Abstract
Objective
The purpose of this study was to determine if ischemic compression therapy over the bladder area results in clinically important changes among female patients with stress and mixed (stress and urge) incontinence.
Methods
One group of patients (n = 24) received ischemic compression therapy directed over the bladder area (experimental group). The control group (n = 9) received ischemic compression therapy directed toward structures of the hip joint. Changes in urinary incontinence symptoms were monitored using a 2-part questionnaire: the urogenital distress inventory and the incontinence impact questionnaire. Patients' perceived amelioration (improvement) was quantified using a scale divided from 0% to 100%.
Results
Mean scores for the first questionnaire (urogenital distress inventory + incontinence impact questionnaire, 19 questions) were 23.3 vs 25.3 at baseline and 10.2 vs 22.2 after 15 treatments for the experimental and control group, respectively. The experimental group scores were 6.9 at 30 days after the last treatment and 11.3 at the 6-month follow-up. The perceived percentages of amelioration after 15 treatments were 69% vs 32% for the experimental and control group, respectively. The experimental group scores were 73% at 30 days after the last treatment and 60% at the 6-month follow-up.
Conclusions
In this study, ischemic compression directed toward elicited trigger points over bladder area was found to be an effective treatment of patients presenting symptoms of urinary incontinence. Improvement in symptoms was still present in follow-up at 6 months.
Key indexing terms: Urinary incontinence, Chiropractic, Myofascial pain syndromes
Introduction
Urinary incontinence (UI) is defined as the inability to control micturition. Urinary incontinence may be transient or permanent, and it can be caused by a range of urinary tract dysfunctions. In adults, 80% of UI cases occur among women; and 15% to 30% of women will have UI in the course of their lifetime.1,2 Aging favors incontinence because of muscular system weakening and bladder capacity reduction.2 Women who had children using natural childbirth strategies are especially at risk because the birthing process places stress on the pelvic muscles.2,3 Those who have incontinence may feel very much isolated and often experience feelings of failure and shame. More than half of the women with UI do not seek treatment and are subject to feelings of embarrassment, powerlessness, and frustration.2
Prolapsus of the bladder and uterus is frequently accompanied by UI. However, a distinction should be made between the former anomalies and benign UI. Patients who succeed in regaining continence through a program of exercises nevertheless remain burdened with the same prolapsus.4 It may also be worth noting that treatment of UI in North America generates more costs than dialyses and coronary bypasses combined.5
Ninety-eight percent of UI patients experience either stress incontinence or urge incontinence: 49% have stress incontinence; 22% have urge incontinence; and 29% have both, that is, mixed incontinence.3,6
Stress incontinence can be defined as involuntary urine loss in the course of physical efforts such as coughing, laughing, and exercising, which all increase intraabdominal pressure. Stress incontinence is not accompanied by any feeling of urgency; and indeed, the leakage is often unnoticed. Urine loss is generally greater during the day and has no relationship to the amount of urine actually in the bladder.1,3 Urethral hypermobility and/or intrinsic sphincter deficiency is commonly present and often has been caused by weakness of the pelvic floor musculature due to childbirth, abdominal obesity, or postmenopausal urogenital atrophy.2,7 Stress incontinence is often triggered by the birthing of the first baby and is commonly exacerbated by subsequent births.3 Urge incontinence is an involuntary loss of urine preceded by a sensation of urgency and followed by uncontrollable micturition. It may be triggered by sudden laughter, the noise of a key in a door, the sight of water, or the sound of a running faucet. The sensation of urgency is sharp, and the patients often cannot make it to the rest room in time. There may be a very significant loss of urine, even complete voiding of the bladder. Urge incontinence is due to bladder wall hyperactivity or instability and central nervous system disorders (eg, parkinsonism, stroke). Urge incontinence may be caused by past or current infection, anxiety, or the normal aging process.2,3,6,8,9 Urge incontinence is more common among the elderly, whereas stress incontinence is more frequently found in younger women.3 Finally, overflow incontinence involves leakage of urine due to an overdistended bladder commonly resulting from outlet obstruction (eg, prostatic hyperplasia) or neurogenic causes. Functional incontinence is the loss of urine caused by the inability to reach a toilet in time.7
