Abstract
The etiology of cervical artery dissection (CAD) is unclear, although a number of risk factors have been reported to be associated with the condition. On rare occasions, patients experience CAD after cervical spine manipulation, making knowledge about the cervical arteries, the predisposing factors, and the pathogenesis of the condition of interest to chiropractors. This commentary reports on the relevant anatomy of the cervical arteries, developmental features of CAD, epidemiology of the condition, and mechanisms of dissection. The analysis of CAD risk factors is confusing, however, because many people are exposed to mechanical events and known pathophysiological associations without ever experiencing dissection. No cause-and-effect relationship has been established between cervical spine manipulation and CAD, but it seems that cervical manipulation may be capable of triggering dissection in a susceptible patient or contributing to the evolution of an already existing CAD. Despite the many risk factors that have been proposed as possible causes of CAD, it is still unknown which of them actually predispose patients to CAD after cervical spine manipulation.
Key indexing terms: Vertebral artery dissection, Risk, Neck, Manipulation, Chiropractic
Introduction
The etiology of cervical artery dissection (CAD) is, for the most part, unclear; and what has been proposed as an explanation for its pathogenesis is largely hypothetical.1 Furthermore, when dealing with a particular case of CAD, the pathogenesis is especially speculative.2 Nevertheless, a number of risk factors have been reported to be associated with the condition, including connective tissue abnormalities, hypertension, recent infection, migraine headache, the use of oral contraceptives, and others. Of special interest to chiropractors is the role cervical spine manipulation (CSM) plays, if any, in the pathogenesis of CAD. Indeed, patients do experience CAD on rare occasions after CSM, making knowledge about the cervical arteries, the predisposing factors, and the pathogenesis of the condition important for chiropractors.
Anatomy of the cervical arteries
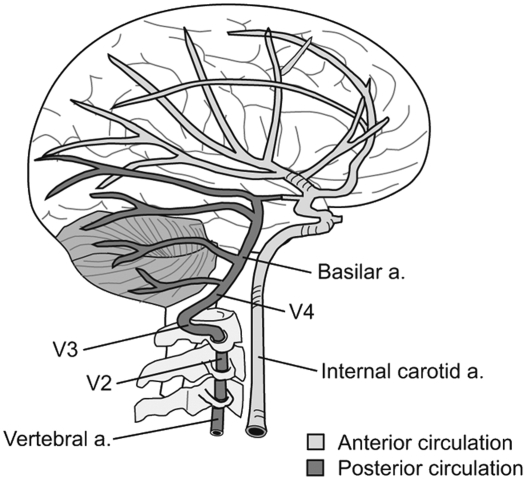
The understanding of CAD is greatly enhanced by having a basic grasp of the relevant anatomy. A pair of vertebral arteries (VAs) and a pair of internal carotid arteries (ICAs) pass through the cervical region to supply the brain with blood. The ICAs and their branches are often referred to as the anterior circulation because they supply blood to the anterior portion of the brain. The vertebral and basilar arteries comprise the vertebrobasilar system, which is referred to as the posterior circulation because it supplies blood to the posterior brain (Fig 1).3
Fig 1.
The ICAs supply blood to the anterior portion of the brain, and the vertebral and basilar arteries supply the posterior brain.
The VA typically emerges from the subclavian artery and can be divided into 4 segments. The first segment is the prevertebral segment, also known as V1, which lies between the longus colli and the anterior scalene muscles before entering the transverse foramen of C6. The second segment, referred to as the cervical segment or V2, passes through the transverse foramina of C6 through C1 to become the atlantal segment or V3 when it exits through the transverse foramen of C1. After exiting the C1 transverse foramen, the atlantal segment abruptly transitions from a vertical pathway to a horizontal orientation. It is as this point where the artery is thought to be most susceptible to injury related to sudden or extreme head movement.4 The atlantal segment is enclosed in a muscular sheath and passes through a groove behind the C1 articular process before entering the cranium through the atlantooccipital membrane and the dura mater. The VA is fixed to the inner surface of the tunnel formed by the transverse foramina by a continuous layer of collagen.5 The final segment is the intracranial segment or V4, which travels upward across the medulla to the pontomedullary junction. At that point, it joins with the opposite VA to become the basilar artery. The basilar artery extends distally to form the posterior inferior and anterior inferior cerebellar arteries, the internal auditory artery, the superior cerebellar artery, the posterior cerebral artery, and numerous medullary and pontine branches. The posterior inferior cerebellar artery frequently develops differently, however, emerging as a branch of the VA before the formation of the basilar artery.
The ICA arises from the common carotid artery at the level it bifurcates into external and internal branches. Like the VA, it is made up of 4 segments. The cervical segment ascends vertically through the neck, situated posterior to the external carotid artery. The cervical portion of the ICA lies below the sternocleidomastoid muscles and is separated from the external carotid artery by the styloglossus and stylopharyngeal muscles. It is located anterior to the longus cervicis muscle and the transverse processes of the upper 3 or 4 cervical vertebrae.6 After entering the carotid canal at the base of the skull, the ICA is known as the petrous segment until it passes through the skull and becomes the cavernous segment. The artery's final subdivision is the supraclinoid segment.3 The ICA is freely moveable within its cervical pathway, but becomes fixed to the surface of the bone as it enters the carotid canal above the atlas.7
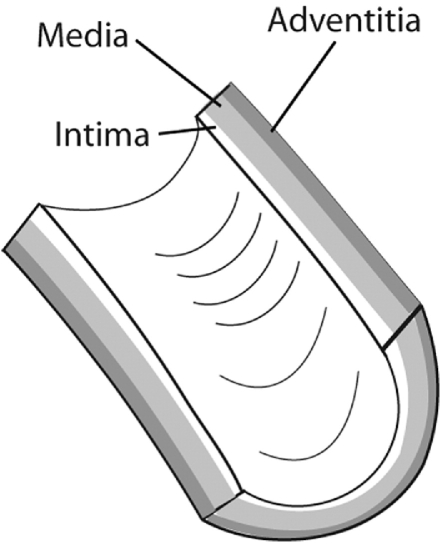
The cervical arteries are made up of 3 layers: the tunica intima, the tunica media, and the tunica adventitia, which is the outermost layer (Fig 2). The tunica intima is the innermost layer of the artery that makes up the vessel lining. It is composed of squamous endothelial cells supported by a thin layer of connective tissue. This layer is thinner and more fragile than the others, making it more susceptible to tearing.8 Consequently, it is the typical site of an initial defect that forms in a developing dissection. The tunica media is the middle muscular layer, which is also the thickest layer. Under autonomic control, muscular contractions can alter the diameter of the vessel. These contractions may develop into spasms, which are thought to occur with enough force to block the arterial lumen and interrupt the blood flow. The tunica adventitia is the outermost layer that mainly consists of longitudinally arranged collagen fibers. This layer merges with bone surfaces at various points along the course of the arteries, which anchors their position. The artery wall itself receives a supply of blood by way of the vasa vasorum, which extends to the outer stratum of the tunica media.
Fig 2.
The tunica intima, the tunica media, and the tunica adventitia form the 3 layers of the cervical arteries.
