Abstract
Embryonic vision is generated and maintained by spontaneous neuronal activation patterns, yet extrinsic stimulation also sculpts sensory development. Because the sensory and motor systems are interconnected in embryogenesis, how extrinsic sensory activation guides multimodal differentiation is an important topic. Further, it is unknown whether extrinsic stimulation experienced near sensory sensitivity onset contributes to persistent brain changes, ultimately affecting postnatal behavior. To determine the effects of extrinsic stimulation on multimodal development, we delivered auditory stimulation to bobwhite quail groups during early, middle, or late embryogenesis, and then tested postnatal behavioral responsiveness to auditory or visual cues. Auditory preference tendencies were more consistently toward the conspecific stimulus for animals stimulated during late embryogenesis. Groups stimulated during middle or late embryogenesis showed altered postnatal species-typical visual responsiveness, demonstrating a persistent multimodal effect. We also examined whether auditory-related brain regions are receptive to extrinsic input during middle embryogenesis by measuring postnatal cellular activation. Stimulated birds showed a greater number of ZENK-immunopositive cells per unit volume of brain tissue in deep optic tectum, a midbrain region strongly implicated in multimodal function. We observed similar results in the medial and caudomedial nidopallia in the telencephalon. There were no ZENK differences between groups in inferior colliculus or in caudolateral nidopallium, avian analog to prefrontal cortex. To our knowledge, these are the first results linking extrinsic stimulation delivered so early in embryogenesis to changes in postnatal multimodal behavior and cellular activation. The potential role of competitive interactions between the sensory and motor systems is discussed.
Keywords: embryogenesis, extrinsic stimulation, multimodal behavior, midbrain, cellular activation
INTRODUCTION
It is well established that sensory function is sculpted in part by extrinsic activation during mammalian postnatal development (Wiesel and Hubel, 1963; von Melchner et al., 2000; Crowley and Katz, 2002; de Villers-Sidani et al., 2007). A less well-established but equally interesting finding is that some avian species (the precocial chicken, except where noted) become differentially responsive to extrinsic sensory activation toward the end of middle embryogenesis. In particular, a transition from reliance on intrinsic (spontaneous) to extrinsic auditory neuronal activation occurs in a graded fashion from embryonic age (E)12–E16 (Levic et al., 2007), culminating in differential, experience-dependent activation at ≈E16 (based on a 21-day incubation period, Jones et al., 2006). Robust spontaneous retinal activation is maintained later in embryogenesis (≈E13–E18, Wong et al., 1998). However, other indices of visual plasticity are evident earlier, which are coincident with the auditory transition to extrinsic activation. Specifically, massive retinal ganglion cell apoptosis (≈E11–E16, Hughes and McLoon, 1979; as cited in Mey and Thanos, 2000) and gradual retraction of direct (≈E10–E16, Williams and McLoon, 1991) and indirect (≈E9–E16, Wizenmann and Thanos, 1990) transient retinotectal projections co-occur with a wave of apoptosis throughout optic tectum (≈E15 and later, Zhang and Galileo, 1998). Like audition, vision is also receptive to external activation during this time (≈E15–E16, Heaton, 1973, in bobwhite quail; Saunders et al., 1973).
Optic tectum plasticity induction during middle embryogenesis raises the question of whether extrinsic auditory stimulation might affect orienting behaviors driven by the visual system, and induce related brain changes. Deep tectal stratum griseum centrale (SGC) neurons have wide dendritic fields in the superficial retinorecipient tectal layers and send ascending fibers to the visual thalamic nucleus rotundus (Luksch et al., 1998). Projections from auditory inferior colliculus to SGC are evident in mid-embryogenesis (semi-altricial owl, Nieder et al., 2003). Adult chicken SGC neurons show auditory response properties (Cotter, 1976), and adult pigeons possess both inferior collicular and tectobulbar efferents (Hunt and Künzle, 1976; Hellmann et al., 2004). Relatedly, projections from SGC and its neighbor, stratum album centrale (SAC), send sensory input to contralateral premotor craniocervical neurons in the brainstem reticular formation to control neck movements in adult mallard (Tellegen et al., 1998), and are evident as early as E5 in chicken (Kroger and Schwarz, 1990). An early wave of massive apoptosis occurs in SGC (≈E7.5–E8, Zhang and Galileo, 1998) before the formation of the later-developing retinorecipient layers, reflecting a phase of nonvisual tectum circuit refinement that may allow for primitive activation and strengthening of aural-motor excitatory connectivity prior to that of visual-motor coupling. Here we show that, like for many earlier studies, a prenatally unmanipulated bobwhite quail hatchling group shows attraction to a conspecific call compared with a heterospecific call 48 h after hatching. Extrinsic auditory stimulation delivered to bobwhite quail during late embryogenesis increases group-level consistency of postnatal auditory directed filial responsiveness. For visual tests, unmanipulated hatchlings and those aurally stimulated during early embryogenesis showed species-typical visually directed preferences for a conspecific hen 72 h after hatching. In contrast, auditory stimulation delivered during middle or late embryogenesis was linked to group-level shifts in proportions of visually directed responsiveness to chance levels. Also, cellular activation was greater in two of three SGC subregions, as well as the medial nidopallium (MN) and caudomedial nidopallium (NCM) for birds stimulated during middle embryo-genesis.
MATERIALS AND METHODS
Animals
Subjects were 294 bobwhite quail embryos (Colinus virginianus) incubated at 37.6°C and 73−78% humidity for the entire incubation period (E0–E23). Thereafter, hatchlings had free access to food and water under natural lighting conditions, and were housed with conspecific hatchlings from the same experimental group. Most animals underwent simultaneous choice tests; the remaining six underwent histology for detection of ZENK-immunopositive (IP) cells in several brain regions.
Embryonic Stimulation
We delivered auditory stimulation near the end of early (E8–E9), middle (E15–E16), or late (E22–E23) embryogenesis. A fourth unstimulated group served as a control. We drew embryos from a communally incubated batch of eggs, and separately exposed subgroups to their particular modified stimulation regimes in a different incubator. After stimulation offset, we returned embryo subgroups to the communal incubator. Control embryos remained in the communal incubator for the duration of embryogenesis.
