Abstract
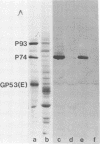
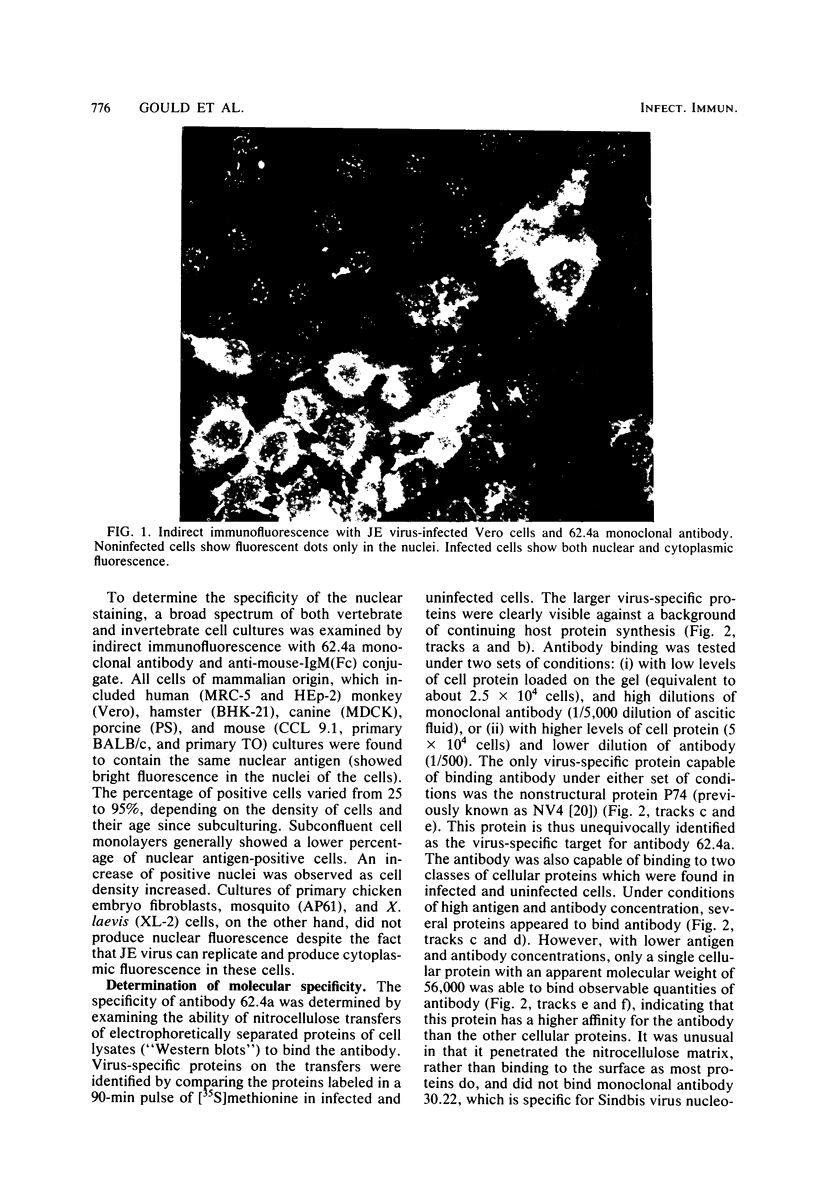
An immunoglobulin M (IgM) class monoclonal antibody raised against Japanese encephalitis virus reacted with an epitope on the nonstructural virus protein P74 (NV4 in the old nomenclature) of several flaviviruses and also with an antigen present in the nuclei of a variety of mammalian cell types. This antigen had a characteristic granular distribution by immunofluorescence and may correspond to a polypeptide of molecular weight 56,000 seen in nitrocellulose transfers of sodium dodecyl sulfate-polyacrylamide gels. Cross-reactivity with nuclear antigen was also occasionally observed in the IgM antibody fraction of mice early after infection with Japanese encephalitis virus and also in acute sera from some clinical cases of encephalitis containing IgM antibody to Japanese encephalitis virus.
