Abstract
Objective
To examine the expression of toll-like receptors (TLR) by primary human Fallopian tube epithelial cells (FTEC) and to determine whether exposure to the TLR3 agonist poly(I:C) would induce an antiviral response.
Design
Tissue culture study.
Setting
University Medical Center.
Patient(s)
Pre-menopausal women undergoing hysterectomy.
Intervention(s)
Primary human FTEC were grown to confluence and high transepithelial resistance and treated with TLR agonists. Conditioned media was collected and RNA was extracted and analyzed for the expression of cytokines, chemokines and antimicrobial genes.
Main Outcome Measure(s)
RNA was analyzed by real-time RT-PCR and protein levels were assessed by ELISA.
Result(s)
The FTEC were demonstrated to express TLR1-9 but not 10. Treatment of FTEC with TLR3 agonist poly(I:C) resulted in increased expression of IL-8, TNF-α, human β-defensin 2, interferon beta, and interferon stimulated genes myxovirus resistance gene 1, 2′,5′-oligoadenylate synthetase, and protein kinase R. Additionally, FTEC exposed to poly(I:C) also resulted in the induction of TLR 2, 3, and 7.
Conclusion(s)
Our results suggest that FTEC are sensitive to viral infection and/or exposure to viral dsRNA and can respond by secreting proinflammatory cytokines that mediate the initiation of an inflammatory response as well as expressing genes that can directly inhibit viral replication.
Keywords: Antiviral, epithelial cells, innate immunity, Fallopian tube, toll-like receptors
INTRODUCTION
Epithelial cells of the female reproductive tract (FRT) are the first line of defense against invading pathogens that might cause sexually transmitted diseases (1). Understanding the role of the FRT in immune surveillance and host defense is crucial for the development of effective mucosal vaccines as well as therapies for ongoing infections. Known to be an efficient physical barrier to infection, epithelial cells are in constant contact with the normal flora of the FRT and must discriminate between commensal organisms and pathogens (2). For this purpose, epithelial cells have evolved specialized innate immune antimicrobial functions as well as the ability to modulate the recruitment and activity of immune cells of both the innate and adaptive immune systems (3).
Toll-like receptors (TLR) are germ-line encoded receptors that are important mediators of microbial recognition. TLR recognize conserved pathogen-associated molecular patterns (PAMP) synthesized by microorganisms but not by the host. Members of the TLR family (TLR 1-11), recognize distinct PAMP produced by various bacterial, fungal, and viral pathogens. The recognition of bacterial PAMP, such as lipopolysaccharide (LPS), peptidoglycan, and flagellin, are mediated by TLR1, 2, 4, 5, and 6 (4–9). TLR7 and TLR8 recognize nucleotide derivatives, such as imiquimod, resiquimod, and loxoribine as well as self and viral single stranded RNA (ssRNA) (10, 11) TLR9 binds unmethylated DNA CpG, found in bacteria (12–15). TLR3 recognizes viral dsRNA, and can also be stimulated by cellular mRNA, potentially from apoptotic or necrotic cells (16, 17). The specific ligands for TLR10 and TLR11 have yet to be identified (18, 19).
Epithelial cells of the human FRT have been shown to express a variety of TLR, including TLR1–3, 5, and 6 by vaginal and cervical epithelial cell lines and TLR1–3 and TLR6 by primary endocervical epithelial cells (20). Immunostaining of whole tissue sections obtained from the FRT have also demonstrated the expression of TLR 1-6 in vivo (21). Although epithelial cells of the lower FRT are relatively well-characterized in terms of their innate immune responses, the upper tract has traditionally been considered “sterile” to invading pathogens and immune molecules that exist in the lower tract. However, studies have demonstrated that macrospheres deposited in the cervix move through the cervix and uterus into the Fallopian tubes within minutes of deposition, supporting the theory that pathogens in the vagina and cervix can routinely reach the uterus and the Fallopian tubes (22). In fact, studies in our laboratory have previously demonstrated that the uterine epithelial cell (UEC) line ECC-1, as well as human primary UEC, express TLR1–9 and are responsive to specific TLR agonists (23, 24).