We used a bobwhite quail embryonic contentment vocalization recording for our prenatal auditory stimulation (see Fig. 1) by continuously looping the call over 10-min intervals every hour for the 24-h period of stimulation (240 min of exposure). These vocalizations are typically produced during the last 24−36 h of incubation, following movement of the embryo into the air space, and facilitate synchronous hatching among embryos (Vince, 1964). Because our recording involved continuous looping of the call bout depicted in Figure 1, the delivered auditory stimulation was drastically stereotyped compared with naturalistic calls produced by fellow embryos, which are much more variable in intensity, pitch, prosody, and frequency. Recorded vocalization amplitude was set to peak at A-weighted 70 dB SPL directly under the sound source.
Figure 1.
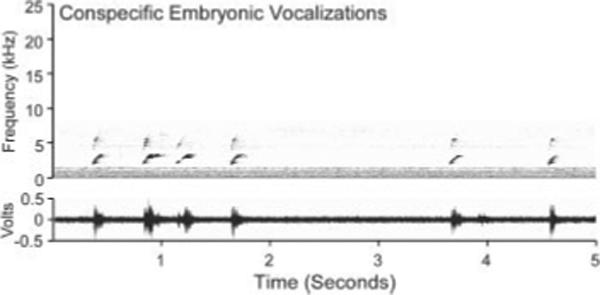
Spectrogram of the species-specific embryonic contentment call played to bobwhite quail embryos. Vocalizations contained no harmonics and ranged from 2 to 6 kHz. The dominant frequency ranged from ∼2 to 3.8 kHz.
Filial Simultaneous Choice Tests
We conducted simultaneous choice tests after hatching to investigate whether prenatally delivered auditory stimulation modified hatchlings’ species-specific auditory (n = 138) or visual (n = 150) recognition. Hatchlings from all conditions were group-exposed to the testing arena for 5 min, 24 h before choice tests to reduce freezing behavior. Behavioral testing apparatus and procedures are described in more detail elsewhere (Markham et al., 2006). For all tests, stimulus location was counterbalanced across subjects. Hatchlings were tested individually and only once.
One group underwent a simultaneous auditory choice test between the conspecific bobwhite and heterospecific scaled quail (Callipepla squamata) maternal assembly calls (see Fig. 2). Call amplitude was set to peak at A-weighted 65 dB SPL from the start location. Calls were continuously looped and simultaneously played for the duration of the test. We conducted auditory tests at post-natal age (P)48, a point when bobwhite quail prefer a conspecific call compared with a heterospecific call without any prior exposure (Lickliter and Virkar, 1989). A second group of hatchlings underwent simultaneous visual choice tests. These were identical to auditory tests, except that they involved a choice between taxidermically prepared models of the bobwhite quail and scaled quail hens. We used these hens to provide naturalistic visual stimuli during the tests. However, it must be noted that when luminosity was measured from the start location after the data were collected, the conspecific mount emitted six fewer lumens than the heterospecific mount when compared with the black backdrop alone for one approach area. Also, visual tests were multisensory, because earlier research indicates that auditory stimulation is required for visual approach elicitation (Lickliter and Virkar, 1989). Thus, visual tests included simultaneous delivery of the same conspecific bobwhite maternal assembly call via each speaker directly behind each hen. We conducted visual tests at P72, a time when unmanipulated lab-reared bobwhite quail chicks typically prefer the bobwhite quail hen compared with the scaled quail hen (Lickliter and Virkar, 1989).
Figure 2.
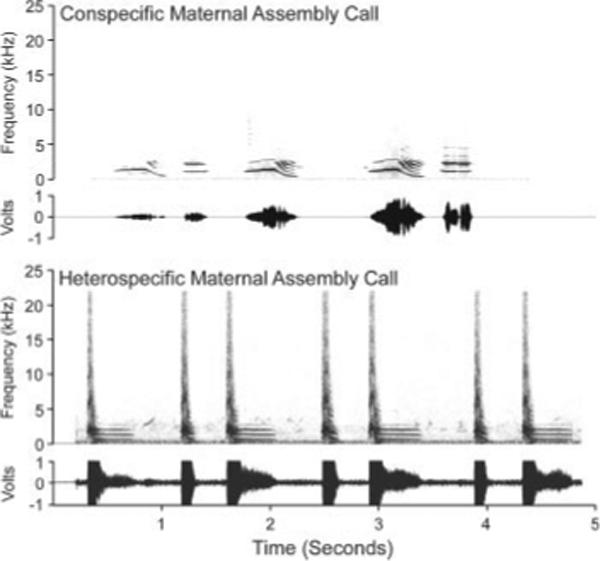
Spectrograms of the conspecific and heterospecific calls used in auditory choice tests. Although the two calls are similar in frequency range and harmonic structure, the heterospecific call has a more stereotyped prosody, a sharp broad-spectrum bark at the beginning of each call, and a notable lack of intrasyllabic inflection compared with the conspecific call. Both calls contain harmonics in the same frequency range as the embryonic contentment call in Figure 1.
Filial Choice Analysis
Choice data included latency to enter, and duration of time spent in each approach area. For within-group comparisons, we used Wilcoxon signed-ranks tests on the raw data (in sec) because typically they do not follow a Gaussian function (Lickliter and Hellewell, 1992). We generated scores for analysis of these data both within-(binomials, expected proportion = 0.5) and between-groups (χ2). Any subject that approached an area received a First Approach score (Conspecific; Heterospecific). Additionally, any subjects remaining in an approach area for more than twice as long as the opposing area received an individual Duration Preference score for the stimulus presented within. Birds not meeting this criterion were scored as no Duration Preference and were excluded from Duration Preference proportion analyses. Birds spending less than 10 sec in both approach areas were scored as duration nonresponders and excluded from all preference and raw statistical analyses.
Auditory-Related Cellular Activation
To investigate the effects of extrinsic embryonic auditory stimulation on the developmental trajectory of postnatal cellular activation, we exposed bobwhite quail embryos to either augmented auditory stimulation at the end of middle embryogenesis (E15–E16), or no embryonic stimulation. Thereafter, we exposed all hatchlings to identical postnatal auditory stimulation, and processed the brains for ZENK protein expression. ZENK is an inducible transcription factor that shows selectively robust expression to conspecific song in several auditory forebrain nuclei of many avian species, including ground feeding birds (Long and Salbaum, 1998; Terpstra et al., 2005).
To induce ZENK protein expression, animals from both conditions were communally isolated in a sound- and light-attenuated chamber, 24 h after hatching. Hatchlings were exposed to postnatal auditory stimulation in the chamber at the end of the isolation period. Auditory stimulation consisted of three different adult conspecific calls, peaking at A-weighted 70 dB SPL (Fig. 2 shows a spectrogram of one call). The calls were looped in succession for a 15 sec interval, followed by 45 sec of silence for 30 min. Subjects remained in the isolation chamber for 30−45 min after stimulation offset (adapted from Mello and Ribeiro, 1998).
