Abstract
Social relations between humans critically depend on our affective experiences of others. Oxytocin enhances prosocial behavior, but its effect on humans' affective experience of others is not known. We tested whether oxytocin influences affective ratings, and underlying brain activity, of faces that have been aversively conditioned. Using a standard conditioning procedure, we induced differential negative affective ratings in faces exposed to an aversive conditioning compared with nonconditioning manipulation. This differential negative evaluative effect was abolished by treatment with oxytocin, an effect associated with an attenuation of activity in anterior medial temporal and anterior cingulate cortices. In amygdala and fusiform gyrus, this modulation was stronger for faces with direct gaze, relative to averted gaze, consistent with a relative specificity for socially relevant cues. The data suggest that oxytocin modulates the expression of evaluative conditioning for socially relevant faces via influences on amygdala and fusiform gyrus, an effect that may explain its prosocial effects.
Keywords: oxytocin, amygdala, fusiform gyrus, face stimuli, gaze, affective value
Introduction
Social animals need to strike a balance between approach and avoidance behavior toward others. Although avoidance may diminish risk of harm, approach is necessary for social activities, including mating, protection of offspring, hunting, and group formation. Oxytocin, a nanopeptide produced within hypothalamic paraventricular nuclei, modulates these processes in animals (Insel et al., 2001; Young, 2002; Debiec, 2005; Lim and Young, 2006) and facilitates prosocial behavior by both increasing approach but also suppressing avoidance (Bartz and Hollander, 2006; Hammock and Young, 2006; Carter, 2007; Heinrichs and Gaab, 2007). In humans, the state of our social relations is often reflected in how we emotionally experience others (Singer et al., 2006). Thus, an oxytocin effect in humans should be evident in altered behavior (Kosfeld et al., 2005) and also in how we affectively experience others. Here, we specifically tested whether affective ratings of faces are modulated by oxytocin treatment while indexing the associated neuronal correlates of this effect.
Both threat (Phelps, 2006) and social (Haxby et al., 2002; Adolphs and Spezio, 2006) signals activate amygdala, which in turn modulates cortical areas involved in emotional and social processing. One expression of this is augmentation of activity in fusiform face area (FFA) to fearful facial expressions (Vuilleumier and Pourtois, 2007), in addition to influences on subcortical and brainstem structures involved in behavioral fear responses (Davis and Whalen, 2001). The amygdala contains dense concentrations of oxytocin receptors (Insel and Shapiro, 1992; Veinante and Freund-Mercier, 1997) which regulate its activity (Huber et al., 2005). Monogamous species, compared with polygamous species, differ in concentrations of oxytocin receptors in several brain regions, including the amygdala (Insel and Shapiro, 1992). An effect on amygdala may provide a potential mechanism whereby oxytocin influences prosocial behavior, specifically by suppressing social avoidance responses (Bartz and Hollander, 2006; Hammock and Young, 2006; Carter, 2007; Heinrichs and Gaab, 2007).
In humans, it has been shown previously that oxytocin attenuates neural responses to aversive pictures, including emotional face expressions (Kirsch et al., 2005; Domes et al., 2007). The behavioral relevance of this attenuation and its implications for social cues remain unaddressed. In this study, we assessed both the neural effects of oxytocin and its impact on affective responses to faces associated with fear as a function of their social relevance. Our experiment involved presentation of face stimuli that had previously been fear conditioned (CS+) or not (CS−) by pairing with shocks. We then assessed whether oxytocin had any effect on the conditioning-induced change in affective ratings of faces, specifically evaluative conditioning effects, as our primary outcome measure. We used stimuli that were matched closely in visual input but differed in social relevance, with direct gaze conveying a more salient social signal (Haxby et al., 2002). Our key hypothesis was that oxytocin, but not placebo, treatment would attenuate negative affective ratings of CS+ relative to CS− faces, an effect associated with modulation of amygdala and FFA responses.
Materials and Methods
Subjects.
Thirty right-handed healthy male subjects were included in the study, which was approved by the local ethical committee (Department of Neurology and Neurosurgery, University College London, London, UK). The subjects had no history of mental or psychiatric disorder. Before the study, subjects provided written informed consent. Three subjects were excluded from the analysis, two because they showed a high degree of drowsiness and had closed eyes in the scanner and one because of movement artifacts in the imaging data.
Experimental design.
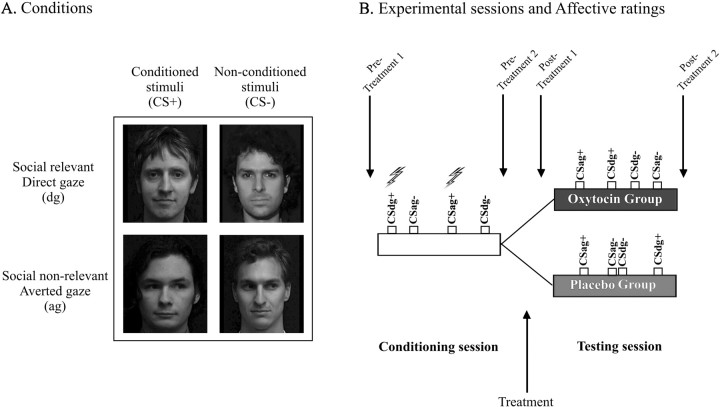
Subjects were first fear conditioned while brain activity was measured using functional magnetic resonance imaging (fMRI) (data reported elsewhere). The conditioned stimuli consisted of four faces of different identities, two with direct gaze and two with averted gaze (taken from George et al., 2001) (Fig. 1). Two of the faces (CS+) [one with direct gaze (CSdg+) and one with averted gaze (CSag+)] were paired with an electric shock [unconditioned stimulus (UCS)] with a 50% contingency. The other two faces (CS−) [one with direct gaze (CSdg−) and one with averted gaze (CSag−)] were never paired with shock.
Figure 1.