Stimulation of human intestinal and pulmonary epithelial cells as well as cell lines from the reproductive tract with TLR agonists has been shown to induce proinflammatory cytokines and chemokines. TLR2 and TLR5 agonists induce the production of IL-6, CCL20/MIP-3α, CXCL8/IL-8, CXCL1/GROα, and CCL2/MCP-1 (7, 25–27). TLR4 and TLR9 agonists induce epithelial cells to produce IL-6 and IL-8 (28). In addition, stimulation of epithelial cells has also been shown to induce the expression of small antimicrobial peptides called defensins (26).
Defensins are cysteine-rich, cationic peptides expressed by leukocytes and epithelial cells of mammals and birds which play an important role in innate host defense against pathogens by virtue of their antibacterial, antifungal and antiviral activities (reviewed in (29, 30)). Human β-defensins (HBD1–4), are largely expressed in various epithelial tissues, including skin, lung, and FRT (30–36).. The roles that defensins play in defense of the host from pathogens are multifaceted, ranging from direct killing of invading microbes to linking innate and acquired immunities through attracting immune cells to the site of pathogenic invasion (31, 32, 35–39).. Although defensins have inhibitory effects on certain viruses, broad antiviral innate immunity is thought to be typically mediated by type I interferons (IFN).
Interferons play key roles in mediating antiviral and antigrowth responses and in modulating immune responses (40). Type I interferons (IFN-α/β) are potent antiviral cytokines and modulators of the immune system. They are induced by viral infection or by dsRNA, a by-product of viral replication, and lead to the production of a broad range of antiviral proteins and immunoactive cytokines. To date, three antiviral pathways have been firmly established. These are the protein kinase R (PKR) (41), the 2′,5′-OAS/RNaseL system (42) and the Mx proteins (43). Mice lacking any one of these components show increased susceptibility to viral infections (44). MxA proteins are interferon-induced GTPases that appear to detect viral infection by sensing the presence of nucleocapsid-like structures and sequestering them so that assembly of new viruses is inhibited (43).. 2′,5′-OAS is induced by type I interferons and activated in the presence of dsRNA. Once activated, 2′,5′-OAS polymerizes ATP into 2′,5′-linked oligoadenylates that are specific activators of a latent endoribonuclease, RNase L (45). RNase L degrades viral and cellular RNAs resulting in inhibition of protein synthesis (46).. The PKR pathway is also activated in response to viral dsRNA. Upon exposure to dsRNA, PKR autophosphorylates and subsequently phosphorylates its substrates, one of which is eIF2 (eukaryotic initiation factor 2), leading to inhibition of protein synthesis (47). In addition, PKR also acts as a signaling transducer by initiating a signaling cascade that result in the production of interferons. Interferons, in turn, activate cellular signaling pathways that culminate in the nucleus with the up-regulation of interferon-stimulated genes, mediators of antiviral, antiproliferative and pro-apoptotic activity (47). The PKR mediated pathway of viral inhibition is extremely efficient and therefore many viruses have evolved immune evasion strategies to by-pass this pathway. Adenovirus (48), herpes simplex virus (49), and HIV (50) are few such examples. Although all three pathways are considered extremely important in controlling viral replication, cells from triple knock-out mice lacking PKR, RNaseL and Mx still exhibit limited IFN-induced antiviral state, indicating that additional antiviral pathways exist (51).
Previously, we characterized the human uterine cell line ECC-1 as well as primary human uterine epithelial cells in terms of their capabilities for innate immune responses (23, 24). The objective of this study was to extend previous findings and examine epithelial cells in a different upper FRT compartment, the Fallopian tube. Specifically, we attempted to determine whether the FTEC are capable of responding to invading pathogens. We approached this by identifying the TLR expressed by FTEC and by determining whether exposure of epithelial cells to the TLR3 agonist poly(I:C) results in the secretion of proinflammatory cytokines. In addition, the expression of antimicrobial defensins and antiviral genes was also examined following exposure of FTEC to poly(I:C).
MATERIALS AND METHODS
Source of Fallopian Tube Tissue
Human Fallopian tube tissue was obtained immediately following surgery from premenopausal women who had undergone hysterectomies at Dartmouth-Hitchcock Medical Center (Lebanon, NH, USA). All tissues used in the study were collected from patients with benign conditions such as fibroids with no pathological lesions observed in the Fallopian tubes after examination by a pathologist. Samples were generally taken from the ampullary region of the Fallopian tubes. A total of seven different patients were used for this study.