ZENK Immunocytochemistry
After postnatal ZENK-inducing stimulation, subjects were deeply anesthetized by intraperitoneal injection of pentobarbitol, transcardially perfused with 0.9% saline followed by ice-cold 4% paraformaldehyde in phosphate-buffered saline, and their brains postfixed in 4% paraformaldehyde for 2 days at 4°C. Brains were then transferred to 2% paraformaldehyde, 15% sucrose at 4°C for 2 days. We parasagittally sectioned the left hemisphere in 40-μm increments on a sliding freezing microtome. Tissues were incubated as follows: (1) 30 min at room temperature (RT) in diluted hydrogen peroxide blocking solution; (2) overnight at 4°C in primary anti-ZENK antibody solution consisting of polyclonal anti-rabbit EGR-1 antiserum (Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:10,000 in 0.3% Triton X-100 PBS (primary); (3) 1 h at RT in biotin-conjugated anti-rabbit antiserum (1:200; Vector Laboratories, Burlingame, CA; secondary); (4) 1 h at RT in a solution of avidinbiotin complex (ABC Elite, Vector Laboratories), 0.025% 3′3 diaminobenzadine (DAB), and 0.3% hydrogen peroxide (tertiary). Tissues from different conditions were processed in the same staining dish to prevent differential rates of oxidation from confounding visualization of the probe. Alternate sections from each brain were stained with cresyl violet for morphological analysis.
ZENK Image Analysis and Cell Quantification
We first performed a qualitative survey at low magnification to identify brain regions that might contribute to the differences obtained in our behavioral tests. We observed no ZENK-immunopositive (IP) cells in entopallium and Field L2, the respective telencephalic targets of the tectofugal visual and auditory pathways. However, there were many labeled cells in higher sensory areas, including several auditory-related regions. This corroborates earlier findings that (a) isolated birds exposed to auditory stimulation do not show ZENK expression in primary sensory telencephalic regions (Mello and Ribeiro, 1998), and ZENK expression is differentially robust to conspecific auditory stimulation in our gallinaceous hatchlings. We counted ZENK-IP cells from five brain regions: internal auditory nucleus of inferior colliculus, or mesencephalicus lateralis pars dorsalis (MLd); SGC in optic tectum; MN, a telencephalic association area and part of the imprinting complex; NCM, a higher auditory area; and caudolateral nidopallium (NCL), the avian analog to prefrontal cortex.
To locate a given area of interest, we viewed adjacent Nissl-stained sections through a Leica MZ6 dissection microscope for detection of conservative morphological landmarks and distance from midline (Bayle et al., 1974; Kuenzel and Masson, 1988). We counted cells from one 125 × 125 μm2 (or 0.0156 mm2) sample frame per section for each region except for SGC, which we divided into three subregions (see Fig. 3). During counting, each SGC subregion was treated as a separate brain region. If a section was missing, we counted cells from the next adjacent ZENK-IP section. Slides were coded for blind cell counts across conditions. To avoid biasing the results, we counted all cells showing nucleation of ZENK protein, regardless of intensity. Counts were made via the optical-disector method, using the upper and left boundaries of the sampling frame and the top of the section as exclusion planes in the X-, Y-, and Z-planes, respectively (adapted from Harding et al., 1994). Apart from cells in the Z-exclusion plane, we counted all ZENK-IP cells throughout the entire section depth at 336× final magnification. Because we did not count from consecutive sections, we calculated coefficient of error (CE) using the conventional method that assumes independent samples (West and Gundersen, 1990). The overall CE median was 0.11.
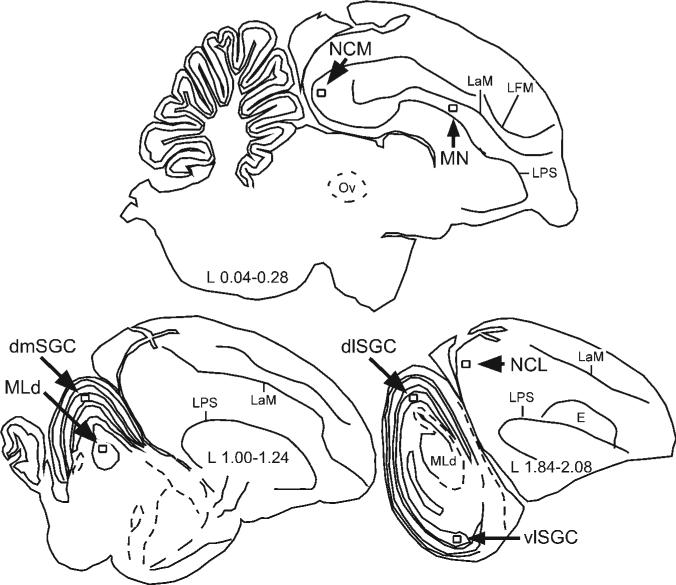
Figure 3.
Schematic parasagittal sections depicting locations of the counting frames (to scale) for neural regions of interest, including stratum griseum centrale (SGC) subregions in the optic tectum: mesencephalis lateralis pars dorsalis (MLd); ventrolateral SGC (vlSGC), dorsolateral SGC (dlSGC), and dorsomedial SGC (dmSGC); medial nidopallium (MN); caudomedial nidopallium (NCM); and caudolateral nidopallium (NCL). The laminae pallio-subpallialis (LPS) and mesopallialis (LaM) served as morphological landmarks for frame placement.
Estimates of ZENK-IP Cell Number per Unit of Tissue Volume
Using the Cavalieri method, we estimated sample volume as the product of the sample frame area (0.0156 mm2), the distance between section planes (0.12 mm), and the number of sections sampled (2). This yielded a summed sample volume, or ΣVFRAME = 0.003744 mm3. We then estimated ZENK-IP cell number per sample volume based on the formula NVCELLS = ΣQ−/ΣVFRAME, where ΣQ− is the sum of ZENK-IP cells counted (adapted from West and Gundersen, 1990). We performed paired-samples t-tests on NVCELLS between groups for each region.
RESULTS
Postnatal Auditory Responsiveness
A total of 118 chicks showed auditory responsiveness. For within-group analyses, Wilcoxon signed-ranks tests on the raw data indicate a shorter latency and longer duration to the conspecific call compared with the heterospecific call for birds in all groups (see Fig. 4). Proportions for First Approach and Duration Preference data are shown in Table 1. Binomials indicate all stimulated groups showed a greater proportion of First Approaches to the conspecific call. Duration Preference analyses indicate a greater proportion of preferences for the conspecific call in all groups, including controls.