A, Stimuli. Four different face identities were used randomized over subjects across the four conditions defined by social relevance (direct vs averted gaze) and fear conditioning (CS+ vs CS−). B, Design. In a conditioning session, the CS+ faces were paired with shock with a 50% contingency. After conditioning, subjects were treated with either oxytocin or placebo. Treatment effects on affective associations to the faces were assessed in a testing session including likeability ratings and fMRI measurement of brain activity.
After fear conditioning, subjects were removed from the scanner and instructed how to inhale a nasal spray that contained either placebo or oxytocin (oxytocin group: n = 15 subjects, mean age of 25.5 years, age range of 19–40 years; placebo group: n = 12 subjects, mean age of 24.2 years, age range of 19–39 years). Subjects were randomly assigned to each group in a double-blind design such that none of the investigators knew which treatment was assigned to each subject. The treatments were coded, and the codes were opened only on completion of the entire experiment. It has been shown previously that nanopeptides pass the blood–brain barrier reliably after intranasal application (Born et al., 2002). Several studies using this method have reported oxytocin-dependent effects on behavior or brain function (Pitman et al., 1993; Heinrichs et al., 2003, 2004; Kirsch et al., 2005; Kosfeld et al., 2005; Domes et al., 2007a). Subjects administered the spray four times with a delay of 45 s between administrations, each administration consisting of one inhalation of the spray into each nostril. Every inhalation contains ∼4 IU such that subjects in the oxytocin group received 32 IU of oxytocin in total.
Forty-five minutes after drug treatment, subjects were brought back into the scanner, shock electrodes were applied, and they were tested again. Neuronal activation to face presentations was measured using fMRI, providing an indirect index of the strength of the aversive associations to the different CS. During this testing phase, subjects were shown the same four faces as during conditioning but without aversive reinforcement through a UCS. Each face appeared 15 times for 990 ms each, with a jittered intrastimulus time between 9000 and 12,600 ms. Order of faces was pseudorandomized (with the restriction that the same condition could not appear three times in a row). The faces were randomly presented either in the center or 5 mm to the right or to the left of the center. The subject had to indicate where the face was shown as fast and accurately as possible using three different keys on a right-hand button box. This cognitive task was intended to ensure subjects would be attentive to the stimuli and to provide a measure of conditioning-induced changes in reaction time (RT). Skin conductance was measured continuously from two electrodes on the index and middle fingers of the left hand, using an AT64 SCR apparatus (Autogenic Systems). Both RT changes and skin conductance responses (SCRs) to CS presentations have been used previously as measures of fear conditioning and its expression (Gottfried and Dolan, 2004; Phelps et al., 2004; Kalisch et al., 2006; Milad et al., 2007). Total duration of testing was 12 min.
Our primary outcome was affective ratings in response to presentation of faces that were exposed to a fear conditioning and nonconditioning manipulation (Fig. 1). Before conditioning (pretreatment 1), subjects were instructed to indicate how sympathetic each face was on a 0–100 visual-analog scale in which 0 meant that that they did not perceive them as sympathetic at all and 100 meant that they perceived them as the most sympathetic person they could imagine. The subjects again completed the same rating after conditioning but before treatment (pretreatment 2) and twice after treatment, once directly before the testing session (posttreatment 1) and once directly after the testing session (posttreatment 2) (Fig. 1). We defined an index of evaluative conditioning as a change in likeability of CS− minus the change in likeability of CS+ (because we expected the conditioning procedure to entail a decrease in likeability of CS+ vs CS− faces). The pretreatment change in affective ratings was thus defined as (ratings of CS− after the conditioning phase vs ratings of CS− before the conditioning phase) versus (ratings of CS+ after the conditioning phase vs ratings of CS+ before the conditioning). The evaluative conditioning index for “posttreatment 1” rating was defined as (ratings of CS− after the treatment but before testing phase vs ratings of CS− before the conditioning phase) versus (ratings of CS+ after the treatment but before testing phase vs ratings of CS+ before conditioning phase). Similarly, the evaluative conditioning index for “posttreatment 2” rating was defined as (ratings of CS− after treatment and the testing phase vs ratings of CS− before the conditioning phase) versus (ratings of CS+ after treatment and the testing phase vs ratings of CS+ before the conditioning phase).
Subjects rated their subjective mood on a visual-analog scale featuring 17 pairs of words (supplemental Table 1, available at www.jneurosci.org as supplemental material) once before conditioning (pretreatment 1) and once after treatment directly before testing (posttreatment 1). They also rated adverse effects on a seven-item physical symptoms rating scale (supplemental Table 2, available at www.jneurosci.org as supplemental material) once before conditioning (pretreatment 1), once after treatment directly before testing (posttreatment 1), and once after testing (posttreatment 2). A fear-related effect on SCR was defined as the SCR response for CS+ (CSdg+ and CSag+) versus CS− (CSdg− and CSag−). SCRs were Z-normalized to reduce interindividual variability (Kalisch et al., 2006). The significance of SCR and RT effects was tested using parametric statistics, whereas the significance of subjective effects was assessed nonparametrically. Because we performed a nonparametric analysis on the affective ratings, we primarily focused on treatment differences in general effects of conditioning and did not include gaze in the model. However, in SCR and reaction time analysis, we included also gaze in our ANOVA.
fMRI scanning and data analysis.
The imaging data (T2*-weighted echo planar images) measuring blood oxygen level-dependent contrast were acquired using a 1.5 tesla Siemens Sonata system. We used a sequence with axial slices tilted by 30° and a flip angle of 90° that reduces signal dropout attributable to susceptibility-induced field inhomogeneities in amygdala and orbitofrontal cortex (Obfc) (Deichmann et al., 2002). Our field of view covered the whole brain in 44 planes. The repetition time was set to 3.96 s (90 ms per slice) and echo time to 50 ms in a single session of 12 min, resulting in 179 volumes.
Images were processed using SPM5 (www.fil.ion.ucl.ac.uk/spm) (Ashburner et al., 2004). Scans were realigned, normalized, and spatially smoothed by an 8 mm full-width half-maximum Gaussian kernel. A high-pass filter (with a cutoff at 128 s) was applied to the time series. The data were then analyzed in an event-related manner.
