Abstract
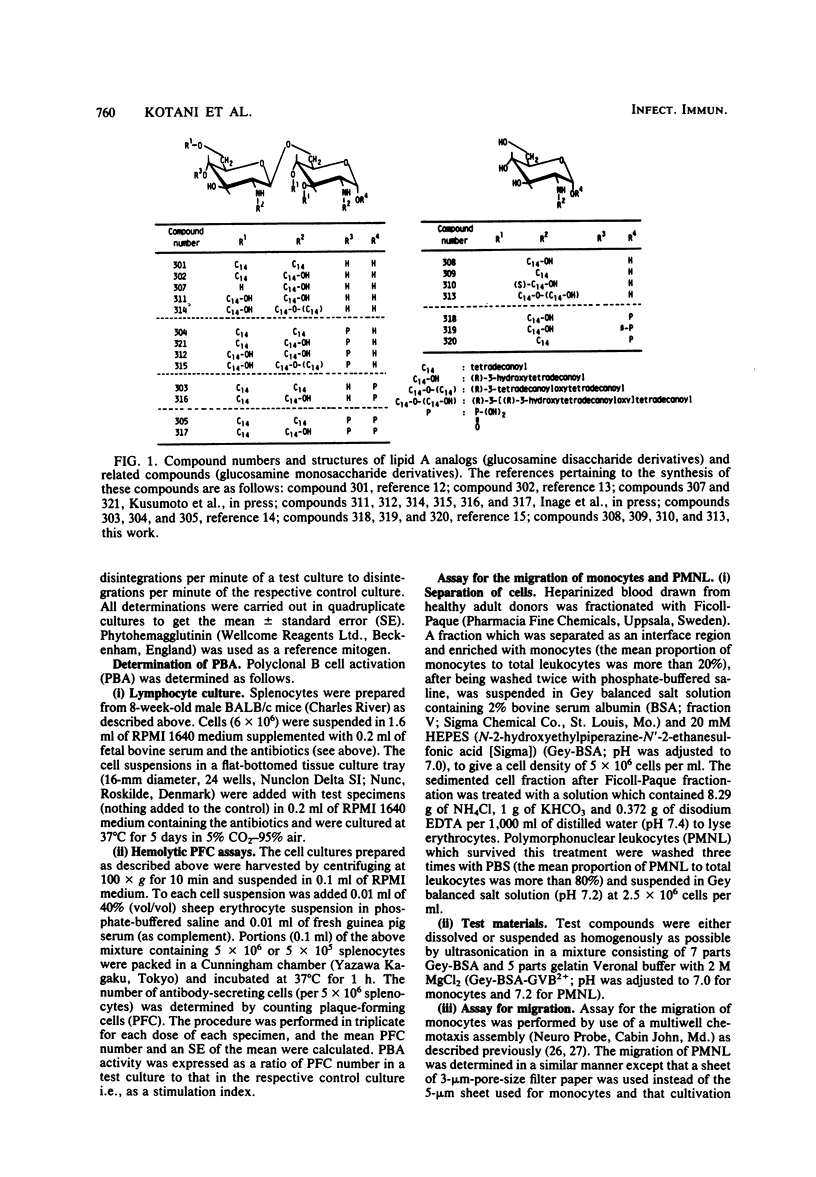
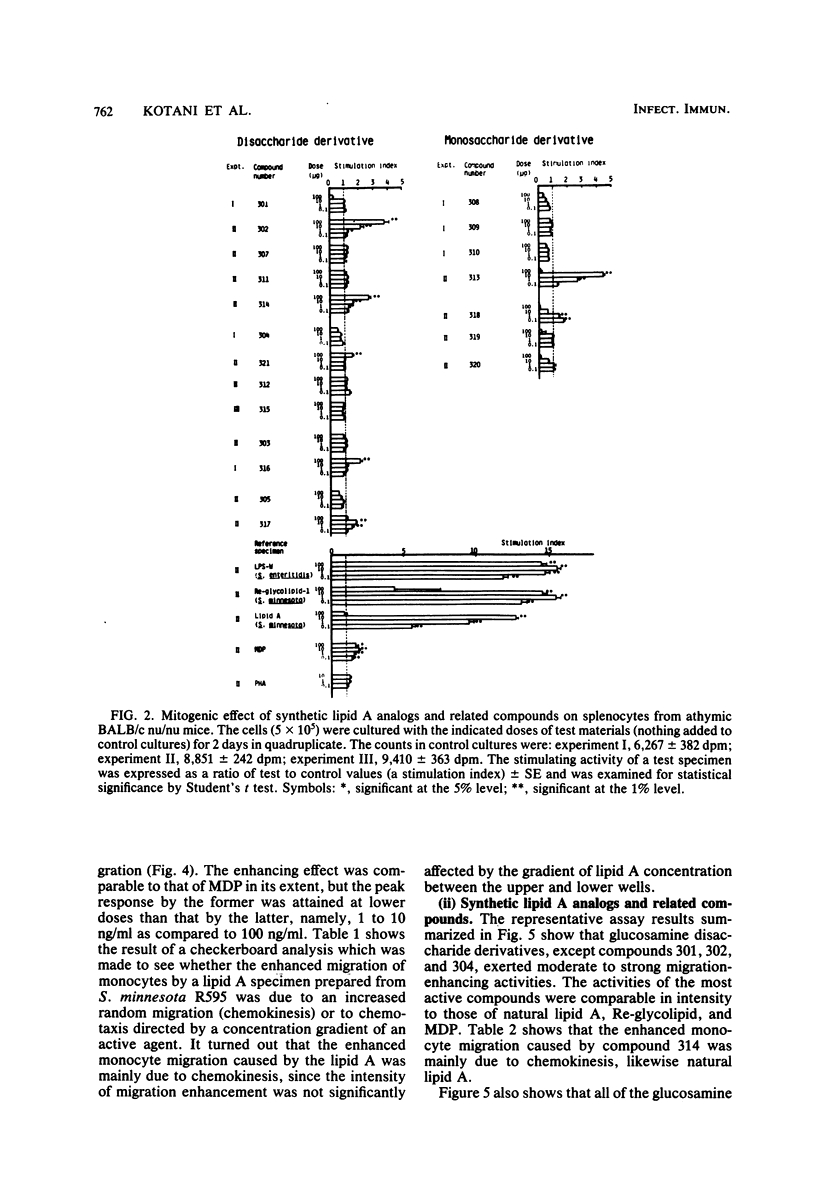
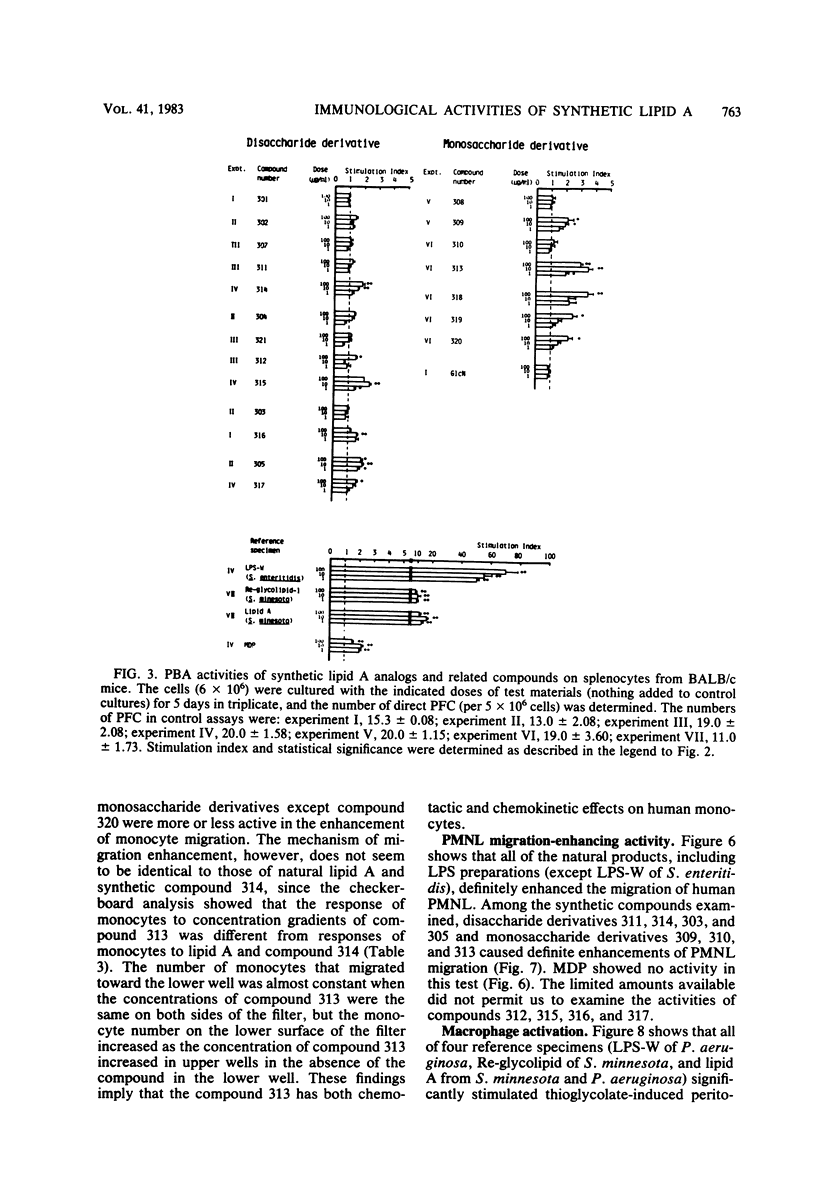
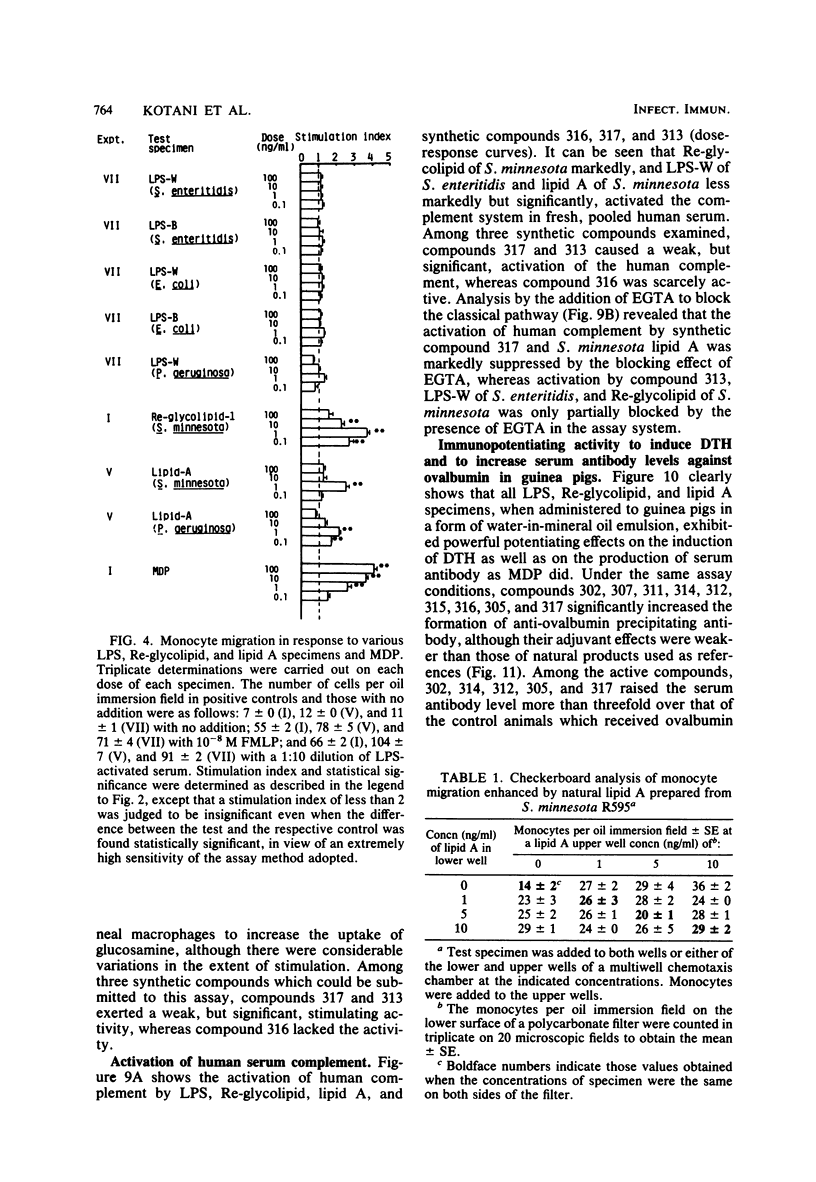
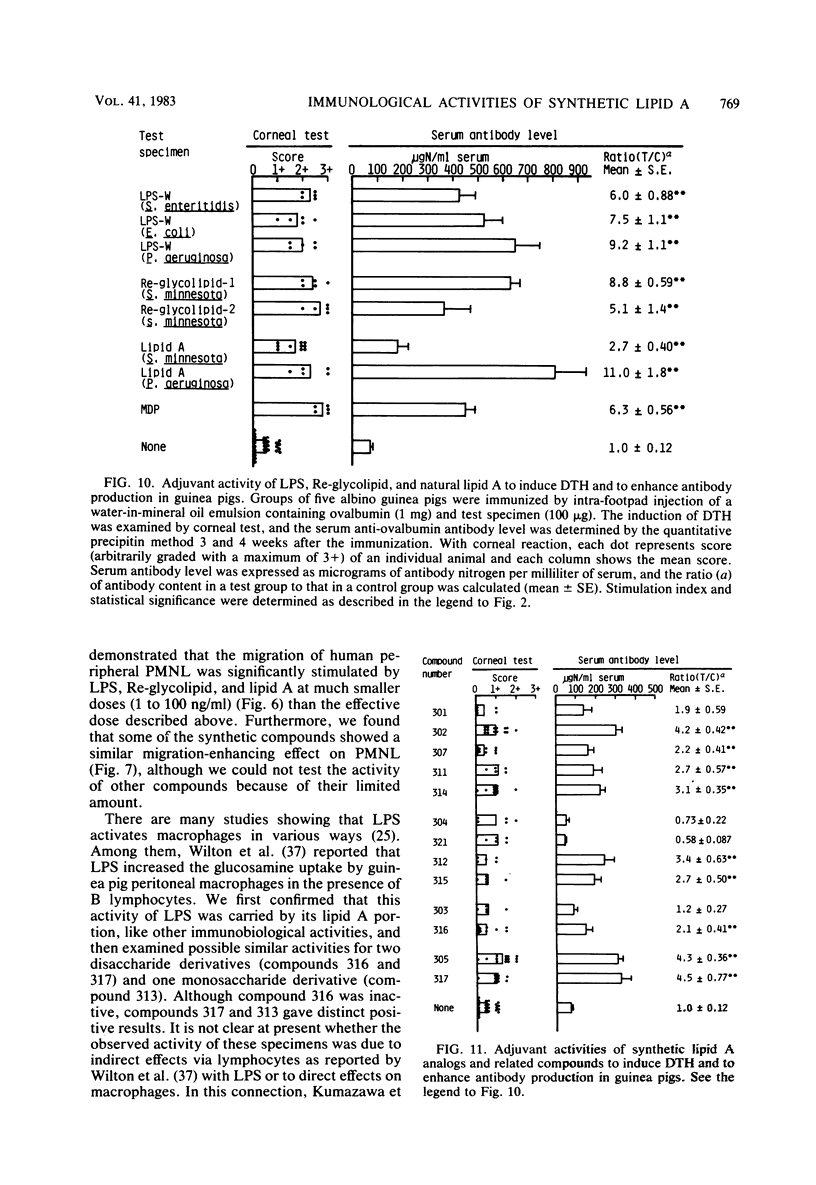
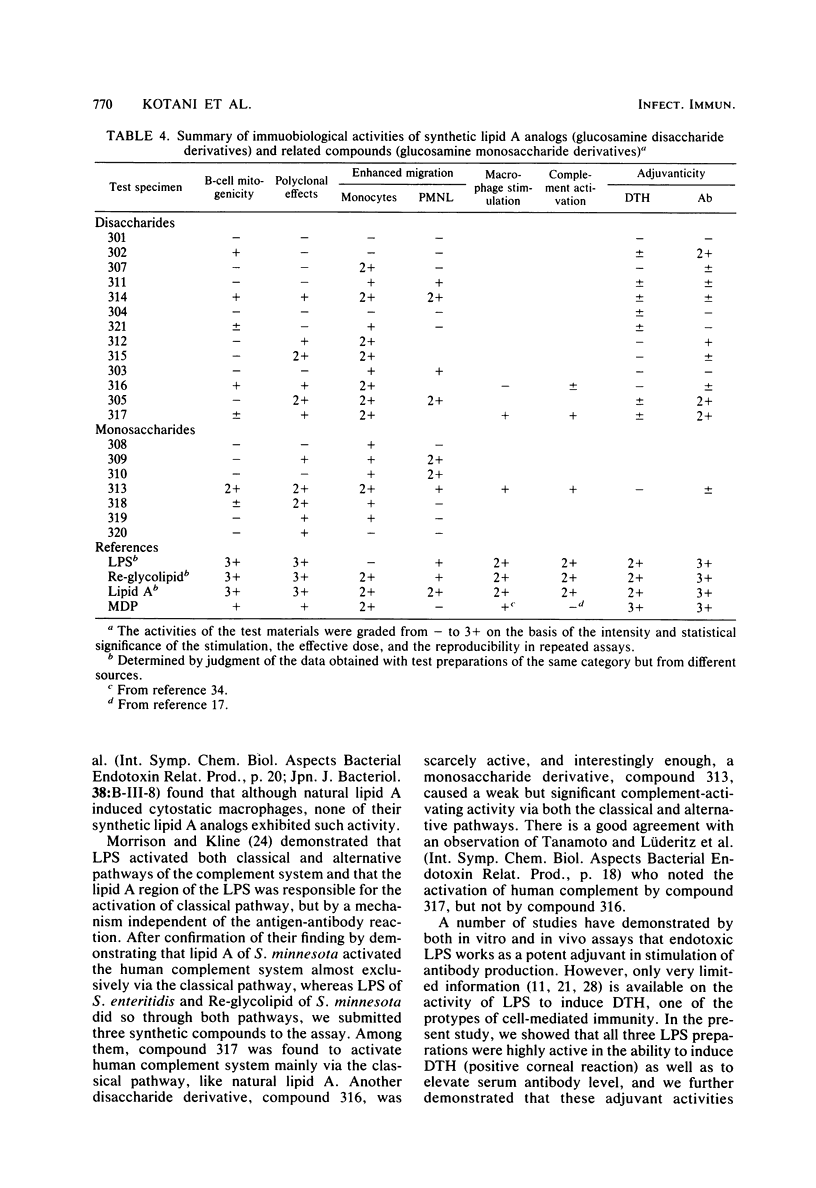
Thirteen acylated and phosphorylated derivatives of beta-1,6-linked glucosamine disaccharide (lipid A analogs), which were synthesized after the structural model of Salmonella-type lipid A, and seven similar derivatives of glucosamine monosaccharide (lipid A-related compounds) were studied for their immunobiological activities. These included mitogenicity and polyclonal B cell activation enhancement of migration of monocytes and polymorphonuclear leukocytes derived from human peripheral blood, stimulation of guinea pig peritoneal macrophages, activation of human complement, and stimulation of serum antibody production and induction of delayed-type hypersensitivity against ovalbumin in guinea pigs. Comparisons were made with lipid A, RE-glycolipid, lipopolysaccharide of natural sources, and a well-known synthetic adjuvant, N-acetylmuramyl-L-alanyl-D-isoglutamine. Some of the lipid A analogs were found to manifest