Abstract
Pre-eclampsia is a common serious disorder of human pregnancy, which is associated with significant maternal and perinatal morbidity and mortality. The suspected aetiology of pre-eclampsia is complex, with susceptibility being attributable to multiple environmental factors and a large genetic component. Recently, we reported significant linkage to chromosome 2q22 in 34 Australian/New Zealand (Aust/NZ) pre-eclampsia/eclampsia families, and activin A receptor type IIA (ACVR2A) was identified as a strong positional candidate gene at this locus. In an attempt to identify the putative risk variants, we have now comprehensively re-sequenced the entire coding region of the ACVR2A gene and the conserved non-coding sequences in a subset of 16 individuals from these families. We identified 45 single nucleotide polymorphisms (SNPs), with 9 being novel. These SNPs were genotyped in our total family sample of 480 individuals from 74 Aust/NZ pre-eclampsia families (including the original 34 genome-scanned families). Our best associations between ACVR2A polymorphisms and pre-eclampsia were for rs10497025 (P = 0.025), rs13430086 (P = 0.010) and three novel SNPs: LF004, LF013 and LF020 (all with P = 0.018). After correction for multiple hypothesis testing, none of these associations reached significance (P > 0.05). Based on these data, it remains unclear what role, if any, ACVR2A polymorphisms play in pre-eclampsia risk, at least in these Australian families. However, it would be premature to rule out this gene as significant associations between ACVR2A SNPs and pre-eclampsia have recently been reported in a large Norwegian (HUNT) population sample.
Keywords: activin, ACVR2A, genetic association, pre-eclampsia, SNP
Introduction
Pre-eclampsia is a complex and serious disorder of human pregnancy diagnosed by the new onset of hypertension and proteinuria in the latter half of pregnancy (Witlin and Sibai, 1997; Roberts et al., 2003; Roberts and Gammill, 2005). The disorder is pregnancy specific and reverses after delivery. The heritable nature of pre-eclampsia has long been recognized (Chesley et al., 1968) with susceptibility to the pre-eclampsia/eclampsia syndrome being attributable to a large genetic component (Salonen Ros et al., 2000; Nilsson et al., 2004; Carr et al., 2005) that involves both maternal and fetal contributions (Lie et al., 1998). Pre-eclampsia occurs in all major ethnic groups and affects ∼5% of all pregnancies. It is a major cause of maternal mortality, accounting for 50 000 maternal deaths yearly in developing countries (Duley, 1992). Although diagnosed by hypertension and proteinuria, it is a multisystemic disorder affecting virtually all organ systems. There is no reliable predictive test, nor is there an effective therapy other than delivery, to cure this serious condition. The identification of the genetic determinants that underlie susceptibility to pre-eclampsia is likely to be crucial to understanding the disease process and to the development of effective screening tests and treatments. Defining the allelic architecture of pre-eclampsia, therefore, remains a priority in obstetric research.
Certain pathogenic features of pre-eclampsia such as endothelial dysfunction and inflammation, and the risk factors for pre-eclampsia are very similar to those of later life cardiovascular disease. Not surprisingly, pre-eclampsia is associated with an increased risk of later life cardiovascular disease (Chesley, 1975; Jonsdottir et al., 1995; Irgens et al., 2001; Funai et al., 2005). This risk is highest for certain subsets [pre-eclampsia occurring before 34 weeks (Irgens et al., 2001) and recurrent pre-eclampsia (Chesley, 1975; Funai et al., 2005)] but even pre-eclampsia that occurs in the first pregnancy at term and never recurs is associated with increased later life cardiovascular disease (Funai et al., 2005). Furthermore, women who have had pre-eclampsia, when examined several years later, manifest changes which were present when they were pre-eclamptic and which indicate increased risk for cardiovascular disease. Thus, previously pre-eclamptic women, even those with normal blood pressure, have higher blood pressures than women who have had only normal pregnancies (Agatisa et al., 2004). Similarly, endothelial dysfunction (Chambers et al., 2001; Agatisa et al., 2004), abnormal lipids (Hubel et al., 2000) and insulin resistance (Laivuori et al., 2000) are more common in previously pre-eclamptic women. It is likely that identification of genetic factors important in pre-eclampsia will also provide insight into genetic factors relevant to cardiovascular disease in women.
Linkage analysis in affected families has been the strategy employed in several large international efforts, including our own, to identify the maternal genetic contributions to susceptibility (Arngrímsson et al., 1999; Moses et al., 2000; Lachmeijer et al., 2001; Laivuori et al., 2003). In our previous genome-wide linkage scan of 34 Australian/New Zealand (Aust/NZ) pre-eclampsia families, we detected significant linkage to chromosome 2q22 (Moses et al., 2000; Fitzpatrick et al., 2004). Fine mapping of the 2q locus in combination with an objective positional candidate gene prioritization strategy combining bioinformatics and gene expression profiling subsequently identified the activin A receptor type IIA (ACVR2A) gene as a high priority positional candidate (Fitzpatrick et al., 2004; Moses et al., 2006). ACVR2A is a key receptor for the cell signalling protein, activin A, which has long been recognized as an important regulator of human pregnancy (Peng et al., 1993). Activin A is also a recognized biological marker of pre-eclampsia and is known to be elevated in the maternal serum of women with pre-eclampsia (Grobman and Wang, 2000; Muttukrishna et al., 2000; Zwahlen et al., 2007). Preliminary association analysis using five known and validated single nucleotide polymorphisms (SNPs) at approximately equidistant intervals spanning the ACVR2A gene provided strong evidence of association for one SNP with pre-eclampsia (Moses et al., 2006).
In this study, we have extended our characterization of ACVR2A as a potential genetic risk factor for pre-eclampsia. First, we comprehensively re-sequenced the ACVR2A gene in affected individuals from our originally genome-scanned 34 Aust/NZ families to identify the complement of polymorphisms most likely to be causal. We then performed a series of association tests with these polymorphisms in our now larger family material of 74 Aust/NZ pre-eclampsia families. A high-density SNP map of 35 SNPs was also used to identify linkage disequilibrium (LD) patterns across the ACVR2A locus.
Materials and Methods
Subjects
All DNA samples were obtained from families recruited over a 15-year period through the Royal Women's Hospital and the Monash Medical Centre in Melbourne, Australia, through print and electronic advertisements in Sydney, Australia, and through the National Women's Hospital in Auckland, New Zealand. The extended cohort consisted of 480 individuals from 74 pre-eclampsia families, including the 34 families whom we have studied to date (Moses et al., 2000; Fitzpatrick et al., 2004; Moses et al., 2006). For the purposes of this manuscript, we shall hereafter refer to the original 34 pre-eclampsia families used to identify the 2q22 locus (Moses et al., 2000) as ‘The 34 Family Cohort’ and the now extended 74 pre-eclampsia families as ‘The 74 Family Cohort’. The 74 Family Cohort included 20 women with eclampsia, 120 women with severe pre-eclampsia, 56 women who had hypertensive first pregnancies without proteinuria (mild pre-eclampsia) and 90 women who had normotensive first pregnancies. The composition of the familial material according to the severe and mild diagnostic criteria is presented in Table I.