We modeled conditions for each subject within a fixed-effects general linear model. The resulting beta estimate maps were then taken to a second-level group analysis, and the significance of contrasts of interest was assessed within a random-effects framework to allow statistical inference across the population. On the second level, we used unpaired two-sample t tests to assess the difference in activations between the oxytocin and the placebo groups.
Our focus of interest in this study was a network of predefined regions involved in processing of fear-related stimuli and faces that included amygdala, FFA, insula, anterior cingulate cortex (ACC), and Obfc (Phelps, 2006; Vuilleumier and Pourtois, 2007). We report all activations in these regions as significant when p < 0.001 uncorrected, except for the amygdala, in which we applied a region of interest [(±24, 3, −24); radius, 8 mm] based on a previous study on oxytocin modulation of fear processing (Kirsch et al., 2005) and performed a small volume correction with a threshold of p < 0.05.
Results
Oxytocin effects on affective evaluations
In line with previous studies in which oxytocin was administrated externally (Pitman et al., 1993; Heinrichs et al., 2003, 2004; Kirsch et al., 2005; Kosfeld et al., 2005; Domes et al., 2007a), we did not observe any significant effect on mood ratings (supplemental Table 1, available at www.jneurosci.org as supplemental material). Oxytocin induced no adverse effects over the course of the experiment (supplemental Table 2, available at www.jneurosci.org as supplemental material).
Changes in likeability ratings for faces, induced by fear conditioning, de facto reflecting evaluative conditioning (see Materials and Methods) constituted our primary outcome measure. After conditioning (pretreatment 2), faces paired with shock (CS+) were perceived as less sympathetic, whereas faces never paired with shock (CS−) were perceived as more sympathetic relative to ratings acquired before conditioning (pretreatment 1) (see supplemental data, available at www.jneurosci.org as supplemental material). Within the oxytocin assigned group, four subjects showed no effect of conditioning on affective ratings. To ensure homogeneity of treatment groups, all additional analysis was performed only on “responders” to our conditioning manipulation (oxytocin group: n = 11 subjects, mean age of 25 years, age range of 19–40 years; placebo group: n = 12 subjects, mean age of 25.5 years, age range of 19–39 years). However, for completeness, we also performed an analysis that included all subjects, which showed that removing these four subjects had no impact on overall results (supplemental data, available at www.jneurosci.org as supplemental material).
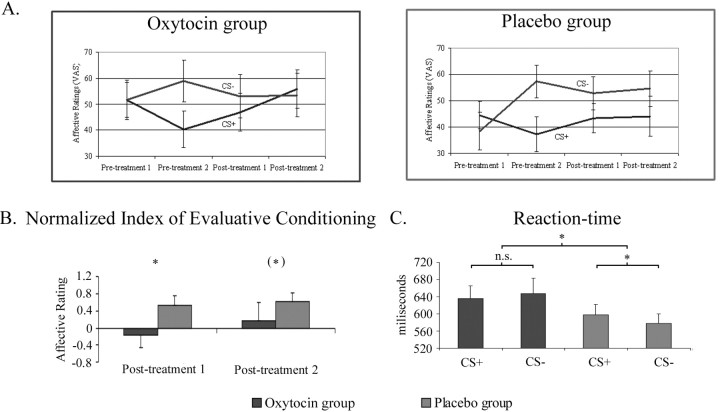
Figure 2A shows the evolution of affective ratings over time in the two treatment groups. The evaluative conditioning index (see Materials and Methods) was significantly greater in the placebo compared with the oxytocin group at posttreatment 1 (oxytocin group average ± SD, 5.273 ± 18.03; placebo group average ± SD, 15.58 ± 18.08; Wilcoxon's signed-rank test, Z = −2.156, p < 0.05) and posttreatment 2 time points (oxytocin group average ± SD, −2.454 ± 7.610; placebo group average ± SD, 14.95 ± 20.30; Wilcoxon's signed-rank test, Z = −2.124, p < 0.05). These results indicate that an induced evaluative change after conditioning was attenuated by oxytocin.
Figure 2.
A, Absolute likeability ratings in the oxytocin (left) and placebo (right) groups before and after conditioning (pretreatment 1 and 2) and before and after testing (posttreatment 1 and 2). B, Reduced likeability-based normalized evaluative conditioning index after oxytocin treatment. C, Reaction times to CS+ and CS− stimuli during testing in the two treatment groups. Slowing of CS+ reaction times characteristic of evaluative conditioning recall was abolished by oxytocin. Error bars indicate SE. *, Significant; (*), threshold significance; n.s., nonsignificant. For p values, see Results.
A closer analysis of these data indicated variability in how subjects rated the faces. Consequently, we performed an analysis in which the pretreatment conditioning-induced change in affective ratings was normalized to 1 (Fig. 2B). Thus, change in ratings after administration of oxytocin was now expressed as the degree of evaluative conditioning effect remaining after treatment (for design, see Fig. 1B). This normalization, which controls for skewing of data, showed a significant difference between oxytocin and placebo groups in that posttreatment affective ratings, whereby the conditioning effects were significantly stronger in the placebo group before the testing (fMRI extinction) session (oxytocin group average ± SD, −0.157 ± 1.002; placebo group average ± SD, 0.522 ± 0.747; Wilcoxon's signed-rank test, Z = −1.723, p < 0.05), whereas the effects showed a trend level difference after the testing session (oxytocin group average ± SD, 0.187 ± 1.338; placebo group average ± SD, 0.6148 ± 0.739; Wilcoxon's signed-rank test, Z = −1.477, p < 0.075). The results indicate that an index of evaluative conditioning of faces was attenuated by oxytocin. Post hoc, we tested whether oxytocin had an overall effect on ratings, regardless of the whether the stimulus was CS+ or CS− and found no such evidence [before testing condition (posttreatment 1): Wilcoxon's signed-rank test, Z = −0.348, p < 0.733; after testing condition (posttreatment 2): Wilcoxon's signed-rank test, Z = −0.319, p < 0.766].
