Abstract
Diapause in overwintering adult female Culex pipiens mosquitoes plays an important role in the transmission of West Nile and other encephalitis-inducing flaviviruses. To investigate the dynamic metabolic processes that control Cx. pipiens diapause, we used radioactive tracer techniques with [14C]-glucose to investigate the metabolic fate and flux of glucose in adult mosquitoes reared under diapause (18°C, short day) and nondiapause (27°C, long day) conditions. We found that by 72 hours post-14C-labeling of 1-day-old mosquitoes, the diapause-destined mosquitoes had converted 46% more 14C-labled glucose into 14C-labled lipid than mosquitoes reared under nondiapausing conditions. When 5-day-old mosquitoes were fed [14C]-glucose, and then switched to water only, the nondiapausing mosquitoes oxidized nearly three times more 14C-labled glycogen and lipid by day 7 than diapausing mosquitoes. This increased energy expenditure in nondiapausing mosquitoes is most likely due to temperature- and light-dependent increases in the basal metabolic rate. Amongst the diapausing mosquitoes we analyzed over a subsequent 7 week period, we found that the amount of 14C-labeled glycogen decreased steadily for the first month of diapause, whereas, 14C-labeled-lipid levels were not significantly decreased until after day 35 of diapause, indicating that flux through glycogenolysis is higher than lipolysis during the first month of diapause. Lastly, our analysis revealed that 38% of the initial 14C-labled lipid that was synthesized during the adult pre-diapause phase was still present following the first gonotrophic cycle. About 33% of this remaining 14C-labeled lipid was localized to the newly developed eggs, suggesting that lipid sparing processes during a minimal 7 week long diapause may enhance egg production.
Keywords: metabolic flux, metabolic fate, diapause, energy metabolism, gonotrophic cycle
1. Introduction
Since the outbreak of West Nile virus (WNV) encephalitis in the New York City area in 1999 (Asnis et al., 2000), it has spread across the United States (Sejvar 2007). Mosquitoes are WNV carriers that become infected when they feed on infected birds. Infected mosquitoes can then spread WNV to humans and other animals. One of the common WNV mosquito vectors is Culex pipiens which harbors WNV when overwintering as diapausing adults, and can transmit the pathogen upon blood feeding during the post-diapause period (Nasci et al., 2001, Dohm et al., 2002). Cx. pipiens is able to transmit other flaviviruses that cause human and animal encephalitis and these viruses have also been shown to survive diapause in overwintering mosquitoes (Bailey et al., 1978). Investigation of physiological and biochemical processes that govern Cx. pipiens diapause would significantly improve our understanding of viral transmission in this important biological vector and may provide novel targets for disease control.
Insect diapause is a dynamic process with at least three main phases: pre-diapause, diapause, and post-diapause (Eldridge 1966, Danks 2000, Kostal 2006). Nulliparous and inseminated Cx. pipiens females enter adult diapause in response to short day length and low temperature experienced during larval and pupal development (Spielman 2001). Overwintering females do not take blood (Mitchell and Briegal, 1989a), and fail to use blood for producing lipid reserves for survival during diapause (Mitchell and Briegal, 1989b). Metabolic studies revealed that during the first few weeks post-eclosion, the diapause females increase the frequency of sugar feeding (Bowen 1992). Such a modified feeding behavior might represent a metabolic switch from blood feeding to sugar gluttony as shown by Robich and Denlinger (2005). This same study also found that fatty acid synthase levels were upregulated and associated with the accumulation of lipid reserves in diapause-destined female Cx. pipiens. Previous metabolic studies of diapause in Cx. pipiens, demonstrated that during the first week post-eclosion, the diapause-destined mosquito accumulates 1.7 times more lipid, and 2.3 more carbohydrate, than nondiapause mosquitoes (Mitchell and Briegal, 1989b). However, these studies could not distinguish between a reorganization of lipid reserves from the larval stage, and/or an increase in de novo synthesis from sugar meals during the adult pre-diapause phase. Moreover, altered metabolic flux through lipid and glycogen biosynthetic pathways in diapausing mosquitoes, and changes in the basal metabolic rates (energy expenditure) under the two distinct environmental conditions, were not addressed.
Physiological changes in diapausing Cx. pipiens mosquitoes have also been studied. For example, follicular development is incomplete in diapausing adult females and they remain teneral until the completion of diapause (Sanburg et al., 1973, Spielman et al., 1973). A more recent study showed that insulin signaling is involved in the regulation of follicular arrest during diapause in Cx. pipiens (Sim and Denlinger, 2008). Once diapause is broken by exposure to a longer photoperiod with an increased incubation temperature, Cx. pipiens females will take a blood meal and lay eggs in about three weeks (Mitchell and Briegal, 1989b). It is unknown if any of the lipid reserve formed in the adult pre-diapause phase is available to support the post-diapause gonotrophic cycle in blood fed Cx. pipiens.