Table I.
Composition of the familial material according to the severe and mild diagnostic criteria.
| No. of affected members | Severe pre-eclampsia | Mild pre-eclampsia |
|---|---|---|
| 1 | 28 | 10 |
| 2 | 27 | 29 |
| 3 | 15 | 21 |
| 4 | 2 | 9 |
| 5 | 1 | 3 |
| 6 | 0 | 1 |
| 7 | 0 | 0 |
| 8 | 0 | 1 |
Entries are the number of families containing the indicated number of affected members.
Diagnosis was based on clinical assessment, using the criteria of the Australasian Society for the Study of Hypertension in Pregnancy (Brown et al., 1993). Women were considered to have severe pre-eclampsia if they had either (i) an increase from baseline systolic blood pressure of ≥25 mmHg, and/or diastolic pressure of ≥15 mmHg; or (ii) the presence of systolic pressure of ≥140 mmHg, and/or diastolic pressure of ≥90 mmHg. These levels had to occur on at least two occasions 6 h or more apart. Proteinuric levels had to exceed 0.3 g/l in a 24 h specimen, or the dipstick proteinuria score was ≥2+ in random urine collection. Women who met these criteria and experienced convulsions or unconsciousness in the prenatal period were classified as having eclampsia. Women with the pattern of hypertension outlined above but with no proteinuria were classified as mild pre-eclampsia. Because pre-eclampsia is considered a disease of first pregnancies (Chesley et al., 1968; Fisher et al., 1981), only those women with the above-mentioned features in their first pregnancies were included. Women with pre-existing hypertension or other medical conditions known to predispose them to pre-eclampsia (e.g. renal disease, diabetes, twin pregnancies or fetal chromosomal abnormalities) were excluded. Ethics approval was obtained from the Research and Ethics committees of The Royal Women's Hospital, Melbourne, Australia, and informed written consent was obtained from family members. Ethical approval for genotyping and statistical analysis of The 74 Family Cohort was granted from the Institutional Review Board, The University of Texas Health Science Center at San Antonio, TX, USA.
Subject selection for re-sequencing
For the re-sequencing of the ACVR2A gene, we selected 16 unrelated pre-eclamptic individuals from a subset of 16 families from The 34 Family Cohort that were the main contributors to the 2q22 linkage signal. Hence, by using affected individuals from these families, we increased the chances of identifying the majority of relevant functional variation.
Genomic DNA reference sequence
Sequence information for use as a reference template and primer design for ACVR2A re-sequencing was obtained from The NCBI Human Genome Database Build 34 (http://www.ncbi.nlm.nih.gov). We completely re-sequenced 1.5 kb of the proximal promoter, all coding regions, the 3′UTR and evolutionarily conserved non-coding regions considered most likely to harbour regulatory variation. Conserved regions were identified using a comparative genomics strategy that involved BLAST sequence comparison of human genomic DNA sequence against mouse genomic DNA sequence (http://www.ncbi.nlm.nih.gov/BLAST). Genomic sequence showing greater than ∼70% homology was considered to be conserved between the two species (Prabhakar et al., 2006).
Primer design
All gene sequences were analysed for repetitive DNA elements using Repeat Masker (http://www.repeatmasker.org) to facilitate primer design. Using Primer3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi), primers were designed to between 20 and 30 bp in length, with an annealing temperature between 55 and 60°C and within 1°C of each other.
Gene re-sequencing
PCRs were performed with 20 ng genomic DNA in a 20 µl reaction containing 0.5 U of AmpliTaq Gold DNA polymerase (Applied Biosystems, Foster City, CA, USA), 1× GeneAMP PCR buffer (Amersham Biosciences, Piscataway, NJ, USA), 0.25 mM dNTP, 2.5 mM MgCl2 and 100 ng each of sense and anti-sense primers. PCR amplification was carried out on a Perkin Elmer GeneAmp 9700 thermal cycler (Applied Biosystems). Amplification was performed with an initial denaturation step at 95°C for 1 min, then 35 cycles of 95°C for 30 s, a primer pair specific annealing temperature (55–60°C) for 30 s and 74°C for 45 s, then a final extension step of 74°C for 2 min.
PCR products were purified using ExoSAP-IT (Amersham Biosciences) according to the manufacturers' instructions and purified PCR products were used as a template for sequencing reactions.
Cycle sequencing was performed on both sense and anti-sense DNA strands in a 20 µl reaction. The sequencing reactions contained 2.5 µl of purified PCR product, 2 µl ABI Prism Big Dye Terminators version 3.1 (Applied Biosystems), 1× ABI Prism Big Dye Terminator Buffer (Applied Biosystems) and 60 ng of either sense or anti-sense primer. Sequence template amplification was performed on a Perkin Elmer GeneAmp 9700 thermal cycler (Applied Biosystems). The cycling conditions were standard for all purified PCR products and involved an initial denaturation step of 95°C for 5 min, then 30 cycles of 95°C for 10 s and 50°C for 5 s, then a final extension at 60°C for 4 min. Sequencing products were precipitated by the addition of four volumes of 75% isopropanol. Excess isopropanol was removed and the precipitate was allowed to dry at 80°C for several minutes. Samples were analysed using the commercial DNA sequencing service of the Australian Genome Research Facility, Melbourne, Australia. DNA sequence polymorphisms were then identified visually using the chromatogram viewing program Chromas 1.44 (http://www.technelysium.com.au/chromas14x.html).
SNP genotyping
The Illumina GoldenGate® SNP Genotyping Assay (Illumina Inc., San Diego, CA, USA) was employed for all genotyping. This assay does not accommodate the genotyping of insertion/deletion variants and consequently only the 45 SNPs that were identified by re-sequencing were subjected to Illumina's SNP Assay Design Tool. An assay was unable to be designed for one SNP (rs13034494). In total, 44 SNPs were successfully designed for inclusion into an Illumina custom SNP panel for genotyping on an Illumina 8 × 12 Sentrix Array Matrix (SAM) (Table II). Each SAM was imaged on the Illumina BeadStation 500GX BeadArray Reader using Illumina BeadScan image data acquisition software (version 2.3.0.13). SNP genotype clustering and individual sample genotype calls were interrogated using the Illumina BeadStudio software, Genotyping Module (version 2.3.41).
Table II.