Oxytocin effects on RTs and SCRs
Gaze did not have any effect on RT in an initial mixed three-way ANOVA (the two other factors were conditioning and treatment). For simplicity, we collapsed gaze conditions and performed a mixed ANOVA with within-subject factor fear conditioning (CS+ and CS−) and between-subject factor treatment (oxytocin and placebo) (Fig. 2C). This analysis showed a significant conditioning × treatment interaction (F(1,22) = 5.234; p < 0.05). The interaction was driven by a differential slowing of RTs to the CS+ (average ± SD RT, 597.4 ± 86.4 ms) versus CS− (average ± SD, r = 577.6 ± 75.7 ms) in the placebo group that was not present in the oxytocin group (average ± SD RT for CS+: 636.6 ± 96.8 ms; average ± SD RT for CS−: 647.9 ± 118.5 ms) (Fig. 2C). Slowing of CS+ relative to CS− RTs during a testing phase after conditioning has been reported previously (Kalisch et al., 2006) and is likely to reflect interference of emotion on a simultaneous cognitive task (Mathews et al., 1997). The results further confirm an attenuation of evaluative conditioning by oxytocin. There was no main effect of treatment for RT (F = 1.96; p = 0.176). SCRs appeared to habituate quickly during the testing session for most subjects, and no differential (CS+ vs CS−) effects of conditioning were observed, again in agreement with our previous study (Kalisch et al., 2006) in which fear memory recall at test was accompanied by differential RT, but not SCR, effects.
Effects of oxytocin on evaluative fear processing in fMRI
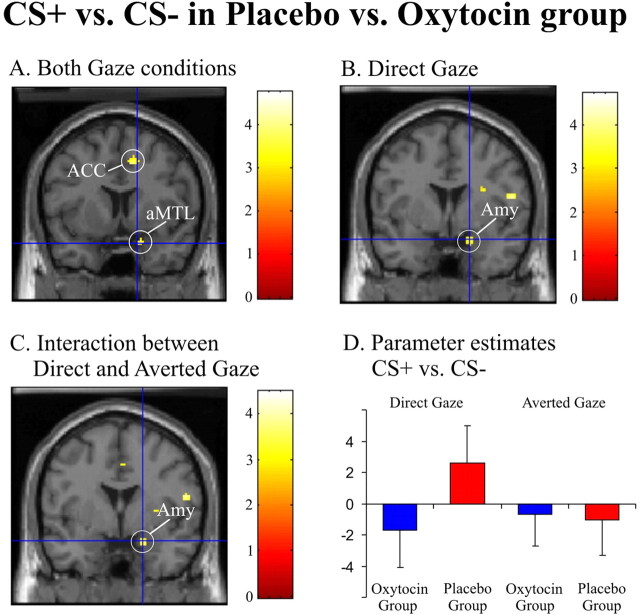
The main effects of evaluative fear conditioning (CS+ > CS−) during the testing session in the two treatment groups are shown in Table 1. In the placebo group, we observed increased activity in the extended/dorsal amygdala and in other regions previously shown to be involved in fear conditioning and extinction such as insula, Obfc, and ACC (Gottfried and Dolan, 2004; Phelps et al., 2004; Kalisch et al., 2006; Milad et al., 2007). Activation of these regions [apart from an activation of rostral ACC (rACC) and Obfc] was not observed in the oxytocin group. Crucially, a significant evaluative conditioning × treatment interaction [(CS+ > CS−)placebo > (CS+ > CS−)oxytocin] was evident in an anterior medial temporal cortex (with a maximum in piriform cortex just anterior to amygdala but extending into amygdala proper) and in the ACC, with the placebo group showing higher activation (Table 1; Fig. 3A).
Table 1.
Main effects in fMRI of fear conditioning (CS+ > CS−) in placebo and oxytocin groups and their interaction
| Placebo group |
Oxytocin group |
Difference placebo versus oxytocin group |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Coordinates | t value | p value | Coordinates | t value | p value | Coordinates | t value | p value | |
| Dorsal/Ext Amy | 18, 2, −8 | 3.68 | <0.001 | 20, 0, −8 | 2.75 | NS | |||
| Ant MTL | 16, 8, −24 | 3.70 | <0.05* | ||||||
| R Insula | 48, 4, 6 | 3.48 | 0.001 | ||||||
| L Insula | −36, 16, −10 | 3.72 | <0.001 | ||||||
| vmPFC | 14, 46, −10 | 3.50 | 0.001 | 16, 46, −10 | 3.66 | 0.001 | |||
| vmPFC | 20, 56, −6 | 3.77 | <0.001 | ||||||
| R lObfc | 44, 54, −12 | 4.51 | <0.001 | 20, 56, −6 | 5.00 | <0.001 | |||
| L lObfc | −40, 52, −6 | 3.80 | <0.001 | ||||||
| L ACC | −14, 14, 40 | 3.63 | <0.001 | ||||||
| R ACC | 10, 10, 42 | 3.95 | <0.001 | 10, 10, 44 | 4.75 | <0.001 | |||
| R rACC | −12, 34, 12 | 3.58 | <0.001 | 6, 46, 16 | 3.57 | <0.001 | |||
| vlPFC | 48, 46, 2 | 3.64 | <0.001 | −50, 32, 8 | 4.13 | <0.001 | |||
Dorsal Amy, Dorsal amygdala; Ext Amy, extended amygdala; Ant MTL, anterior medial temporal lobe; vmPFC, ventromedial prefrontal cortex; vlPFC, ventrolateral prefrontal cortex; rACC, rostral anterior cingulate cortex; R, right; L, left; NS, nonsignificant. *Small volume corrected.
Figure 3.