Full text
PDF


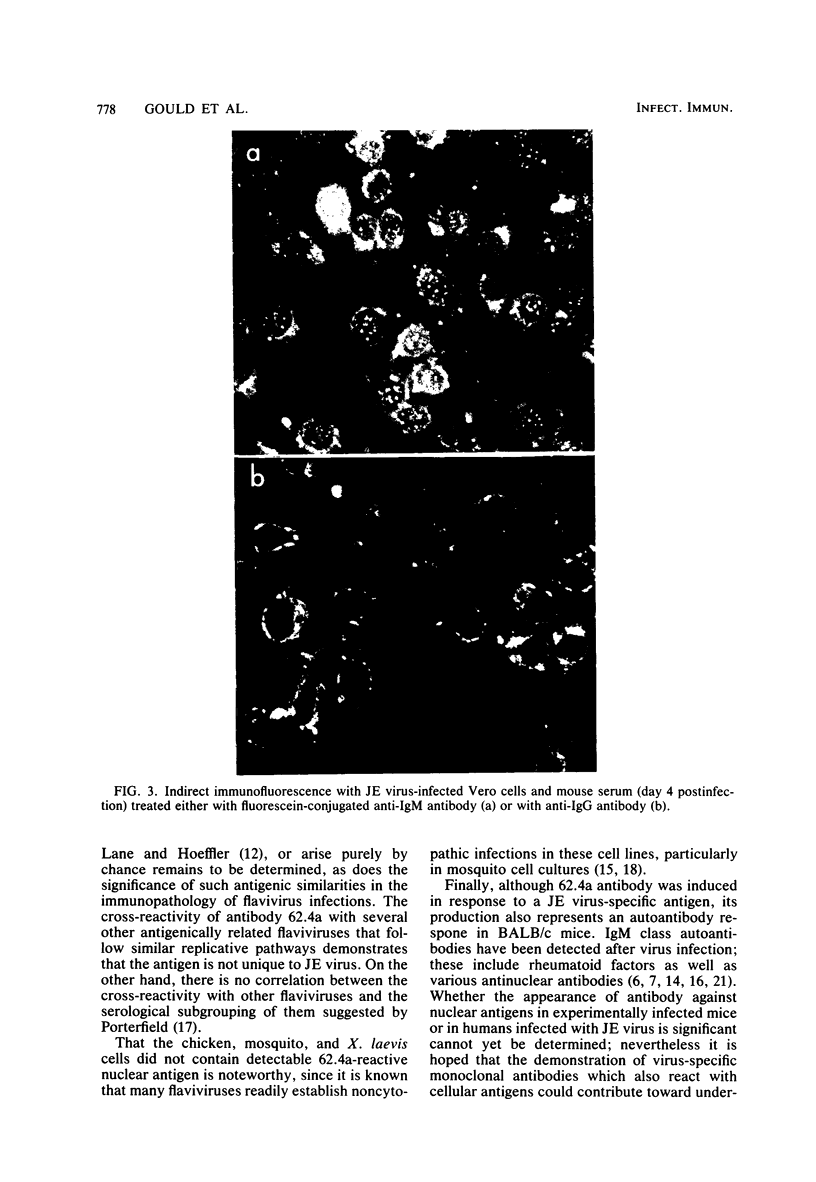

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chanas A. C., Gould E. A., Clegg J. C., Varma M. G. Monoclonal antibodies to Sindbis virus glycoprotein E1 can neutralize, enhance infectivity, and independently inhibit haemagglutination or haemolysis. J Gen Virol. 1982 Jan;58(Pt 1):37–46. doi: 10.1099/0022-1317-58-1-37. [DOI] [PubMed] [Google Scholar]
- Clegg J. C. Glycoprotein detection in nitrocellulose transfers of electrophoretically separated protein mixtures using concanavalin A and peroxidase: application to arenavirus and flavivirus proteins. Anal Biochem. 1982 Dec;127(2):389–394. doi: 10.1016/0003-2697(82)90192-0. [DOI] [PubMed] [Google Scholar]
- Crawford L., Leppard K., Lane D., Harlow E. Cellular proteins reactive with monoclonal antibodies directed against simian virus 40 T-antigen. J Virol. 1982 May;42(2):612–620. doi: 10.1128/jvi.42.2.612-620.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRESNER E., TROMBLY P. The latex-fixation reaction in nonrheumatic diseases. N Engl J Med. 1959 Nov 12;261:981–988. doi: 10.1056/NEJM195911122612001. [DOI] [PubMed] [Google Scholar]
- De Madrid A. T., Porterfield J. S. The flaviviruses (group B arboviruses): a cross-neutralization study. J Gen Virol. 1974 Apr;23(1):91–96. doi: 10.1099/0022-1317-23-1-91. [DOI] [PubMed] [Google Scholar]
- JOHNSON R. E., HALL A. P. Rubella arthritis; report of cases studied by latex tests. N Engl J Med. 1958 Apr 10;258(15):743–745. doi: 10.1056/NEJM195804102581506. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kos K. A., Osborne B. A., Goldsby R. A. Inhibition of group B arbovirus antigen production and replication in cells enucleated with cytochalasin B. J Virol. 1975 Apr;15(4):913–917. doi: 10.1128/jvi.15.4.913-917.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Hoeffler W. K. SV40 large T shares an antigenic determinant with a cellular protein of molecular weight 68,000. Nature. 1980 Nov 13;288(5787):167–170. doi: 10.1038/288167a0. [DOI] [PubMed] [Google Scholar]