Location and description of polymorphisms identified by the re-sequencing of ACVR2A
| Variant | Chr locn (bp) | Function | Polymorphism | MAF |
|---|---|---|---|---|
| rs1424954 | 148317264 | 5′UTR | G→A | 0.330 |
| rs13224 | 148319154 | 5′UTR | NP | 0 |
| rs7572676 | 148320092 | Intron | NP | 0 |
| LF001 | 148323680 | Intron | C→A | 0.224 |
| rs1364658 | 148324278 | Intron | C→G | 0.328 |
| LF021 | 148324711 | Intron | T indel | No assay design |
| LF005 | 148327708 | Intron | G indel | No assay design |
| rs6747792 | 148327998 | Intron | A→C | 0.224 |
| LF002 | 148328072 | Intron | NP | 0 |
| rs1014064 | 148328624 | Intron | A→G | 0.332 |
| LF003 | 148330129 | Intron | A→G | 0.014 |
| rs13034494 | 148332000 | Intron | C→T | Failed assay design |
| rs12998729 | 148333762 | Intron | G→A | 0.328 |
| rs17741978 | 148333850 | Intron | C→G | 0.224 |
| rs1364657 | 148334737 | Intron | G→A | 0.055 |
| rs7582403 | 148335224 | Intron | A→G | 0.354 |
| rs1895694 | 148336216 | Intron | A→G | 0.369 |
| rs2113794 | 148339447 | Intron | A→C | 0.369 |
| LF004 | 148340383 | Intron | G→A | 0.012 |
| LF008 | 148343404 | Intron | GCTTATTC indel | No assay design |
| rs17742134 | 148343866 | Intron | G→A | 0.224 |
| rs929939 | 148343998 | Intron | C→A | 0.342 |
| rs1424943 | 148344632 | Intron | NP | 0 |
| rs6713811 | 148348005 | Intron | G→A | 0.369 |
| rs1424941 | 148359588 | Intron | G→A | 0.224 |
| rs2161983 | 148365856 | Intron | G→A | 0.342 |
| rs1128919 | 148373587 | Synonymous | G→A | 0.331 |
| rs2288190 | 148374059 | Intron | G→A | 0.415 |
| LF016 | 148374101 | Intron | T→C | Genotyping failed |
| rs3754541 | 148375065 | Intron | G→A | 0.224 |
| rs10497025 | 148378672 | Intron | G→C | 0.267 |
| rs1227307 | 148378775 | Intron | NP | 0 |
| rs13008497 | 148383231 | Intron | G→A | 0.328 |
| rs1469211 | 148383305 | Intron | G→A | 0.224 |
| LF012 | 148385604 | Intron | G→A | 0.012 |
| LF013 | 148388204 | Intron | A→G | 0.012 |
| rs3768687 | 148388490 | Intron | G→A | 0.332 |
| LF020 | 148390323 | Intron | A→G | 0.012 |
| rs13026650 | 148390671 | Intron | G→A | 0.332 |
| LF014 | 148390982 | Intron | NP | 0 |
| LF015 | 148391000 | Intron | AAG indel | No assay design |
| rs3764955 | 148391267 | Intron | G→C | 0.328 |
| rs7601098 | 148393084 | Intron | G→C | 0.055 |
| rs3820716 | 148396730 | Intron | A→G | 0.425 |
| rs2303392 | 148396897 | Intron | C→G | 0.342 |
| rs1803029 | 148401443 | 3′UTR | NP | 0 |
| rs13430086 | 148403531 | 3′UTR | T→A | 0.310 |
| rs17692648 | 148404201 | 3′UTR | A→C | 0.214 |
| rs1047081 | 148404773 | 3′UTR | NP | 0 |
Chr, chromosome; locn, location; bp, base pair; MAF, minor allele frequency; NP, non-polymorphic; indel, insertion/deletion.
Genotype cleaning
Mistyping analysis was conducted using SimWalk2 (Sobel and Lange, 1996). Mendelian discrepancies and spurious recombinations were removed by blanking genotypes identified by SimWalk2 as having a high probability of being in error.
Statistical analyses
Allele frequency calculations, tests for deviations from Hardy–Weinberg and pattern assessment of LD among genotyped ACVR2A SNPs were performed using the statistical computer software SOLAR (Almasy and Blangero, 1998). SNP association analysis was conducted with SOLAR's quantitative trait linkage disequilibrium (QTLD) procedure. This procedure performs a test for population stratification and two commonly used association tests: measured genotype analysis (Boerwinkle et al., 1986) and the quantitative transmission disequilibrium test (QTDT) (Abecasis et al., 2000). It also performs a variant of the QTDT, the QTLD test (Havill et al., 2005), which achieves greater power by utilizing genotype information from founders but is not applicable if population stratification is present. In this study, the statistical significance of SNP association was determined by the QTLD test, unless there was evidence of stratification, in which case the QTDT was used. The imputation of missing genotypes was performed from the haplotype estimation procedure in SimWalk2. SNP association analyses were performed using both a general diagnostic criteria (where women with mild pre-eclampsia, severe pre-eclampsia or eclampsia were coded as affected) and a strict diagnostic criteria (where only women with severe pre-eclampsia or eclampsia were coded as affected).
Results
ACVR2A re-sequencing
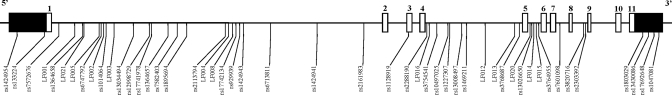
The genomic sequence of ACVR2A spans 83.3 kb, of which we have re-sequenced ∼40 kb that incorporates 1.5 kb of the proximal promoter, all coding regions, the 3′UTR and non-coding intragenic DNA conserved between human and mouse. We identified a total of 49 gene polymorphisms (Fig. 1) and at the time of discovery, 20 of these variants were identified as being novel. Subsequently, 7 of these have been deposited on the public databases (i.e. NCBI's dbSNP) leaving 13 novel variants. All but four variants identified were SNPs and the remainder were small (1–8 bp) insertion/deletion variants (Table II).
Figure 1.
Schematic representation of the ACVR2A gene. The open boxes represent the 11 numbered exons and the solid boxes represent the 5′ and 3′ untranslated regions, respectively. The location of the 49 polymorphisms identified by re-sequencing are positioned below. The polymorphisms are labelled with their unique identifiers [rs-numbered SNPs deposited in NCBI's dbSNP or LF-numbered variants (SNPs or insertion deletions) assigned by the investigators to novel polymorphisms].
ACVR2A SNP genotyping
We used the Illumina GoldenGate® SNP Genotyping Assay to genotype the 44 successfully designed SNPs in all 480 members of The 74 Family Cohort. One SNP (rs13034494) failed assay design and the four insertion deletion variants identified were not designed due to the constraints of the chosen genotyping assay. All but one (LF016) of the designed SNPs were successfully genotyped and of these, eight SNPs were non-polymorphic (Table II). One of the eight non-polymorphic SNPs, LF014, was subsequently determined to be non-polymorphic after genotypes were blanked due to Mendelian inheritance discrepancies. We observed a very high individual genotyping success rate (97.9–99.4%) and all polymorphic SNPs conformed to Hardy–Weinberg expectations (P > 0.05). All non-polymorphic SNPs were omitted from subsequent SNP association and LD analyses.
