07)79004-9. [DOI] [PubMed] [Google Scholar]
- Karpus WJ, Gould KE, Swanborg RH. CD4+ suppressor cells of autoimmune encephalomyelitis respond to T cell receptor-associated determinants on effector cells by interleukin-4 secretion. Eur. J. Immunol. 1992;22:1757–1763. doi: 10.1002/eji.1830220714. [DOI] [PubMed] [Google Scholar]
- Kriesel JD, Sibley WA. Editorial: The case for rhinoviruses in the pathogenesis of multiple sclerosis. Mult. Scler. 2005;11:1–4. doi: 10.1191/1352458505ms1128ed. [DOI] [PubMed] [Google Scholar]
- Kriesel JD, White A, Hayden FG, Spruance SL, Petajan J. Multiple sclerosis attacks are associated with picornavirus infections. Mult. Scler. 2004;10:145–148. doi: 10.1191/1352458504ms1005oa. [DOI] [PubMed] [Google Scholar]
- Kumar V, Maglione J, Thatte J, Pederson B, Sercarz E, Ward ES. Induction of a type 1 regulatory CD4 T cell response following Vβ8.2 DNA vaccination results in immune deviation and protection from experimental autoimmune encephalomyelitis. Int. Immunol. 2001;13:835–841. doi: 10.1093/intimm/13.6.835. [DOI] [PubMed] [Google Scholar]
- Kumar V, Sercarz E. Dysregulation of potentially pathogenic self reactivity is crucial for the manifestation of clinical autoimmunity. J. Neurosci. Res. 1996;45:334–339. doi: 10.1002/(SICI)1097-4547(19960815)45:4<334::AID-JNR2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF. Epidemiologic evidence for multiple sclerosis as an infection. Clin. Microbiol. Rev. 1993;6:382–427. doi: 10.1128/cmr.6.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leachman SA, Shylankevich M, Slade MD, Levine D, Sundaram RK, Xiao W, Bryan M, Zelterman D, Tiegelaar RE, Brandsma JL. Ubiquitin-fused and/or multiple early genes from cottontail rabbit papillomavirus as DNA vaccines. J. Virol. 2002;76:7616–7624. doi: 10.1128/JVI.76.15.7616-7624.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifert JA, Rodriguez-Carreno MP, Rodriguez F, Whitton JL. Targeting plasmid-encoded proteins to the antigen presentation pathways. Immunol. Rev. 2004;199:40–53. doi: 10.1111/j.0105-2896.2004.0135.x. [DOI] [PubMed] [Google Scholar]
- Libbey JE, Fujinami RS. Are virus infections triggers for autoimmune disease? Clin. Microbiol. Newslett. 2002;24:73–76. doi: 10.1016/S0196-4399(02)80019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Fujinami RS. Viral demyelinating disease in experimental animals. In: Herndon RM, editor. Multiple Sclerosis: Immunology, Pathology and Pathophysiology. New York: Demos; 2003. pp. 125–133. [Google Scholar]
- Liu WJ, Zhao K-N, Gao FG, Leggatt GR, Fernando GJP, Frazer IH. Polynucleotide viral vaccines: codon optimisation and ubiquitin conjugation enhances prophylactic and therapeutic efficacy. Vaccine. 2002;20:862–869. doi: 10.1016/s0264-410x(01)00406-6. [DOI] [PubMed] [Google Scholar]
- Marrie RA, Wolfson C, Sturkenboom MC, Gout O, Heinzlef O, Roullet E, Abenhaim L. Multiple sclerosis and antecedent infections: a case-control study. Neurology. 2000;54:2307–2310. doi: 10.1212/wnl.54.12.2307. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y. New approach to immunotherapy against organ-specific autoimmune diseases with T cell receptor and chemokine receptor DNA vaccines. Curr. Drug Targets. Immune. Endocr. Metabol. Disord. 2005;5:73–77. doi: 10.2174/1568008053174732. [DOI] [PubMed] [Google Scholar]
- McClain MA, Gatson NN, Powell ND, Papenfuss TL, Gienapp IE, Song F, Shawler TM, Kithcart A, Whitacre CC. Pregnancy suppresses experimental autoimmune encephalomyelitis through immunoregulatory cytokine production. J. Immunol. 2007;179:8146–8152. doi: 10.4049/jimmunol.179.12.8146. [DOI] [PubMed] [Google Scholar]
