Abstract
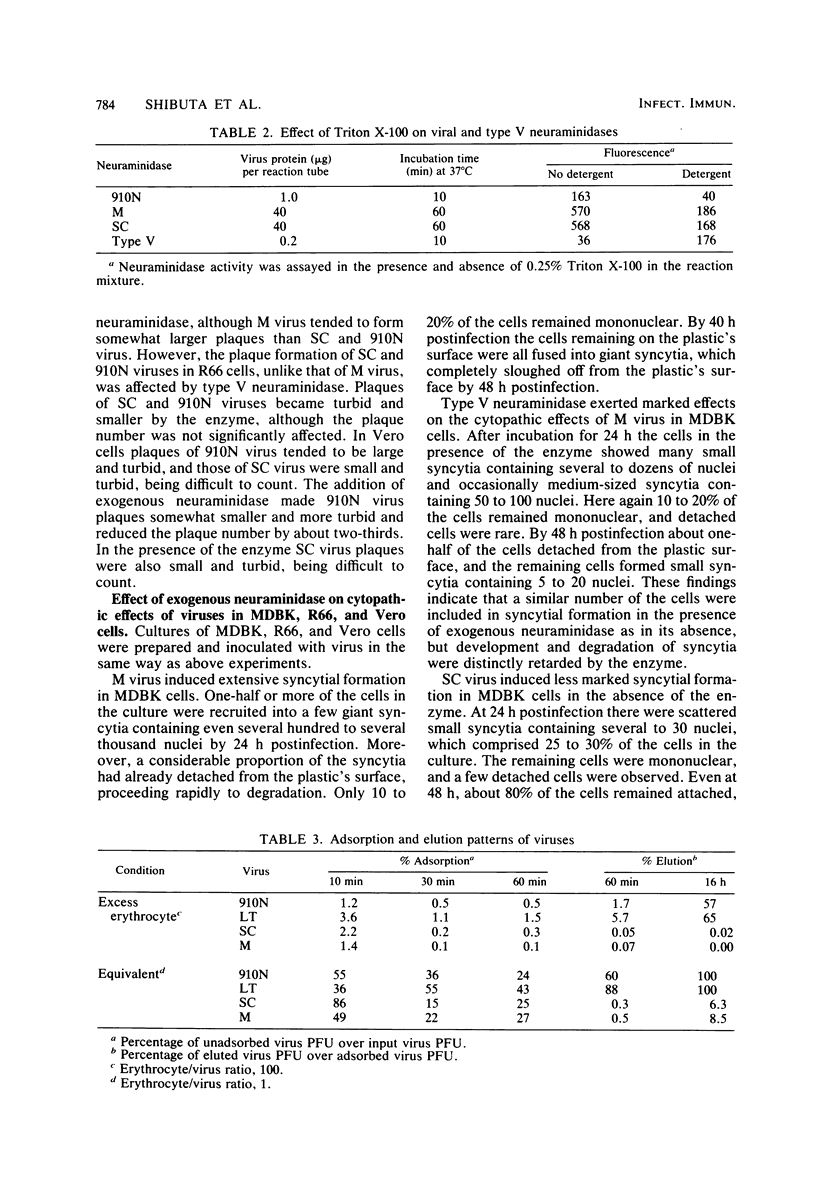
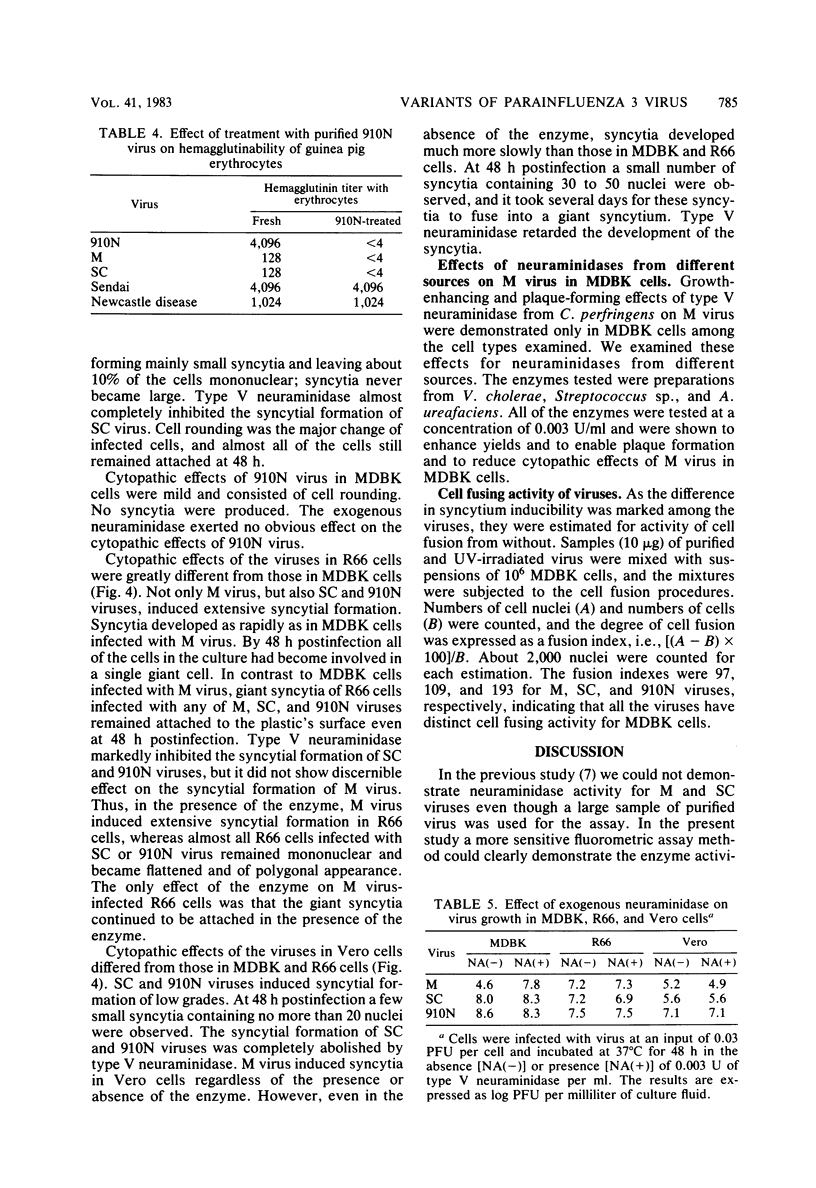
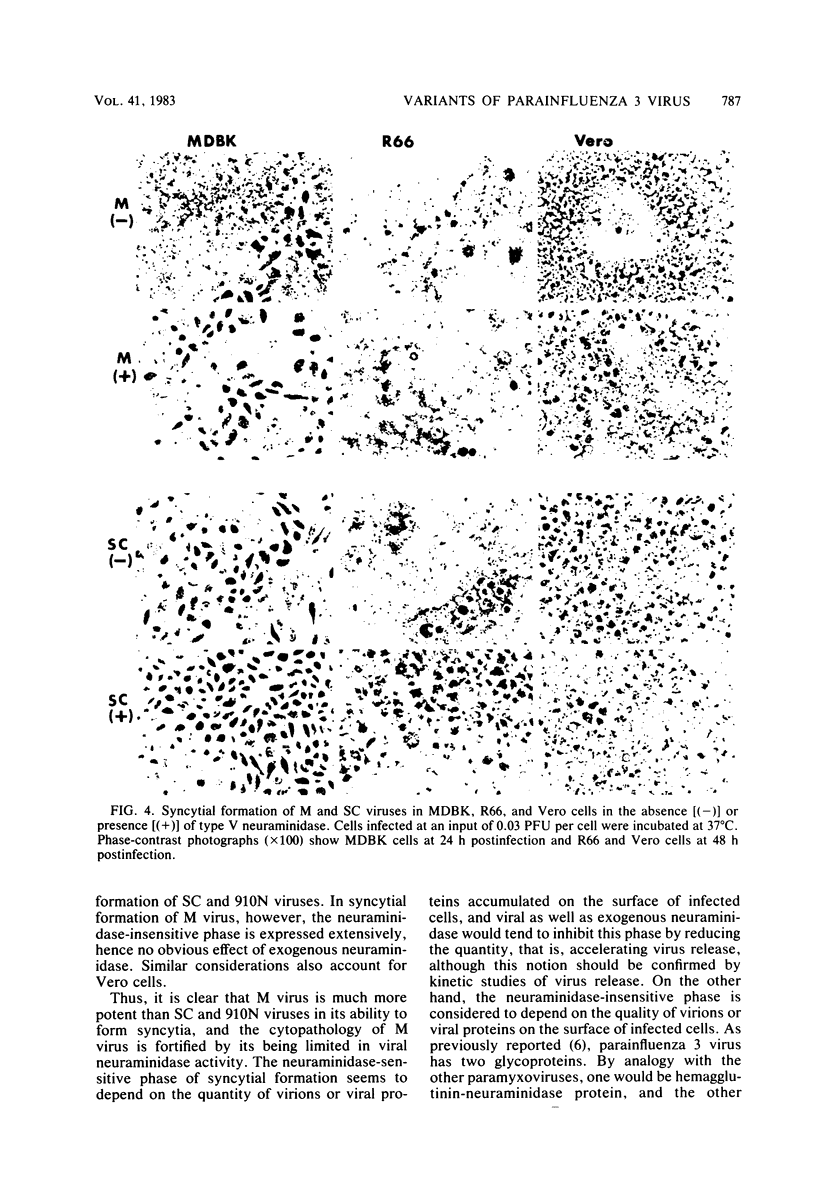
By a sensitive fluorometric assay method, we could definitely demonstrate neuraminidase activity for two variants of parainfluenza 3 virus, M and SC, which were previously shown to have no detectable neuraminidase activity. The enzyme activities of these viruses were very similar to each other, showing a much lower catalytic rate, a much higher Km value, and a more acidic pH optimum than those of the virus variants of high neuraminidase activity, 910N, LT, and MR. M and SC viruses eluted from guinea pig erythrocytes very poorly, whereas 910N and LT viruses eluted readily. M virus required the aid of a bacterial neuraminidase for effective growth and plaque formation in MDBK cells, but the virus grew well and formed plaques in R66 and Vero cells without the enzyme. SC virus required no exogenous neuraminidase for growth in all of these cell types. Depending on cell type, SC virus induced slight to extensive syncytial formation which was greatly inhibited by exogenous neuraminidase. In contrast, M virus induced extensive syncytial