Figure 4.
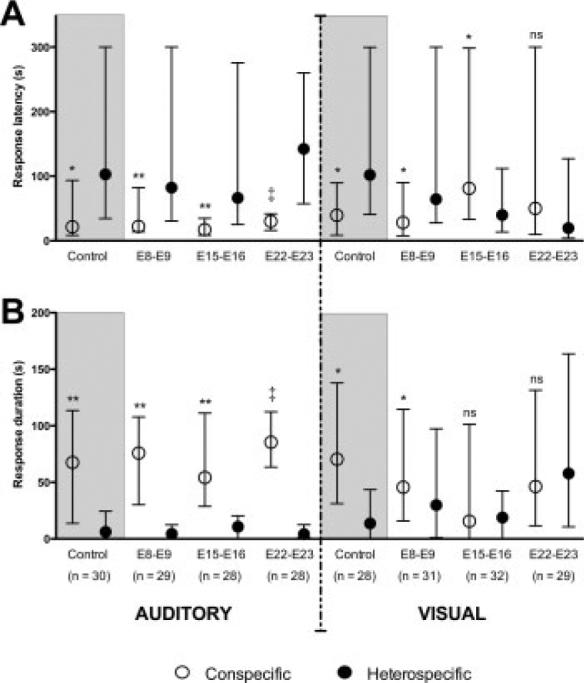
Effects of differently timed embryonic auditory stimulation on animals’ choice for filial auditory or visual stimuli. Control groups are shaded to aid visual comparison of within-group measures. A: For auditory latency, hatchlings in all four groups showed a faster response to the conspecific stimulus, and this effect was particularly robust for the E22–E23 group. In contrast, only animals from the control and E8–E9 groups showed a faster response to the conspecific stimulus in the visual choice tests. The E15–E16 group showed a faster response to the heterospecific stimulus. B: Duration scores are closely in line with latency data in response direction and strength, with the exception that the E15–E16 group showed no statistically significant difference for either stimulus in visual tests. Data signify median with interquartile range. Significance values represent those obtained from Wilcoxon signed-ranks tests, *p < 0.05; **p < 0.01; ‡p < 0.0001; ns, not significantly different.
Table 1.
Auditory Within-Group Results
| Control | E8-E9 | E15-E16 | E22-E23 | |
|---|---|---|---|---|
| First Approach | ||||
| Conspecific | 0.65 | 0.80* | 0.71* | 0.83* |
| Heterospecific | 0.35 | 0.20 | 0.29 | 0.17 |
| Duration Preference Score | ||||
| Conspecific | 0.72* | 0.86* | 0.80* | 1.00* |
| Heterospecific | 0.28 | 0.14 | 0.20 | 0.00 |
p < 0.05.
For between-group analyses, we performed twoway likelihood ratio chi-square (χ2) tests (Table 2) to evaluate proportion homogeneity between four groups (Control, E8–E9, E15–E16, and E22–E23) for two measures: First Approach (Conspecific vs. Heterospecific) and Duration Preference (Conspecific vs. Heterospecific). We used the Gamma test (G-test, or γ in the Tables) as our measure of association because of its appropriateness for ordinal data (Siegel and Castellan, 1988). For the auditory data, the omnibus χ2 was significant for Duration Preference only, for which we conducted post-hoc pairwise comparisons using Holm's sequential Bonferroni method to adjust for family wise error. Statistics indicate the evidence is sufficient to reject the hypothesis that the groups possess homogeneous proportions for only two comparisons. Specifically, proportions are likely heterogeneous between the Control and E22–E23 groups, and the E15–E16 and E22–E23 groups. G-tests reflect strong positive associations for all statistically significant heterogeneous pairwise comparisons.
Table 2.
Auditory Between-Group Results
| Comparison | χ2 (df, N/n) | p-value | α | Significance | γ |
|---|---|---|---|---|---|
| First Approach | |||||
| Omnibus | 3.285 (3, 118) | 0.349 | 0.05 | NS | +0.206 |
| Duration Preference Score | |||||
| Omnibus | 11.924 (3, 109) | 0.038 | 0.05 | * | +0.475 |
| Control vs. E22-E23 | 11.460 (1, 55) | 0.001 | 0.008 | * | +1.000 |
| E15-E16 vs. E22-E23 | 7.697 (1, 51) | 0.006 | 0.01 | * | +1.000 |
| E8-E9 vs. E22-E23 | 5.401 (1, 55) | 0.020 | 0.0125 | NS | +1.000 |
| Control vs. E8-E9 | 1.707 (1, 58) | 0.191 | 0.017 | NS | +0.408 |
| Control vs. E15-E16 | 0.426 (1, 54) | 0.514 | 0.025 | NS | +0.208 |
| E8-E9 vs. E15-E16 | 0.371 (1, 54) | 0.542 | 0.05 | NS | −0.220 |
Postnatal Visual Responsiveness
The same statistical tests were performed for identically generated scores from visual test data, with the addition of a No Duration Preference comparison (Preference vs. No Preference). A total of 126 chicks responded. For First Approach and Duration Preference binomials, only the unstimulated and early stimulated (E8–E9) groups showed a proportion weighted in the direction of the conspecific bobwhite hen (Table 3). Interestingly, despite the fact that the later stimulated groups showed no directional preference for either hen, most birds still showed an individual preference for one side, spending twice as much time in one approach area as the opposing one (reflected in the low proportion of No Duration Preference scores). Wilcoxon signed-ranks tests on raw data were generally consistent with First Approach and Duration Preference tests for the two hens (see Fig. 4), with the exception that the E15–E16 group showed a faster response to the heterospecific hen.
Table 3.
Visual Within-Group Results
| Control | E8-E9 | E15-E16 | E22-E23 | |
|---|---|---|---|---|
| First Approach | ||||
| Conspecific | 0.74* | 0.71* | 0.34 | 0.38 |
| Heterospecific | 0.26 | 0.29 | 0.66 | 0.62 |
| Duration Preference Score | ||||
| Conspecific | 0.78* | 0.83* | 0.46 | 0.36 |
| Heterospecific | 0.22 | 0.17 | 0.54 | 0.64 |
| No Duration Preference | ||||
| Preference | 0.96* | 0.77* | 0.88* | 0.96* |
| No Preference | 0.04 | 0.23 | 0.12 | 0.04 |
p < 0.05.