To elucidate the dynamics of metabolic processes that control diapause in Cx. pipiens, and to track the metabolic fate of glucose during sugar feeding in adult pre-diapause mosquitoes, we have performed a series of radioactive trace-labeling studies in which uniformly-labeled [14C]-glucose was fed for 24 hours to Cx. pipiens females that had been reared from the second larval instar stage onward under diapause or nondiapause conditions. By analyzing the amount of glucose-derived 14C contained in glycogen, lipid, protein, and amino acid pools at various time points before and after diapause, and comparing these data to that from nondiapausing mosquitoes, we have been able to determine the metabolic fate of this carbon in terms energy expenditure and flux through metabolite pools. Results from these quantitative studies may provide biochemical insights into this important physiological process in Cx. pipiens and perhaps lead to novel strategies for vector control.
2. Materials and methods
2.1. Rearing of mosquitoes
We used a Cx. pipiens strain (Buckeye strain) obtained from Dr. David Denlinger (Ohio State University) that was established in September 2000 from larvae collected in Columbus, Ohio (Robich and Denlinger, 2005, Robich et al., 2007). The stock colony was maintained with a 10% sucrose solution at 27°C, 75% relative humidity (RH), and a photoperiod of 15h:9h (light:dark). Larvae were reared in a 9″×13″ plastic tray containing 350 ml of deionized H2O, and fed with a 1:1 mixture of TetraMin fish flakes (Doctors Foster & Smith, Thinelander, WI) and crushed Prolab 2500 rodent diet (PMI Nutrition International, Brentwood, MI). The larvae were maintained at ~200–250 insects per tray.
2.2. Inducement and termination of diapause
Following the protocol of Robich et al. (2007), eggs and the first instar larvae were kept under normal colony conditions (nondiapause) until the second instar stage and then moved to a CARON 6010 Environmental Test Chamber (Caron Products & Services, Inc., Marietta, Ohio) under the diapause conditions of 18°C, 75% RH and a photoperiod of 9h:15h (light:dark). These diapause-destined adult females were maintained with 10% sucrose solution for 7 days after eclosion. To confirm diapause status, primary follicles and germarium lengths were dissected and compared between diapause and non-diapause females according to the methods described by Spielman et al. (1973). To terminate diapause, the diapause females were moved to nondiapause conditions and maintained with a 10% sucrose solution.
2.3. Experimental design
To compare the differences in formation and conversion of energy reserves from a sugar meal between nondiapause and diapause-destined females during the adult pre-diapause phase (after eclosion), 1-day old adult females were starved for 24 h and then fed with [14C]-glucose for 24 h. The 14C-labeled mosquitoes were then maintained on unlabeled glucose for 5 more days. Mosquitoes were sampled every 24 h and whole body extracts were processed to obtain 14C-labeled glycogen, lipid, protein, and amino acids. These 1-day-old 14C-labeled mosquitoes are referred to as the D-1 cohort in all experiments. In a second experiment designed to characterize the utilization of energy reserves from the adult pre-diapause phase, during and after diapause, 5-day old sugar-fed pre-diapause and nondiapause females were starved for 24 h and then fed with 14C-glucose for 24 h, followed by feeding for 24 h with an unlabeled 10% sucrose solution. These 14C-labeled mosquitoes are referred to as the D-5 cohort in all experiments. The D-5 mosquitoes were maintained with water only under diapause or nondiapause conditions and only live mosquitoes were sampled at each time point. After 49 days under diapause conditions with water only feeding, the D-5 mosquitoes were transferred to a chamber maintained at nondiapausing conditions. These mosquitoes were fed an unlabeled 10% sucrose solution for two weeks, followed by feeding a chick blood meal (Pel-Freez Arkansas LLC, Rogers, AR).
2.4. Metabolic labeling with [14C]-glucose
The [14C]-glucose labeling protocol for D-1 and D-5 mosquitoes was conducted as previously described (Zhou et al., 2004b). Briefly, 200 1-day old or 5-day old adult female Cx. pipiens mosquitoes were placed individually in 10-ml plastic scintillation vials sealed with a nylon mesh. Prior to feeding with [14C]-glucose, they were deprived of sugar and water for 24 h. Forty microcuries of uniformly-labeled [14C]-glucose (specific activity: 11.5 GBq/mmol, 311 mCi/mmol, American Radiolabeled Chemicals, Inc., St. Louis, MO) were added to an 1.5-ml Eppendorf tube, dried under N2 gas, and then dissolved in 995 μl of 10% sucrose solution and 5 μl of 1:30 diluted blue food dye (Assorted Food colors & Egg Dye, McCormick & Co., Inc., Hunt valley, MD). A 5 μl drop of the mixture was placed on the nylon mesh (0.2 μCi/female) and the mosquitoes were allowed to feed on the sucrose drop in their respective environmental chambers (T: 18C or 27C, RH: 75%, light:dark set at 9h:15h or 15h:9h). At 4, 8, and 12 h post-initial feeding, another 5 μl of unlabeled 10% sucrose was placed on the same site of the nylon mesh where the first sucrose drop was placed in order to dissolve any remaining [14C]-glucose that had dried on the nylon mesh. This procedure facilitated the recovery of [14C]-glucose in the mosquitoes. At 24 h post-initial feeding, the completely fed mosquitoes, as indicated by the presence of blue food dye, were transferred into a regular adult mosquito cage (18 cm diameter × 19 cm depth and covered with nylon mesh) and marked as Day 0, while the partially fed mosquitoes were eliminated from the experiment. At 0, 1, 2, 3, 4, and 5 days post-labeling in the D-1 experiment, and at 0, 1, 3, 5, 7, 14, 21, 28, 35, 42, and 49 days post-labeling in the D-5 experiment, pools of 14C-labeled females were collected and dried at 100°C for 1 h and used separately for the microseparation of mosquito glycogen, lipid, protein, and amino acids. The dried mosquito samples were crushed with a glass rod in appropriate volume of saturated Na2SO4 in H2O and methanol, then extracted with methanol:chloroform (1:1 v/v) into fractions containing protein, glycogen, total lipids, and mixture of amino acids and sugars. The fraction containing total lipids was passed through a column containing silicic acid to remove phospholipids from triacylglycerols. The combined aqueous extracts, which contain simple sugars and the amino acids, were mixed with cation-exchange resin to separate amino acids and sugars. The recovery of each group of metabolites was more than 99%, which is similar to our previous 14C-labeling study in Ae. aegypti (Zhou et al., 2004a).