Most scientific studies on UI perceive and classify UI as a symptom, which is why most investigations are pursued on the sole basis of questionnaires.6 The goals of most therapy are to relieve urinary symptoms and increase functional capacity of the bladder. Common conservative therapies for stress incontinence include weight loss for the obese, drug therapy, electrical stimulation, cough treatment, and physical transactions to forestall urinary leakage, such as contraction of the pelvic muscles, crossing of the legs, and use of tampons.5 Exercises to strengthen the pelvic muscles will substantially reduce incontinence in those women who are sufficiently motivated and willing to carry them out anywhere between 30 and 200 times a day. Unfortunately, these exercises must be continued for an indefinite period.5 Surgical options depend on the etiology and are usually regarded as the last therapeutic option. Recent studies have reported results related to the use of various techniques for lifting the urethra in stress incontinence. Long-term follow-ups of 3 commonly used surgical procedures—laparoscopic colposuspension, vaginal needle suspension, and abdominal colposuspension—yielded only a 20% to 50% satisfactory outcome.10-12
The urge to urinate and excessively frequent micturition may be caused by trigger points (TrPs) located in the lower abdominal muscles, and those TrPs may both weaken and provoke a partial contraction of these muscles.13 Based on this assumption, the present study investigated the potential value of treating TrPs elicited over bladder area on patients with UI.
The objective of this study was to investigate the possible changes in patients with chronic UI receiving (a) ischemic compression over bladder area and (b) ischemic compression directed to another unrelated area of the pelvis. Clinical effectiveness was monitored using a 2-part self-reporting questionnaire. Measuring both qualitative and quantitative outcomes has become increasingly important in clinical trials over the past few years,14 and the use of self-rated questionnaires is a convenient method to gather such information. We hypothesized that patients receiving ischemic compression therapy over the bladder area would experience a significant reduction in UI symptoms.
Methods
Participants
This prospective randomized clinical trial was conducted in a private clinic in Trois-Rivières, Québec, and was approved by the ethics board of l'Ordre des Chiropraticiens du Québec. An advertisement was placed in a local newspaper on 2 occasions inviting women with UI to apply for participation in a clinical trial. All participants had to meet the following criteria: (a) female, (b) age between 20 and 55 years, (c) experiencing UI on a daily basis for at least 3 months, and (d) not diagnosed with urinary tract pathologies. Moreover, patients weighing more than 200 pounds, patients who had major abdominal surgery, and patients presenting excessive prolapsed genitalia were all excluded from the trial. The selected participants were informed that they would be receiving a course of 15 chiropractic treatments at no cost and should agree to complete the questionnaires. The first 33 women who responded to the advertisement and met the inclusion criteria were asked to meet with the investigator. They were also asked to read and sign an informed consent form. Participants were told that there were 2 potential ways to treat UI and that the investigator wished to investigate the effectiveness of each approach. They were also told that the patients would be randomly allocated to one of the 2 possible treatment methods. All 33 participants were evaluated and treated by the same chiropractor and were not informed of their treatment regimen (experimental vs control group).
Randomization
The random allocation was managed by an independent research assistant. Upon recruitment, each participant was assigned a random allocation number generated from a random table (2/3-1/3). Two thirds were even numbers and one thirds odd numbers, which were mixed in an opaque envelope. The assistant would pull a number and assign the patient as follows: an even number in the experimental group and an odd number in the control group. The 33 subjects were divided into 2 groups. The experimental group (n = 24) aged 36 to 52 years (mean age, 44.4 years) was found to have an average history of UI of 5.8 years (range, 7 months to 10 years). The control group (n = 9) aged 33 to 50 years (mean age, 41.6 years) was found to have a history of UI averaging 6.1 years (range, 3-10 years). In both groups, if a patient reported having UI for a period exceeding 10 years, the 10-year upper limit was used to calculate the average duration of UI. In the experimental group, 12 had stress incontinence and 12 had mixed (stress and urge) incontinence. In the control group, 4 had stress incontinence and 5 had mixed incontinence. Participants' characteristics are presented in Table 1.