the mitogenic, polyclonal B cell-activating macrophage-stimulating, complement-activating, and immunostimulating activities, although the observed activities were generally far less than those of natural products in intensity and efficiency. Other immunobiological effects exhibited by most of the synthetic lipid A analogs were the enhancement of migration of monocytes and polymorphonuclear leukocytes. It is premature to draw definite conclusions on structure-activity relationships, since a few compounds which were active in some assay systems were scarcely active in other assays. However, an indisputable fact was that beta-1,6-glucosamine disaccharide 1 alpha,4'-diphosphate, which carries two amide-bound (R)-3-hydroxytetradecanoyl and three ester-bound tetradecanoyl residues, and thus has the structure most closely resembling natural lipid A among test compounds in this study, was definitely active in all of the present assay systems. However, its potency was generally much less than natural products. Some of glucosamine monosaccharide derivatives, especially N-(R)-3-[(R)-3-hydroxytetradecanoyloxy]tetradecanoyl glucosamine, also exerted all of the in vitro activities described above. This fact suggests that a glucosamine disaccharide structure may not necessarily be a prerequisite as far as the in vitro immunobiological activities tested are concerned.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Petit J. F., Lefrancier P., Lederer E. Muramyl peptides. Chemical structure, biological activity and mechanism of action. Mol Cell Biochem. 1981 Dec 4;41:27–47. doi: 10.1007/BF00225295. [DOI] [PubMed] [Google Scholar]
- Adamu S. A., Sperry J. F. Polymorphonuclear neutrophil chemotaxis induced and inhibited by Bacteroides spp. Infect Immun. 1981 Sep;33(3):806–810. doi: 10.1128/iai.33.3.806-810.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedid L., Audibert F., Johnson A. G. Biological activities of muramyl dipeptide, a synthetic glycopeptide analogous to bacterial immunoregulating agents. Prog Allergy. 1978;25:63–105. [PubMed] [Google Scholar]
- Egawa K., Kasai N. Endotoxic glycolipid as a potent depressor of the hepatic drug-metabolizing enzyme systems in mice. Microbiol Immunol. 1979;23(2):87–94. doi: 10.1111/j.1348-0421.1979.tb00444.x. [DOI] [PubMed] [Google Scholar]