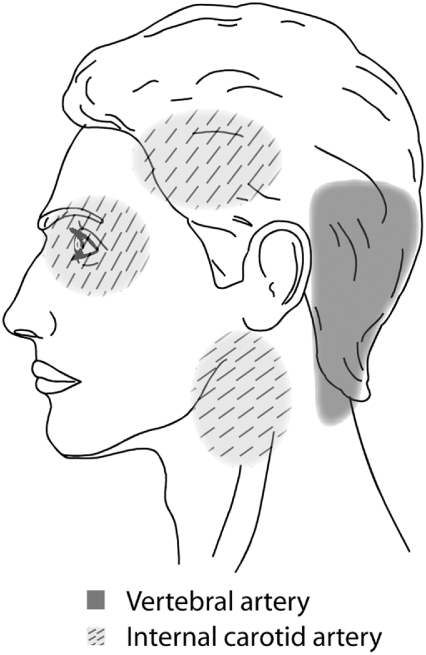
The cervical arteries are innervated with pain-sensitive nerve fibers that may generate neck pain and headache when provoked. Several studies have shown that pain is typically the first symptom associated with CAD,9,10 and a recent descriptive study involving 245 CAD patients reported that 8% of them presented with head or neck pain as their only symptom.11 In this study, pain-only CAD patients were mostly composed of VA or multiple artery dissection cases, with only 3 cases of ICA dissection. Pain related to CAD frequently occurs suddenly and is of severe intensity, often described by patients as being different from any previous pain. Accordingly, the clinical manifestations of CAD typically include severe head and neck pain that involves mostly the ipsilateral occipitocervical area when the VA is affected12 or the periorbital, frontal, and upper cervical region when the ICA is involved (Fig 3).13 These symptoms may or may not be followed by ischemic involvement in the brain, cerebellum, or brain stem. The interval of time between the initial pain of CAD and ischemic symptoms is quite variable, however, with reports ranging from almost immediately to several weeks.
Fig 3.
Regions of the head and neck where pain is commonly found in CAD patients.
Characteristics of CAD
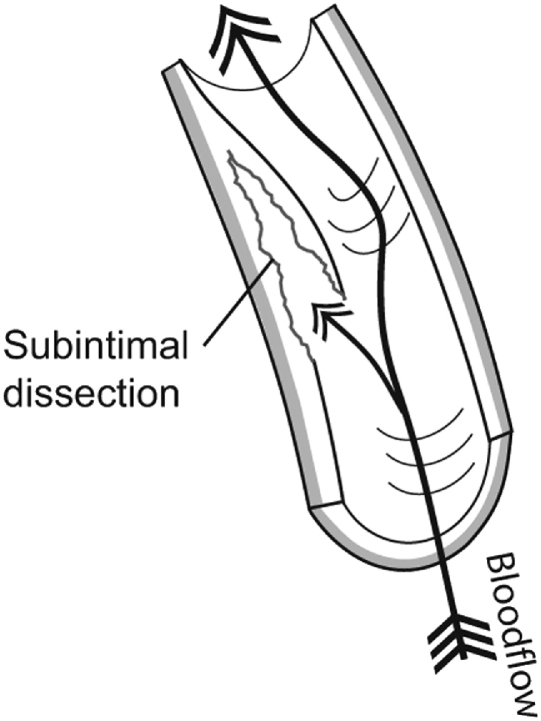
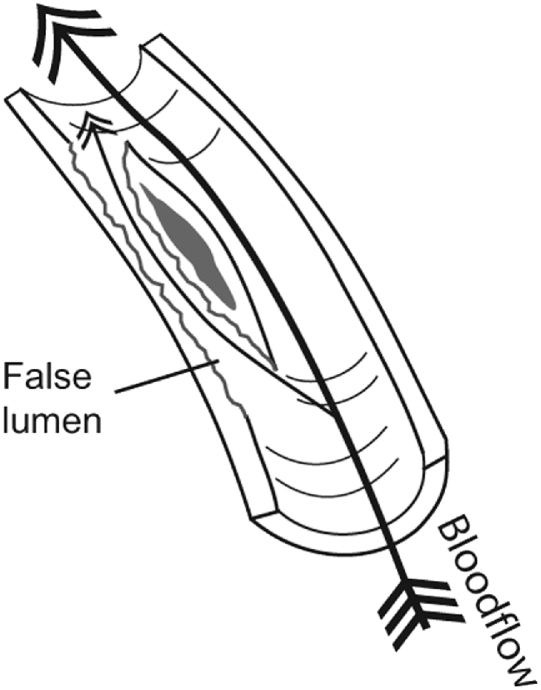
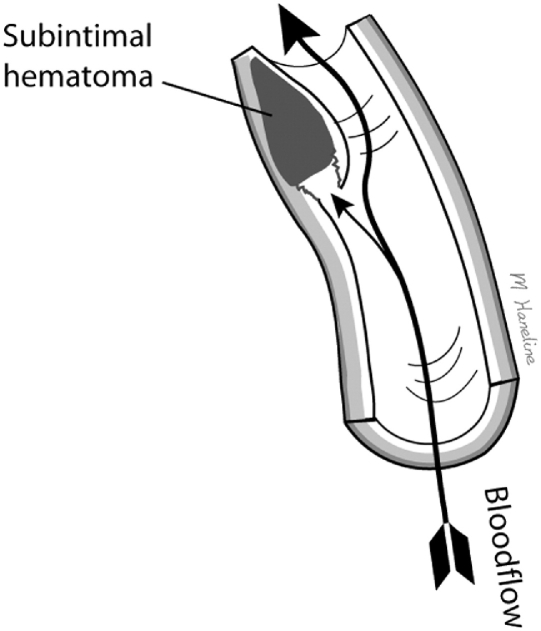
Arterial dissection is an uncommon vascular wall condition that typically involves a tear at some point in the artery's lining and the formation of an intimal flap, which allows blood to penetrate into the muscular portion of the vessel wall. Blood flowing between the layers of the torn blood vessel may cause the layers to separate from each other, resulting in arterial narrowing or even complete obstruction of the lumen (Fig 4). Moreover, pulsatile pressure damages the muscular layer, resulting in a splitting or dissection of the intimal and medial layers that may extend along the artery variable distances, usually in the direction of blood flow.14,15 Another way for dissection to occur involves a primary intramural hemorrhage of the vasa vasorum, which builds pressure between the intimal and medial layers and may eventually rupture into the vessel's true lumen.16-19 Occasionally, a double lumen (also known as false lumen) is formed when the subintimal hemorrhage ruptures back into the arterial lumen distally (Fig 5).
Fig 4.
Cervical artery dissection typically involves an initial tear in the artery lining. Blood then penetrates into the muscular portion of the vessel wall and may cause the layers to separate from each other forming a subintimal dissection.
Fig 5.
A double lumen is formed when a subintimal hemorrhage ruptures back into the arterial lumen distally.
The underlying cause of intimal tears is uncertain, although some experts maintain that because tearing occurs, previous trauma was necessarily involved.20 On the other hand, intimal tears are common in cases of spontaneous CAD in which no known trauma occurred before the dissection. A number of risk factors that instigate structural abnormalities of the arterial wall may increase its susceptibility to mechanical stress and predispose these patients to dissection.