Table 1.
Baseline characteristic of the experimental and control groups
| Group | n | Age (y) | Symptom Duration | UDI + IIQ | UI Types |
|---|---|---|---|---|---|
| Experimental | 24 | 44.4 (36-52) | 5.8 y (7 mo to 10 y) | 23.3 | 12 SI, 12 MI |
| Control | 9 | 41.6 (33-50) | 6.1 y (3-10 y) | 25.3 | 4 SI, 5 MI |
SI, Stress incontinence; MI, mixed incontinence.
Crossover
Thirty days after the 15 initial treatments, the 9 participants of the control group were invited to receive 15 more treatments, this time in the bladder area; 8 accepted.
Outcome measures
For this study, the severity of symptoms related to UI was measured using a modified 2-part self-rating questionnaire: the urogenital distress inventory (UDI) and the incontinence impact questionnaire (IIQ).15 When used together, the UDI and IIQ are thought to provide detailed information on the impact of incontinence on the day-to-day life of persons with UI.15,16 Some questions like the ones pertaining to the sexual function of the patients were not used. They were developed with the goal of self-administration by patients. The use of questionnaires for the purpose of gathering information about a person's UI symptoms has recently been acknowledged as the best means to do so.10
The first questionnaire used in this study had 2 parts: The first part consisted of UDI and was made up of 7 questions. The second part, IIQ, had 12 questions. Both parts required the participants to rank on a scale of 0 to 3 points the impact of UI symptoms on their lifestyle. In addition, patients were asked to rate improvement in their symptoms on a scale of 0% to 100%. The patients completed the questionnaires at different intervals throughout the trial. Data from the questionnaires were collected at baseline after 15 treatments, 30 days after the 15 initial treatments, and 6 months later. All of the 33 patients who participated in the trial completed the questionnaires after the initial 15 treatments.
Six months after the last treatment, questionnaires and improvement scales were mailed to all of the participants, who completed them (with one exception at 6 months) and returned them in a self-addressed, postage-paid envelope.
Clinical interventions
The control treatment consisted of ischemic compression therapy over TrPs located around the hip area (gluteus maximus, medius, and minimus), starting with a light pressure and gradually increasing the pressure to the participant's maximum pain tolerance level. The duration of the compression varied from 5 to 15 seconds; participants with more TrPs received shorter-lasting compression because there is a limit to the amount of pain that a patient can tolerate during a single visit. The duration of the compression was monitored to make the control treatment tolerable for all participants.
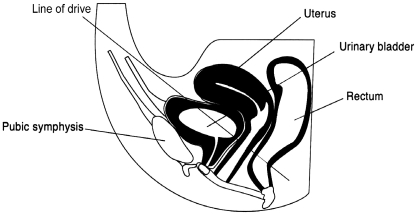
The experimental treatment consisted of ischemic compression therapy on the TrPs located over the bladder area. The pressure was applied behind the pubis with a line of drive directed toward the coccyx. Fig 1 illustrates this technique. The hyperirritable foci were palpated over an area approximately 6 cm wide by 2 cm deep. The thumb tip pressure, one thumb on the other, was applied on hyperirritable points (1-3 points), starting with a light pressure and gradually increasing the pressure to the participant's maximum pain tolerance level. The patient had to have a mild reaction coupled with a medium-strength facial muscle contraction.17 The duration of the compression was 15 seconds at each TrP. Treatments were given 3 times a week for a period of 5 weeks. A trial flowchart is presented in Fig 2.
Fig 1.
Illustration of the experimental treatment consisting of ischemic compression therapy on TrPs located over the bladder area. The pressure is applied behind to the pubis with a line of drive directed toward the coccyx.