LD (association) analysis
Under the mild diagnostic criteria for pre-eclampsia, nominal association was seen with two SNPs, rs10497025 (QTLD P = 0.025) and rs13430086 (QTDT P = 0.010) (Table III). Under the strict diagnostic criteria for pre-eclampsia, we observed nominal associations with three novel SNPs, LF004, LF013 and LF020 (all QTLD P-values = 0.018) (Table IV). To correct for multiple hypothesis testing, we used SOLAR to simulate a quantitative trait with a heritability of 0.51 (Moses et al., 2006) and assigned affection status to a set number of affected women with the highest simulated trait values. The number of affected women was determined from our 74 Family Cohort (n = 140 women denoted as having severe pre-eclampsia). The QTLD analysis procedure was repeated 10 000 times to our simulated trait and an experiment-wide P-value for each SNP was determined at an α of 0.05. The experiment-wide P-values for our nominally associated SNPs failed to satisfy the statistical significance threshold (P > 0.05).
Table III.
Association analyses of SNPs identified by re-sequencing of ACVR2A using the ‘Mild diagnostic criteria’.
| SNP | Population stratification | QTDT | QTLD |
|---|---|---|---|
|
P-values | |||
| rs1424954 | 0.477 | 0.166 | 0.128 |
| LF001 | 1.000 | 1.000 | 0.217 |
| rs1364658 | 0.381 | 0.172 | 0.134 |
| rs6747792 | 0.829 | 0.784 | 0.217 |
| rs1014064 | 0.478 | 0.166 | 0.128 |
| LF003 | 0.847 | 0.542 | 0.278 |
| rs12998729 | 0.381 | 0.172 | 0.134 |
| rs17741978 | 1.000 | 1.000 | 0.217 |
| rs1364657 | 0.477 | 0.210 | 0.625 |
| rs7582403 | 0.128 | 0.116 | 0.240 |
| rs1895694 | 0.087 | 0.078 | 0.128 |
| rs2113794 | 0.087 | 0.078 | 0.128 |
| LF004 | 0.293 | 0.757 | 0.149 |
| rs17742134 | 1.000 | 1.000 | 0.217 |
| rs929939 | 1.000 | 0.452 | 0.156 |
| rs6713811 | 0.060 | 0.079 | 0.132 |
| rs1424941 | 1.000 | 1.000 | 0.217 |
| rs2161983 | 0.339 | 0.169 | 0.136 |
| rs1128919 | 0.478 | 0.166 | 0.128 |
| rs2288190 | 0.253 | 0.101 | 0.133 |
| rs3754541 | 1.000 | 1.000 | 0.217 |
| rs10497025 | 0.157 | 0.054 | 0.025 |
| rs13008497 | 0.381 | 0.172 | 0.134 |
| rs1469211 | 1.000 | 1.000 | 0.217 |
| LF012 | 0.602 | 0.978 | 0.284 |
| LF013 | 0.293 | 0.757 | 0.149 |
| rs3768687 | 0.477 | 0.166 | 0.128 |
| LF020 | 0.960 | 1.000 | 0.150 |
| rs13026650 | 0.477 | 0.166 | 0.128 |
| rs3764955 | 0.381 | 0.172 | 0.134 |
| rs7601098 | 0.477 | 0.210 | 0.625 |
| rs3820716 | 0.183 | 0.221 | 0.216 |
| rs2303392 | 0.323 | 0.168 | 0.135 |
| rs13430086 | 0.023 | 0.010 | 0.035 |
| rs17692648 | 0.959 | 0.662 | 0.141 |
Table IV.
Association analyses of SNPs identified by re-sequencing of ACVR2A using the ‘Strict diagnostic criteria’.
| SNP | Population stratification | QTDT | QTLD |
|---|---|---|---|
|
P-values | |||
| rs1424954 | 0.406 | 0.427 | 0.412 |
| LF001 | 0.054 | 0.129 | 0.642 |
| rs1364658 | 0.337 | 0.428 | 0.423 |
| rs6747792 | 0.007 | 0.074 | 0.670 |
| rs1014064 | 0.406 | 0.427 | 0.412 |
| LF003 | 0.983 | 0.425 | 0.127 |
| rs12998729 | 0.337 | 0.428 | 0.423 |
| rs17741978 | 0.054 | 0.129 | 0.642 |
| rs1364657 | 0.729 | 1.000 | 0.818 |
| rs7582403 | 0.071 | 0.286 | 0.671 |
| rs1895694 | 0.041 | 0.175 | 0.344 |
| rs2113794 | 0.041 | 0.175 | 0.344 |
| LF004 | 0.941 | 0.226 | 0.018 |
| rs17742134 | 0.054 | 0.129 | 0.642 |
| rs929939 | 0.476 | 0.727 | 0.674 |
| rs6713811 | 0.115 | 0.329 | 0.346 |
| rs1424941 | 0.054 | 0.129 | 0.642 |
| rs2161983 | 0.387 | 0.614 | 0.578 |
| rs1128919 | 0.406 | 0.427 | 0.412 |
| rs2288190 | 0.870 | 0.852 | 0.886 |
| rs3754541 | 0.054 | 0.129 | 0.642 |
| rs10497025 | 0.318 | 0.471 | 0.504 |
| rs13008497 | 0.337 | 0.428 | 0.423 |
| rs1469211 | 0.054 | 0.129 | 0.642 |
| LF012 | 0.656 | 0.696 | 0.132 |
| LF013 | 0.941 | 0.226 | 0.018 |
| rs3768687 | 0.406 | 0.427 | 0.412 |
| LF020 | 0.944 | 0.226 | 0.018 |
| rs13026650 | 0.406 | 0.427 | 0.412 |
| rs3764955 | 0.337 | 0.428 | 0.422 |
| rs7601098 | 0.729 | 1.000 | 0.818 |
| rs3820716 | 0.017 | 0.070 | 0.185 |
| rs2303392 | 0.277 | 0.683 | 0.573 |
| rs13430086 | 0.010 | 0.051 | 0.273 |
| rs17692648 | 0.068 | 0.109 | 0.722 |
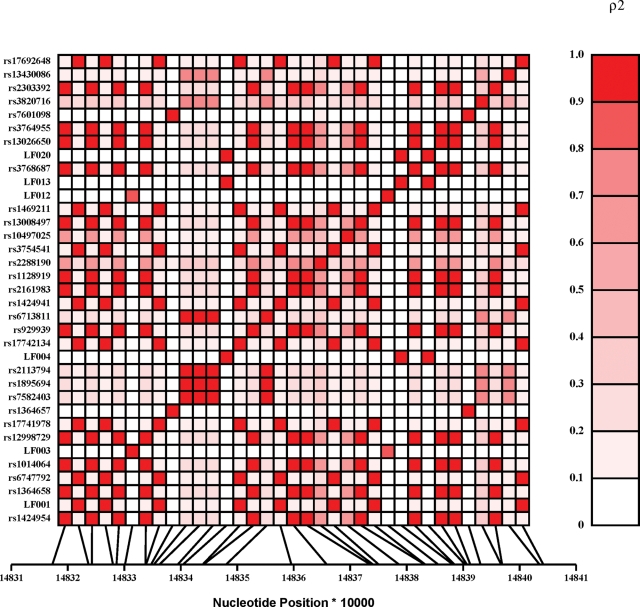
Figure 2 depicts a variable pattern of LD across ACVR2A as measured by the squared value of the pair-wise correlation among intragenic genotypes (ρ2). From this plot, a very strong correlation (ρ2 > 0.9) is observed among the three novel SNPs (LF004, LF013 and LF020) displaying a nominal association with severe pre-eclampsia. These SNPs are not correlated with any other variant tested in ACVR2A suggesting that one, or a combination of all three of these SNPs, may have a functional influence on the gene. Given the intronic position of these SNPs and the relatively small re-sequencing sample data set (n = 16), we cannot exclude, however, the possibility of these SNPs being in LD with another as yet unidentified putative functional SNP. The imputation of missing genotypes using haplotype estimation procedures did not reveal any additional association information (data not shown).