For between-group analyses, omnibuses suggested proportion heterogeneity among groups for First Approach and Duration Preference, but not for No Duration Preference comparison data (Table 4). Pairwise comparisons indicate sufficient evidence to reject the homogeneity assumption for the same group comparisons for both data sets with statistically significant omnibuses. For brevity, we report here in the text only those groups for which there was no evidence suggesting heterogeneity in the pairwise comparisons. Specifically, there was no heterogeneity between the Control and E8–E9 groups, or between the E15–E15 and E22–E23 groups. G-tests indicate negative association for all pairwise comparisons reflecting heterogeneity.
Table 4.
Visual Between-Group Results
| Comparison | χ2 (df, N/n) | p-value | α | Significance | γ |
|---|---|---|---|---|---|
| First Approach | |||||
| Omnibus | 17.251 (3, 126) | 0.001 | 0.05 | * | −0.468 |
| Control vs. E15-E16 | 10.352 (1, 63) | 0.001 | 0.008 | * | −0.692 |
| E8-E9 vs. E15-E16 | 8.875 (1, 66) | 0.003 | 0.01 | * | −0.642 |
| Control vs. E22-E23 | 8.042 (1, 60) | 0.004 | 0.0125 | * | −0.649 |
| E8-E9 vs. E22-E23 | 6.567 (1, 63) | 0.009 | 0.017 | * | −0.594 |
| Control vs. E8-E9 | 0.105 (1, 65) | 0.745 | 0.025 | NS | −0.090 |
| E15-E16 vs. E22-E23 | 0.083 (1, 61) | 0.773 | 0.05 | NS | +0.077 |
| Duration Preference Score | |||||
| Omnibus | 18.000 (3, 107) | 0.000 | 0.05 | * | −0.539 |
| E8-E9 vs. E22-E23 | 12.726 (1, 52) | 0.000 | 0.008 | * | −0.800 |
| Control vs. E22-E23 | 10.250 (1, 55) | 0.001 | 0.01 | * | −0.726 |
| E8-E9 vs. E15-E16 | 7.971 (1, 52) | 0.005 | 0.0125 | * | −0.705 |
| Control vs. E15-E16 | 5.867 (1, 55) | 0.015 | 0.017 | * | −0.603 |
| E15-E16 vs. E22-E23 | 0.666 (1, 56) | 0.415 | 0.025 | NS | −0.219 |
| Control vs. E8-E9 | 0.251 (1, 51) | 0.617 | 0.05 | NS | +0.176 |
| No Preference Comparisons | |||||
| Omnibus | 7.765 (3, 120) | 0.051 | 0.05 | NS | +0.106 |
ZENK-IP Cell Number
We conducted paired-samples t-tests to evaluate differences in ZENK-IP cell number per unit volume of tissue between groups for each brain region (see Fig. 5). ZENK-IP cell number was significantly greater for the stimulated group compared with the control group in four brain regions: dorsolateral SGC (dlSGC), t(2) 6.433, p = 0.023; dorsomedial SGC (dmSGC), t(2) = 24.401, p = 0.002; MN, t(2) = 5.429, p = 0.032; and NCM, t(2) = 5.562, p = 0.031. There were no labeled cell number differences between groups in MLd, ventrolateral SCG (vlSGC), or NCL. Implications of these results are discussed in more detail below.
Figure 5.
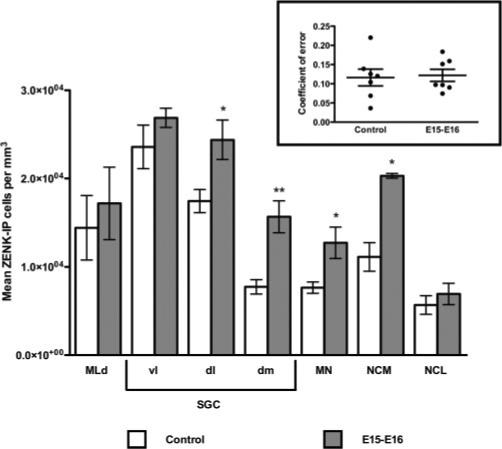
ZENK-IP cell number per unit tissue volume for each brain region of interest, for birds receiving prenatal stimulation during E15–E16, or no prenatal stimulation (controls). ZENK-IP cell number was significantly greater for dlSGC, dmSGC, MN, and NCM. Significance reflects values from paired-samples t-tests, *p < 0.05; **p < 0.01. Inset: coefficient of error for number of cells counted per brain, per region; dots are mean CEs per brain region, lines are means and SEMs per group.
DISCUSSION
Extrinsic Embryonic Auditory Stimulation Facilitates Auditory Filial Approach
Generally speaking, auditory choice results reflect typical responsiveness for all groups, with a few subtle yet important differences. Raw latency and duration analyses indicate birds in all groups responded faster to, and spent more time around the conspecific call compared with the heterospecific call. By contrast, First Approach binomials indicate the control group proportion did not differ from chance. This may signify that the egg handling during transfer between hatchers for stimulation purposes somehow facilitated only stimulated embryos’ development. Of course, it may also be the result of the auditory stimulation itself, but because the cochlear hair cells are not mature at E8–E9, this is unlikely. Further, the control group First Approach proportion trend was toward the conspecific call, and shared homogeneity with the stimulated groups. We posit that the within-group First Approach homogeneity for controls may be due to small effect size. Duration Preference binomials indicate the proportion of birds preferring the conspecific call was significantly greater than chance for all groups, and statistical significance differs by orders of magnitude in some cases. A between-group omnibus indeed suggests heterogeneity. Pairwise comparisons reveal the E22–E23 group to be most consistently heterogeneous compared with other groups. Overall, we interpret this as evidence for the Vince phenomenon, wherein auditory stimulation in late embryogenesis facilitates development as indicated by hatching synchronicity among differently aged bobwhite quail embryos (Vince, 1964).
Extrinsic Embryonic Auditory Stimulation Produces Divergent Visual Responsiveness
Only control and E8–E9 groups show species-typical visual responsiveness in within-group analyses. Control group results support previous findings. Cochlear mechanotransduction is absent before E11 in chick (Si et al., 2003), and it probably emerges slightly later for bobwhite quail due to their longer incubation time. Thus, it is logical that birds stimulated during E8–E9 responded similarly to controls. In contrast, there is an unexpected trend in the E15–E16 and E22–E23 within-group analyses toward heterospecific as opposed to conspecific attraction, with significance for the E15–E16 latency result. Perhaps either greater luminosity (see Methods) or novelty is the attractive feature during visual tests for later-stimulated groups. Specifically, it is possible that birds cannot see the darker hen against the black backdrop, and can only see the lighter heterospecific hen, inferring deficient visual function. On the other hand, support for the novelty hypothesis would suggest that vision is developmentally advanced for birds in one or both groups, further illustrating the Vince phenomenon. The prospect of novelty attraction is not outlandish in the context of previous research (Jackson and Bateson, 1974). Our hatchlings spent 3 days prior to tests becoming familiar with precocial conspecifics bearing plumage characteristics similar to those of the conspecific mount, and emitting calls with pros-ody similar to that played behind both mounts. It is possible that both phenomena are at play based on timing.