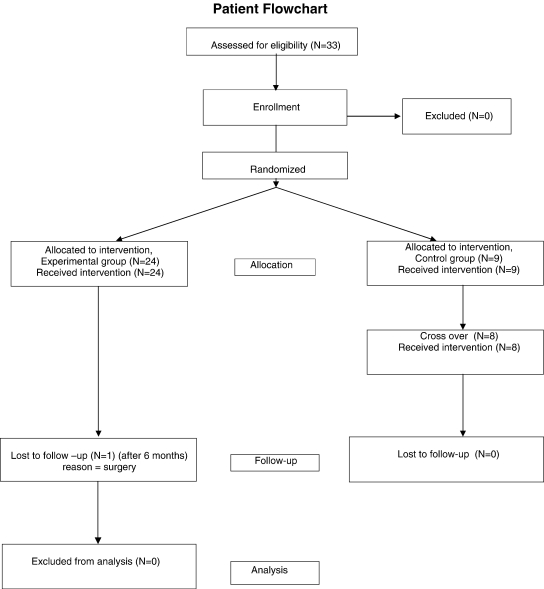
Fig 2.
Patient flowchart for the trial.
Statistical analysis
Results from the 33 patients were analyzed according to the “intention to treat” approach.
The questionnaire scores were first submitted to a repeated-measure 2-way analysis of variance (ANOVA) (2 group × 2 time intervals.). This analysis tested for the main effect of treatment (experimental or control), the main effect of time intervals (baseline evaluation and after 15 treatments), and the interaction. A t test for independent samples was used to compare the perceived amelioration percentage after 15 treatments. To test for the effects of experimental treatment over the different time intervals, a repeated-measure 1-way ANOVA was performed. When a main effect of time intervals was observed for the experimental group, post hoc comparisons were performed using Tukey tests. For all analyses, statistical significance was set at P < .05.
Results
The 33 participants were recruited, treated, and evaluated between January 2001 and July 2002. All 33 patients received the prescribed 15 treatments. All the participants in the experimental group (n = 24) completed the questionnaires after 15 treatments, 30 days after the treatments, and 6 months later, except for one patient who did not complete the questionnaires at 6 months because she had a hysterectomy. All the participants in the control group (n = 9) completed the questionnaires after 15 treatments and, in the case of the crossover group (n = 8), after 30 treatments (one patient refused to receive the 15 more treatments). Patients' characteristics at baseline evaluation were similar in both groups (independent t test, P > .05; Table 1).
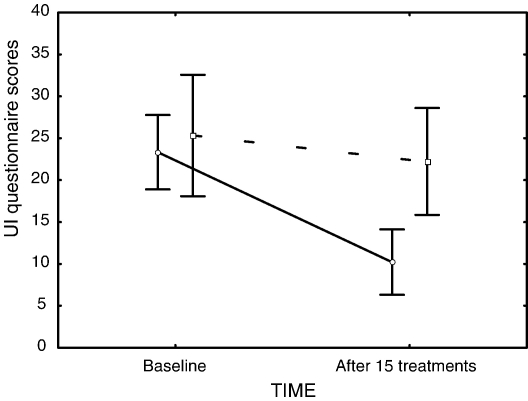
The mean scores and standard deviations for the UI questionnaire are presented in Table 2. A significant group × time interval interaction was observed (F1,31 = 13.551, P < .001). A main effect of time intervals showed a significant decrease of UI symptoms after 15 treatments (F1,31 = 35.62, P < .001); and post hoc analysis revealed that only the experimental group showed a significant decrease from 23.3 (95% confidence interval (CI), 18.9-27.8) to 10.2 (95% CI, 6.3-14.1) in UI symptoms after 15 treatments (Tukey P < .001). Fig 3 illustrates these results.
Table 2.