- Fensom A. H., Gray G. W. The chemical composition of the lipopolyacarideof Pseudomonas aeruginosa. Biochem J. 1969 Sep;114(2):185–196. doi: 10.1042/bj1140185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Rietschel E. T., Lüderitz O., Westphal O. Interaction of lipopolysaccharides and lipid A with complement. Eur J Biochem. 1971 Mar 1;19(1):143–152. doi: 10.1111/j.1432-1033.1971.tb01298.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Rietschel E. T., Lüderitz O., Westphal O., Kim Y. B., Watson D. W. Biological activities of lipid A complexed with bovine-serum albumin. Eur J Biochem. 1972 Dec 4;31(2):230–233. doi: 10.1111/j.1432-1033.1972.tb02524.x. [DOI] [PubMed] [Google Scholar]
- Inal S., Nagaki K., Ebisu S., Kato K., Kotani S. Activation of the alternative complement pathway by water-insoluble glucans of streptococcus mutans: the relation between their chemical structures and activating potencies. J Immunol. 1976 Oct;117(4):1256–1260. [PubMed] [Google Scholar]
- Kawasaki A. [Activation of human complement system by bacterial cell walls, their water-soluble enzymatic extracts and related synthetic compounds]. Osaka Daigaku Shigaku Zasshi. 1982 Jun;27(1):46–61. [PubMed] [Google Scholar]