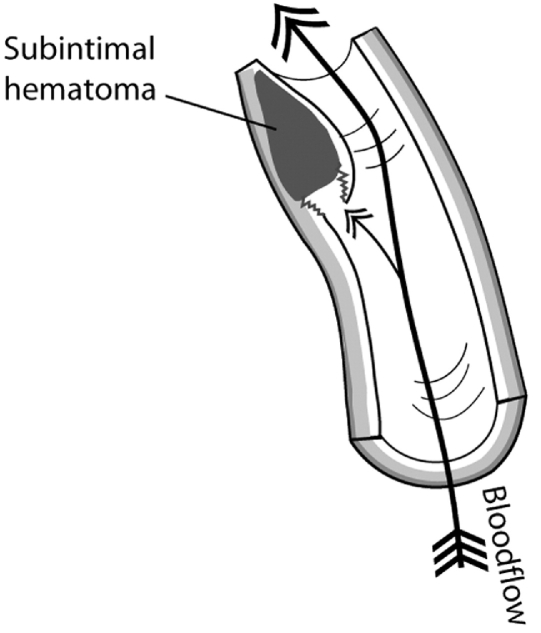
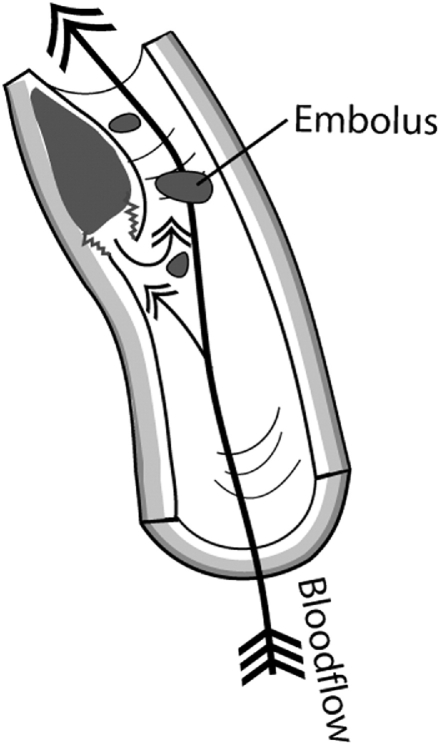
Subintimal hematoma develops after the intimal lining separates from the media and blood begins to accumulate in the vessel wall (Fig 6). The accrued blood soon develops into a thrombus and deforms the intima into the arterial lumen. Blood flow in the cervical arteries can be obstructed directly by the subintimal hematoma or emboli may detach from a thrombus and travel distally to obstruct the progressively smaller vessels in the brain, resulting in a stroke (Fig 7). Indeed, it is the release of emboli that most commonly causes brain ischemia secondary to CAD.21 A subintimal hematoma can also be caused by a primary intramural hemorrhage of the vasa vasorum without an initial intimal tear.
Fig 6.
Subintimal dissection with thrombus formation.
Fig 7.
Emboli may detach from a primary thrombus and travel distally to obstruct progressively smaller vessels in the brain.
Subadventitial hematoma occurs when a dissection penetrates through the tunica media into the subadventitial plane, resulting in the accumulation of blood between these layers. Instead of deforming the arterial lumen, as in subintimal dissection, the vessel's outer wall expands outward and a pseudoaneurysm develops (Fig 8). This deformity is observed regularly in CAD patients. In fact, a recent study involving 71 CAD patients reported that 35 (49.3%) of them had pseudoaneurysms.22 Interestingly, none of these 35 patients had transient ischemic attack, stroke, aneurysmal rupture, or clinical symptoms suggestive of vessel compression.
Fig 8.
Subadventitial dissection occurs when blood penetrates through the tunica media into the subadventitial plane. Blood accumulating between these layers deforms the tunica adventitia outward, producing a pseudoaneurysm.
Epidemiological features
Internal carotid artery dissections occur about 3 to 5 times more frequently than those involving the VA,17 although VA dissections are more likely to be associated with rapid head movements.23 The male-to-female ratio for the incidence of CAD is roughly equivalent, and the mean age of CAD patients is 46.3 years.24 The reported incidence is 2.6 to 2.9 per 100 000 for ICA dissection25,26 and 1 to 1.5 per 100 000 for VA dissection.2 Using the lower incidence estimates, there are more than 7000 cases of ICA dissection and about 3000 involving the VA that occur in the United States each year.27 Furthermore, cervical dissections represent the underlying etiology in approximately 20% of ischemic strokes that occur in patients 30 to 45 years age, compared with 2% or 2.5% in older patients.26 Given that the earliest symptoms of CAD often include headache and neck pain, some of these patients will undoubtedly present to chiropractic offices for treatment before a stroke is recognizable. Cervical spine manipulation performed at this point may be blamed for causing the full manifestation of the stroke, whether or not a causative relationship truly existed.
The outcome of CAD is unpredictable, with some patients obtaining a full recovery, some having permanent neurologic deficits, and a small number dying. For instance, a series of 126 CAD patients revealed that after a maximum of 6 months, 88 patients made an excellent recovery, 22 had a mild to moderate handicap, 15 were severely disabled, and 1 died.28 Another study that provided follow-up data on 46 manipulation-related CAD patients reported that at 1 year after stroke, 8 patients experienced a complete recovery, 36 had neurologic deficits, and 2 died. Long-term neurologic problems in these patients included incoordination, numbness, speech or swallowing dysfunction, and visual disturbances.29
Mechanisms of dissection
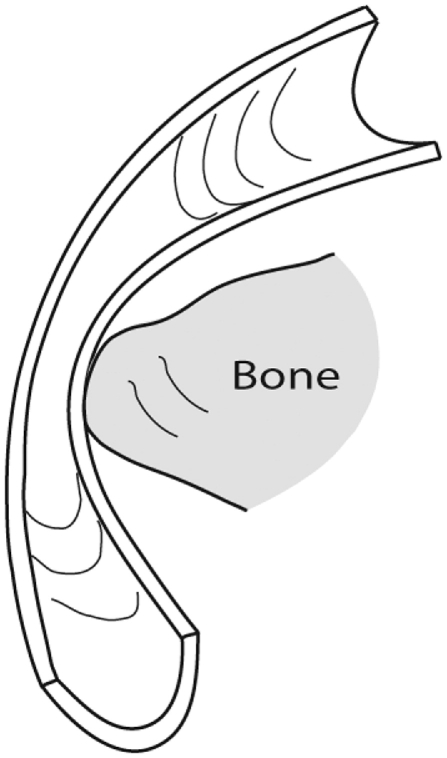
The VA seems most susceptible to strain in the atlantal segment as it traverses the C1-2 articulation,30 where it seems most at risk during rotational movements.6 The proposed mechanism of arterial strain during neck rotation involves the ipsilateral C1-2 joint being fixed while the contralateral side of C1 is propelled forward, which compresses and stretches the artery. The ICA is most susceptible to strain in the upper cervical region when the head and neck are in combined rotation or lateral flexion and simultaneous hyperextension.8,31 This position has been theorized to fix the otherwise freely moveable ICA against the anterior surface of C1 or C2, which makes it susceptible to injury when a sufficient amount of force is applied (Fig 9).32
Fig 9.
The cervical arteries are compressed and stretched when the neck is rotated and/or extended sufficiently.
Temporary arterial spasm, alone or with other lesions, has been suggested as a possible etiologic factor in postmanipulation vertebrobasilar ischemia.33 However, this mechanism is theoretical; and no objective evidence is available to support its existence.34 Frisoni and Anzola35 offered several plausible reasons why the connection between arterial spasm and vertebrobasilar ischemia is unlikely: first of all, angiographic evidence of arterial spasm consists of the narrowing of the vessel lumen that overlaps with evidence of dissection; second, spasm due to angiography is short-lived and seldom, if ever, results in arterial thrombosis; and third, no cases with macroscopically and microscopically intact vessels are available. Therefore, the authors felt that when stenosis of the VA is present on imaging studies, it most likely represents dissection rather than spasm.