Data from the first questionnaire (UDI + IIQ)
| Group | n | At Baseline | After 15 Treatments | 30 d After the 15th Treatment | 6 mo After the Last Treatment |
|---|---|---|---|---|---|
| Experimental | 24 | 23.3 (10.7) | 10.2 (8.4) | 6.9 (4.8) | 11.3 (11.1) |
| Control | 9 | 25.3 (10.7) | 22.2 (11.8) | ||
| Crossover | 8 | 25.3 (13.2) | 21.91 (13.6) | 19.9 (13.6) | 20.41 (10.9) |
Numbers inside parentheses indicate the standard deviation.
Fig 3.
Mean scores for the UDI and IIQ combined at baseline and after 15 treatments for both groups. The vertical bars denote 0.95 CIs, the solid line represents the experimental group, and the dashed line represents the control group.
The perceived percentages of amelioration are presented in Table 3. For this dependent variable, the t test for independent samples yielded a significant difference between the 2 groups (P < .001).
Table 3.
Patients' perceived percentage of amelioration in UI symptoms
| Group | n | After 15 Treatments | 30 d After the 15th Treatment | 6 mo After the Last Treatment |
|---|---|---|---|---|
| Experimental | 24 | 69% (25) | 73% (23.5) | 60% (29.5) |
| Control | 9 | 21% (32.2) | 26% (27.5) | |
| Crossover | 8 | 24% (32.2) | 31% (32.3) |
Numbers inside parentheses indicate the standard deviation.
Finally, when the repeated-measure 1-way ANOVA was performed for the experimental group, it showed a persistence of UI symptoms reduction and perceived amelioration over time. Post hoc analysis revealed that a significant reduction in UI questionnaire score persisted at 30 days and 6 months after the last treatment (Tukey P < .001). At 30 days and 6 months, the UI questionnaire scores were 6.9 (95% CI, 4.6-8.6) and 11.3 (95% CI, 6.6-16.1), respectively.
For the 8 patients who received 30 treatments (15 control + 15 experimental), the results were as follows: for the first questionnaire, the average ran from 25.3 at baseline to 20.6 after 30 treatments; for the second questionnaire, it was 48% amelioration after 30 treatments.
There were no notable adverse effects after these ischemic compressions, except for a slight sensitivity that was felt by some patients at the beginning of treatments. This was remedied by reducing the force of the thumb applications in the next few visits.
Discussion
Management considerations
Currently, there are many therapeutic strategies to manage UI. Some of them are behavioral, some pharmaceutical, and some invasive. The choice of treatment largely depends on the underlying cause of UI, as well as on patients' motivation and ability to use conservative therapies. Retzky suggested that more than 90% of cases of UI can be successfully cured or improved by nonsurgical, conservative care.18 Kegel was the first to advocate pubococcygeal retraining for stress incontinence.19 The patient must be first taught how to draw in either the vagina or the anal sphincter without actively contracting the abdomen, buttock, or thigh muscles. Typically, patients begin with 10 contractions for 10 seconds each, 3 or 4 times a day. The number of sets can be increased over time.19 The mainstay of medical management of UI has been the use of pharmaceuticals. Although evidence of drug efficacy for stress incontinence is limited, anticholinergic agents such as oxybutynin and tolterodine may be acceptable options for urge incontinence.
Theoretical role of chiropractic care
As physicians, chiropractors may have a significant role to play in the investigation and application of alternative treatment strategies. They are qualified to carry out clinical diagnoses, to recommend conservative treatments, and to make the appropriate referrals; and they may be particularly well prepared through theory to be clinical researchers.4,8 For many patients, noninvasive and nonpharmacological approaches may be used to complement or as an alternative to more traditional medical management of UI. Many drugs, such as anticholinergics, have a long list of potential adverse effects. Thus, a more conservative, hands-on approach may be warranted and is something that should be considered wherever clinically feasible.