Figure 2.
Linkage disequilibrium (LD) plot of all genotyped ACVR2A SNPs. The metric to the right of the plot (ρ2) indicates the strength of the correlation among genotypes at each SNP locus with 0.0 indicating no correlation (i.e. LD) and 1.0 indicating a perfect correlation (i.e. complete LD).
Discussion
In this study, we have identified, by re-sequencing, novel intronic SNPs in the ACVR2A gene which are in LD with each other and which are associated with pre-eclampsia. These results are consistent with our previous preliminary association analysis of the ACVR2A gene (Moses et al., 2006), where known SNPs in ACVR2A displayed association in a subset of families (The 34 Family Cohort) of the larger family cohort used in this current study (The 74 Family Cohort). The fact that these three SNPs have no correlation with any other SNP tested suggests that this association would not have been detected using commercial SNP arrays or experiments designed using SNPs from the public databases. At present, it is not clear how these variants influence the expression or function of ACVR2A. Even though no correlation is seen with this haplotype and any other SNP tested, it is still not possible to determine whether they are in LD with more distant variants without deeper re-sequencing efforts.
We were not able to replicate the rs1424954 SNP association previously reported by Moses et al. (2006). This association was detected in the 34 Family Cohort used for the original chromosome 2 localization (Moses et al., 2000). These families were chosen for the genome-wide linkage scan as they were mostly large three-generation pedigrees with multiple members affected. Increasing sample size by the addition of extra families has the potential to increase power to detect variants with small genetic effects (Terwilliger and Goring, 2000). However, this also introduces the potential to dilute genetic effects by introducing greater genetic heterogeneity. The additional 40 families included in this study were smaller pedigrees with fewer affected and these may have diluted the effect of the rs1424954 polymorphism and introduced additional functional variation detected here. In addition, the five SNPs chosen from the previous pilot study were tag SNPs not chosen for any potential functional contribution. In this current study, the re-sequencing of conserved gene regions was more likely to identify polymorphisms to be functional.
Most studies looking for genetic variation affecting common disease focus on alleles altering the coding sequence of genes. However, studies now often identify non-coding variation influencing human disease [e.g. Type II diabetes and serum triglycerides (Saxena et al., 2007), schizophrenia (Law et al., 2006) and psoriasis (Capon et al., 2004)]. This is also consistent with what is known of the genetics that underlie similar phenotypes in other organisms (Hendrich and Willard, 1995; Mackay, 1996). The idea that common non-coding SNPs are responsible for many complex human disorders fits well in explaining traits that typically arise after decades of life, such as diabetes or cardiovascular disease, or, in the case of pre-eclampsia, only during pregnancy. Regulatory regions may be particularly relevant to chronic diseases where response to homeostatic epistasis or environmental variation results in gradual accumulation of damage over many years before reaching a critical threshold (Weiss and Terwilliger, 2000). In the case of pre-eclampsia, the effects could be brought about due to the increased physiological demands of pregnancy.
The three novel intronic SNPs that initially indicated association with pre-eclampsia in this present study are all in regions not previously recognized as having regulatory function. If these conserved regions are unidentified regulatory sequences, then these polymorphisms could have the potential to affect the transcription of ACVR2A. This would be consistent with the perturbations seen in the circulating levels of activin A during pre-eclamptic pregnancies (described below) and with emerging knowledge of the complexity of transcriptional regulation. For example, a recent study by Carninci et al. (2005) identified hundreds of transcription sites, many located within internal exons and introns of protein coding genes, indicating that promoter sites are common and that transcriptional organization is complex. This transcriptional architecture implies that most genomic regions serve multiple functions. This is the reason behind our re-sequencing strategy in this current study, whereby in an effort to capture all potentially functional genetic variation in the ACVR2A gene, all intragenic DNA regions showing evolutionary conservation in addition to the coding, promoter and 3′UTR sequences were re-sequenced, based on the hypothesis that those regions showing evolutionary conservation are more likely to harbour regulatory sequences.
ACVR2A is a receptor for the cell signalling protein activin A that is known to be an important regulator of reproductive function. Activin A belongs to the TGF-β super family of genes and is known to regulate cell differentiation, proliferation and apoptosis and is found in a variety of cells including the endometrium, placenta and vascular endothelium. Activin A has key roles in promoting decidualization of endometrial stromal cells (Jones et al., 2002) and in the regulation of trophoblast differentiation and invasion into the decidua (Caniggia et al., 1997). This means the normal physiological remodelling of the spiral arteries that is reduced in pre-eclampsia is highly dependent on activin A signalling through ACVR2A. Activin A also has an established role in endothelial functioning and vascular homeostasis, where altered ACVR2A binding could result in endothelial dysfunction triggering systemic inflammation, hypertension, proteinuria and oedema.
There have been several studies, looking at maternal serum in pre-eclampsia patients, which have shown increased circulating activin A concentrations compared with gestational matched controls (Petraglia et al., 1995a, b; Muttukrishna et al., 1997; Fraser et al., 1998; Laivuori et al., 1999; Silver et al., 1999; D'Antona et al., 2000). The main source of this increased activin A in maternal blood during pregnancy is thought to originate from the placenta (Petraglia et al., 1987). It is possible that in a proportion of cases, altered ACVR2A expression would have a similar effect as altered activin A expression, resulting in an unbalancing event that in combination with an ischaemic placenta could initiate the pathophysiological cascade leading to the maternal syndrome. Changes in the expression or action of the activin A receptors, specifically ACVR2A, could increase the activity of already increased levels of activin A, exacerbating the endothelial activation. This extends further, explaining why in some women the insufficient modification of spiral arteries and subsequent placental hypoxia and release of activin A are not enough to trigger the maternal syndrome of pre-eclampsia.
