Abstract
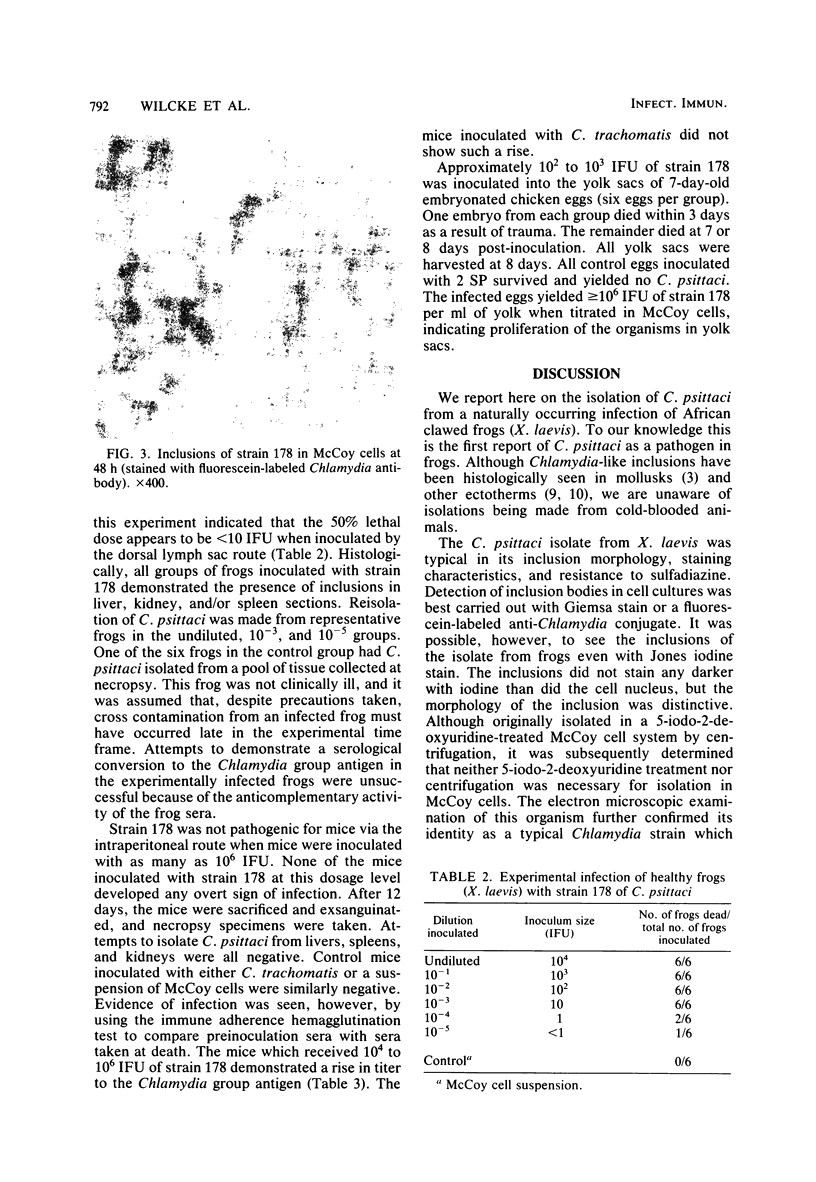
An inclusion-forming agent was isolated from the livers of commercially raised African clawed frogs (Xenopus laevis) involved in an epizootic of high morbidity and mortality. Original isolation was made in McCoy cells. This agent was identified as Chlamydia psittaci based on the formation of typical intracytoplasmic inclusions which developed within 48 h, were not stained by iodine, and were resistant to sulfadiazine. The isolate from one particular frog (designated as strain 178) was further studied and found to be lethal for 7-day-old embryonated chicken eggs after intra-yolk sac inoculation. This strain was demonstrated not to be pathogenic for mice when inoculated intraperitoneally. The cell culture isolate of C. psittaci was transmitted to uninfected X. laevis, causing disease and death.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gordon F. B., Harper I. A., Quan A. L., Treharne J. D., Dwyer R. S., Garland J. A. Detection of Chlamydia (Bedsonia) in certain infections of man. I. Laboratory procedures: comparison of yolk sac and cell culture for detection and isolation. J Infect Dis. 1969 Oct;120(4):451–462. doi: 10.1093/infdis/120.4.451. [DOI] [PubMed] [Google Scholar]
- Harshbarger J. C., Chang S. C. Chlamydiae (with phages), mycoplasmas, and richettsiae in Chesapeake Bay bivalves. Science. 1977 May 6;196(4290):666–668. doi: 10.1126/science.193184. [DOI] [PubMed] [Google Scholar]
- Lennette E. T., Lennette D. A. Immune adherence hemagglutination: alternative to complement-fixation serology. J Clin Microbiol. 1978 Mar;7(3):282–285. doi: 10.1128/jcm.7.3.282-285.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer C. E., Anver M. R., Simmons J. L., Wilcke B. W., Jr, Nace G. W. Spontaneous and experimental infections of Xenopus laevis with Chlamydia psittaci. Lab Anim Sci. 1982 Dec;32(6):680–686. [PubMed] [Google Scholar]
- Schachter J., Caldwell H. D. Chlamydiae. Annu Rev Microbiol. 1980;34:285–309. doi: 10.1146/annurev.mi.34.100180.001441. [DOI] [PubMed] [Google Scholar]
- Shindarov L., Runevski N., Vassileva V. Morphologic and cytochemic studies on the development of Chlamydia psittaci in a tissue culture of a cold-blooded animal. Pathol Microbiol (Basel) 1969;34(6):329–339. doi: 10.1159/000162183. [DOI] [PubMed] [Google Scholar]
- Shindarov L., Runevski N., Vassileva V. Morphologic and cytochemic studies on the development of Chlamydia psittaci in tissue culture of a cold-blooded animal at 20 degrees C. Zentralbl Bakteriol Orig. 1971;216(1):9–14. [PubMed] [Google Scholar]
- Spears P., Storz J. Biotyping of Chlamydia psittaci based on inclusion morphology and response to diethylaminoethyl-dextran and cycloheximide. Infect Immun. 1979 Apr;24(1):224–232. doi: 10.1128/iai.24.1.224-232.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth B. B., Alexander E. R. Isolation of Chlamydia trachomatis by use of 5-iodo-2-deoxyuridine-treated cells. Appl Microbiol. 1974 May;27(5):912–916. doi: 10.1128/am.27.5.912-916.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]