A myofascial TrP describes a zone of intense pain in a hardened muscle band that triggers pain when stimulated.20 An active TrP can be present in the absence of pain. The TrP is persistently hard; it is a contracted band of muscle that contracts sharply when mechanically stimulated by plucking it manually.20 Treatment of the TrP by manual compression results in softening of the taut band and an increase in the pressure pain threshold not seen in control.20 The TrP is associated with muscle weakness, and there is a restoration of muscle strength when the TrP is inactivated.20 Among the most popular methods of treating myofascial pain used by chiropractors is ischemic compression therapy, also known as Nimmo technique, trigger point therapy, or acupressure.21 To our knowledge, only one publication (a case report) reporting the beneficial effect of soft tissue therapy (manual massage) on traumatically induced UI has been published in the literature.22 The National Board of Chiropractic Examiners 2000 Job Analysis reported that more than 90% of chiropractors use TrP therapy for passive adjustive care, 68% use acupressure, and 40% report using Nimmo (receptor tonus technique).21 The Nimmo technique is the most closely related to our approach. It suggests that one should apply manual pressure for 5 to 7 seconds on the TrP involved, a deep pressure, but not so great that the patient will tense the affected muscle.23 The acupressure technique differs in the fact that the pressure is applied on predetermined acupuncture points. This approach may be beneficial for patients with conditions such as shoulder pain, low back pain, and fibromyalgia.24-26 Controlled studies are needed to confirm these findings.
Rationale for ischemic compression therapy in UI
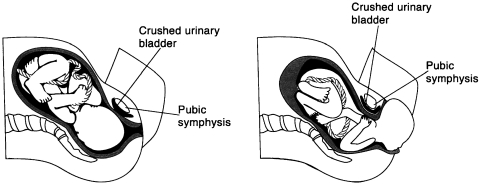
Bladder infection and trauma may cause abnormal functioning.27 The goal of the TrP therapy is to restore normal, at-rest muscle length and a fine range of motion through passive stretching.27 Referred motor activity to the pelvic floor muscles (sphincters) due to TrPs can be the sole or concomitant cause of urinary syndromes.27 In the present trial, the TrPs being localized deep behind the pubis, the authors hypothesize that these TrPs are in the musculature of the bladder wall. The elimination of the TrPs normalizes the tonus of the bladder, its functions, and the contractibility of the lower bladder sphincter. This trial added to another one (also carried out by us) recently published on gastroesophageal reflux disease28 and challenges the belief that TrPs are only seen in striated muscle, the stomach and the bladder walls being essentially composed of smooth muscle. In many cases of female patients with UI, the urinary bladder was crushed between the mother's pubis and the baby during birth. Fig 4 illustrates this phenomenon. This is especially critical when a mother gives birth to a large baby or if the diameter of the mother's pelvic inlet is small. Trauma may be severe enough to produce myofascial TrPs in the bladder area, causing tensions, hyperirritability, and thus a possible malfunction. If the TrPs are severe enough, the incontinence may appear immediately after birthing. If the TrPs are present but not severe enough, the symptoms may appear only when the patient approaches 50 years of age, when the muscles start to weaken naturally. In this trial, 21 of 24 women in the experimental group had experienced vaginal birth, whereas the figure was 9 of 9 in the control group. As urge UI is a bladder problem whereas stress UI is a sphincter problem of the lower urethra,1,2 the ischemic compressions used on the hyperirritable foci (TrPs) located in the bladder area may at the same time compress the TrPs (if any) located at the sphincters' level. This would explain why the same treatment was efficacious for urge UI and for stress UI.
Fig 4.
Illustration of possible mechanisms leading to myofascial TrPs in the bladder area. Giving birth to a large baby and a small pelvic inlet diameter are considered possible risk factors.
In this study, patients' UI symptoms significantly improved with ischemic compression after 15 treatments over the bladder area. Significant results were observed 30 days after receiving the last treatments, when 20 out of 24 patients registered a more than 50% (average, 73%) amelioration of their perceived symptoms. Moreover, in both types of evaluation, the experimental treatment was more than 3 times more effective than the control treatment. In the present trial, when asked about the amelioration of their symptoms, 18 (75%) out of 24 participants in the experimental group indicated a clear improvement within 6 treatments.