Our data were analysed under two different diagnostic criteria. Pre-eclampsia/eclampsia is one of a number of hypertensive disorders that complicate human pregnancy. A particular confounding problem associated with a working definition of pre-eclampsia is whether mild pre-eclampsia (pregnancy-induced hypertension without proteinuria), severe pre-eclampsia (pregnancy-induced hypertension with proteinuria) and eclampsia (severe pre-eclampsia with epileptic seizures) are a continuum of degrees of severity or whether each represents a distinct group.
The severe pre-eclampsia/eclampsia syndrome represents the greatest danger to the health and welfare of the mother and the fetus, whereas pregnancy-induced hypertension is a relatively benign disorder characterized by mild to moderate elevations of blood pressure late in pregnancy that return to normal post-partum (Roberts and Lain, 2002). It has been suggested that women who develop mild pre-eclampsia have a greater future risk of developing essential hypertension later in life than women with severe pre-eclampsia and eclampsia (Adams and Finlayson, 1961; Adams and Macgillivray, 1961). On the other hand, an increased risk of myocardial infarction (Mann et al., 1976) and ischaemic heart disease (Jonsdottir et al., 1995) has been demonstrated in women with severe pre-eclampsia or eclampsia but not in pregnancy-induced hypertension. The subgroups clearly share several pathological features, but differing future risk profiles suggest that the severe and mild phenotypes may have divergent aetiologies. This may explain the differing genetic associations identified between these two diagnostic groups in this study.
It has been suggested that pregnancy can unmask a woman's potential for disease, providing a window to her long-term health and presenting opportunities for primary prevention (Sattar and Greer, 2002). A major problem in the prevention of vascular disease has been the difficulty in identifying individuals at risk at an early enough stage for them to benefit from intervention such as modification of their lifestyle. If pre-eclampsia is confirmed as an indicator of increased vascular risk in mothers, the identification of genetic factors underlying susceptibility provides a great opportunity for screening and early intervention strategies for cardiovascular disease.
In this study, we identified, by re-sequencing, three novel, rare intronic SNPs in ACVR2A which are in strong LD with each other and which, on first assessment, showed significant evidence of association with pre-eclampsia, although this was not confirmed after correction for multiple testing. There are, however, compelling reasons not to rule out a role for this gene in genetic susceptibility to pre-eclampsia, including the biological role that activin A is known to play in the establishment of pregnancy (Petraglia et al., 1993; Caniggia et al., 1997; Jones et al., 2002), its apparent utility as a biological marker of pre-eclampsia (Petraglia et al., 1995a, b; Muttukrishna et al., 1997; Fraser et al., 1998; Laivuori et al., 1999; Silver et al., 1999; D'Antona et al., 2000) and the recent report of association between ACVR2A SNPs and pre-eclampsia in an independent Norwegian population (Roten et al., 2008). Taken together, this body of data should encourage further sufficiently powered studies to confirm the existence of risk polymorphisms in this gene, which may help to identify high-risk individuals and guide new diagnostic procedures or novel therapies for the treatment of pre-eclampsia.
Funding
This work was supported by the National Institutes of Health, USA [R01HD049847 to E.K.M., S.P.B. and J.B., MH059490 to J.B. and C06 RR017515]; and the San Antonio Area Foundation [Semp Russ Foundation to M.P.J.]. E.F. is the recipient of the Royal Women's Hospital Postgraduate Research Degree Scholarship. M.P.J. is the recipient of a Cowles Postdoctoral Fellowship.
Acknowledgements
We thank all the pre-eclampsia families whose participation made this work possible and gratefully acknowledge the support of the clinicians and research midwives who contributed to this study.
References
- Abecasis GR, Cookson WO, Cardon LR. Pedigree tests of transmission disequilibrium. Eur J Hum Genet. 2000;8:545–551. doi: 10.1038/sj.ejhg.5200494. [DOI] [PubMed] [Google Scholar]
- Adams EM, Finlayson A. Familial aspects of pre-eclampsia and hypertension in pregnancy. Lancet. 1961;2:1375–1378. doi: 10.1016/s0140-6736(61)91197-7. [DOI] [PubMed] [Google Scholar]
- Adams EM, Macgillivray I. Long-term effect of preeclampsia on blood-pressure. Lancet. 1961;2:1373–1375. doi: 10.1016/s0140-6736(61)91196-5. [DOI] [PubMed] [Google Scholar]
- Agatisa PK, Ness RB, Roberts JM, Costantino JP, Kuller LH, McLaughlin MK. Impairment of endothelial function in women with a history of preeclampsia: an indicator of cardiovascular risk. Am J Physiol Heart Circ Physiol. 2004;286:H1389–H1393. doi: 10.1152/ajpheart.00298.2003. [DOI] [PubMed] [Google Scholar]
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arngrímsson R, Sigurard ttir S, Frigge ML, Bjarnad ttir RI, Jonsson T, Stefansson H, Baldursdottir A, Einarsdottir AS, Palsson B, Snorradottir S, et al. A genome-wide scan reveals a maternal susceptibility locus for pre-eclampsia on chromosome 2p13. Hum Mol Genet. 1999;8:1799–1805. doi: 10.1093/hmg/8.9.1799. [DOI] [PubMed] [Google Scholar]
- Boerwinkle E, Chakraborty R, Sing CF. The use of measured genotype information in the analysis of quantitative phenotypes in man. I. Models and analytical methods. Ann Hum Genet. 1986;50:181–194. doi: 10.1111/j.1469-1809.1986.tb01037.x. [DOI] [PubMed] [Google Scholar]
- Brown MA, Gallery EDM, Gatt SP, Leslie G, Robinson J. Management of hypertension in pregnancy: executive summary. Med J Aust. 1993;158:700–702. [PubMed] [Google Scholar]
- Caniggia I, Lye SJ, Cross JC. Activin is a local regulator of human cytotrophoblast cell differentiation. Endocrinology. 1997;138:3976–3986. doi: 10.1210/endo.138.9.5403. [DOI] [PubMed] [Google Scholar]