- Lane D., Koprowski H. Molecular recognition and the future of monoclonal antibodies. Nature. 1982 Mar 18;296(5854):200–202. doi: 10.1038/296200a0. [DOI] [PubMed] [Google Scholar]
- Langenhuysen M. M. Antibodies against -globulin after blood transfusion and cytomegalovirus-infection. Clin Exp Immunol. 1971 Sep;9(3):393–398. [PMC free article] [PubMed] [Google Scholar]
- Leake C. J., Varma M. G., Pudney M. Cytopathic effect and plaque formation by arboviruses in a continuous cell line (XTC-2) from the toad Xenopus laevis. J Gen Virol. 1977 May;35(2):335–339. doi: 10.1099/0022-1317-35-2-335. [DOI] [PubMed] [Google Scholar]
- Markenson J. A., Daniels C. A., Notkins A. L., Hoofnagle J. H., Gerety J., Barker L. F. The interaction of rheumatoid factor with hepatitis B surface antigen-antibody complexes. Clin Exp Immunol. 1975 Feb;19(2):209–217. [PMC free article] [PubMed] [Google Scholar]
- Pudney M., Varma M. G., Leake C. J. Establishment of a cell line (XTC-2) from the South African clawed toad, Xenopus laevis. Experientia. 1973 Apr 15;29(4):466–467. doi: 10.1007/BF01926785. [DOI] [PubMed] [Google Scholar]
- SVEC K. H., DINGLE J. H. THE OCCURENCE OF RHEUMATOID FACTOR IN ASSOCIATION WITH ANTIBODY RESPONSE TO INFLUENZA A2(ASIAN) VIRUS. Arthritis Rheum. 1965 Aug;8:524–529. doi: 10.1002/art.1780080406. [DOI] [PubMed] [Google Scholar]
- Shapiro D., Kos K. A., Russell P. K. Protein synthesis in Japanese encephalitis virus-infected cells. Virology. 1973 Nov;56(1):95–109. doi: 10.1016/0042-6822(73)90290-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma M. G., Pudney M., Leake C. J., Peralta P. H. Isolations in a mosquito (Aedes pseudoscutellaris) cell line (Mos. 61) of yellow fever virus strains from original field material. Intervirology. 1975;6(1):50–56. doi: 10.1159/000149453. [DOI] [PubMed] [Google Scholar]