Mechanical mechanisms
Traumatic vs spontaneous dissections
There are many reports in the literature of CAD occurring subsequent to trauma of varied degrees of severity. For instance, a retrospective analysis of 80 patients with vertebrobasilar ischemia that was determined to be caused by frank neck trauma reported that 70 of the cases were related to motor vehicle accidents.36 However, CADs more commonly occur after very minor trauma and even everyday activities that most people would consider to be nontraumatic. Furthermore, in some cases, no potentially traumatic activity at all could be identified. The latter variety of CADs would be considered spontaneous.18 A review article that considered CAD studies that involved 5 or more patients during the 10-year period between 1994 and 2003 reported that 371 of 606, or 61% of them, were spontaneous.37 The remaining 39% were associated with trivial or other trauma, which included manipulation in 9% of the total cases.
Frisoni and Anzola35 suggested 3 phases of evolution as a possible explanation for the pathogenesis of vertebrobasilar ischemia after neck motion. In the first phase, there is damage to the tunica intima or tunica media of one or both VAs. This phase may remain subclinical, or it may progress to the latter phases. In the second phase, immediate symptoms develop after neck motion because of a dissection that blocks the vessel's lumen. The third phase is characterized by progressive or delayed symptoms that are the result of thrombus formation or a slowly progressive dissection. This model may help explain why some patients have reported immediate dissection-related symptoms after cervical manipulation, whereas others have developed symptoms gradually over time. At the present time, this proposal has only theoretical support and remains speculative.
Trivial trauma and potential mechanical triggers
Traumatic dissections are typically caused by an obvious external injury, for instance, whiplash or blunt trauma. Spontaneous dissections, on the other hand, can be triggered by trivial trauma associated with abrupt movement or unusual positioning of the head and neck. Examples of trivial trauma include backing up a car, coughing, vomiting, unusual sleeping positions, having one's hair washed at a beauty salon, and rhythmic movement of the head and neck to music.38-40 The full range of trivial trauma encompasses countless everyday activities that involve head and neck movement. An article by Rome41 listed 68 activities that have been implicated in the development of CAD. Strenuous physical exertion has also been reported as a risk factor for developing CAD. Bear in mind, however, that many CADs occur spontaneously; and to add to the confusion, the etiologies of trivial trauma–related and spontaneous dissections are sometimes pooled when cases are reported in the literature. Indeed, if trivial events such as turning one's head while driving and coughing are capable of precipitating CADs, the distinction between spontaneous and traumatic events becomes that much more subtle and elusive.
The distinction between spontaneous dissection and dissection due to trivial trauma is ambiguous, being based primarily on the ability of the attending physician to elicit information about an associated potentially causal event in the patient history. However, because CADs have been reported in association with many commonplace activities, many CAD patients, if queried sufficiently, will be able to identify a suspicious activity in the days or weeks before the dissection. Because of this uncertainty, researchers sometimes disagree about how to classify the various CAD etiologies. To confound the issue even more, the clinical presentation of CAD patients with spontaneous vs trivial trauma–related etiologies does not differ, making them virtually impossible to distinguish based on examination.23,42 Another complicating factor is the variable time interval between injury and the onset of frank CAD symptoms. Although most of the time the onset is minutes to hours, there are reports in the literature of CAD occurring months after a traumatic event.43,44
Given that most CADs are not related to trauma or even abrupt head movements, one must wonder whether a clear cause-and-effect relationship can be established in cases that involve mechanical triggers. Typically, all that exists is a temporal relationship between some event and the development of CAD, which by itself cannot support causation. In any disease, there must be a precipitating cause, something that must take place before the disease can occur. A precipitating cause is sometimes related to one factor, although it may consist of multiple components. A mechanical trigger would generally be considered a component cause but not a precipitating cause of a particular CAD.45
Cervical spine manipulation has been reported as a CAD trigger in a number of case reports and case series and a few case-control studies, for the most part involving the VA. However, these study designs are not capable of determining whether one event caused the other. Thus, although on rare occasions CAD is associated with CSM, the basis for this association is not clear, especially when one considers that patients often seek treatment because of headache and neck pain, for example, 92% of patients in one study.46 These symptoms, at least in some cases, may actually have been caused by a CAD in progress. Subsequent manipulation may have had nothing to do with the development of ischemic neurologic signs, or it may have served as a trigger to provoke the condition. Nevertheless, the other 8% in this study who did not have head or neck pain yet still developed postmanipulation CAD are unexplained by this rationale. Incidence estimates of manipulation-related CAD range from 1 in 400 00047 to 1 in 5.85 million cervical manipulations.48 Probably the most commonly quoted statistic is 1 in 1 million.49
Because of the anatomical relationship between the arteries and bone structures in the upper cervical spine, rotational manipulation has been suspected as being responsible for most manipulation-related CADs.50 In addition, the histories of many manipulation-related CADs that have been reported in the literature have indicated that the patient was manipulated with the neck in rotation. Nonetheless, a study that considered 64 CAD cases that were temporally associated with CSM found that CAD-related strokes followed any form of standard cervical manipulation, including rotation, extension, lateral flexion, neutral position, and even nonforce manipulations. The authors concluded that CAD should be considered a random and unpredictable complication of any neck movement including cervical manipulation.46
Pathophysiological mechanisms
Several researchers have suggested that CAD patients are predisposed to dissection because of some type of underlying arterial abnormality.51-53 This opinion is based on several observations: first, most CADs are not related to trauma and simply occur spontaneously; second, CAD patients frequently have coexisting physiological abnormalities, such as hypertension, recent infection, migraine headache, and several others; third, the average person is exposed to trivial events involving the neck on a daily basis, yet most of them do not develop CAD. In fact, very few people ever experience CAD, perhaps only 3 or 4 per year in a city of 100 000 residents; and those that do tend to have certain risk factors more frequently than the unaffected population. Assessing the contribution of these individual risk factors is complicated, however, because many CADs occur in people who are in otherwise good health and who have not been exposed to trauma. Furthermore, assigning causation to any one of these risk factors in any particular patient with CAD is problematic because many people have CAD risk factors but do not develop dissection.
A recent systematic review by Rubinstein et al54 considered studies that have investigated CAD risk factors and found that nearly all of them were influenced by a variety of biases. The authors concluded that they found little evidence to support the presumed risk associated with these potential risk factors. Accordingly, the results of studies that deal with CAD risk factors should be interpreted with caution. Furthermore, even when methodologically strong studies point to risk factors for various conditions, they are often proven to be wrong in subsequent research.55 With this in mind, Table 1 presents a list of some commonly reported pathophysiological risk factors associated with CAD.
Table 1.