- Capon F, Allen MH, Ameen M, Burden AD, Tillman D, Barker JN, Trembath RC. A synonymous SNP of the corneodesmosin gene leads to increased mRNA stability and demonstrates association with psoriasis across diverse ethnic groups. Hum Mol Genet. 2004;13:2361–2368. doi: 10.1093/hmg/ddh273. [DOI] [PubMed] [Google Scholar]
- Carninci P, Kasukawa T, Katayama S, Gough J, Frith MC, Maeda N, Oyama R, Ravasi T, Lenhard B, Wells C, et al. The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- Carr DB, Epplein M, Johnson CO, Easterling TR, Critchlow CW. A sister's risk: family history as a predictor of preeclampsia. Am J Obstet Gynecol. 2005;193:965–972. doi: 10.1016/j.ajog.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Chambers JC, Fusi L, Malik IS, Haskard DO, De Swiet M, Kooner JS. Association of maternal endothelial dysfunction with preeclampsia. JAMA. 2001;285:1607–1612. doi: 10.1001/jama.285.12.1607. [DOI] [PubMed] [Google Scholar]
- Chesley LC. Cardiovascular changes in pregnancy. Obstet Gynecol Annu. 1975;4:71–97. [PubMed] [Google Scholar]
- Chesley LC, Annitto JE, Cosgrove RA. The familial factor in toxemia of pregnancy. Obstet Gynecol. 1968;32:303–311. [PubMed] [Google Scholar]
- D'Antona D, Reis FM, Benedetto C, Evans LW, Groome NP, de Kretser DM, Wallace EM, Petraglia F. Increased maternal serum activin A but not follistatin levels in pregnant women with hypertensive disorders. J Endocrinol. 2000;165:157–162. doi: 10.1677/joe.0.1650157. [DOI] [PubMed] [Google Scholar]
- Duley L. Maternal mortality associated with hypertensive disorders of pregnancy in Africa, Asia, Latin America and the Caribbean. Br J Obstet Gynaecol. 1992;99:547–553. doi: 10.1111/j.1471-0528.1992.tb13818.x. [DOI] [PubMed] [Google Scholar]
- Fisher KA, Luger A, Spargo BH, Lindheimer MD. Hypertension in pregnancy: clinical-pathological correlations and remote prognosis. Medicine (Baltimore) 1981;60:267–276. [PubMed] [Google Scholar]
- Fitzpatrick E, Goring HH, Liu H, Borg A, Forrest S, Cooper DW, Brennecke SP, Moses EK. Fine mapping and SNP analysis of positional candidates at the preeclampsia susceptibility locus (PREG1) on chromosome 2. Hum Biol. 2004;76:849–862. doi: 10.1353/hub.2005.0017. [DOI] [PubMed] [Google Scholar]
- Fraser RF, 2nd, McAsey ME, Coney P. Inhibin-A and pro-alpha C are elevated in preeclamptic pregnancy and correlate with human chorionic gonadotropin. Am J Reprod Immunol. 1998;40:37–42. doi: 10.1111/j.1600-0897.1998.tb00386.x. [DOI] [PubMed] [Google Scholar]
- Funai EF, Friedlander Y, Paltiel O, Tiram E, Xue X, Deutsch L, Harlap S. Long-term mortality after preeclampsia. Epidemiology. 2005;16:206–215. doi: 10.1097/01.ede.0000152912.02042.cd. [DOI] [PubMed] [Google Scholar]
- Grobman WA, Wang EY. Serum levels of activin A and inhibin A and the subsequent development of preeclampsia. Obstet Gynecol. 2000;96:390–394. doi: 10.1016/s0029-7844(00)00926-1. [DOI] [PubMed] [Google Scholar]
- Havill LM, Dyer TD, Richardson DK, Mahaney MC, Blangero J. The quantitative trait linkage disequilibrium test: a more powerful alternative to the quantitative transmission disequilibrium test for use in the absence of population stratification. BMC Genet. 2005;6(Suppl 1):S91. doi: 10.1186/1471-2156-6-S1-S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrich BD, Willard HF. Epigenetic regulation of gene expression: the effect of altered chromatin structure from yeast to mammals. Hum Mol Genet. 1995;4:1765–1777. doi: 10.1093/hmg/4.suppl_1.1765. [DOI] [PubMed] [Google Scholar]
- Hubel CA, Snaedal S, Ness RB, Weissfeld LA, Geirsson RT, Roberts JM, Arngrimsson R. Dyslipoproteinaemia in postmenopausal women with a history of eclampsia. BJOG. 2000;107:776–784. doi: 10.1111/j.1471-0528.2000.tb13340.x. [DOI] [PubMed] [Google Scholar]
- Irgens HU, Reisaeter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. Br Med J. 2001;323:1213–1217. doi: 10.1136/bmj.323.7323.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RL, Salamonsen LA, Findlay JK. Activin A promotes human endometrial stromal cell decidualization in vitro. J Clin Endocrinol Metab. 2002;87:4001–4004. doi: 10.1210/jcem.87.8.8880. [DOI] [PubMed] [Google Scholar]
- Jonsdottir LS, Arngrimsson R, Geirsson RT, Sigvaldason H, Sigfusson N. Death rates from ischemic heart disease in women with a history of hypertension in pregnancy. Acta Obstet Gynecol Scand. 1995;74:772–776. doi: 10.3109/00016349509021195. [DOI] [PubMed] [Google Scholar]
- Lachmeijer AM, Arngrimsson R, Bastiaans EJ, Frigge ML, Pals G, Sigurdardottir S, Stefansson H, Palsson B, Nicolae D, Kong A, et al. A genome-wide scan for preeclampsia in the Netherlands. Eur J Hum Genet. 2001;9:758–764. doi: 10.1038/sj.ejhg.5200706. [DOI] [PubMed] [Google Scholar]
- Laivuori H, Kaaja R, Turpeinen U, Stenman UH, Ylikorkala O. Serum activin A and inhibin A elevated in pre-eclampsia: no relation to insulin sensitivity. Br J Obstet Gynaecol. 1999;106:1298–1303. doi: 10.1111/j.1471-0528.1999.tb08185.x. [DOI] [PubMed] [Google Scholar]
- Laivuori H, Kaaja R, Koistinen H, Karonen SL, Andersson S, Koivisto V, Ylikorkala O. Leptin during and after preeclamptic or normal pregnancy: its relation to serum insulin and insulin sensitivity. Metabolism. 2000;49:259–263. doi: 10.1016/s0026-0495(00)91559-2. [DOI] [PubMed] [Google Scholar]
- Laivuori H, Lahermo P, Ollikainen V, Widen E, Haiva-Mallinen L, Sundstrom H, Laitinen T, Kaaja R, Ylikorkala O, Kere J. Susceptibility loci for preeclampsia on chromosomes 2p25 and 9p13 in Finnish families. Am J Hum Genet. 2003;72:168–177. doi: 10.1086/345311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AJ, Lipska BK, Weickert CS, Hyde TM, Straub RE, Hashimoto R, Harrison PJ, Kleinman JE, Weinberger DR. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5′ SNPs associated with the disease. Proc Natl Acad Sci USA. 2006;103:6747–6752. doi: 10.1073/pnas.0602002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie RT, Rasmussen S, Brunborg H, Gjessing HK, Lie-Nielsen E, Irgens LM. Fetal and maternal contributions to risk of pre-eclampsia: population based study. Br Med J. 1998;316:1343–1347. doi: 10.1136/bmj.316.7141.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay TF. The nature of quantitative genetic variation revisited: lessons from Drosophila bristles. Bioessays. 1996;18:113–121. doi: 10.1002/bies.950180207. [DOI] [PubMed] [Google Scholar]