2.5. Blood feeding and egg sampling of 49 day old Cx. pipiens mosquitoes following diapause
Following 49 days of growth under diapause conditions, the surviving mosquitoes were transferred to the nondiapause growth chamber and maintained with 10% sucrose solution feeding for 2 weeks. Fourteen days after switching the mosquitoes to nondiapause growth conditions (63 days post-labeling), the mosquitoes were fed with chick blood in a membrane feeder using 37°C pre-warmed blood. For egg collection, each blood fed mosquito was placed in a plastic water cup containing a 30-ml beaker with water. The cup was sealed with a piece of mesh. Eggs were collected at 120 hour post feeding. The spent females were dissected to collect any eggs retained in ovaries. The collected eggs and the carcasses were used for the microseparation of glycogen, lipid, protein and amino acids (Zhou et al., 2004a, Zhou et al., 2004b).
2.6. Statistical analysis
Data were obtained from three separate experimental samples and the various biochemical products were expressed as DPM/mosquito or relative DPM as noted (table 1). One-way ANOVA with Tukey’s multiple comparison test was used for comparison of various indices between nondiapause and adult diapause-destined or diapause females. The significance level was set at p≤0.05. All the statistical analyses and graphs were prepared using Graph Pad Prism 4 (Graph Pad Prism Software, Inc., San Diego, CA) and SPSS for Windows (v11.5) (SPSS Inc., IL).
Table 1.
Summary of [14C]-glucose metabolism in diapausing D-5 mosquitoes.
| Diapause (%) |
Post-Diapause (%) |
Gonotrophic Cycle (%) |
||||
|---|---|---|---|---|---|---|
| T0 | T7 | T49 | T63 | T68C | T68E | |
| Glycogen | 100.0 | 89.3 | 11.8 | 7.7 | 4.3 | ND |
| Lipid | 100.0 | 181.0 | 53.7 | 58.6 | 25.7 | 12.6 |
| Protein | 100.0 | 23.3 | 13.8 | 7.9 | 8.3 | 1.3 |
| Amino Acids | 100.0 | 9.2 | 3.6 | 1.7 | 0.9 | ND |
T0, day that mosquitoes were transferred to diapause conditions and switched to water only feeding; T68C, carcass; T68E, eggs; ND, not determined.
3. Results
3.1. Fate of [14C]-glucose during the adult pre-diapause phase
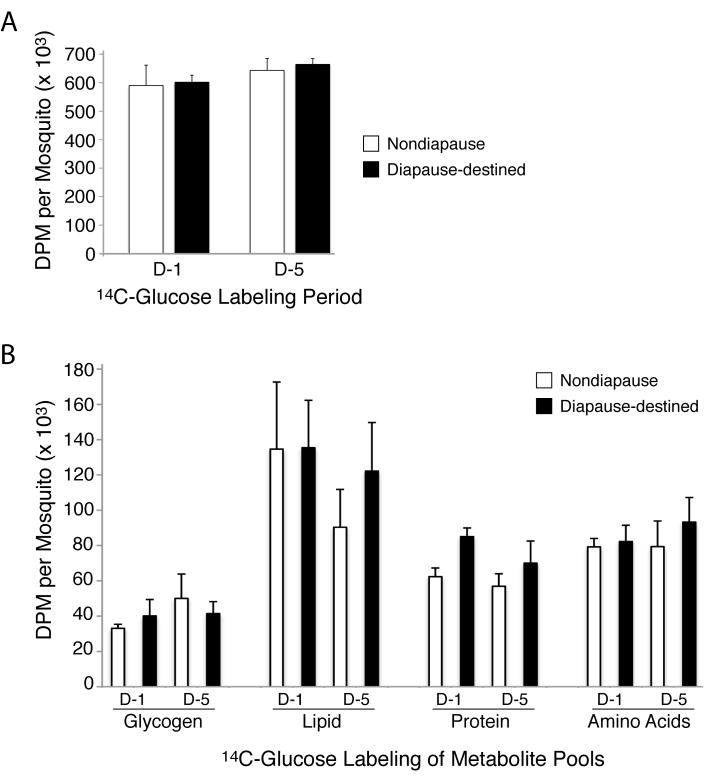
Since it is possible that changes in metabolic flux are initiated by exposure to diapause conditions during the larval stage, and that metabolic changes occur in newly emerged adults that are affected by diapause conditions, we determine the fate of sugar-derived carbon in nondiapause and diapause-destined 1-day old (D-1) and 5-day old (D-5) female mosquitoes using a radioactive trace-labeling approach with uniformly-labeled [14C]-glucose. As shown in figure 1A, there was no significant difference between the nondiapause and diapause-destined D-1 and D-5 mosquitoes in terms of total [14C]-glucose uptake (figure 1A), suggesting that glucose feeding during the 24 hour trace-labeling period in each of these mosquito groups was similar. Moreover, biochemical fractionation revealed no significant difference between the amount of 14C-labeled glycogen, lipid, protein, or amino acids in nondiapause or diapause-destined mosquitoes at the end of the 24 hour labeling period, regardless of their age at the time of labeling (D-1 or D-5). Taken together, these data indicate that accumulated differences in total glycogen and lipid stores in diapausing mosquitoes (Mitchell et al., 1989b), is not due to differential metabolic flux at day 1 and day 5 post-eclosion.