Relative multimodal activation is a third alternative mechanism underlying our visual chance-level analyses. The E22–E23 findings are discordant with observations that the same calls delivered day earlier (E21–E22) enhance visual filial responsiveness in bobwhite quail (relative to results from other experiments, Sleigh and Lickliter, 1996, p. 331). Embryos are receptive to extrinsic visual input through the eggshell late in development (Koshiba et al., 2003), and extrinsic, stereotyped auditory stimulation may facilitate evoked audiovisual coupling during this time, when there is low motor activation (early in E21 in bobwhite quail, Freeman and Vince, 1974). On the other hand, spontaneous motor activation during middle embryogenesis (Hamburger et al., 1965), and hatching motor movement just prior to birth (and after movement quiescence, Hamburger and Oppenheim, 1967), coupled with the same auditory stimulation may result in enhanced aural-motor coupling at the expense of the relatively less activated visual system. This hypothesis is further buttressed by results from postnatal stimulation experiments. Hatchlings engage in much motor movement. Bobwhite quail postnatally exposed to conspecific contentment calls while group-reared under normal lighting conditions show species-typical visual responsiveness (McBride and Lickliter, 1994). Here, the birds were exposed to concomitant and robust auditory, visual, and motor peripheral and central nervous activation. By comparison, postnatal auditory stimulation experienced in darkness, wherein auditory and motor activation are contiguous in the absence of visual activation, makes chickens subsequently less responsive to a flashing light (Bateson and Seaburne-May, 1973).
We have further preliminary support for our relative multimodal activation hypothesis. Although both E15–E16 and E22–E23 groups showed no attraction to the conspecific mount in visual tests, individuals still spent more time in one approach area. These results suggest that birds’ behavior in these groups was not based on visual discrimination, but rather due to equal attraction to the identical auditory stimulation played behind the hen mounts, resulting in random approach elicitation to one of the two areas between birds. Clearly, the role of relative multimodal coincident activation in producing differential behavioral responsiveness requires further testing.
Mid-Embryogenic Auditory Stimulation Increases Postnatal Cellular Activation
There were more ZENK-IP cells in two dorsal SGC subregions of prenatally stimulated birds, but not in a more ventrocaudal portion. Because both groups received identical postnatal ZENK-inducing stimulation, more postnatal ZENK expression in dorsal SGC for the prenatally stimulated group indicates it was somehow activated by auditory input. This is supported by the absence of ZENK-IP in superficial, solely retinorecipient tectal layers. Also, we counted ZENK-IP large nucleoli in SGC. These nucleoli may reside in quail cells homologous to pigeon SGC large multipolar premotor neurons (Hellmann et al., 2004). Sensory activation is strongly linked to translation of ZENK mRNA induced by motor activation in avian brain tissue (Whitney and Johnson, 2005). Perhaps some of our labeled SGC cells engage in both motor and auditory function. Subregional SGC cellular activation differences for the prenatally stimulated group likely mirror differently timed rostrocaudal and spatiotemporal optic tectum development (as evinced by oligodendrocytic activation, Galileo, 2003), along with different ventrodorsal functional subdomains (mallard duck, Tellegen et al., 1998; pigeon, Hell-mann et al., 2004).
There was also greater ZENK-IP cell number per unit volume in the auditory-related telencephalic regions MN and NCM for prenatally stimulated birds compared with unstimulated birds. MN and NCM neurons are robustly responsive to conspecific vocalizations in ground feeding birds (Thode et al., 2005). Our results are also concordant with ZENK expression analyses in adult female songbirds (Woolley and Doupe, 2008). Specifically, stereotyped song elicits more ZENK expression than less-stereotyped song in caudomedial mesopallium (a subregion of the imprinting complex, like MN), and our stimulation was highly stereotyped. Also, unfamilar song elicits more ZENK expression in songbird NCM than familiar song. The auditory stimulation was novel to our embryos. Here, greater cellular activation may reflect the presence of more newborn cells available for auditory recruitment into MN and possibly NCM. Postnatal call exposure facilitates the production of new neurons in chick intermediate mesopallium (Komissarova and Anokhin, 2008).
In contrast, though there was robust ZENK expression in MLd, between-group differences were absent. Again, this result replicates similar findings in adult female songbirds (Woolley and Doupe, 2008), and is in agreement with findings that MLd follows strict, nonspatial and tonotopic tuning characteristics in the juvenile barn owl (Knudsen and Konishi, 1978). Here, the presence of many ZENK-IP cells may reflect increases in synaptic efficacy between MLd and its postsynaptic targets, perhaps while MLd maintains its structural integrity. Finally, NCL receives indirect input from SGC via the visual nucleus rotundus and entopallium. We observed no differences in cellular activation in this mammalian pre-frontal cortex analog (Diekamp et al., 2002). It may only become attuned to auditory activation for the purpose of working memory, a process we did not manipulate.
Throughout this article we refer to our ZENK-IP results as evidence of cellular rather than neuronal activation. Although ZENK is tightly linked to neuronal activation (Knapska and Kaczmarek, 2004), it also plays a strong role in inflammation (Howe et al., 2006). Because we did not double-label the tissue in this experiment, identification of perikarya surrounding ZENK-IP nucleoli was not possible. Perhaps extrinsic embryonic auditory experience prolonged survival of more auditory-related cells at the time of stimulation, and then postnatal auditory experience induced apoptosis and/or process degeneration due to extrinsically induced circuit refinement. Here, some or all of the labeled cells we observed could have been glia activated for phagocytosis.