formation in all these cells regardless of the presence or absence of exogenous neuraminidase, although development and disintegration of the syncytia were more or less retarded by the enzyme, especially in MDBK cells. These results indicate that M virus possesses highly potent inducibility of syncytial formation which is further fortified by being low in viral neuraminidase activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Merz D. C., Wolinsky J. S. Biochemical features of mumps virus neuraminidases and their relationship with pathogenicity. Virology. 1981 Oct 15;114(1):218–227. doi: 10.1016/0042-6822(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Myers R. W., Lee R. T., Lee Y. C., Thomas G. H., Reynolds L. W., Uchida Y. The synthesis of 4-methylumbelliferyl alpha-ketoside of N-acetylneuraminic acid and its use in a fluorometric assay for neuraminidase. Anal Biochem. 1980 Jan 1;101(1):166–174. doi: 10.1016/0003-2697(80)90056-1. [DOI] [PubMed] [Google Scholar]
- Palese P., Schulman J. L., Bodo G., Meindl P. Inhibition of influenza and parainfluenza virus replication in tissue culture by 2-deoxy-2,3-dehydro-N-trifluoroacetylneuraminic acid (FANA). Virology. 1974 Jun;59(2):490–498. doi: 10.1016/0042-6822(74)90458-9. [DOI] [PubMed] [Google Scholar]
- Shibuta H., Adachi A., Kanda T., Matumoto M. Experimental parainfluenzavirus infection in mice: fatal illness with atrophy of thymus and spleen in mice caused by a variant of parainfluenza 3 virus. Infect Immun. 1982 Feb;35(2):437–441. doi: 10.1128/iai.35.2.437-441.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuta H., Kanda T., Adachi A., Yogo Y. Characterization of bovine parainfluenza virus type 3. Microbiol Immunol. 1979;23(7):617–628. doi: 10.1111/j.1348-0421.1979.tb00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuta H., Kanda T., Hazama A., Adachi A., Matumoto M. Parainfluenza 3 virus: plaque-type variants lacking neuraminidase activity. Infect Immun. 1981 Oct;34(1):262–267. doi: 10.1128/iai.34.1.262-267.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Torsch V. M., Berg R., Murphy B. R., Lee Y. C. Fluorometric assay for measurement of viral neuraminidase--application to the rapid detection of influenza virus in nasal wash specimens. J Infect Dis. 1980 Oct;142(4):516–523. doi: 10.1093/infdis/142.4.516. [DOI] [PubMed] [Google Scholar]