All human subjects work was carried out with the approval of the Dartmouth College Institutional Review Board. Approval to use tissues was previously obtained from the Committee for the Protection of Human Subjects (CPHS). The authors had no conflicts of interests.
Isolation of FTEC
Epithelial cells were isolated as previously described (52, 53). Briefly, tissues were minced under sterile conditions into 1- to 2-mm fragments and subjected to enzymatic digestion using an enzyme mixture that contained final concentrations of 3.4 mg/ml pancreatin (Invitrogen Life Technologies, Carlsbad, CA, USA), 0.1 mg/ml hyaluronidase (Worthington Biochemical, Lakewood, NJ, USA), 1.6 mg/ml collagenase (Worthington Biochemical), and 2 mg/ml D-glucose, in 1x HBSS (Invitrogen Life Technologies). Enzymes were chosen to maximize digestion of the extracellular matrix while minimizing digestion of cell surface antigens. After enzymatic digestion for 2 h at 37°C, cells were dispersed through a 250-μm mesh screen, washed, and resuspended in DMEM/F12 complete medium. Complete medium was supplemented with 20 mM HEPES, 2 mM L-glutamine (all from Invitrogen Life Technologies), 50 mg/ml primocin (Invivogen, San Diego, CA, USA), and 10% defined FBS (HyClone, Logan, UT, USA) and did not contain phenol red.
Epithelial cell sheets were separated from stromal cells by filtration through a 20-μm nylon mesh filter (Small Parts, Miami Lakes, FL, USA). Epithelial sheets were retained on the 20-μm filter, while stromal cells passed through. Epithelial sheets were recovered by rinsing and backwashing the filter with complete medium. Epithelial sheets were collected, centrifuged at 500 × g for 10 min, and analyzed for cell number and viability prior to resupension in a small volume of complete medium. By this procedure we have isolated epithelial cells that stain positive for the epithelial antigens Ber-EP4 and cytokeratin and negative for CD4, CD45, and vimentin (54).
Cell Culture
To establish a cell culture system of polarized human FTEC with both apical and basolateral compartments, the human FTEC were cultured in Human Extracellular Matrix (Becton Dickenson, Franklin Lakes, NJ, USA) coated Falcon cell culture inserts in 24-well culture plates (Fisher Scientific, Pittsburgh, PA, USA). For these experiments, apical and basolateral compartments had 300 and 850 μl of complete medium, respectively. The medium was changed every 2 days. Following a 24 h incubation with various TLR agonists: ultra pure LPS from Salmonella minnesota (List Biological Laboratories, Campbell, CA, USA), 1 μg/ml; poly(I:C) (Invivogen), 25 μg/ml; zymosan from Saccharomyces cerevisiae (Invivogen), 100 ng/ml. FTEC apical and basolateral conditioned media were collected and centrifuged for 5 min at 10,000 × g and stored at -80°C until needed.
Measurement of Transepithelial Resistance (TER)
As an indicator of tight junction formation of epithelial cell monolayers, TER was periodically assessed using an EVOM electrode and Voltohmmeter (World Precision Instruments, Sarasota, FL, USA), as described previously (55). TER is a functional measurement of the integrity of tight junctions in an epithelial monolayer. The presence of contaminating non-epithelial cells in culture interferes with the formation of tight junctions and therefore prevents an increase in TER. The TER therefore is also an indicator for the purity of epithelial cell monolayers (54).
Measurement of IL-8, TNF-α and HBD2 Secretion
Concentrations of IL-8 and TNF-α in the apical and basolateral supernatants from human primary FTEC were each determined with an ELISA test kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s protocol. Concentrations of HBD2 in the apical and basolateral media were also determined by ELISA (Peprotech, Rocky Hill, NJ, USA). The IL-8, TNF-α, and HBD2 ELISAs had minimum detection levels of 2.3 pg/well. Standards for each ELISA were resuspended in cell culture medium. Amounts of cytokines and HBD2 were quantified based on a standard curve after OD measurements at 450 nm on an ELISA reader (Dynex, Chantilly, VA, USA).