Conclusion and Future Directions
To our knowledge, this is the first demonstration that stimulation delivered during middle embryogenesis can have enduring effects on postnatal multimodal behavior and the developmental trajectory of cellular activation in the brain, despite the highly plastic nature of the nervous system during early development. Clever experiments are needed to isolate the hypotheses we generated from our data. Specifically, is luminosity preference, novelty preference, or relative multimodal activation the predominating factor impinging on our observed behavioral differences? Do the underlying mechanisms of the extrinsic sensory effects differ based on timing? Investigation of physiological and neural mechanisms underlying these behavioral phenomena would in part help to answer these questions. Perhaps repetitive and restricted extrinsic auditory activation delivered during midembryogenesis can rescue some tectal neurons normally slated for an apoptotic fate in an activity-dependent manner, for use in audition. Along with the possibility of newborn cell induction and migration, and phagocytosis, this putative mechanism is not necessarily exclusive to region or timing. Further, nor are the two mechanisms mutually exclusive from one another. In sum, how extrinsic auditory activation during embryogenesis can influence the sculpting of neuronal circuits in the deeper tectal layers is an important question germane to the development of the neural substrates underlying midbrain multisensory and sensorimotor function.
Acknowledgments
The authors especially thank Tadd Patton for assistance with tissue processing and manuscript review; Peter Navarro and Isaac Tourgmann for assistance with data collection; and Theresa Jones for technical assistance and use of Neurolucida. Experiments were approved in advance by the FIU IACUC and complied with the “Principles of Animal Care” publication No. 86−23, revised 1985, of the National Institutes of Health.
Contract grant sponsor: NIH; contract grant numbers: MH62225, HD048423.
REFERENCES
- Bateson PP, Seaburne-May G. Effects of prior exposure to light on chicks’ behaviour in the imprinting situation. Anim Behav. 1973;21:720–725. doi: 10.1016/s0003-3472(73)80097-1. [DOI] [PubMed] [Google Scholar]
- Bayle JD, Ramade F, Oliver J. Stereotaxic topography of the brain of the quail (Coturnix coturnix japonica). J Physiol Paris. 1974;68:219–241. [PubMed] [Google Scholar]
- Cotter JR. Visual and nonvisual units recorded from the optic tectum of Gallus domesticus. Brain Behav Evol. 1976;13:1–21. doi: 10.1159/000123798. [DOI] [PubMed] [Google Scholar]
- Crowley JC, Katz LC. Ocular dominance development revisited. Curr Opin Neurobiol. 2002;12:104–109. doi: 10.1016/s0959-4388(02)00297-0. [DOI] [PubMed] [Google Scholar]
- de Villers-Sidani E, Chang EF, Bao S, Merzenich MM. Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat. J Neurosci. 2007;27:180–189. doi: 10.1523/JNEUROSCI.3227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekamp B, Kalt T, Güntürkün O. Working memory neurons in pigeons. J Neurosci. 2002;22:RC210. doi: 10.1523/JNEUROSCI.22-04-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman BM, Vince MA. Development of the Avian Embryo. Chapman and Hall; London: 1974. [Google Scholar]
- Galileo DS. Spatiotemporal gradient of oligodendrocyte differentiation in chick optic tectum requires brain integrity and cell-cell interactions. Glia. 2003;41:25–37. doi: 10.1002/glia.10163. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Balaban M, Oppenheim R, Wenger E. Periodic motility of normal and spinal chick embryos between 8 and 17 days of incubation. J Exp Zool. 1965;159:1–13. doi: 10.1002/jez.1401590102. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Oppenheim R. Prehatching motility and hatching behavior in the chick. J Exp Zool. 1967;166:171–203. doi: 10.1002/jez.1401660203. [DOI] [PubMed] [Google Scholar]
- Harding AJ, Halliday GM, Cullen K. Practical considerations for the use of the optical disector in estimating neuronal number. J Neurosci Methods. 1994;51:83–89. doi: 10.1016/0165-0270(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Heaton MB. Early visual function in bobwhite and Japanese quail embryos as reflected by pupillary reflex. J Comp Physiol Psychol. 1973;84:134–139. doi: 10.1037/h0035055. [DOI] [PubMed] [Google Scholar]
- Hellmann B, Güntürkün O, Manns M. Tectal mosaic: Organization of the descending tectal projections in comparison to the ascending tectofugal pathway in the pigeon. J Comp Neurol. 2004;472:395–410. doi: 10.1002/cne.20056. [DOI] [PubMed] [Google Scholar]
- Howe CL, Mayoral S, Rodriguez M. Activated microglia stimulate transcriptional changes in primary oligodendrocytes via IL-1β. Neurobiol Dis. 2006;23:731–739. doi: 10.1016/j.nbd.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Hughes WF, McLoon SC. Ganglion cell death during normal retinal development in the chick: Comparisons with cell death induced by early target field destruction. Exp Neurol. 1979;66:587–601. doi: 10.1016/0014-4886(79)90204-8. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Künzle H. Observations on the projections and intrinsic organization of the pigeon optic tectum: An autoradiographic study based on anterograde and retrograde, axonal and dendritic flow. J Comp Neurol. 1976;170:153–172. doi: 10.1002/cne.901700203. [DOI] [PubMed] [Google Scholar]
- Jackson PS, Bateson PP. Imprinting and exploration of slight novelty in chicks. Nature. 251:1974, 609–610. doi: 10.1038/251609a0. [DOI] [PubMed] [Google Scholar]
- Jones TA, Jones SM, Paggett KC. Emergence of hearing in the chicken embryo. J Neurophysiol. 2006;96:128–141. doi: 10.1152/jn.00599.2005. [DOI] [PubMed] [Google Scholar]
- Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog Neurobiol. 2004;74:183–211. doi: 10.1016/j.pneurobio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Konishi M. Space and frequency are represented separately in auditory midbrain of the owl. J Neurophysiol. 1978;41:870–884. doi: 10.1152/jn.1978.41.4.870. [DOI] [PubMed] [Google Scholar]
- Komissarova NV, Anokhin KV. Effects of an imprinting procedure on cell proliferation in the chick brain. Neurosci Behav Physiol. 2008;38:289–296. doi: 10.1007/s11055-008-0041-z. [DOI] [PubMed] [Google Scholar]
- Koshiba M, Nakamura S, Deng C, Rogers LJ. Light-dependent development of asymmetry in the ipsilateral and contralateral thalamofugal visual projections of the chick. Neurosci Lett. 2003;336:81–84. doi: 10.1016/s0304-3940(02)01162-x. [DOI] [PubMed] [Google Scholar]