Figure 1.
Total 14C incorporation and metabolite distribution in D-1 and D-5 mosquitoes. A. Total consumed radioactivity within the first 24 h of [14C]-glucose feeding in D-1 and D-5 nondiapausing and adult diapause-destined Cx. pipiens. B. Total 14C-labeling of glycogen, lipid, protein, and amino acids pools within the first 24 h of [14C]-glucose feeding in D-1 and D-5 nondiapausing and diapause-destined Cx. pipiens. Data are presented as mean ± SEM of disintegrations per minute (DPM) per mosquito from three independent experiments. One-way ANOVA with Tukey’s multiple comparison test was used for statistical comparison between nondiapause and diapause-destined mosquitoes. No two-way comparisons were found to be statistically significant at the level of p≤0.05.
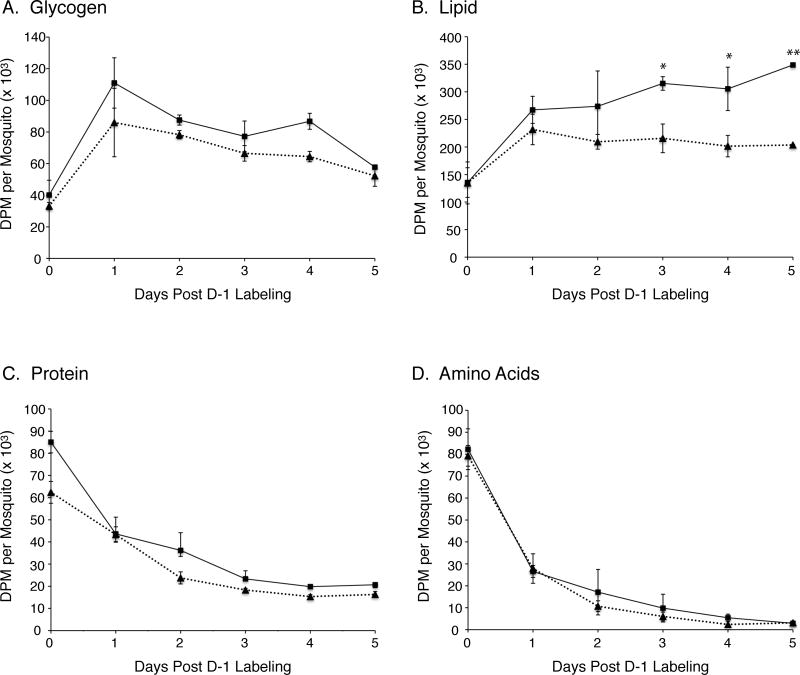
We next examined the fate of 14C-glucose in D-1 nondiapause and diapause-destined mosquitoes during the 5 day pre-diapause phase after eclosion. The data in figure 2 reveal that there were no significant differences between nondiapause and diapause-destined mosquitoes in the amount of 14C-labeled glycogen, protein, and amino acid over this time period, however, the level of 14C-labeled lipid was 46%, 51%, and 71% higher in the diapause-destined mosquitoes on days 3, 4, and 5, respectively. Since the total [14C]-glucose uptake was the same in both sets of mosquitoes during the 24 hr trace-labeling period (figure 1A), and our analysis only tracks 14C-labeled metabolites, these results suggest that diapause-destined adult Cx pipiens mosquitoes may upregulate lipid synthesizing pathways during the pre-diapause phase after eclosion as previously proposed (Robich and Denlinger, 2005). In addition, it is likely that nondiapause mosquitoes have significantly higher basal metabolic rates and energy demands due to environmental differences in temperature and daylight length than. This would result in a preferential depletion of lipid stores through fatty acid oxidation in the nondiapause mosquitoes compared to diapause-destined mosquitoes.
Figure 2.