Oxytocin modulates processing of socially relevant fear stimuli in the amygdala. A, Neural responses to CS+ relative to CS− face stimuli are stronger in the placebo than in the oxytocin group in anterior medial temporal lobe (aMTL) and ACC [(CS+ > CS−)placebo > (CS+ > CS−)oxytocin: (x, y, z) = (16, 8, −24); t = 3.70 and (x, y, z) = (10, 10, 44); t = 4.75, respectively]. B, Similar treatment effects were observed in the amygdala (Amy) when restricting the analysis to faces displaying direct gaze only [(CSdg+ > CSdg−)placebo > (CSdg+ > CSdg−)oxytocin: (x, y, z) = (18, 2, −20); t = 3.45]. C, In a three-way ANOVA, a comparison of direct- versus averted-gaze faces as a function of fear processing showed larger activation for direct gaze [(CSdg+ > CSdg−) > (CSag+ > CSag−) in the placebo group than the oxytocin group: (x, y, z) = (20, 0, −20); t = 3.55]. All activations are superimposed on a mean structural image and thresholded at p = 0.005. D, Parameter estimates characterizing the three-way interaction between fear conditioning, gaze, and treatment. Error bars indicate SE.
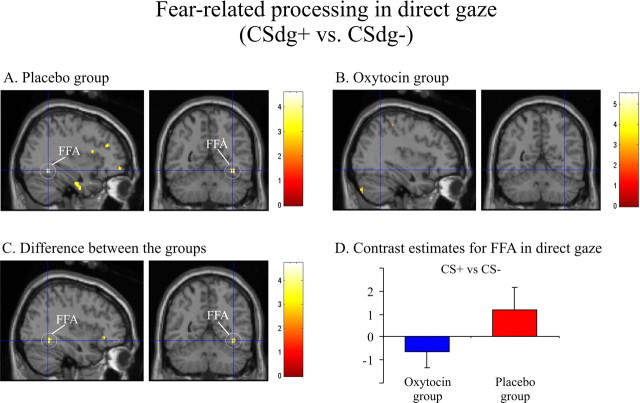
Simple main effects of evaluative fear conditioning for faces displaying direct gaze (CSdg+ > CSdg−) are shown in Table 2. In the placebo group, we observed increased activity in caudal ACC, right FFA (Fig. 4), and at trend level significance in bilateral amygdala. In the oxytocin group, we observed activation in caudal ACC and ventrolateral prefrontal cortex (vlPFC). A significant fear conditioning × treatment interaction [(CSdg+ > CSdg−)placebo > (CSdg+ > CSdg−)oxytocin) was observed in the right amygdala, caudal, rostral, and subgenual ACC, and right FFA, with the placebo group again showing higher activation (Table 2; Figs. 3B, 4C). Simple main effects of fear conditioning for the faces displaying averted gaze (CSag+ > CSag−) are shown in Table 3. The insula was activated in both groups. No significant evaluative fear conditioning × treatment interaction [(CSag+ > CSag−)placebo > (CSag+ > CSag−)oxytocin] was observed in insula, FFA, amygdala, or caudal ACC.
Table 2.
Simple main effects of fear conditioning for direct gaze faces (CSdg+ > CSdg−) in the placebo and oxytocin groups
| Placebo group |
Oxytocin group |
Difference placebo versus oxytocin group |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Coordinates | t value | p value | Coordinates | t value | p value | Coordinates | t value | p value | |
| Amy | 20, 0, −18 | 2.63 | NS | 18, 2, −20 | 3.45 | 0.05* | |||
| Amy | −14, −8, −22 | 2.65 | NS | ||||||
| R Insula | 40, 32, −8 | 3.30 | NS | ||||||
| L Insula | |||||||||
| cACC | 16, 10, 52 | 3.95 | <0.001 | 16, 2, 36 | 4.48 | <0.001 | 14, 8, 46 | 4.01 | <0.001 |
| cACC | −16, 10, 46 | 3.50 | 0.001 | −16, 10, 44 | 3.58 | <0.001 | |||
| subgACC | 14, 44, −8 | 4.20 | <0.001 | ||||||
| vlPFC | 48, 48, 4 | 3.89 | <0.001 | 48, 48, 4 | 4.73 | <0.001 | |||
| vlPFC | −50, 32, 10 | 4.72 | <0.001 | −50, 32, 6 | −5.01 | <0.001 | |||
| mObfc | 18, 54, −6 | 4.60 | <0.001 | ||||||
| R FFA | 36, −54, −12 | 4.47 | <0.001 | 38, −54, −12 | 3.71 | <0.001 | |||
Amy, Amygdala; cACC, caudal anterior cingulate cortex; subgACC, subgenual ACC; vlPFC, ventrolateral prefrontal cortex; mObfc, medial orbitofrontal cortex; FFA, fusiform face area; R, right; L, left; NS, nonsignificant. *Small volume corrected.
Figure 4.
Oxytocin modulates processing of socially relevant fear-conditioned stimuli in the fusiform gyrus. A–C, Processing of conditioned faces displaying direct gaze (CSdg+ > CSdg−) in the FFA in the placebo group [(x, y, z) = (36, −54, −12); t = 4.47] (A) and in the oxytocin group (same coordinate, no activation in FFA) (B) and the difference between the treatment groups [(x, y, z) = (38, −54, −12); t = 3.71] (C). D, Parameter estimates for the interaction in C. Error bars indicate SE. Activations are shown thresholded at p = 0.005.
Table 3.
Simple main effects of fear conditioning for averted gaze faces (CSag+ > CSag−) in the placebo and oxytocin groups
| Placebo group |
Oxytocin group |
Difference placebo versus oxytocin group |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Coordinates | t value | p value | Coordinates | t value | p value | Coordinates | t value | p value | |
| Dorsal/Ext Amy | 22, −4, −10 | 3.26 | NS | 20, −2, −10 | 2.17 | NS | |||
| R Insula | 40, 22, 2 | 4.18 | <0.001 | 34, 20, 2 | 3.29 | NS | |||
| L Insula | −32, 20, 6 | 4.89 | <0.001 | ||||||
| rACC/vmPFC | 12, 48, 18 | 4.20 | <0.001 | −8, 50, 6 | −3.49 | 0.001 | |||
| dmPFC | |||||||||
| mObfc | 20, 60, −4 | 4.74 | <0.001 | 18, 44, −16 | 3.42 | 0.001 | |||
| lObfc | −30, 42, −12 | 3.39 | 0.001 | ||||||
| lObfc | 28, 56, 8 | −3.46 | 0.001 | ||||||
| vlPFC | 32, 60, 2 | 3.55 | <0.001 | −40, 36, 0 | −3.69 | 0.001 | |||
Dorsal Amy, Dorsal amygdala; Ext Amy, extended amygdala; rACC, rostral anterior cingulate cortex; vmPFC, ventromedial prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; mObfc, medial orbitofrontal cortex; lObfc, lateral orbitofrontal cortex; vlPFC, ventrolateral prefrontal cortex; R, right; L, left; NS, nonsignificant.