Pathophysiological risk factors for CAD
| Risk Factor | Basis |
|---|---|
| Connective Tissue Abnormalities52,56 | Skin Biopsies of 68% of 25 and 55% of 65 CAD Patients Showed Evidence of Irregular Collagen Fibrils and Fragmentation of Elastic Fibers That Is Thought to Weaken the Arterial Wall. |
| Vascular Type IV Ehlers-Danlos Syndrome52,57,58 and Marfan Syndrome59 | These Inherited Connective Tissue Disorders Affect the Skin, Joints, and the Walls of Blood Vessels. The Incidence of CAD Has Been Reported to Be Higher in Patients With These Conditions. |
| Recent Infection60-62 | Case-Control Studies Point to Recent Infection as a Potential Trigger of CAD, Possibly Related to Arterial Wall Damage Caused by Proteolytic, Oxidative, or Autoimmune Defects. Furthermore, the Incidence of CAD Has Been Reported to Be Higher During Certain Seasons, Which May Be Related to the Higher Incidence of Upper Respiratory Tract Infections During the Winter. |
| Hyperhomocysteinemia63-66 | Especially Apparent in Patients With Total Plasma Levels That Exceed 12 μmol/L. Associated Arterial Wall Abnormalities May Increase the Artery's Susceptibility to Mechanical Stress. |
| Fibromuscular Dysplasia28,67 | Affects the ICA More Commonly Than the VA. Present in Up to 23% of ICA Dissection Patients, Making It the Most Frequently Reported Associated Abnormality. Characterized by Irregular Segments of Stricture and Dilation in the Vessel. |
| Cystic Medial Necrosis2 | Focal Degeneration of the Elastic Tissue and Muscle of the Tunica Media, With the Development of Mucoid Material. There Is a Breakdown of the Collagen, Elastin, and Smooth Muscle, and an Increase in the Artery's Ground Substance. |
| Type 1 Osteogenesis Imperfecta68 | Interferes With the Production of Type 1 Collagen. In Some Cases, Collagen Synthesis Is Decreased, Whereas in Others, Structurally Defective Collagen Is Produced. |
| Anatomical Abnormalities12,69-72 | May Result in Blood Flow Disturbance Leading to Insufficient Collateral Circulation. The Atlantal Segment of the VA Is Commonly Anomalous. Arterial Redundancies (eg, Coils, Kinks, and Loops) and Increased Diameter of the Common Carotid Artery Are More Common in Patients With ICA Dissection. |
| Hypertension14,73-75 | Several Studies Have Pointed to Hypertension as a CAD Risk Factor. A Well-Designed Case-Control Study Reported a Statistically Significant Association in the Subgroup of VA Dissection Patients, but Not in the Overall CAD Group. |
| History of Migraine76-78 | Robust Odds Ratios Have Been Generated in Several Case-Control Studies Pointing to This Association—7.41 (95% CI 3.11-17.64) in 1 Study. |
| Autosomal Dominant Polycystic Kidney Disease79,80 | Has Been Reported in Association With CAD by Several Authors, Although the Frequency of This Association and the Mechanism Involved Are Unknown. |
| Antithyroid Autoimmunity81,82 | A Recent Case-Control Study Reported the Presence of Antithyroid Autoimmunity in 31.0% of 29 CAD Patients, Compared With 6.9% of 29 Non-CAD Stroke Patients (P = .041). There Have Also Been Recent Case Reports of ICA Dissection in Patients With Graves Disease. Immunologic Mechanisms May Contribute to the Vascular Damage That Is Thought to Initiate CADs. |
| EE Genotype of the E469K ICAM-1 Polymorphism83 | The EE Genotype Gives Rise to a Proinflammatory Tendency in Patients That May Predispose Them to Developing CAD. |
| Methylenetetrahydrofolate Reductase C677T Genotype63,65,66,84 | Mutation of This Genotype Leads to Elevated Serum Levels of Homocysteine. The Issue Is Controversial Because Studies Have Not Been in Agreement. |
| α-1-Antitrypsin Deficiency85-87 | Researchers Have Theorized That This Deficiency May Lead to a Fragile Vessel Wall That Is Predisposed to Dissection, but There Is Little Evidence to Support This Relationship. Only a Few Small Studies Have Reported on This Association, and Their Results Are Conflicting. |
| Oral Contraceptive Use60,77,78 | Studies That Have Considered This Issue Are in Conflict. Only 1 Small Study Showed That Current Use of Oral Contraceptives Was Associated With CAD. The Consensus of Researchers Is That No Good Evidence Exists Supporting This Association. |
| Cardiovascular Risk Factors10,14,61,78,88 | May Actually Be Protective. Atherosclerotic Changes, Hypercholesterolemia, Advanced Age, and Diabetes Are Reported to Be Either Not Associated or Significantly Less Prevalent in CAD Patients. |
CI indicates confidence interval; ICAM-1, intracellular adhesion molecule 1.
Conclusion
The cervical arteries are susceptible to dissection in association with a variety of trivial events. This association is confusing, however, because people normally encounter innumerable trivial events during their lifetime without ever experiencing dissection. Moreover, patients do not live in a vacuum and are typically exposed to other potentially contributory incidents before and after an event that is suspected of causing a particular CAD. For instance, they turn their heads to back up cars, have their hair washed at a beauty shop, practice yoga, sleep on their stomachs, get angry, or experience some other event that could have been the true cause of the dissection. Furthermore, because most CADs develop in the absence of any discernible mechanical event, it is very difficult to indict a particular incident or factor as the cause of a particular stroke.
A number of pathophysiological risk factors have been described in the literature that are thought to contribute to the development of CAD. Like trivial events, not all risk factors are present in every patient; and some patients experience CAD without any of them. The most compelling risk factors have to do with connective tissue abnormalities, which may contribute to a weakening of the vascular wall, making it more susceptible to tearing. Indeed, many researchers think that an underlying arterial abnormality must be present for dissection to occur. Included in this line of reasoning is the fact that elevated homocysteine levels, linked to arterial weaknesses both in vitro and in vivo, are known to activate proteolytic enzymes and interfere with the cross-linking of the collagen.89 Both these phenomena compromise the integrity of the arterial wall. Nonetheless, the exact pathogenesis of CAD is uncertain. Its etiology is probably multifactorial and related to a variety of arteriopathies that are produced by an assortment of genetic and environmental factors.63,90
No certain cause-and-effect relationship has been established between CSM and CAD, but it seems that cervical manipulation may be capable of triggering dissection in a susceptible patient or contributing to the evolution of an already existing CAD. Despite the many risk factors that have been proposed as possible causes of CAD, it is still unknown which of them definitely predispose patients to CAD after CSM. Thus, it is not possible at this time to accurately identify patients at risk of CAD before CSM.