Time course of 14C-labeled glycogen, lipid, protein, and amino acid metabolism in D-1 mosquitoes during the adult pre-diapause phase. Data are presented as mean ± SEM of DPM/mosquito from three independent experiments. Results from nondiapause mosquitoes are shown as a dotted line and diapause-destined mosquitoes as a solid line. A) Data from 14C-glycogen pools. B) Data from 14C-lipid pools. C) Data from 14C-protein pools. D) Data from 14C-amino acid pools. One-way ANOVA with Tukey’s multiple comparison test was used for statistical comparison between nondiapause and diapause-destined mosquitoes, showing p≤0.05 as (*) and p≤0.01 as (**).
3.2. Energy metabolism during the early and late diapause phases
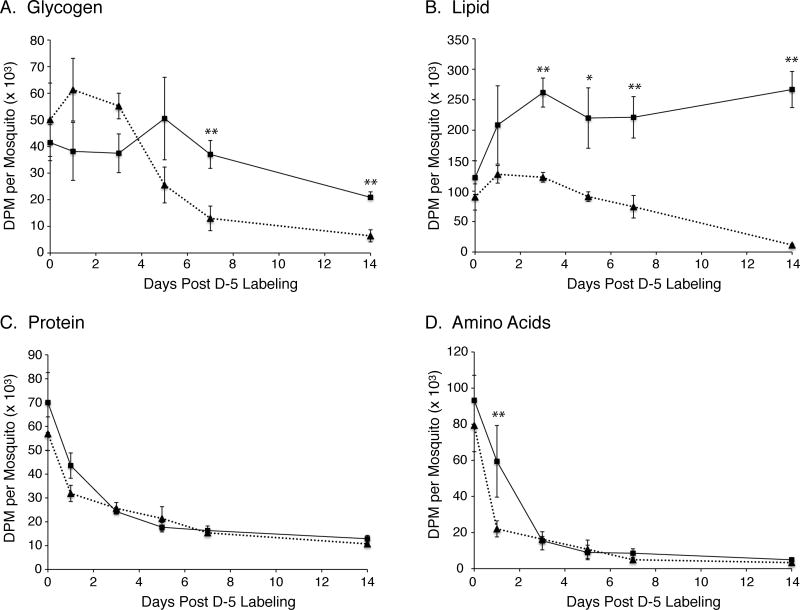
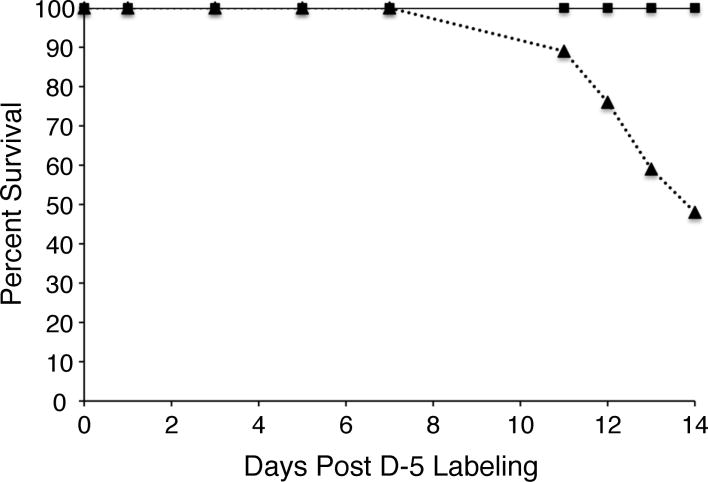
Once we validated that the diapause-destined mosquitoes were indeed converted to a diapause physiological state by examining primary follicles and germarium lengths (Spielman and Wang, 1973), the D-5 nondiapause and diapause mosquitoes were switched to water only feeding (starvation). At various time points, pools of mosquitoes were sampled and levels of whole body 14C-labeled glycogen, lipid, protein, and amino acids were determined. As shown in figure 3, the levels of 14C-labeled glycogen and lipid decreased dramatically in the nondiapause mosquitoes compared to the diapausing mosquitoes, demonstrating that differences in the environmental growth conditions during the pre-diapause phase was sufficient to alter the metabolic dynamics. Moreover, by day 14 of starvation, the nondiapause mosquitoes had nearly exhausted all of the 14C-labeled glycogen and lipid they had accumulated, which would explain the high mortality rate in this group of mosquitoes (figure 4). With regard to lipid metabolism, it can be seen in table 1 that the amount of 14C-labeled lipid increased by almost two-fold in the diapausing mosquitoes during this early diapause phase, while the amount 14C-labeled protein and amino acid decreased by ~80% and ~90%, respectively. This result suggests that metabolic processes in diapausing mosquitoes that stimulate lipogenesis using glucose-derived carbon must be scavenging carbon from glucogenic and ketogenic amino acids that were initially labeled with 14C through the glucose-alanine cycle (Felig 1973).
Figure 3.
Time course of 14C-labeled glycogen, lipid, protein, and amino acid metabolism in D-5 mosquitoes during the early diapause phase. Data are presented as mean ± SEM of DPM/mosquito from three independent experiments. Results from nondiapause mosquitoes are shown as a dotted line and diapause-destined mosquitoes as a solid line. A) Data from 14C-glycogen pools. B) Data from 14C-lipid pools. C) Data from 14C-protein pools. D) Data from 14C-amino acid pools. One-way ANOVA with Tukey’s multiple comparison test was used for statistical comparison between nondiapause and diapause-destined mosquitoes, showing p≤0.05 as (*) and p≤0.01 as (**).