It can be conjectured that activity elicited by socially relevant cues, in our experiment direct as opposed to averted-gaze faces, should be more susceptible to oxytocin. Therefore, we examined for a three-way interaction between fear conditioning (CS+ and CS−), treatment (oxytocin and placebo), and social relevance (direct gaze and averted gaze). There was a significant interaction in right amygdala, driven by enhanced responses to fear-conditioned faces with direct gaze in the placebo group (Table 4; Fig. 3C,D). The right FFA, rACC, and vlPFC showed a similar interaction (Table 4).
Table 4.
Interaction between faces displaying direct and averted gaze in the placebo and oxytocin group [(CSdg+ vs CSdg−) vs (CSag+ vs CSag−)]
| Placebo group |
Oxytocin group |
Difference placebo > oxytocin group |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Coordinates | t value | p value | Coordinates | t value | p value | Coordinates | t value | p value | |
| Amy | 20, 0, −20 | 3.94 | <0.05* | 20, 0, −20 | 3.55 | <0.05* | |||
| Amy | −18, −8, −26 | 2.56 | NS | ||||||
| R Insula | 30, 30, −10 | 3.66 | <0.001 | ||||||
| R FFA | 42, −50, −14 | −4.58 | <0.001 | 42, −50, −14 | 3.59 | <0.001 | |||
| cACC | 14, 0, 40 | 3.89 | <0.001 | ||||||
| −12, −2, 48 | 4.04 | <0.001 | |||||||
| mObfc | 16, 44, −18 | 3.89 | <0.001 | ||||||
| rACC/vmPFC | 4, 62, 0 | −5.60 | <0.001 | 4, 62, 0 | 4.16 | <0.001 | |||
| vlPFC | 34, 62, 4 | 3.73 | <0.001 | ||||||
| vlPFC | 46, 50, 4 | 4.37 | <0.001 | ||||||
Amy, Amygdala; FFA, fusiform face area; cACC, caudal anterior cingulate cortex; mObfc, medial orbitofrontal cortex; rACC, rostral anterior cingulate cortex; vmPFC, ventromedial prefrontal cortex; vlPFC, ventrolateral prefrontal cortex; R, right; NS, nonsignificant. *Small volume corrected.
Discussion
Animal studies have shown that oxytocin is involved in regulating social interactions, mediating increased approach behavior toward conspecifics (Lim and Young, 2006). Oxytocin is also implicated in inhibition of fear-related processes (Debiec, 2005). It has been hypothesized that these two effects are functionally related and that oxytocin mediates its prosocial behavior partly through suppression of avoidance-related processes (Lim and Young, 2006). One possibility is that oxytocin influences fear-related social stimuli more than fear-related nonsocial stimuli. Although social cues are mostly conveyed through the olfactory system in rodents, in which the oxytocin system has been most extensively studied, in humans social cues depend on the visual system, as exemplified by face processing (Haxby et al., 2002; Adolphs et al., 2005; Lim and Young, 2006). Moreover, because social-affective responses are modified with respect to our experience of others (Singer et al., 2006), we conjectured that oxytocin might modulate this dimension. This suggests that oxytocin effects on fear-related social stimuli should be evident in attenuated affective ratings and attenuated brain responses within regions processing socially relevant stimuli (i.e., faces).
The best characterization of postconditioning change in affective ratings and their modulation by oxytocin is that mediated by evaluative conditioning (De Houwer et al., 2001). Our demonstration of an attenuation in affective ratings for fear-related faces by oxytocin is in line with the hypothesis that oxytocin-mediated prosocial processes involve a suppression of aversive associations to specific stimuli (Lim and Young, 2006). It has been shown previously that oxytocin has prosocial effects in humans, as in oxytocin treatment influencing trust behavior in economic games (Kosfeld et al., 2005), modulating inferences regarding others' mental states (Domes et al., 2007a), and reducing stress in social interactions (Pitman et al., 1993; Heinrichs et al., 2003; Domes et al., 2007a). Importantly, in our study, oxytocin had no effect on mood, in line with previous studies (Pitman et al., 1993; Heinrichs et al., 2003, 2004; Kirsch et al., 2005; Kosfeld et al., 2005; Domes et al., 2007a), but did impact on acquired negative affective ratings related to social stimuli.
We observed a significant effect of oxytocin on the amygdala, a region implicated in fear processing, including fear learning (Phelps, 2006). The amygdala also plays a key role in processing social cues such as direction of eye gaze, manifest in an enhanced amygdala response to direct compared with averted gaze (Kawashima et al., 1999; George et al., 2001; Haxby et al., 2002; Adolphs et al., 2005). These two dimensions, fear and social cue processing, interact in the amygdala as when a face signals threat (Vuilleumier and Pourtois, 2007) and in judgment of untrustworthiness (Winston et al., 2002). The fact that the amygdala expresses high concentrations of oxytocin receptors (Insel and Shapiro, 1992; Veinante and Freund-Mercier, 1997; Huber et al., 2005), which act by inhibiting activity in the basolateral amygdala through the influence of GABA (Huber et al., 2005), provides a likely mechanisms by which oxytocin might induce specific effects on socially related fear (Debiec, 2005).