- McRae BL, Vanderlugt CL, Dal Canto MC, Miller SD. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J. Exp. Med. 1995;182:75–85. doi: 10.1084/jem.182.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz LM, McGuinness SD, Harris C. Urinary tract infections may trigger relapse in multiple sclerosis. Axone. 1998;19:67–70. [PubMed] [Google Scholar]
- Miller A, Lider O, Roberts AB, Sporn MB, Weiner HL. Suppressor T cells generated by oral tolerization to myelin basic protein suppress bothin vitro and in vivo immune responses by the release of transforming growth factor βafter antigen-specific triggering. Proc. Natl. Acad. Sci. USA. 1992;89:421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakoshi A, Yoon WK, Jee Y, Matsumoto Y. Characterization of the antigen specificity and TCR repertoire, and TCR-based DNA vaccine therapy in myelin basic protein-induced autoimmune encephalomyelitis in DA rats. J. Immunol. 2003;170:6371–6378. doi: 10.4049/jimmunol.170.12.6371. [DOI] [PubMed] [Google Scholar]
- Nicholson SM, Dal Canto MC, Miller SD, Melvold RW. Adoptively transferred CD8+ T lymphocytes provide protection against TMEV-induced demyelinating disease in BALB/c mice. J. Immunol. 1996;156:1276–1283. [PubMed] [Google Scholar]
- Panitch HS. Influence of infection on exacerbations of multiple sclerosis. Ann. Neurol. 1994;36 Suppl:S25–S28. doi: 10.1002/ana.410360709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pender MP. Experimental autoimmune encephalomyelitis. In: Pender MP, McCombe PA, editors. Autoimmune Neurological Disease. Cambridge, UK: Cambridge University Press; 1995. pp. 26–88. [Google Scholar]
- Pickart CM. Back to the future with ubiquitin. Cell. 2004;116:181–190. doi: 10.1016/s0092-8674(03)01074-2. [DOI] [PubMed] [Google Scholar]
- Racke MK, Bonomo A, Scott DE, Cannella B, Levine A, Raine CS, Shevach EM, Röcken M. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J. Exp. Med. 1994;180:1961–1966. doi: 10.1084/jem.180.5.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp NS, Gilroy J, Lerner AM. Role of bacterial infection in exacerbation of multiple sclerosis. Am. J. Phys. Med. Rehabil. 1995;74:415–418. doi: 10.1097/00002060-199511000-00004. [DOI] [PubMed] [Google Scholar]
- Riedl P, Reimann J, Schirmbeck R. Complexes of DNA vaccines with cationic, antigenic peptides are potent, polyvalent CD8(+) T-cell-stimulating immunogens. Methods Mol. Med. 2006;127:159–169. doi: 10.1385/1-59745-168-1:159. [DOI] [PubMed] [Google Scholar]
- Robinson WH, Fontoura P, Lee BJ, de Vegvar HEN, Tom J, Pedotti R, DiGennaro CD, Mitchell DJ, Fong D, Ho PPK, Ruiz PJ, Maverakis E, Stevens DB, Bernard CCA, Martin R, Kuchroo VK, van Noort JM, Genain CP, Amor S, Olsson T, Utz PJ, Garren H, Steinman L. Protein microarrays guide tolerizing DNA vaccine treatment of autoimmune encephalomyelitis. Nat. Biotechnol. 2003;21:1033–1039. doi: 10.1038/nbt859. [DOI] [PubMed] [Google Scholar]