TaqMan Real-time RT-PCR
Real-time RT-PCR was done with a two-step protocol as described previously (56). Total RNA was isolated from cells using TRIzol Reagent according to the manufacturer’s recommendations (Invitrogen Life Technologies) and purified with RNeasy columns (Qiagen, Valencia, CA, USA). Coincident with RNA purification was on-column DNase digestion using the RNase-Free DNase set (Qiagen). For each specimen, 400 ng of total RNA was reverse-transcribed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s recommendations in a 20-μl volume. Relative mRNA expression levels of genes of interest were measured using the 5′ fluorogenic nuclease assay in real-time quantitative PCR using TaqMan chemistry on the ABI 7300 Prism real-time PCR instrument (Applied Biosystems, Foster City, CA, USA). The HBD2, IFN-β, 2′,5′-OAS, MxA, PKR, TLR2, 3, 4, 5, 7 and 9, and β-actin primer/MGB probe sets were obtained from Applied Biosystems assays-on-demand (ID nos. Hs00823638, Hs00277188, Hs00242943, Hs00182073, Hs00169345, Hs00152932, Hs00152933, Hs00152939, Hs00152825, Hs00152971, Hs00152973, and 4333762T, respectively). PCR was conducted using the following cycle parameters: 95°C, 12 min for 1 cycle (95°C, 20 s; 60°C, 1 min), for 40 cycles. Analysis was conducted using the sequence detection software supplied with the ABI 7300. The software calculates the threshold cycle (Ct) for each reaction and this was used to quantify the amount of starting template in the reaction. The Ct values for each set of duplicate reactions were averaged for all subsequent calculations. A difference in Ct values (ΔCt) was calculated for each gene by taking the mean Ct of each gene of interest and subtracting the mean Ct for the housekeeping gene β-actin for each cDNA sample. Assuming that each reaction functions at 100% PCR efficiency, a difference of one Ct represents a 2-fold difference. Relative expression levels were expressed as a fold-increase in mRNA expression and calculated using the formula 2−Δ ΔCt.
Statistics
The data are presented as the mean ± SE. A two-tailed paired t test or a one-way ANOVA with Bonferonni’s post-test was performed using GraphPad InStat version 3.0a (GraphPad Software, San Diego, CA, USA). A p value of <0.05 was taken as indicative of statistical significance.
RESULTS
Human FTEC Express TLR1-9
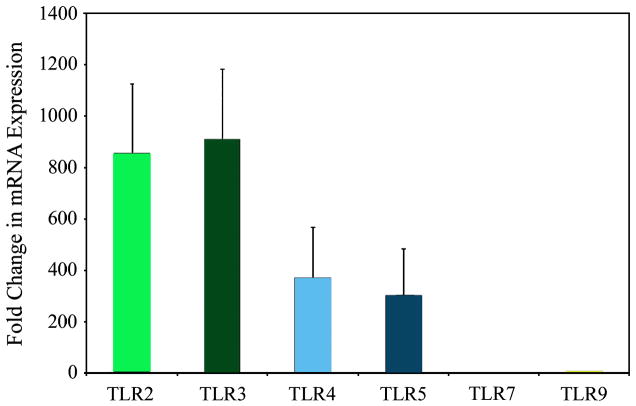
FTEC were tested for the expression of TLR1–10 by RT-PCR. FTEC were isolated from hysterectomy tissue samples and grown on cell inserts until confluent and polarized. The formation of tight junctions, as indicated by transepithelial resistance (TER) measurements of >500 ohm/well or greater (background resistance approximately 150 ohms/well), is an indication of epithelial cell integrity and verification that a polarized monolayer has been formed. Once sufficient TER was achieved, total RNA was isolated and mRNA expression of TLR was examined. By RT-PCR, we detected the expression of TLR1-9 mRNA (data not shown). Next, we performed real-time RT-PCR for quantitative comparison of the relative TLR expression by the FTEC. As shown in Fig. 1, the relative TLR expression levels were highest for TLR2 and 3 followed by TLR4 and 5 and then followed by TLR7 and 9. Expression of these TLRs was observed in FTEC preparations from six patients.
Figure 1. Constitutive mRNA expression of TLR by FTEC.