- Mann JI, Doll R, Thorogood M, Vessey MP, Waters WE. Risk factors for myocardial infarction in young women. Br J Prev Soc Med. 1976;30:94–100. doi: 10.1136/jech.30.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses EK, Lade JA, Guo G, Wilton AN, Grehan M, Freed K, Borg A, Terwilliger JD, North R, Cooper DW, et al. A genome scan in families from Australia and New Zealand confirms the presence of a maternal susceptibility locus for pre-eclampsia, on chromosome 2. Am J Hum Genet. 2000;67:1581–1585. doi: 10.1086/316888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses EK, Fitzpatrick E, Freed KA, Dyer TD, Forrest S, Elliott K, Johnson MP, Blangero J, Brennecke SP. Objective prioritization of positional candidate genes at a quantitative trait locus for pre-eclampsia on 2q22. Mol Hum Reprod. 2006;12:505–512. doi: 10.1093/molehr/gal056. [DOI] [PubMed] [Google Scholar]
- Muttukrishna S, Child TJ, Groome NP, Ledger WL. Source of circulating levels of inhibin A, pro alpha C-containing inhibins and activin A in early pregnancy. Hum Reprod. 1997;12:1089–1093. doi: 10.1093/humrep/12.5.1089. [DOI] [PubMed] [Google Scholar]
- Muttukrishna S, North RA, Morris J, Schellenberg JC, Taylor RS, Asselin J, Ledger W, Groome N, Redman CW. Serum inhibin A and activin A are elevated prior to the onset of pre-eclampsia. Hum Reprod. 2000;15:1640–1645. doi: 10.1093/humrep/15.7.1640. [DOI] [PubMed] [Google Scholar]
- Nilsson E, Salonen Ros H, Cnattingius S, Lichtenstein P. The importance of genetic and environmental effects for pre-eclampsia and gestational hypertension: a family study. BJOG. 2004;111:200–206. doi: 10.1111/j.1471-0528.2004.00042x.x. [DOI] [PubMed] [Google Scholar]
- Peng C, Huang TH, Jeung EB, Donaldson CJ, Vale WW, Leung PC. Expression of the type II activin receptor gene in the human placenta. Endocrinology. 1993;133:3046–3049. doi: 10.1210/endo.133.6.8243335. [DOI] [PubMed] [Google Scholar]
- Petraglia F, Sawchenko P, Lim AT, Rivier J, Vale W. Localization, secretion, and action of inhibin in human placenta. Science. 1987;237:187–189. doi: 10.1126/science.3299703. [DOI] [PubMed] [Google Scholar]
- Petraglia F, Anceschi MM, Calza L, Garuti GC, Fusaro P, Giardino L, Genazzani AR, Vale W. Inhibin and activin in human fetal membranes: evidence for a local effect on prostaglandin release. J Clin Endocrinol Metab. 1993;77:542–548. doi: 10.1210/jcem.77.2.8345060. [DOI] [PubMed] [Google Scholar]
- Petraglia F, Aguzzoli L, Gallinelli A, Florio P, Zonca M, Benedetto C, Woodruff K. Hypertension in pregnancy: changes in activin A maternal serum concentration. Placenta. 1995;a 16:447–454. doi: 10.1016/0143-4004(95)90102-7. [DOI] [PubMed] [Google Scholar]
- Petraglia F, De Vita D, Gallinelli A, Aguzzoli L, Genazzani AR, Romero R, Woodruff TK. Abnormal concentration of maternal serum activin-A in gestational diseases. J Clin Endocrinol Metab. 1995;b 80:558–561. doi: 10.1210/jcem.80.2.7852520. [DOI] [PubMed] [Google Scholar]
- Prabhakar S, Poulin F, Shoukry M, Afzal V, Rubin EM, Couronne O, Pennacchio LA. Close sequence comparisons are sufficient to identify human cis-regulatory elements. Genome Res. 2006;16:855–863. doi: 10.1101/gr.4717506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46:1243–1249. doi: 10.1161/01.HYP.0000188408.49896.c5. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Lain KY. Recent Insights into the pathogenesis of pre-eclampsia. Placenta. 2002;23:359–372. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension. 2003;41:437–445. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- Roten LT, Johnson MP, Forsmo S, Fitzpatrick E, Dyer TD, Brennecke SP, Blangero J, Moses EK, Austgulen R. Association between the candidate susceptibility gene ACVR2A on chromosome 2q22 and pre-eclampsia in a large Norwegian population-based study (the HUNT study) Eur J Hum Genet. 2008;17:250–257. doi: 10.1038/ejhg.2008.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen Ros H, Lichtenstein P, Lipworth L, Cnattingius S. Genetic effects on the liability of developing pre-eclampsia and gestational hypertension. Am J Med Genet. 2000;91:256–260. [PubMed] [Google Scholar]
- Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? Br Med J. 2002;325:157–160. doi: 10.1136/bmj.325.7356.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- Silver HM, Lambert-Messerlian GM, Star JA, Hogan J, Canick JA. Comparison of maternal serum total activin A and inhibin A in normal, preeclamptic, and nonproteinuric gestationally hypertensive pregnancies. Am J Obstet Gynecol. 1999;180:1131–1137. doi: 10.1016/s0002-9378(99)70606-x. [DOI] [PubMed] [Google Scholar]
- Sobel E, Lange K. Descent graphs in pedigree analysis: applications to haplotyping, location scores, and marker-sharing statistics. Am J Hum Genet. 1996;58:1323–1337. [PMC free article] [PubMed] [Google Scholar]
- Terwilliger JD, Goring HH. Gene mapping in the 20th and 21st centuries: statistical methods, data analysis, and experimental design. Hum Biol. 2000;72:63–132. [PubMed] [Google Scholar]
- Weiss KM, Terwilliger JD. How many diseases does it take to map a gene with SNPs? Nat Genet. 2000;26:151–157. doi: 10.1038/79866. [DOI] [PubMed] [Google Scholar]
- Witlin AG, Sibai BM. Hypertension in pregnancy: current concepts of preeclampsia. Annu Rev Med. 1997;48:115–127. doi: 10.1146/annurev.med.48.1.115. [DOI] [PubMed] [Google Scholar]
- Zwahlen M, Gerber S, Bersinger NA. First Trimester Markers for Pre-Eclampsia: Placental vs. Non-Placental Protein Serum Levels. Gynecol Obstet Invest. 2007;63:15–21. doi: 10.1159/000094672. [DOI] [PubMed] [Google Scholar]