For the crossover group, the results are quite disappointing. They are not consistent in comparison with the experimental group. It is rather hard to explain why the treatment that was effective with the experimental treatment was not effective when control patients crossed over to the experimental treatment. It could be that after having received 15 control treatments without any amelioration, the patients were rather discouraged and had acquired negative expectations of the results they could look forward to after 15 more treatments, especially because these participants had had this problem for an average of 6.1 years.
Study limitations
The experimental and the control groups were not equal in number because in a practice-based trial, it is psychologically much harder on the practitioner to treat a control group than an experimental group.
All patients were evaluated and treated by the same doctor of chiropractic, and having only one treating doctor may have introduced a treatment bias. The total number of patients was rather small. Cointervention was not accounted for because all control group participants were offered 15 experimental treatments after the 15 control treatments. This strategy was chosen because practice-based studies that provide a group of patients with control treatment only may be ethically questionable. The authors elected to offer the control group the same treatment as the experimental group after the 15th visit, explaining why data between groups could not be compared after that period. Because of the long-term nature of the control group patients' condition (mean duration, 6.1 years), it was unlikely that any significant natural improvement would have occurred during the time scale of the study.
However, ramifications of treatment bias, the inherent flaws in using a “medium-strength fascial muscle contraction” as an outcome for the experimental group to determine manual pressure, and the distinct possibility that subjects in either group had varying degrees of TrP activity are also limitations to this study.
Conclusions
To our knowledge, the present study is the first to evaluate the effect of ischemic compression therapy over the bladder area in female patients with UI. The findings of this pilot study suggest that UI may be successfully managed with a conservative approach using myofascial techniques such as ischemic compression. This therapeutic approach may be combined with other clinical strategies used to manage UI, including Kegel exercises, biofeedback techniques, and pharmacotherapy. It could also be compared with these strategies. Large-scale randomized controlled trials are needed to assess the validity of our findings. Future studies should include a larger patient population, long-term follow-up, more than one treating practitioner, and a blinded evaluator.
Acknowledgment
The authors would like to thank Dr Brien J Gleberzon, DC, for his assistance in preparing this manuscript.
References
- 1.Fanti A., Kachak Newman D., Collins J. US Department of Health and Human Services; Rockville: 1996. Quick reference guide for clinicians. Managing acute and chronic urinary incontinence. [AHCPR Publication no. 96-0686] [Google Scholar]
- 2.Simon H., Cannista S., Etkin M. Nidus Information services; New York: 1999. Well connected, urinary incontinence, report 50. [Google Scholar]
- 3.Genadry R.R. Evaluation and conservative management of women with stress urinary incontinence. Md Med J. 1995;44:31–35. [PubMed] [Google Scholar]
- 4.Chapman-Smith David. Le traitement chiropratique des dysfonctionnements viscéraux. The Chiropractic Report, Toronto dec. 1995. P:5.
- 5.Resnick N.M. Urinary incontinence. Lancet. 1995;346:94–99. doi: 10.1016/s0140-6736(95)92117-6. [DOI] [PubMed] [Google Scholar]
- 6.Hampel C., Wienhold D., Benken N. Prevalence and natural history of female incontinence. Urology. 1997;32(Suppl 2):3–12. [PubMed] [Google Scholar]
- 7.Grymonpre R.E. Urinary incontinence in adults. In: Gray J., editor. Therapeutic choices. 4th ed. Canadian Pharmacists Association; Ottawa: 2003. p. 546. [Google Scholar]
- 8.Keating J.C., Schulte E.A., Miller E. Conservative care of urinary incontinence in the elderly. J Manipulative Physiol Ther. 1988;11:300–308. [PubMed] [Google Scholar]
- 9.Berg I., Fielding D., Meadow R. Psychiatric disturbance, urgency, and bacteria in children with day and night wetting. Arch Dis Child. 1977;52:651–657. doi: 10.1136/adc.52.8.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das S. Comparative outcome analysis of laparoscopic colposuspension, abdominal colposuspension and vaginal needle suspension for female urinary incontinence. J Urol. 1998;160:368–371. [PubMed] [Google Scholar]
- 11.Saidi M.H., Gallagher M.S., Skop Pi. Extraperitoneal laparoscopic colposuspension: short-term cure rate, complications, and duration of hospital stay in comparison with Burch colposuspension. Am Coll Obstet Gynecol. 1998;92:619–621. doi: 10.1016/s0029-7844(98)00266-x. [DOI] [PubMed] [Google Scholar]
- 12.Drutz H.P., Alnaif B. Surgical management of pelvic organ prolapse and stress urinary incontinence. Clin Obstet Gynecol. 1998;41:786–793. doi: 10.1097/00003081-199809000-00034. [DOI] [PubMed] [Google Scholar]
- 13.Travel JG, Simons DG. Myofascial pain and dysfunction. The trigger point manual, vol 1. Williams and Wilkins, Baltimore; 1983, P 15, 671.