Real-time RT-PCR was used to determine relative levels of expression of TLR2, 3, 4, 5, 7, and 9 normalized against an endogenous control β-actin. The data was further normalized against TLR7, which was the lowest expressing gene. Results are a mean of six patient samples.
Effects of TLR Agonists Poly(I:C), LPS, and Zymosan on Apical and Basolateral Secretion of IL-8 and TNF-α by FTEC
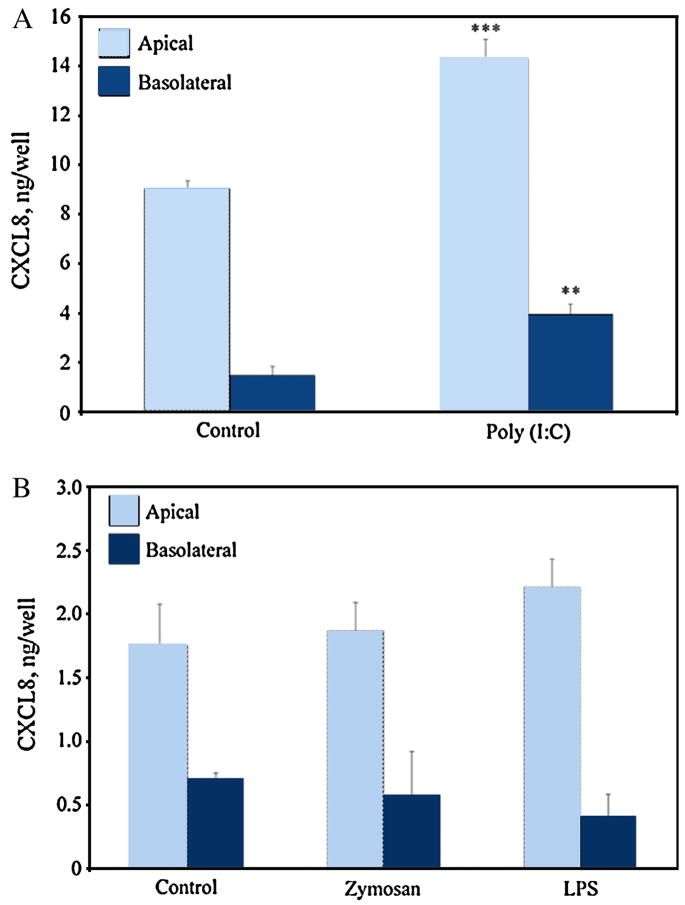
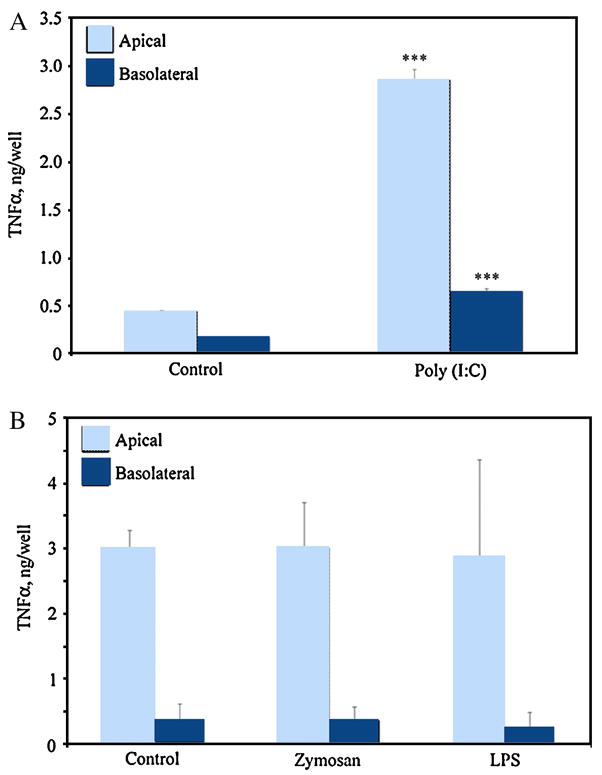
To determine whether the TLR expressed by FTEC were functional and therefore capable of initiating an innate immune response, we treated primary FTEC with agonists for TLR2 (zymosan), TLR3 (poly(I:C)) and TLR4 (LPS). Cells were grown to confluence until polarization as indicated by high TER values. Cells were then apically treated with TLR agonists for 24 h, after which the apical and basolateral media were collected and assayed for the proinflammatory cytokines IL-8 and TNF-α by ELISA. As shown in Figure 2A, poly(I:C) treatment of FTEC (n=5 experiments) resulted in a significantly greater secretion of IL-8 both apically and basolaterally, than that seen with untreated controls. However, when FTEC (n=4 experiments) were treated with zymosan and LPS, no significant increase in IL-8 secretion was observed either in the apical or the basolateral media (Figure 2B). A similar pattern was observed when the TLR agonist treated FTEC were assayed for TNF-α secretion (Figure 3). Poly(I:C) treatment increased TNF-α secretion both apically and basolaterally (Figure 3A), whereas LPS and zymosan treatments showed no significant effects (Figure 3B). These data suggest that FTEC are selectively responsive to specific TLR agonists.