References
- 1.Thanvi B., Munshi S.K., Dawson S.L., Robinson T.G. Carotid and vertebral artery dissection syndromes. Postgrad Med J. 2005;81(956):383–388. doi: 10.1136/pgmj.2003.016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schievink W.I. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898–906. doi: 10.1056/NEJM200103223441206. [DOI] [PubMed] [Google Scholar]
- 3.Netter F. Ciba Pharmaceutical Company; Summit: 1983. Volume 1: nervous system. Part 1: anatomy and physiology. [Google Scholar]
- 4.Reddy M., Reddy B., Schoggl A., Saringer W., Matula C. The complexity of trauma to the cranio-cervical junction: correlation of clinical presentation with Doppler flow velocities in the V3-segment of the vertebral arteries. Acta Neurochir (Wien) 2002;144(6):575–580. doi: 10.1007/s007010200078. [DOI] [PubMed] [Google Scholar]
- 5.Chopard R.P., de Miranda Neto M.H., Lucas G.A., Chopard M.R. The vertebral artery: its relationship with adjoining tissues in its course intra and inter transverse processes in man. Rev Paul Med. 1992;110(6):245–250. [PubMed] [Google Scholar]
- 6.Davis J.M., Zimmerman R.A. Injury of the carotid and vertebral arteries. Neuroradiology. 1983;25(2):55–69. doi: 10.1007/BF00333294. [DOI] [PubMed] [Google Scholar]
- 7.Ringel S.P., Harrison S.H., Norenberg M.D., Austin J.H. Fibromuscular dysplasia: multiple “spontaneous” dissecting aneurysms of the major cervical arteries. Ann Neurol. 1977;1(3):301–304. doi: 10.1002/ana.410010320. [DOI] [PubMed] [Google Scholar]
- 8.Stringer W.L., Kelly D.L. Traumatic dissection of the extracranial internal carotid artery. Neurosurgery. 1980;6(2):123–130. doi: 10.1227/00006123-198002000-00002. [DOI] [PubMed] [Google Scholar]
- 9.de Bray J.M., Penisson-Besnier I., Dubas F., Emile J. Extracranial and intracranial vertebrobasilar dissections: diagnosis and prognosis. J Neurol Neurosurg Psychiatry. 1997;63(1):46–51. doi: 10.1136/jnnp.63.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumgartner R.W., Arnold M., Baumgartner I. Carotid dissection with and without ischemic events: local symptoms and cerebral artery findings. Neurology. 2001;57(5):827–832. doi: 10.1212/wnl.57.5.827. [DOI] [PubMed] [Google Scholar]
- 11.Arnold M., Cumurciuc R., Stapf C., Favrole P., Berthet K., Bousser M.-G. Pain as the only symptom of cervical artery dissection. J Neurol Neurosurg Psychiatry. 2006;77(9):1021–1024. doi: 10.1136/jnnp.2006.094359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Triano J., Kawchuk G.N., editors. Current concepts in spinal manipulation and cervical arterial incidents. NCMIC Group Inc; West Des Moines (Iowa): 2005. [Google Scholar]
- 13.Haneline M., Lewkovich G. Identification of internal carotid artery dissection in chiropractic practice. J Can Chiropr Assoc. 2004;48(3):206–210. [PMC free article] [PubMed] [Google Scholar]
- 14.Mokri B., Sundt T.M., Houser O.W., Piepgras D.G. Spontaneous dissection of the cervical internal carotid artery. Ann Neurol. 1986;19(2):126–138. doi: 10.1002/ana.410190204. [DOI] [PubMed] [Google Scholar]
- 15.Zetterling M., Carlstrom C., Konrad P. Internal carotid artery dissection. Acta Neurol Scand. 2000;101(1):1–7. doi: 10.1034/j.1600-0404.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- 16.Ehrenfeld W.K., Wylie E.J. Spontaneous dissection of the internal carotid artery. Arch Surg. 1976;111(11):1294–1301. doi: 10.1001/archsurg.1976.01360290128019. [DOI] [PubMed] [Google Scholar]
- 17.Hart R.G., Easton J.D. Dissections of cervical and cerebral arteries. Neurol Clin. 1983;1(1):155–182. [PubMed] [Google Scholar]
- 18.Kochan JP, Kanamalla US. Carotid and vertebral artery dissection. eMedicine.com [Web page]. June 23, 2006. Available from: http://www.emedicine.com/radio/topic132.htm. Accessed August 12, 2006.
- 19.Volker W., Besselmann M., Dittrich R. Generalized arteriopathy in patients with cervical artery dissection. Neurology. 2005;64(9):1508–1513. doi: 10.1212/01.WNL.0000159739.24607.98. [DOI] [PubMed] [Google Scholar]
- 20.Beatty R.A. Dissecting hematoma of the internal carotid artery following chiropractic cervical manipulation. J Trauma. 1977;17(3):248–249. doi: 10.1097/00005373-197703000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Benninger D.H., Georgiadis D., Kremer C., Studer A., Nedeltchev K., Baumgartner R.W. Mechanism of ischemic infarct in spontaneous carotid dissection. Stroke. 2004;35(2):482–485. doi: 10.1161/01.STR.0000109766.27393.52. [DOI] [PubMed] [Google Scholar]
- 22.Touze E., Randoux B., Meary E., Arquizan C., Meder J.-F., Mas J.-L. Aneurysmal forms of cervical artery dissection: associated factors and outcome. Stroke. 2001;32(2):418–423. doi: 10.1161/01.str.32.2.418. [DOI] [PubMed] [Google Scholar]
- 23.Prabhakar S., Bhatia R., Khandelwal N., Lal V., Das C.P. Vertebral artery dissection due to indirect neck trauma: an underrecognised entity. Neurol India. 2001;49(4):384–390. [PubMed] [Google Scholar]
- 24.Schievink W.I. The treatment of spontaneous carotid and vertebral artery dissections. Curr Opin Cardiol. 2000;15(5):316–321. doi: 10.1097/00001573-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Schievink W.I., Mokri B., Whisnant J.P. Internal carotid artery dissection in a community. Rochester, Minnesota, 1987-1992. Stroke. 1993;24(11):1678–1680. doi: 10.1161/01.str.24.11.1678. [DOI] [PubMed] [Google Scholar]
- 26.Giroud M., Fayolle H., Andre N. Incidence of internal carotid artery dissection in the community of Dijon. J Neurol Neurosurg Psychiatry. 1994;57(11):1443. doi: 10.1136/jnnp.57.11.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haneline M.T., Croft A.C., Frishberg B.M. Association of internal carotid artery dissection and chiropractic manipulation. Neurolog. 2003;9(1):35–44. doi: 10.1097/01.nrl.0000038583.58012.10. [DOI] [PubMed] [Google Scholar]
- 28.Dziewas R., Konrad C., Drager B. Cervical artery dissection-clinical features, risk factors, therapy and outcome in 126 patients. J Neurol. 2003;250(10):1179–1184. doi: 10.1007/s00415-003-0174-5. [DOI] [PubMed] [Google Scholar]
- 29.Haldeman S., Kohlbeck F.J., McGregor M. Unpredictability of cerebrovascular ischemia associated with cervical spine manipulation therapy: a review of sixty-four cases after cervical spine manipulation. Spine. 2002;27(1):49–55. doi: 10.1097/00007632-200201010-00012. [DOI] [PubMed] [Google Scholar]
- 30.Hufnagel A., Hammers A., Schonle P.W., Bohm K.D., Leonhardt G. Stroke following chiropractic manipulation of the cervical spine. J Neurol. 1999;246(8):683–688. doi: 10.1007/s004150050432. [DOI] [PubMed] [Google Scholar]