- Kim Y. B., Watson D. W. Biologically active endotoxins from Salmonella mutants deficient in O- and R-polysaccharides and heptose. J Bacteriol. 1967 Nov;94(5):1320–1326. doi: 10.1128/jb.94.5.1320-1326.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Narita T., Stewart-Tull D. E., Shimono T., Watanabe Y. Immunoadjuvant activities of cell walls and their water-soluble fractions prepared from various gram-positive bacteria. Biken J. 1975 Jun;18(2):77–92. [PubMed] [Google Scholar]
- Lüderitz O., Galanos C., Lehmann V., Mayer H., Rietschel E. T., Weckesser J. Chemical structure and biological activities of lipid A's from various bacterial families. Naturwissenschaften. 1978 Nov;65(11):578–585. doi: 10.1007/BF00364907. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Kline L. F. Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS). J Immunol. 1977 Jan;118(1):362–368. [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Kotani S., Fukuda K., Tsukamoto Y., Mori M., Kusumoto S., Shiba T. Stimulation of migration of human monocytes by bacterial cell walls and muramyl peptides. Infect Immun. 1982 Dec;38(3):817–824. doi: 10.1128/iai.38.3.817-824.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Kotani S., Kusumoto S., Shiba T. Possible chemotaxis of human monocytes by N-acetylmuramyl-L-alanyl-D-isoglutamine. Infect Immun. 1983 Jan;39(1):449–451. doi: 10.1128/iai.39.1.449-451.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M., Nakashima I., Kato N. Adjuvant action of bacterial lipopolysaccharide in induction of delayed-type hypersensitivity to protein antigens. Cell Immunol. 1982 Jan 1;66(1):111–120. doi: 10.1016/0008-8749(82)90162-9. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Wollenweber H. W., Zähringer U., Lüderitz O. Lipid A, the lipid component of bacterial lipopolysaccharides: relation of chemical structure to biological activity. Klin Wochenschr. 1982 Jul 15;60(14):705–709. doi: 10.1007/BF01716559. [DOI] [PubMed] [Google Scholar]
- Rosner M. R., Tang J., Barzilay I., Khorana H. G. Structure of the lipopolysaccharide from an Escherichia coli heptose-less mutant. I. Chemical degradations and identification of products. J Biol Chem. 1979 Jul 10;254(13):5906–5917. [PubMed] [Google Scholar]