- 14.Bolton J.E. The evidence of evidence-based medicine: what counts and doesn't count? J Manipulative Physiol Ther. 2002;24:362–366. doi: 10.1067/mmt.2001.115259. [DOI] [PubMed] [Google Scholar]
- 15.Shumaker S.A., Wyman J.F., Vebersax J.S. Health-related quality of life measures for women with urinary incontinence: the incontinence impact questionnaire and the urogenital distress inventory. Qual Life Res. 1994;3:291–306. doi: 10.1007/BF00451721. [DOI] [PubMed] [Google Scholar]
- 16.Wyman J.F., Harkins S.C., Taylor J.R. Psychosocial impact of urinary incontinence in women. Obstet Gynecol. 1987;70:378–381. [PubMed] [Google Scholar]
- 17.Mayer J.J., Zachman Z.J., Keating J.C. Effectiveness of chiropractic management for patellofemoral syndrome's symptomatic control phase: a single subject experiment. J Manipulative Physiol Ther. 1990;13:539–549. [PubMed] [Google Scholar]
- 18.Retzky S.S. Incontinence: usually stress or urge. DO. 1999;40:62–65. [Google Scholar]
- 19.Kegel A.H. Stress incontinence of urine in women: physiologic treatment. Int Surg. 1956;25:487–499. [PubMed] [Google Scholar]
- 20.Gerwin R.D., Dommerholt J., Shah J.P. An expansion of Simons' integrated hypothesis of trigger point formation. Curr Pain Headache Rep. 2004;8:468–475. doi: 10.1007/s11916-004-0069-x. [DOI] [PubMed] [Google Scholar]
- 21.Christensen MG. Job analysis of chiropractic. National Board of Chiropractic Examiners 2000. Greeley, Co: 78-9.
- 22.Stude D.E., Bergmann T.F., Finer B.A. A conservative approach for a patient with traumatically induced urinary incontinence. J Manipulative Physiol Ther. 1998;21:363–367. [PubMed] [Google Scholar]
- 23.Cohen J.H., Schneider M. Receptor-tonus technique: an overview. Chiropr Tech. 1990;2:13–16. [Google Scholar]
- 24.Hains G. Chiropractic management of shoulder pain and dysfunction of myofascial origin using ischemic compression techniques. J Can Chiropr Assoc. 2002;46:192–200. [Google Scholar]
- 25.Hains G., Hains F. Combined ischemic compression and spinal manipulation in the treatment of fibromyalgia. J Manipulative Physiol Ther. 2000;24:225–230. [PubMed] [Google Scholar]
- 26.Hains G. Locating and treating low back pain of myofascial origin by ischemic compression. J Can Chiropr Assoc. 2002;46:257–263. [Google Scholar]
- 27.Doggweiler-Wiygul R. Urologic myofascial pain syndromes. Curr Pain Headache Rep. 2004;8:445–451. doi: 10.1007/s11916-004-0065-1. [DOI] [PubMed] [Google Scholar]
- 28.Hains G., Hains F., Descarreaux M. Gastroesophageal reflux disease, spinal manipulative therapy and ischemic compression: a preliminary study. J Am Chiropr Assoc Online. 2007;44:7–19. [Google Scholar]