- 31.Fabian T.C., Patton J.H., Croce M.A., Minard G., Kudsk K.A., Pritchard F.E. Blunt carotid injury. Importance of early diagnosis and anticoagulant therapy. Ann Surg. 1996;223(5):513–522. doi: 10.1097/00000658-199605000-00007. [discussion 515-522] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lepojarvi M., Tarkka M., Leinonen A., Kallanranta T. Spontaneous dissection of the internal carotid artery. Acta Chir Scand. 1988;154(10):559–566. [PubMed] [Google Scholar]
- 33.Di Fabio R.P. Manipulation of the cervical spine: risks and benefits. Phys Ther. 1999;79(1):50–65. [PubMed] [Google Scholar]
- 34.Mann T., Refshauge K.M. Causes of complications from cervical spine manipulation. Aust J Physiother. 2001;47(4):255–266. doi: 10.1016/s0004-9514(14)60273-7. [DOI] [PubMed] [Google Scholar]
- 35.Frisoni G., Anzola G. Vertebrobasilar ischemia after neck motion. Stroke. 1991;22(11):1452–1460. doi: 10.1161/01.str.22.11.1452. [DOI] [PubMed] [Google Scholar]
- 36.Beaudry M., Spence J.D. Motor vehicle accidents: the most common cause of traumatic vertebrobasilar ischemia. Can J Neurol Sci. 2003;30(4):320–325. doi: 10.1017/s0317167100003024. [DOI] [PubMed] [Google Scholar]
- 37.Haneline M.T., Lewkovich G.N. An analysis of the etiology of cervical artery dissections: 1994 to 2003. J Manipulative Physiol Ther. 2005;28(8):617–622. doi: 10.1016/j.jmpt.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 38.Ast G., Woimant F., Georges B., Laurian C., Haguenau M. Spontaneous dissection of the internal carotid artery in 68 patients. Eur J Med. 1993;2(8):466–472. [PubMed] [Google Scholar]
- 39.Kumar S.D., Kumar V., Kaye W. Bilateral internal carotid artery dissection from vomiting. Am J Emerg Med. 1998;16(7):669–670. doi: 10.1016/s0735-6757(98)90172-3. [DOI] [PubMed] [Google Scholar]
- 40.Foye P.M., Najar M.P., Camme A.A. Pain, dizziness, and central nervous system blood flow in cervical extension: vascular correlations to beauty parlor stroke syndrome and salon sink radiculopathy. Am J Phys Med Rehabil. 2002;81(6):395–399. doi: 10.1097/00002060-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Rome P. Perspectives: an overview of comparative considerations of cerebrovascular accidents. Chirpr J Aust. 1999;29:87–102. [Google Scholar]
- 42.Biousse V., D'Anglejan-Chatillon J., Touboul P.J., Amarenco P., Bousser M.G. Time course of symptoms in extracranial carotid artery dissections. A series of 80 patients. Stroke. 1995;26(2):235–239. doi: 10.1161/01.str.26.2.235. [DOI] [PubMed] [Google Scholar]
- 43.Pica R.A., Rockwell B.H., Raji M.R., Dastur K.J., Berkey K.E. Traumatic internal carotid artery dissection presenting as delayed hemilingual paresis. AJNR Am J Neuroradiol. 1996;17(1):86–88. [PMC free article] [PubMed] [Google Scholar]
- 44.Norris J.W., Beletsky V., Nadareishvili Z.G. Sudden neck movement and cervical artery dissection. The Canadian Stroke Consortium. CMAJ. 2000;163(1):38–40. [PMC free article] [PubMed] [Google Scholar]
- 45.Hill M.D. Cervical artery dissection, imaging, trauma and causal inference. Can J Neurol Sci. 2003;30(4):302–304. doi: 10.1017/s0317167100002985. [DOI] [PubMed] [Google Scholar]
- 46.Haldeman S., Kohlbeck F.J., McGregor M. Stroke, cerebral artery dissection, and cervical spine manipulation therapy. J Neurol. 2002;249(8):1098–1104. doi: 10.1007/s00415-002-0783-4. [DOI] [PubMed] [Google Scholar]
- 47.Dvorak J., Orelli F.V. How dangerous is manipulation to the cervical spine? Manual Med. 1985;2(1):1–4. [Google Scholar]
- 48.Haldeman S., Carey P., Townsend M., Papadopoulos C. Arterial dissections following cervical manipulation: the chiropractic experience. CMAJ. 2001;165(7):905–906. [PMC free article] [PubMed] [Google Scholar]
- 49.Hosek R.S., Schram S.B., Silverman H., Myers J.B., Williams S.E. Cervical manipulation. JAMA. 1981;245(9):922. [PubMed] [Google Scholar]
- 50.Klougart N., Leboeuf-Yde C., Rasmussen L.R. Safety in chiropractic practice. Part II: treatment to the upper neck and the rate of cerebrovascular incidents. J Manipulative Physiol Ther. 1996;19(9):563–569. [PubMed] [Google Scholar]
- 51.Haneline M., Triano J. Cervical artery dissection. A comparison of highly dynamic mechanisms: manipulation versus motor vehicle collision. J Manipulative Physiol Ther. 2005;28(1):57–63. doi: 10.1016/j.jmpt.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 52.Brandt T., Orberk E., Weber R. Pathogenesis of cervical artery dissections: association with connective tissue abnormalities. Neurology. 2001;57(1):24–30. doi: 10.1212/wnl.57.1.24. [DOI] [PubMed] [Google Scholar]
- 53.Schievink W.I., Mokri B., Piepgras D.G. Spontaneous dissections of cervicocephalic arteries in childhood and adolescence. Neurology. 1994;44(9):1607–1612. doi: 10.1212/wnl.44.9.1607. [DOI] [PubMed] [Google Scholar]
- 54.Rubinstein S.M., Peerdeman S.M., van Tulder M.W., Riphagen I., Haldeman S. A systematic review of the risk factors for cervical artery dissection. Stroke. 2005;36(7):1575–1580. doi: 10.1161/01.STR.0000169919.73219.30. [DOI] [PubMed] [Google Scholar]
- 55.Taubes G. Epidemiology faces its limits. Science. 1995;269(5221):164–169. doi: 10.1126/science.7618077. [DOI] [PubMed] [Google Scholar]
- 56.Brandt T., Hausser I., Orberk E. Ultrastructural connective tissue abnormalities in patients with spontaneous cervicocerebral artery dissections. Ann Neurol. 1998;44(2):281–285. doi: 10.1002/ana.410440224. [DOI] [PubMed] [Google Scholar]
- 57.Ulbricht D., Diederich N.J., Hermanns-Le T., Metz R.J., Macian F., Pierard G.E. Cervical artery dissection: an atypical presentation with Ehlers-Danlos–like collagen pathology? Neurology. 2004;63(9):1708–1710. doi: 10.1212/01.wnl.0000142970.09454.30. [DOI] [PubMed] [Google Scholar]
- 58.North K.N., Whiteman D.A., Pepin M.G., Byers P.H. Cerebrovascular complications in Ehlers-Danlos syndrome type IV. Ann Neurol. 1995;38(6):960–964. doi: 10.1002/ana.410380620. [DOI] [PubMed] [Google Scholar]
- 59.Sztajzel R., Hefft S., Girardet C. Marfan's syndrome and multiple extracranial aneurysms. Cerebrovasc Dis. 2001;11(4):346–349. doi: 10.1159/000047665. [DOI] [PubMed] [Google Scholar]
- 60.Grau A.J., Brandt T., Buggle F. Association of cervical artery dissection with recent infection. Arch Neurol. 1999;56(7):851–856. doi: 10.1001/archneur.56.7.851. [DOI] [PubMed] [Google Scholar]
- 61.Guillon B., Berthet K., Benslamia L., Bertrand M., Bousser M.G., Tzourio C. Infection and the risk of spontaneous cervical artery dissection: a case-control study. Stroke. 2003;34(7):e79–e81. doi: 10.1161/01.STR.0000078309.56307.5C. [DOI] [PubMed] [Google Scholar]