- Rodriguez F, An L-L, Harkins S, Zhang J, Yokoyama M, Widera G, Fuller JT, Kincaid C, Campbell IL, Whitton JL. DNA immunization with minigenes: Low frequency of memory cytotoxic T lymphocytes and inefficient antiviral protection are rectified by ubiquitination. J. Virol. 1998;72:5174–5181. doi: 10.1128/jvi.72.6.5174-5181.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez F, Zhang J, Whitton JL. DNA immunization: Ubiquitination of a viral protein enhances cytotoxic T-lymphocyte induction and antiviral protection but abrogates antibody induction. J. Virol. 1997;71:8497–8503. doi: 10.1128/jvi.71.11.8497-8503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Lindsley MD, Pierce ML. Role of T cells in resistance to Theiler's virus infection. Microb. Pathog. 1991;11:269–281. doi: 10.1016/0882-4010(91)90031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz PJ, Garren H, Ruiz IU, Hirschberg DL, Nguyen L-VT, Karpuj MV, Cooper MT, Mitchell DJ, Fathman CG, Steinman L. Suppressive immunization with DNA encoding a self-peptide prevents autoimmune disease: modulation of T cell costimulation. J. Immunol. 1999;162:3336–3341. [PubMed] [Google Scholar]
- Sands WA, Bulut V, Severn A, Xu D, Liew FY. Inhibition of nitric oxide synthesis by interleukin-4 may involve inhibiting the activation of protein kinase C epsilon. Eur. J. Immunol. 1994;24:2345–2350. doi: 10.1002/eji.1830241013. [DOI] [PubMed] [Google Scholar]
- Selmaj K, Kowal C, Walczak A, Nowicka J, Raine CS. Naked DNA vaccination differentially modulates autoimmune responses in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2000;111:34–44. doi: 10.1016/s0165-5728(00)00329-5. [DOI] [PubMed] [Google Scholar]
- Sibley WA, Bamford CR, Clark K. Clinical viral infections and multiple sclerosis. Lancet. 1985;1:1313–1315. doi: 10.1016/S0140-6736(85)92801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Molecular mimicry in multiple sclerosis. Autoimmunity. 2006;39:3–8. doi: 10.1080/08916930500484922. [DOI] [PubMed] [Google Scholar]
- Sun D, Klinkert WEF. Functional heterogeneity among CD4+ encephalitogenic T cells in recruitment of CD8+ T cells in experimental autoimmune encephalomyelitis. J. Immunol. 1989;143:2867–2872. [PubMed] [Google Scholar]
- Sun D, Qin Y, Chluba J, Epplen JT, Wekerle H. Suppression of experimentally induced autoimmune encephalomyelitis by cytolytic T-T cell interactions. Nature. 1988;332:843–845. doi: 10.1038/332843a0. [DOI] [PubMed] [Google Scholar]
- Theil DJ, Tsunoda I, Rodriguez F, Whitton JL, Fujinami RS. Viruses can silently prime for and trigger central nervous system autoimmune disease. J. NeuroVirol. 2001;7:220–227. doi: 10.1080/13550280152403263. [DOI] [PubMed] [Google Scholar]
- Thilenius ARB, Sabelko-Downes KA, Russell JH. The role of the antigen-presenting cell in Fas-mediated direct and bystander killing: potential in vivo function of Fas in experimental allergic encephalomyelitis. J. Immunol. 1999;162:643–650. [PubMed] [Google Scholar]
- Tobery TW, Siliciano RF. Targeting of HIV-1 antigens for rapid intracellular degradation enhances cytotoxic T lymphocyte (CTL) recognition and the induction of de novo CTL responses in vivo after immunization. J. Exp. Med. 1997;185:909–920. doi: 10.1084/jem.185.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Fujinami RS. Two models for multiple sclerosis: Experimental allergic encephalomyelitis and Theiler's murine encephalomyelitis virus. J. Neuropathol. Exp. Neurol. 1996;55:673–686. doi: 10.1097/00005072-199606000-00001. [DOI] [PubMed] [Google Scholar]
- Tsunoda I, Kuang L-Q, Fujinami RS. Induction of autoreactive CD8+ cytotoxic cells during Theiler's murine encephalomyelitis virus infection: Implications for autoimmunity. J. Virol. 2002;76:12834–12844. doi: 10.1128/JVI.76.24.12834-12844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Kuang L-Q, Tolley ND, Whitton JL, Fujinami RS. Enhancement of experimental allergic encephalomyelitis (EAE) by DNA immunization with myelin proteolipid protein (PLP) plasmid DNA. J. Neuropathol. Exp. Neurol. 1998;57:758–767. doi: 10.1097/00005072-199808000-00005. [DOI] [PubMed] [Google Scholar]