Two previous human studies have reported reduced fear-related activation of amygdala after oxytocin (Kirsch et al., 2005; Domes et al., 2007). However, unlike the present report, these studies lacked a relevant behavioral measure. Moreover, because social and nonsocial stimuli were not matched, it is not possible to dissociate whether oxytocin has a stronger effect on socially relevant stimuli associated with the same degree of fear. It is this specificity of oxytocin for stimuli with a higher social value that is the novel finding reported here. Specifically, we show attenuated activation of the anterior medial temporal region for fear-related stimuli in the oxytocin group, an effect expressed to a greater degree for faces displaying direct gaze compared with averted gaze, consistent with a specific modulation of socially relevant threat stimuli (Kawashima et al., 1999; George et al., 2001; Haxby et al., 2002; Adolphs et al., 2005). It is important to stress that both averted- and direct-gaze faces are social stimuli. However, in direct gaze, attention is toward the individual, and in averted gaze, the attention is directed toward an extrapersonal spatial location. Thus, although the difference is subtle and the faces are highly matched, it has been suggested that direct gaze is more socially relevant (Haxby et al., 2002).
The amygdala showed significantly stronger activation to fear-related faces displaying direct gaze than averted gaze under placebo, although the behavioral results did not suggest any differences in evaluative conditioning strength. The fact that there is lack of congruency between amygdala and behavioral data means we cannot reach a strong conclusion that amygdala activity drives a difference in affective ratings. There are several possible explanations for the observed differences. First, behavioral effects, like affective ratings or reaction times, are noisy measurements and may not be as sensitive as a direct measure of brain activity (for example, see Meyer-Lindenberg et al., 2005). Thus, it is possible that there is a relationship between amygdala specificity for direct gaze and affective ratings but that this study lacks power to show such a relationship. Another possibility is that the specific modulation of amygdala for direct gaze represents another process unrelated to the change in affective ratings. Other regions such as the ACC that is involved in processing affective and conditioned stimuli (Phelps, 2006) may be important for the observed effects on evaluative conditioning. This region showed a general oxytocin-dependent attenuation for CS+ faces in the present study and contains high concentrations of oxytocin receptors in monogamous species (Insel and Shapiro, 1992). Thus, the effect of oxytocin may be more complex than just involving an amygdala modulation. At the very least, our data suggest that oxytocin is more effective in modulating the amygdala response for socially relevant stimuli because these stimuli are more prone to activate amygdala than less socially relevant stimuli when associated with fear. The alternative hypothesis, namely that both social and nonsocial fear-related stimuli activate amygdala equally but only social-specific stimuli are modulated by oxytocin, seems less likely given the present results.
We also observed fear-related activation in right FFA for faces displaying direct gaze in the placebo group, an effect attenuated in the oxytocin group. It has been suggested previously that the FFA processes face identity (Kawashima et al., 1999; George et al., 2001; Haxby et al., 2002) especially when the face signals threat (Morris et al., 1998; Vuilleumier et al., 2001) and that this interaction between face and fear processing is dependent on amygdala influences (Vuilleumier et al., 2004). Thus, the attenuated FFA activity for fear-related faces in the oxytocin group may be a consequence of attenuated amygdala activity. This finding underlines the fact that oxytocin does not just suppress general fear-related responses but also processing of specific identities associated with threat, in line with evidence that prosocial processes involve suppression of negative associations to specific individuals (Lim and Young, 2006). At first glance, this might seem to conflict with findings that oxytocin receptor knock-out mice have lower social recognition of conspecifics (Ferguson et al., 2002; Bielsky and Young, 2004). However, we note that tasks in these studies addressed approach and not avoidance behavior, raising the possibility that oxytocin induces prosocial behavior not through augmenting social memory related to approach but suppressing social memory related to avoidance. Interestingly, for averted faces, we observed no significant fear-related activation in FFA in the placebo group, nor any difference between the treatment groups. Thus, as for the amygdala, FFA responds more reliably to social relevant cues associated with threat.
Deficits in processing social cues are evident in clinical populations such as Williams syndrome and autism. Both syndromes involve abnormal processing of faces: although amygdala activation is heightened in autism for faces with direct gaze (Dalton et al., 2005), it is suppressed in Williams syndrome for fearful faces (Meyer-Lindenberg et al., 2005). Patients with Williams syndrome show high sociability and empathy (Meyer-Lindenberg et al., 2006), whereas autistic people show impaired social functioning (Hill and Frith, 2003). Moreover, the amount of time autistic subjects fixate on eyes direct gazing at the observer correlates with amygdala and fusiform activity (Dalton et al., 2005). It has been suggested that an underlying cause for deficits in social interaction in autistics is a malfunctioning oxytocin system (Bartz and Hollander, 2006; Hammock and Young, 2006; Carter, 2007; Heinrichs and Gaab, 2007), evidenced in lower oxytocin levels than in normal controls (Modahl et al., 1998; Green et al., 2001) and an association with specific variants of the oxytocin receptor gene (Wu et al., 2005; Ylisaukko-oja et al., 2006). The present study shows that processing of socially relevant cues related to fear are attenuated by oxytocin, raising the issue as to whether oxytocin might improve social interactions in autistics in line with recent data showing that oxytocin apparently alleviates other symptoms in autistic disorder (Bartz and Hollander, 2006).
In conclusion, we show that oxytocin attenuates social fear processing, consistent with animal studies in which this effect is suggested to underlie approach to conspecific individuals and that, in humans, it translates behaviorally into a modulation of evaluative fear-conditioned responses, including a suppression of fear-induced affective ratings. Moreover, we show that the effect of oxytocin on affective face processing is expressed not only within the amygdala, as shown previously, but also by other regions processing specific social cues such as the fusiform gyrus. Finally, we observed that the fMRI effects in these regions were different depending on gaze both during general fear processing and attributable to oxytocin treatment, suggesting that social cues interact with these processes. The findings suggest that oxytocin may have an effect in disorders in which social impairments are a key feature.
Footnotes
This work was supported by a Wellcome Trust Programme Grant (R.J.D.). P.P. was also supported by Hjärnfonden and the Swedish Research Council.