- 62.Grau A.J., Buggle F., Steichen-Wiehn C. Clinical and biochemical analysis in infection-associated stroke. Stroke. 1995;26(9):1520–1526. doi: 10.1161/01.str.26.9.1520. [DOI] [PubMed] [Google Scholar]
- 63.Gallai V., Caso V., Paciaroni M. Mild hyperhomocyst(e)inemia: a possible risk factor for cervical artery dissection. Stroke. 2001;32(3):714–718. doi: 10.1161/01.str.32.3.714. [DOI] [PubMed] [Google Scholar]
- 64.Pezzini A., Del Zotto E., Padovani A. Hyperhomocysteinemia: a potential risk factor for cervical artery dissection following chiropractic manipulation of the cervical spine. J Neurol. 2002;249(10):1401–1403. doi: 10.1007/s00415-002-0851-9. [DOI] [PubMed] [Google Scholar]
- 65.Pezzini A., Del Zotto E., Archetti S. Plasma homocysteine concentration, C677T MTHFR genotype, and 844ins68bp CBS genotype in young adults with spontaneous cervical artery dissection and atherothrombotic stroke. Stroke. 2002;33(3):664–669. doi: 10.1161/hs0302.103625. [DOI] [PubMed] [Google Scholar]
- 66.Konrad C., Muller G.A., Langer C. Plasma homocysteine, MTHFR C677T, CBS 844ins68bp, and MTHFD1 G1958A polymorphisms in spontaneous cervical artery dissections. J Neurol. 2004;251(10):1242–1248. doi: 10.1007/s00415-004-0523-z. [DOI] [PubMed] [Google Scholar]
- 67.Luscher T.F., Lie J.T., Stanson A.W., Houser O.W., Hollier L.H., Sheps S.G. Arterial fibromuscular dysplasia. Mayo Clin Proc. 1987;62(10):931–952. doi: 10.1016/s0025-6196(12)65051-4. [DOI] [PubMed] [Google Scholar]
- 68.Rouviere S., Michelini R., Sarda P., Pages M. Spontaneous carotid artery dissection in two siblings with osteogenesis imperfecta. Cerebrovasc Dis. 2004;17(2-3):270–272. doi: 10.1159/000076967. [DOI] [PubMed] [Google Scholar]
- 69.Haynes M.J. Doppler studies comparing the effects of cervical rotation and lateral flexion on vertebral artery blood flow. J Manipulative Physiol Ther. 1996;19(6):378–384. [PubMed] [Google Scholar]
- 70.Cacciola F., Phalke U., Goel A. Vertebral artery in relationship to C1-C2 vertebrae: an anatomical study. Neurol India. 2004;52(2):178–184. [PubMed] [Google Scholar]
- 71.Barbour P.J., Castaldo J.E., Rae-Grant A.D. Internal carotid artery redundancy is significantly associated with dissection. Stroke. 1994;25(6):1201–1206. doi: 10.1161/01.str.25.6.1201. [DOI] [PubMed] [Google Scholar]
- 72.Guillon B., Tzourio C., Biousse V., Adrai V., Bousser M.G., Touboul P.J. Arterial wall properties in carotid artery dissection: an ultrasound study. Neurology. 2000;55(5):663–666. doi: 10.1212/wnl.55.5.663. [DOI] [PubMed] [Google Scholar]
- 73.Gonzales-Portillo F., Bruno A., Biller J. Outcome of extracranial cervicocephalic arterial dissections: a follow-up study. Neurol Res. 2002;24(4):395–398. doi: 10.1179/016164102101200087. [DOI] [PubMed] [Google Scholar]
- 74.Haldeman S., Kohlbeck F.J., McGregor M. Risk factors and precipitating neck movements causing vertebrobasilar artery dissection after cervical trauma and spinal manipulation. Spine. 1999;24(8):785–794. doi: 10.1097/00007632-199904150-00010. [DOI] [PubMed] [Google Scholar]
- 75.Pezzini A., Caso V., Zanferrari C. Arterial hypertension as risk factor for spontaneous cervical artery dissection. A case-control study. J Neurol Neurosurg Psychiatry. 2006;77(1):95–97. doi: 10.1136/jnnp.2005.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tzourio C., Benslamia L., Guillon B. Migraine and the risk of cervical artery dissection: a case-control study. Neurology. 2002;59(3):435–437. doi: 10.1212/wnl.59.3.435. [DOI] [PubMed] [Google Scholar]
- 77.D'Anglejan-Chatillon J., Ribeiro V., Mas J.L., Youl B.D., Bousser M.G. Migraine—a risk factor for dissection of cervical arteries. Headache. 1989;29(9):560–561. doi: 10.1111/j.1526-4610.1989.hed2909560.x. [DOI] [PubMed] [Google Scholar]
- 78.Pezzini A., Granella F., Grassi M. History of migraine and the risk of spontaneous cervical artery dissection. Cephalalgia. 2005;25(8):575–580. doi: 10.1111/j.1468-2982.2005.00919.x. [DOI] [PubMed] [Google Scholar]
- 79.Larranaga J., Rutecki G.W., Whittier F.C. Spontaneous vertebral artery dissection as a complication of autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1995;25(1):70–74. doi: 10.1016/0272-6386(95)90629-0. [DOI] [PubMed] [Google Scholar]
- 80.Schievink W.I., McDougall C.G., Spetzler R.F. Headache in autosomal dominant polycystic kidney disease due to spontaneous vertebral artery dissection. BNI Quarterly. 1998;14(4) [Google Scholar]
- 81.Pezzini A., Del Zotto E., Mazziotti G. Thyroid autoimmunity and spontaneous cervical artery dissection. Stroke. 2006;37:2375–2377. doi: 10.1161/01.STR.0000236500.15976.f3. [DOI] [PubMed] [Google Scholar]
- 82.Campos C.R., Basso M., Evaristo E.F., Yamamoto F.I., Scaff M. Bilateral carotid artery dissection with thyrotoxicosis. Neurology. 2004;63(12):2443–2444. doi: 10.1212/01.wnl.0000147327.29332.b5. [DOI] [PubMed] [Google Scholar]
- 83.Longoni M., Grond-Ginsbach C., Grau A.J. The ICAM-1 E469K gene polymorphism is a risk factor for spontaneous cervical artery dissection. Neurology. 2006;66(8):1273–1275. doi: 10.1212/01.wnl.0000208411.01172.0b. [DOI] [PubMed] [Google Scholar]
- 84.Kloss M., Wiest T., Hyrenbach S. MTHFR 677TT genotype increases the risk for cervical artery dissections. J Neurol Neurosurg Psychiatry. 2006;77(8):951–952. doi: 10.1136/jnnp.089730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vila N., Millan M., Ferrer X., Riutort N., Escudero D. Levels of alpha1-antitrypsin in plasma and risk of spontaneous cervical artery dissections: a case-control study. Stroke. 2003;34(9):E168–E169. doi: 10.1161/01.STR.0000085085.20390.A3. [DOI] [PubMed] [Google Scholar]
- 86.Konrad C., Langer C., Muller G.A. Protease inhibitors in spontaneous cervical artery dissections. Stroke. 2005;36(1):9–13. doi: 10.1161/01.STR.0000149631.52985.27. [DOI] [PubMed] [Google Scholar]
- 87.Pezzini A., Magoni M., Corda L. Alpha-1-antitrypsin deficiency–associated cervical artery dissection: report of three cases. Eur Neurol. 2002;47(4):201–204. doi: 10.1159/000057899. [DOI] [PubMed] [Google Scholar]
- 88.Ahl B., Bokemeyer M., Ennen J.C., Kohlmetz C., Becker H., Weissenborn K. Dissection of the brain supplying arteries over the life span. J Neurol Neurosurg Psychiatry. 2004;75(8):1194–1196. doi: 10.1136/jnnp.2003.013326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rosner A.L. Spontaneous cervical artery dissections and implications for homocysteine. J Manipulative Physiol Ther. 2004;27(2):124–132. doi: 10.1016/j.jmpt.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 90.Brandt T., Grond-Ginsbach C. Spontaneous cervical artery dissection: from risk factors toward pathogenesis. Stroke. 2002;33(3):657–658. [PubMed] [Google Scholar]