Figure 4.
Nondiapause mosquitoes show a high rate of mortality during the second week of diapause. Number of surviving D-5 mosquitoes from a representative experiment are plotted as a function of days post-labeling. Results from nondiapause mosquitoes are shown as a dotted line and diapause-destined mosquitoes as a solid line.
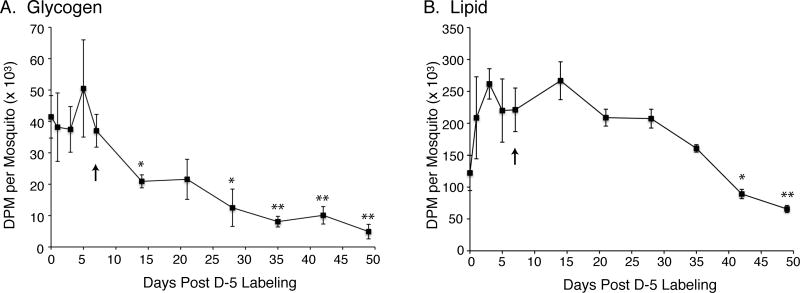
To follow the fate of 14C-labeled glycogen and lipid during the late diapause phase, we sampled mosquitoes every 7 days from day 14 to 49 of starvation as shown in figure 5. Using day 7 of starvation as the baseline for glycogen and lipid reserves during the onset of diapause (arrow in figure 5), we found that 14C-glycogen levels decreased significantly at day 14 (p=0.02), and continued to decline until day 49 when they were only 11.8% of the pre-diapause amount (table 1). In contrast, 14C-lipid levels did not begin to decrease until day 35, and were not significantly lower until day 42 (p=0.01). The observed 79% decrease in 14C-glycogen from day 7 to day 35, compared to 14C-lipid levels which only decreased 28% during this same time period, indicates that glycogen plays an important role in the first ~5 weeks of diapause, whereas, lipid reserves become more important after week ~6. Note that in our experiments, diapause was terminated early at day 49 to retain sufficient levels of 14C for analysis, however, it is likely that most diapausing mosquitoes would have survived an additional 60 days (~120 day diapause phase) based on other studies (Robich and Denlinger, 2005).
Figure 5.
Time course of 14C-labeled glycogen and lipid metabolism in diapausing D-5 mosquitoes during the late diapause phase. Data are presented as mean ± SEM of DPM/mosquito from three independent experiments. A) Data from 14C-glycogen pools. B) Data from 14C-lipid pools. One-way ANOVA with Tukey’s multiple comparison test was used for statistical comparison between day 7 of diapause (arrow) and later time points showing p≤0.05 as (*) and p≤0.01 as (**).
3.3. Lipids synthesized during adult pre-diapause phase are used for egg production in mosquitoes subjected to a 49 day long diapause
Diapause was terminated by transferring mosquitoes to an environmental chamber maintained under nondiapause conditions and providing sugar pads for 14 days. Mosquitoes were then fed a chick blood meal and the amount of 14C-labeled lipid in the eggs and carcass was determined at 120 hours post blood meal feeding. As shown in table 1, we found that of the 38.3% of total 14C-labeled lipid that remained at the end of the gonotrophic cycle, 32.9% of it was localized to the eggs (12.6% of the starting amount). These data indicate that 14C-labeled lipid produced from sugar derived carbon during the adult pre-diapause phase, contributes to egg production under conditions in which the diapause period is short enough to spare sufficient amounts of lipid.
4. Discussion
Sugar meals are critical for building up energy reserves in adult pre-diapause Cx. pipiens mosquitoes because blood feeding behavior is curtailed at this time (Mitchell and Briegel, 1989b, Robich and Denlinger, 2005). Although increases in the total amount of stored glycogen and lipid has been observed in adult pre-diapause mosquitoes (Mitchell et al., 1989b), no studies to date have determined the metabolic fate of these sugar meals, nor if there are any changes in metabolic flux between various metabolite pools as a function of diapause. Based on our previous experience using radioactive trace-labeling methods to study the fate of [14C]-glucose fed to teneral female Ae. aegypti mosquitoes (Zhou et al., 2004b), we have now applied similar quantitative analyses to study metabolism in adult female Cx. pipiens mosquitoes during various phases of diapause. The results of these studies provide the first dynamic picture of energy metabolism in Cx. pipiens as a function of diapause and extend earlier studies that measured the total amount of glycogen and lipid that accumulate in the pre-diapause phase of larvae and adults.
We first investigated the adult pre-diapause phase and found that diapause conditions do not significantly alter either the amount of glucose ingested, or metabolic pattern, compared to mosquitoes reared under nondiapause conditions (figure 1). These results would seem contradictory to the behavioral studies of Bowen (1992), in which it was reported that diapause-destined female Cx. pipiens had an increased frequency of sugar feeding. However, the two studies employed different feeding methods which could explain the results. In the Bowen experiments, the mosquitoes were fed in a continuous ad libitum manner, whereas in our experiments, we only monitored feeding for a 24 h period in 1-day-old or 5-day-old mosquitoes which did not account for possible behavioral differences in feeding on days 2, 3, and 4. Another possibility is that the average glucose meal size in the Bowen study may have been smaller for the diapause-destined mosquitoes, such that more frequent feeding did not actually lead to increased glucose ingestion. One way to distinguish between these two possibilities would be to allow nondiapause and diapause-destined mosquitoes to feed on [14C]-glucose in a continuous ad libitum manner for the entire 5 days and then quantitate the level of 14C label in each group.