- Sveen K. Rabbit polymorphonuclear leukocyte migration in vitro in response to lipopolysaccharides from Bacteroides, Fusobacterium and Veillonella. Acta Pathol Microbiol Scand B. 1977 Dec;85B(6):374–380. doi: 10.1111/j.1699-0463.1977.tb01992.x. [DOI] [PubMed] [Google Scholar]
- Takada H., Tsujimoto M., Kato K., Kotani S., Kusumoto S., Inage M., Shiba T., Yano I., Kawata S., Yokogawa K. Macrophage activation by bacterial cell walls and related synthetic compounds. Infect Immun. 1979 Jul;25(1):48–53. doi: 10.1128/iai.25.1.48-53.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal O., Jann K., Himmelspach K. Chemistry and immunochemistry of bacterial lipopolysaccharides as cell wall antigens and endotoxins. Prog Allergy. 1983;33:9–39. [PubMed] [Google Scholar]
- Wilton J. M., Rosenstreich D. L., Oppenheim J. J. Activation of guinea pig macrophages by bacterial lipopolysaccharide requires bone marrow-derived lymphocytes. J Immunol. 1975 Jan;114(1 Pt 2):388–393. [PubMed] [Google Scholar]
- Yasuda T., Kanegasaki S., Tsumita T., Tadakuma T., Homma J. Y., Inage M., Kusumoto S., Shiba T. Biological activity of chemically synthesized analogues of lipid A. Demonstration of adjuvant effect in hapten-sensitized liposomal system. Eur J Biochem. 1982 May 17;124(2):405–407. [PubMed] [Google Scholar]



