Abstract
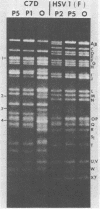
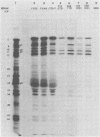
We report on the colonization of murine trigeminal ganglia after sequential infection of mice by herpes simplex viruses (HSVs). In preliminary studies, we have established that whereas the HSV-1(F) strain efficiently colonizes ganglia when inoculated by either the ear or eye routes, the HSV-1 X HSV-2 recombinant C7D colonizes ganglia when inoculated by the eye route only. The experimental design consisted of inoculating the right eye with C7D on day 1 and with HSV-1(F) in both left and right eyes on day 26. Both right and left trigeminal ganglia were removed and analyzed independently for latent virus on day 52. Our studies indicate that HSV-1(F) viruses were recovered from all left trigeminal ganglia but from only a small number of right trigeminal ganglia. Some right trigeminal ganglia yielded no viruses, whereas others yielded both C7D and HSV-1(F) viruses identified on the basis of plaque morphology and restriction enzyme cleavage patterns of viral DNA. The results indicate that more than one virus may colonize the same ganglion and that trigeminal ganglia may be protected from colonization by a superinfecting virus by determinants acting at a local level in the absence of demonstrable virus.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchman T. G., Roizman B., Adams G., Stover B. H. Restriction endonuclease fingerprinting of herpes simplex virus DNA: a novel epidemiological tool applied to a nosocomial outbreak. J Infect Dis. 1978 Oct;138(4):488–498. doi: 10.1093/infdis/138.4.488. [DOI] [PubMed] [Google Scholar]
- Centifanto-Fitzgerald Y. M., Varnell E. D., Kaufman H. E. Initial herpes simplex virus type 1 infection prevents ganglionic superinfection by other strains. Infect Immun. 1982 Mar;35(3):1125–1132. doi: 10.1128/iai.35.3.1125-1132.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Field H. J., Darby G. Pathogenicity in mice of strains of herpes simplex virus which are resistant to acyclovir in vitro and in vivo. Antimicrob Agents Chemother. 1980 Feb;17(2):209–216. doi: 10.1128/aac.17.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Wildy P. The pathogenicity of thymidine kinase-deficient mutants of herpes simplex virus in mice. J Hyg (Lond) 1978 Oct;81(2):267–277. doi: 10.1017/s0022172400025109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Klein R. J. Pathogenetic mechansims of recurrent herpes simplex virus infections. Arch Virol. 1976;51(1-2):1–13. doi: 10.1007/BF01317829. [DOI] [PubMed] [Google Scholar]
- Locker H., Frenkel N. BamI, KpnI, and SalI restriction enzyme maps of the DNAs of herpes simplex virus strains Justin and F: occurrence of heterogeneities in defined regions of the viral DNA. J Virol. 1979 Nov;32(2):429–441. doi: 10.1128/jvi.32.2.429-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofgren K. W., Stevens J. G., Marsden H. S., Subak-Sharpe J. H. Temperature-sensitive mutants of herpes simplex virus differ in the capacity to establish latent infections in mice. Virology. 1977 Jan;76(1):440–443. doi: 10.1016/0042-6822(77)90319-1. [DOI] [PubMed] [Google Scholar]
- Maitland N. J., Smith I. W., Peutherer J. F., Robertson D. H., Jones K. W. Restriction endonuclease analysis of DNA from genital isolates of herpes simplex virus type 2. Infect Immun. 1982 Dec;38(3):834–842. doi: 10.1128/iai.38.3.834-842.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKendall R. R. Efficacy of herpes simplex virus type 1 immunization in protecting against acute and latent infection by herpes simplex virus type 2 in mice. Infect Immun. 1977 May;16(2):717–719. doi: 10.1128/iai.16.2.717-719.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan J. L., Darby G. Herpes simplex virus latency: the cellular location of virus in dorsal root ganglia and the fate of the infected cell following virus activation. J Gen Virol. 1980 Dec;51(Pt 2):233–243. doi: 10.1099/0022-1317-51-2-233. [DOI] [PubMed] [Google Scholar]
- Morse L. S., Buchman T. G., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus DNA. IX. Apparent exclusion of some parental DNA arrangements in the generation of intertypic (HSV-1 X HSV-2) recombinants. J Virol. 1977 Oct;24(1):231–248. doi: 10.1128/jvi.24.1.231-248.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrild B., Ludwig H., Rott R. Identification of a common antigen of herpes simplex virus bovine herpes mammillitis virus, and B virus. J Virol. 1978 Jun;26(3):712–717. doi: 10.1128/jvi.26.3.712-717.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R. W., Walz M. A., Wohlenberg C., Notkins A. L. Latent infection of sensory ganglia with herpes simplex virus: efficacy of immunization. Science. 1975 May 30;188(4191):938–940. doi: 10.1126/science.166432. [DOI] [PubMed] [Google Scholar]
- Roizman B., Tognon M. Restriction enzyme analysis of herpesvirus DNA: stability of restriction endonuclease patterns. Lancet. 1982 Mar 20;1(8273):677–677. [PubMed] [Google Scholar]
- Sekizawa T., Openshaw H., Wohlenberg C., Notkins A. L. Latency of herpes simplex virus in absence of neutralizing antibody: model for reactivation. Science. 1980 Nov 28;210(4473):1026–1028. doi: 10.1126/science.6254149. [DOI] [PubMed] [Google Scholar]
- Smith I. W., Maitland N. J., Peutherer J. F., Robertson D. H. Restriction enzyme analysis of herpesvirus-2 DNA. Lancet. 1981 Dec 19;2(8260-61):1424–1424. doi: 10.1016/s0140-6736(81)92840-3. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Dunstan M. E. Herpes simplex virus thymidine kinase expression in infection of the trigeminal ganglion. Virology. 1979 Dec;99(2):417–422. doi: 10.1016/0042-6822(79)90021-7. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Miller R. L., Rapp F. Trigeminal ganglion infection by thymidine kinase-negative mutants of herpes simplex virus. Science. 1979 Aug 31;205(4409):915–917. doi: 10.1126/science.224454. [DOI] [PubMed] [Google Scholar]
- Wagner M. J., Summers W. C. Structure of the joint region and the termini of the DNA of herpes simplex virus type 1. J Virol. 1978 Aug;27(2):374–387. doi: 10.1128/jvi.27.2.374-387.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren K. G., Koprowski H., Lonsdale D. M., Brown S. M., Subak-Sharpe J. H. The polypeptide and the DNA restriction enzyme profiles of spontaneous isolates of herpes simplex virus type 1 from explants of human trigeminal, superior cervical and vagus ganglia. J Gen Virol. 1979 Apr;43(1):151–171. doi: 10.1099/0022-1317-43-1-151. [DOI] [PubMed] [Google Scholar]
- Wohlenberg C., Openshaw H., Notkins A. L. In vitro system for studying the efficacy of antiviral agents in preventing the reactivation of latent herpes simplex virus. Antimicrob Agents Chemother. 1979 Apr;15(4):625–627. doi: 10.1128/aac.15.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]