- Tsunoda I, Terry EJ, Marble BJ, Lazarides E, Woods CM, Fujinami RS. Modulation of experimental autoimmune encephalomyelitis by VLA-2 blockade. Brain Pathol. 2007;17:45–55. doi: 10.1111/j.1750-3639.2006.00042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Tolley ND, Theil DJ, Whitton JL, Kobayashi H, Fujinami RS. Exacerbation of viral and autoimmune animal models for multiple sclerosis by bacterial DNA. Brain Pathol. 1999;9:481–493. doi: 10.1111/j.1750-3639.1999.tb00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderlugt CL, Neville KL, Nikcevich KM, Eagar TN, Bluestone JA, Miller SD. Pathologic role and temporal appearance of newly emerging autoepitopes in relapsing experimental autoimmune encephalomyelitis. J. Immunol. 2000;164:670–678. doi: 10.4049/jimmunol.164.2.670. [DOI] [PubMed] [Google Scholar]
- Vidalin O, Tanaka E, Spengler U, Trépo C, Inchauspé G. Targeting of hepatitis C virus core protein for MHC I or MHC II presentation does not enhance induction of immune responses to DNA vaccination. DNA Cell Biol. 1999;18:611–621. doi: 10.1089/104454999315024. [DOI] [PubMed] [Google Scholar]
- Waisman A, Ruiz PJ, Hirschberg DL, Gelman A, Oksenberg JR, Brocke S, Mor F, Cohen IR, Steinman L. Suppressive vaccination with DNA encoding a variable region gene of the T-cell receptor prevents autoimmune encephalomyelitis and activates Th2 immunity. Nat. Med. 1996;2:899–905. doi: 10.1038/nm0896-899. [DOI] [PubMed] [Google Scholar]
- Wandinger K, Jabs W, Siekhaus A, Bubel S, Trillenberg P, Wagner H, Wessel K, Kirchner H, Hennig H. Association between clinical disease activity and Epstein-Barr virus reactivation in MS. Neurology. 2000;55:178–184. doi: 10.1212/wnl.55.2.178. [DOI] [PubMed] [Google Scholar]
- Wang L-Y, Fujinami RS. Enhancement of EAE and induction of autoantibodies to T-cell epitopes in mice infected with a recombinant vaccinia virus encoding myelin proteolipid protein. J. Neuroimmunol. 1997;75:75–83. doi: 10.1016/s0165-5728(96)00235-4. [DOI] [PubMed] [Google Scholar]
- Wang L-Y, Theil DJ, Whitton JL, Fujinami RS. Infection with a recombinant vaccinia virus encoding myelin proteolipid protein causes suppression of chronic relapsing-remitting experimental allergic encephalomyelitis. J. Neuroimmunol. 1999;96:148–157. doi: 10.1016/s0165-5728(99)00020-x. [DOI] [PubMed] [Google Scholar]
- Wang Q-M, Sun S-H, Hu Z-L, Zhou F-J, Yin M, Xiao C-J, Zhang J-C. Epitope DNA vaccines against tuberculosis: spacers and ubiquitin modulates cellular immune responses elicited by epitope DNA vaccine. Scand. J. Immunol. 2004;60:219–225. doi: 10.1111/j.0300-9475.2004.01442.x. [DOI] [PubMed] [Google Scholar]
- Whitham RH, Bourdette DN, Hashim GA, Herndon RM, Ilg RC, Vandenbark AA, Offner H. Lymphocytes from SJL/J mice immunized with spinal cord respond selectively to a peptide of proteolipid protein and transfer relapsing demyelinating experimental autoimmune encephalomyelitis. J. Immunol. 1991;146:101–107. [PubMed] [Google Scholar]
- Zhang M, Obata C, Hisaeda H, Ishii K, Murata S, Chiba T, Tanaka K, Li Y, Furue M, Chou B, Imai T, Duan X, Himeno K. A novel DNA vaccine based on ubiquitin-proteasome pathway targeting 'self'-antigens expressed in melanoma/melanocyte. Gene Ther. 2005;12:1049–1057. doi: 10.1038/sj.gt.3302490. [DOI] [PubMed] [Google Scholar]