- Kroger S, Schwarz U. The avian tectobulbar tract: Development, explant culture, and effects of antibodies on the pattern of neurite outgrowth. J Neurosci. 1990;10:3118–3134. doi: 10.1523/JNEUROSCI.10-09-03118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzel WJ, Masson M. A Stereotaxic Atlas of the Brain of the Chick (Gallus domesticus). Johns Hopkins University Press; Baltimore, MD: 1988. [Google Scholar]
- Levic S, Nie L, Tuteja D, Harvey M, Sokolowski BH, Yamoah EN. Development and regeneration of hair cells share common functional features. Proc Natl Acad Sci USA. 2007;104:19108–19113. doi: 10.1073/pnas.0705927104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickliter R, Hellewell TB. Contextual determinants of auditory learning in bobwhite quail embryos and hatchlings. Dev Psychobiol. 1992;25:17–31. doi: 10.1002/dev.420250103. [DOI] [PubMed] [Google Scholar]
- Lickliter R, Virkar P. Intersensory functioning in bobwhite quail chicks: Early sensory dominance. Dev Psychobiol. 1989;22:651–667. doi: 10.1002/dev.420220702. [DOI] [PubMed] [Google Scholar]
- Long KD, Salbaum JM. Evolutionary conservation of the immediate-early gene ZENK. Mol Biol Evol. 1998;15:284–292. doi: 10.1093/oxfordjournals.molbev.a025925. [DOI] [PubMed] [Google Scholar]
- Luksch H, Cox K, Karten HJ. Bottlebrush dendritic endings and large dendritic fields: Motion-detecting neurons in the tectofugal pathway. J Comp Neurol. 1998;396:399–414. [PubMed] [Google Scholar]
- Markham RG, Toth G, Lickliter R. Prenatally elevated physiological arousal interferes with perceptual learning in bobwhite quail (Colinus virginianus) embryos. Behav Neurosci. 2006;120:1315–1325. doi: 10.1037/0735-7044.120.6.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride T, Lickliter R. Specific postnatal auditory stimulation interferes with species-typical visual responsiveness in bobwhite quail chicks. Dev Psychobiol. 1994;27:169–183. doi: 10.1002/dev.420270304. [DOI] [PubMed] [Google Scholar]
- Mello CV, Ribeiro S. ZENK protein regulation by song in the brain of songbirds. J Comp Neurol. 1998;393:426–438. doi: 10.1002/(sici)1096-9861(19980420)393:4<426::aid-cne3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Mey J, Thanos S. Development of the visual system of the chick. I. Cell differentiation and histogenesis. Brain Res Brain Res Rev. 2000;32:343–379. doi: 10.1016/s0165-0173(99)00022-3. [DOI] [PubMed] [Google Scholar]
- Nieder B, Wagner H, Luksch H. Development of output connections from the inferior colliculus to the optic tectum in barn owls. J Comp Neurol. 2003;464:511–524. doi: 10.1002/cne.10827. [DOI] [PubMed] [Google Scholar]
- Saunders JC, Coles RB, Gates GR. The development of auditory evoked responses in the cochlea and cochlear nuclei of the chick. Brain Res. 1973;63:59–74. doi: 10.1016/0006-8993(73)90076-0. [DOI] [PubMed] [Google Scholar]
- Si F, Brodie H, Gillespie PG, Vazquez AE, Yamoah EN. Developmental assembly of transduction apparatus in chick basilar papilla. J Neurosci. 2003;23:10815–10826. doi: 10.1523/JNEUROSCI.23-34-10815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S, Castellan NJ. Nonparametric Statistics for the Behavioral Sciences. McGraw Hill; Boston, MA: 1988. pp. 19–36. [Google Scholar]
- Sleigh MJ, Lickliter R. Type and amount of prenatal stimulation alters perceptual responsiveness in bobwhite quail chicks. Infant Behav Dev. 1996:19. [Google Scholar]
- Tellegen AJ, Karssen AM, Rietveld TM, Dubbeldam JL. A crossed projection from the optic tectum to craniocervical premotor areas in the brainstem reticular formation. An anterograde and retrograde tracing study in the mallard (Anas platyrhynchos L.). Eur J Morphol. 1998;36:227–243. doi: 10.1076/ejom.36.4.227.5823. [DOI] [PubMed] [Google Scholar]
- Terpstra NJ, Bolhuis JJ, Den Boer-Visser AM, Ten Cate C. Neuronal activation related to auditory perception in the brain of a non-songbird, the ring dove. J Comp Neurol. 2005;488:342–351. doi: 10.1002/cne.20592. [DOI] [PubMed] [Google Scholar]
- Thode C, Bock J, Braun K, Darlison MG. The chicken immediate-early gene ZENK is expressed in the medio-rostral neostriatum/hyperstriatum ventrale, a brain region involved in acoustic imprinting, and is up-regulated after exposure to an auditory stimulus. Neuroscience. 2005;130:611–617. doi: 10.1016/j.neuroscience.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Vince MA. Synchronization of hatching in American Bobwhite Quail (Colinus virginianus). Nature. 1964;203:1192–1193. doi: 10.1038/2031192a0. [DOI] [PubMed] [Google Scholar]
- von Melchner L, Pallas SL, Sur M. Visual behaviour mediated by retinal projections directed to the auditory pathway. Nature. 2000;404:871–876. doi: 10.1038/35009102. [DOI] [PubMed] [Google Scholar]
- West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- Whitney O, Johnson F. Motor-induced transcription but sensory-regulated translation of ZENK in socially interactive songbirds. J Neurobiol. 2005;65:251–259. doi: 10.1002/neu.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Effects of visual deprivation on morphology and physiology of cells in the cat's lateral geniculate body. J Neurophysiol. 1963;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- Williams CV, McLoon SC. Elimination of the transient ipsilateral retinotectal projection is not solely achieved by cell death in the developing chick. J Neurosci. 1991;11:445–453. doi: 10.1523/JNEUROSCI.11-02-00445.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wizenmann A, Thanos S. The developing chick isthmo-optic nucleus forms a transient efferent projection to the optic tectum. Neurosci Lett. 1990;113:241–246. doi: 10.1016/0304-3940(90)90591-v. [DOI] [PubMed] [Google Scholar]
- Wong WT, Sanes JR, Wong RO. Developmentally regulated spontaneous activity in the embryonic chick retina. J Neurosci. 1998;18:8839–8852. doi: 10.1523/JNEUROSCI.18-21-08839.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SC, Doupe AJ. Social context-induced song variation affects female behavior and gene expression. PLoS Biol. 2008;6:e62. doi: 10.1371/journal.pbio.0060062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Galileo DS. Widespread programmed cell death in early developing chick optic tectum. Neurore-port. 1998;9:2797–2801. doi: 10.1097/00001756-199808240-00021. [DOI] [PubMed] [Google Scholar]