The only significant difference we observed between the two sets of D-1 mosquitoes during the adult pre-diapause phase was the increase in 14C-labeled lipid in diapause-destined mosquitoes, which began as early as 72 hours post-feeding (figure 2). In terms of increased flux through lipid synthesizing pathways, this result suggests that lipid synthesis is already stimulated in diapause-destined larvae, or that sugar feeding in newly eclosed adults stimulates a metabolic switch that activates lipogenesis. Additional 14C-labeling studies during the larval development of nondiapause and diapause-destined mosquitoes would be needed to address this question. Interestingly, recent studies have shown that upregulation of fatty acid synthase gene expression is associated with diapause in Cx. pipiens (Robich and Denlinger, 2005), and that decreased insulin signaling may be an upstream signal in the initiation of Cx. pipiens diapause (Sim and Denlinger, 2008). Therefore, this proposed lipogenic switch in diapause-destined mosquitoes, could be regulated by insulin signaling in response to circadian rhythm changes or decreased temperature.
One of the most interesting results from our study was the finding that glycogen, not lipid, is the primary energy source during early diapause (figure 5). Indeed, 14C-labeled lipid levels are not reduced significantly until day 35 of diapause, whereas, the level of 14C-labeled glycogen begins to decrease by day 14. In hibernating mammals, fuel metabolism is reorganized so that most organs depend on aerobic lipid oxidation for their energy needs at the onset of hibernation (Carey et al., 2003). However, some hibernating snakes and lizards use hepatic glycogen, not fat, as their main energy source (Abdel-Kader et al., 1995). Therefore, while lipid oxidation during hibernation is primary energy source in mammals, other organisms use glycogenolysis to a greater extent as is the case in Cx. pipiens. Since we found that 14C-labeled glycogen is nearly depleted in Cx. pipiens by day 35 of diapause, which coincides with the time that levels of 14C-labeled lipid first begin to decline, it is tempting to speculate that there is a energy sensing signal, perhaps the AMP-activated protein kinase complex (Towler and Hardie, 2007), that stimulates the switch from glycogenolyis to lipolysis at this time.
We also found that ~33% of the 14C-labeled lipid remaining in the 49 day old diapausing mosquitoes was transferred to eggs following completion of the gonotrophic cycle. This represented 12.6% of the total 14C-labeled lipid synthesized in D-5 mosquitoes during the adult pre-diapause phase (table 1). It is possible that preferential glycogen usage early in the diapause phase, and lipid sparing processes in the later stages of diapause, help to preserve lipid reserves to ensure a more productive gonotrophic cycle in the post-diapause phase. An alternative explanation is that lipid reserves are primarily used for survival during an extended diapause phase, which can be up to 120 days long in Cx. pipiens (Robich and Denlinger, 2005). However, since we terminated diapause at day 49 in our experiment, there was sufficient lipid available to produce more viable eggs in the first gonotrophic cycle than would have been possible if diapause were longer. The relationship between the length of diapause, the amount of lipid reserve available when diapause is terminated, and fecundity, could provide insight into the regulatory processes that govern lipid metabolism and reproduction. It seems likely that insulin or ecdysone signaling could at least be partially involved in coordinating these related physiological processes.
In summary, we have used radioactive trace-labeling methods to identify the metabolic fate of [14C]-glucose fed to adult pre-diapause Cx. pipiens mosquitoes to complement earlier studies that measured bulk increases in glycogen and lipid reserves as a function of the diapause state (Mitchell and Briegel, 1989b). Based on levels of 14C-labeled metabolite pools during various stages of diapause, our results suggest that adjustments in metabolic flux between glycogen and lipid energy reserves are highly regulated as a way to optimize fuel metabolism during diapause. We propose that these tightly controlled metabolic processes prolong survival and enhance reproductive success following the first gonotrophic cycle. Future studies are needed that combine the use of RNAi knock-down strategies of specific genes, with quantitative metabolic 14C-labeling methods, to identify key regulatory control points in the diapause process.