References
- Adolphs R, Spezio M. Role of the amygdala in processing visual social stimuli. Prog Brain Res. 2006;156:363–378. doi: 10.1016/S0079-6123(06)56020-0. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston K, Penny W. Imaging neuroscience: theory and analysis. In: Frackowiak RSJ, Friston K, Frith CD, Dolan RJP, Price CJ, Zeki S, Ashburner J, Penny W, editors. Human brain function. Ed 2. San Diego: Academic; 2004. pp. 599–1104. [Google Scholar]
- Bartz JA, Hollander E. The neuroscience of affiliation: forging links between basic and clinical research on neuropeptides and social behavior. Horm Behav. 2006;50:518–528. doi: 10.1016/j.yhbeh.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25:1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Carter CS. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav Brain Res. 2007;176:170–186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, Alexander AL, Davidson RJ. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Debiec J. Peptides of love and fear: vasopressin and oxytocin modulate the integration of information in the amygdala. BioEssays. 2005;27:869–873. doi: 10.1002/bies.20301. [DOI] [PubMed] [Google Scholar]
- De Houwer J, Thomas S, Baeyens F. Associative learning of likes and dislikes: a review of 25 years of research on human evaluative conditioning. Psychol Bull. 2001;127:853–869. doi: 10.1037/0033-2909.127.6.853. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Josephs O, Hutton C, Corfield DR, Turner R. Compensation of susceptibility-induced BOLD sensitivity losses in echo-planar fMRI imaging. NeuroImage. 2002;15:120–135. doi: 10.1006/nimg.2001.0985. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007a;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Gläscher J, Büchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007b;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Front Neuroendocrinol. 2002;23:200–224. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- George N, Driver J, Dolan RJ. Seen gaze-direction modulates fusiform activity and its coupling with other brain areas during face processing. NeuroImage. 2001;13:1102–1112. doi: 10.1006/nimg.2001.0769. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat Neurosci. 2004;7:1144–1152. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- Green L, Fein D, Modahl C, Feinstein C, Waterhouse L, Morris M. Oxytocin and autistic disorder: alterations in peptide forms. Biol Psychiatry. 2001;50:609–613. doi: 10.1016/s0006-3223(01)01139-8. [DOI] [PubMed] [Google Scholar]
- Hammock EA, Young LJ. Oxytocin, vasopressin and pair bonding: implications for autism. Philos Trans R Soc Lond B Biol Sci. 2006;361:2187–2198. doi: 10.1098/rstb.2006.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biol Psychiatry. 2002;51:59–67. doi: 10.1016/s0006-3223(01)01330-0. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Gaab J. Neuroendocrine mechanisms of stress and social interaction: implications for mental disorders. Curr Opin Psychiatry. 2007;20:158–162. doi: 10.1097/YCO.0b013e3280146a13. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Meinlschmidt G, Wippich W, Ehlert U, Hellhammer DH. Selective amnesic effects of oxytocin on human memory. Physiol Behav. 2004;83:31–38. doi: 10.1016/j.physbeh.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Hill EL, Frith U. Understanding autism: insights from mind and brain. Philos Trans R Soc Lond B Biol Sci. 2003;358:281–289. doi: 10.1098/rstb.2002.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci USA. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Gingrich BS, Young LJ. Oxytocin: who needs it? Prog Brain Res. 2001;133:59–66. doi: 10.1016/s0079-6123(01)33005-4. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci. 2006;26:9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima R, Sugiura M, Kato T, Nakamura A, Hatano K, Ito K, Fukuda H, Kojima S, Nakamura K. The human amygdala plays an important role in gaze monitoring. A PET study. Brain. 1999;122:779–783. doi: 10.1093/brain/122.4.779. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Mathews A, Mackintosh B, Fulcher EP. Cognitive biases in anxiety and attention to threat. Trends Cogn Sci. 1997;1:340–345. doi: 10.1016/S1364-6613(97)01092-9. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Hariri AR, Munoz KE, Mervis CB, Mattay VS, Morris CA, Berman KF. Neural correlates of genetically abnormal social cognition in Williams syndrome. Nat Neurosci. 2005;8:991–993. doi: 10.1038/nn1494. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Mervis CB, Berman KF. Neural mechanisms in Williams syndrome: a unique window to genetic influences on cognition and behaviour. Nat Rev Neurosci. 2006;7:380–393. doi: 10.1038/nrn1906. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Modahl C, Green L, Fein D, Morris M, Waterhouse L, Feinstein C, Levin H. Plasma oxytocin levels in autistic children. Biol Psychiatry. 1998;43:270–277. doi: 10.1016/s0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Büchel C, Frith CD, Young AW, Calder AJ, Dolan RJ. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Lasko NB. Effects of intranasal vasopressin and oxytocin on physiologic responding during personal combat imagery in Vietnam veterans with posttraumatic stress disorder. Psychiatry Res. 1993;48:107–117. doi: 10.1016/0165-1781(93)90035-f. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466–469. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier MJ. Distribution of oxytocin- and vasopressin-binding sites in the rat extended amygdala: a histoautoradiographic study. J Comp Neurol. 1997;383:305–325. [PubMed] [Google Scholar]
- Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O'Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nat Neurosci. 2002;5:277–283. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, Gong X, Zhang Y, Yang X, Zhang D. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biol Psychiatry. 2005;58:74–77. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Ylisaukko-oja T, Alarcón M, Cantor RM, Auranen M, Vanhala R, Kempas E, von Wendt L, Järvelä I, Geschwind DH, Peltonen L. Search for autism loci by combined analysis of Autism Genetic Resource Exchange and Finnish families. Ann Neurol. 2006;59:145–155. doi: 10.1002/ana.20722. [DOI] [PubMed] [Google Scholar]
- Young LJ. The neurobiology of social recognition, approach, and avoidance. Biol Psychiatry. 2002;51:18–26. doi: 10.1016/s0006-3223(01)01268-9. [DOI] [PubMed] [Google Scholar]