Acknowledgments
We thank Dr. David Denlinger, Department of Entomology, The Ohio State University for invaluable help in establishing the Cx. pipiens colony at the University of Arizona. We also thank Ms. Mary Hernandez for her patience in rearing the mosquitoes. This work was supported by the National Institutes of Health grant R01-AI046451 to RLM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Kader A, El-Daly E, Okasha S, Mansour A. Carbohydrate metabolism in Malpolon monspessulanus and Uromastix aegyptius during the entry, deep and arousal phases of hibernation. Journal of Thermal Biology. 1995;20:367–373. [Google Scholar]
- Asnis DS, Conetta R, Teixeira AA, Waldman G, Sampson BA. The West Nile Virus outbreak of 1999 in New York: the Flushing Hospital experience. Clinical Infectious Diseases. 2000;30:413–8. doi: 10.1086/313737. [DOI] [PubMed] [Google Scholar]
- Bailey CL, Eldridge BF, Hayes DE, Watts DM, Tammariello RF, Dalrymple JM. Isolation of St. Louis encephalitis virus from overwintering Culex pipiens mosquitoes. Science. 1978;199:1346–9. doi: 10.1126/science.628843. [DOI] [PubMed] [Google Scholar]
- Bowen MF. Patterns of sugar feeding in diapausing and nondiapausing Culex pipiens (Diptera: Culicidae) females. Journal of Medical Entomology. 1992;29:843–9. doi: 10.1093/jmedent/29.5.843. [DOI] [PubMed] [Google Scholar]
- Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiological Reviews. 2003;83:1153–81. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- Danks HV. Insect cold hardiness: a Canadian perspective. Cryo Letters. 2000;21:297–308. [PubMed] [Google Scholar]
- Dohm DJ, Sardelis MR, Turell MJ. Experimental vertical transmission of West Nile virus by Culex pipiens (Diptera: Culicidae) Journal of Medical Entomology. 2002;39:640–4. doi: 10.1603/0022-2585-39.4.640. [DOI] [PubMed] [Google Scholar]
- Eldridge BF. Environmental Control of Ovarian Development in Mosquitoes of the Culex pipiens Complex. Science. 1966;151:826–828. doi: 10.1126/science.151.3712.826. [DOI] [PubMed] [Google Scholar]
- Felig P. The glucose-alanine cycle. Metabolism. 1973;22:179–207. doi: 10.1016/0026-0495(73)90269-2. [DOI] [PubMed] [Google Scholar]
- Kostal V. Eco-physiological phases of insect diapause. Journal of Insect Physioliology. 2006;52:113–27. doi: 10.1016/j.jinsphys.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Mitchell CJ, Briegel H. Fate of the blood meal in force-fed, diapausing Culex pipiens (Diptera: Culicidae) Journal of Medical Entomology. 1989a;26:332–41. doi: 10.1093/jmedent/26.4.332. [DOI] [PubMed] [Google Scholar]
- Mitchell CJ, Briegel H. Inability of diapausing Culex pipiens (Diptera: Culicidae) to use blood for producing lipid reserves for overwinter survival. Journal of Medical Entomology. 1989b;26:318–26. doi: 10.1093/jmedent/26.4.318. [DOI] [PubMed] [Google Scholar]
- Nasci RS, Savage HM, White DJ, Miller JR, Cropp BC, Godsey MS, Kerst AJ, Bennett P, Gottfried K, Lanciotti RS. West Nile virus in overwintering Culex mosquitoes, New York City, 2000. Emerging Infectious Diseases. 2001;7:742–4. doi: 10.3201/eid0704.010426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robich RM, Denlinger DL. Diapause in the mosquito Culex pipiens evokes a metabolic switch from blood feeding to sugar gluttony. Proceedings of the National Academy of Sciences U S A. 2005;102:15912–7. doi: 10.1073/pnas.0507958102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robich RM, Rinehart JP, Kitchen LJ, Denlinger DL. Diapause-specific gene expression in the northern house mosquito, Culex pipiens L., identified by suppressive subtractive hybridization. Journal of Insect Physiology. 2007;53:235–45. doi: 10.1016/j.jinsphys.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanburg LL, Larsen JR. Effect of photoperiod and temperature on ovarian development in Culex pipiens pipiens. Journal of Insect Physiology. 1973;19:1173–90. doi: 10.1016/0022-1910(73)90202-3. [DOI] [PubMed] [Google Scholar]
- Sejvar JJ. The long-term outcomes of human West Nile virus infection. Clinical Infectious Diseases. 2007;44:1617–24. doi: 10.1086/518281. [DOI] [PubMed] [Google Scholar]
- Sim C, Denlinger DL. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proceedings of the National Academy of Sciences U S A. 2008;105:6777–81. doi: 10.1073/pnas.0802067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman A, Wong J. Studies on autogeny in natural populations of Culex pipiens. 3. Midsummer preparation for hibernation in anautogenous populations. Journal of Medical Entomology. 1973;10:319–24. doi: 10.1093/jmedent/10.4.319. [DOI] [PubMed] [Google Scholar]
- Spielman A. Structure and seasonality of nearctic Culex pipiens populations. Annals of the New York Academy of Sciences. 2001;951:220–34. doi: 10.1111/j.1749-6632.2001.tb02699.x. [DOI] [PubMed] [Google Scholar]
- Zhou G, Flowers M, Friedrich K, Horton J, Pennington J, Wells MA. Metabolic fate of [14C]-labeled meal protein amino acids in Aedes aegypti mosquitoes. Journal of Insect Physiology. 2004a;50:337–49. doi: 10.1016/j.jinsphys.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Zhou G, Pennington JE, Wells MA. Utilization of pre-existing energy stores of female Aedes aegypti mosquitoes during the first gonotrophic cycle. Insect Biochemistry and Molecular Biology. 2004b;34:919–25. doi: 10.1016/j.ibmb.2004.05.009. [DOI] [PubMed] [Google Scholar]