Figure 2. Chemokine secretion by FTEC treated with TLR agonists poly(I:C), zymosan, and LPS.
Following 24 h apical treatment of FTEC to TLR agonists, conditioned media was collected and analyzed for the presence of IL-8 by ELISA. While poly(I:C) stimulation, (A), showed a significant increase in IL-8 secretion both apically and basolaterally, treatment with zymosan and LPS (B) did not show an increase. The p values were calculated using a two-tailed paired t test. ***, Significantly greater (p<0.001) than control; **, p<0.01. Results for (A) are a mean ±SE of five patient samples. Results for (B) are a mean ± SE value of four patient samples.
Figure 3. Cytokine secretion by FTEC treated with TLR agonists poly(I:C), zymosan, and LPS.
Following 24 h apical exposure of FTEC to TLR agonists, conditioned media was collected and analyzed for the presence of TNF-α by ELISA. Significant increase in TNF-α secretion was detected in both the apical and the basolateral conditioned media after poly(I:C) treatment (A), whereas no change in TNF-α secretion was observed after LPS and zymosan treatments (B). The p values were calculated using a two-tailed paired t test. ***, p<0.001. Results are a mean ±SE of five patient samples.
Induction of Beta-defensin 2 Secretion by Poly(I:C) Treatment of FTEC
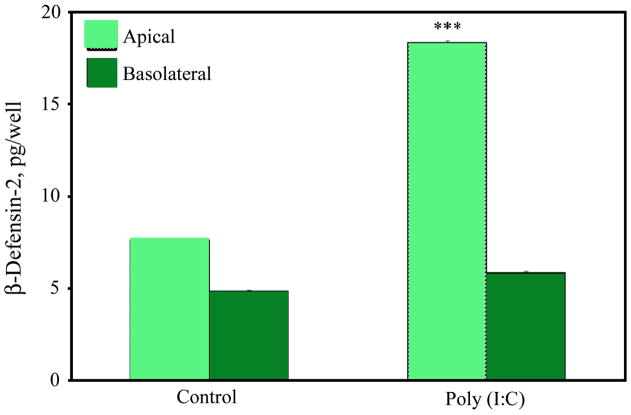
Previously, we have demonstrated that uterine epithelial cells express human beta-defensins 1, 2, and 3 (HBD1-3) and that exposure to poly(I:C) in the apical compartment significantly increased levels of HBD1 and HBD2 proteins (23). Therefore we sought to determine whether FTEC were also capable of increased HBD2 secretion upon stimulation with poly(I:C). FTEC from three patients were grown to confluence and high TER values and apically treated with poly(I:C) for 24 h. Supernatants were tested for HBD2 protein by ELISA. As shown in Figure 4, HBD2 secretion was significantly increased in the apical but not the basolateral compartments. These data suggest that the FTEC are capable of mounting an innate immune response by selective apical secretion of HBD2 when exposed to poly(I:C), an analog for double stranded viral RNA.
Figure 4. Secretion of antimicrobials by FTEC treated with poly(I:C).
FTEC were exposed to poly(I:C) apically for 24 h after which the apical and the basolateral media was analyzed for the presence of HBD2 by ELISA. Significantly increased secretion of HBD2 was observed in the apical but not the basolateral conditioned media upon poly(I:C) treatment. The p values were calculated using a two-tailed paired t test. ***, p<0.001 Results are a mean ±SE of three patient samples.
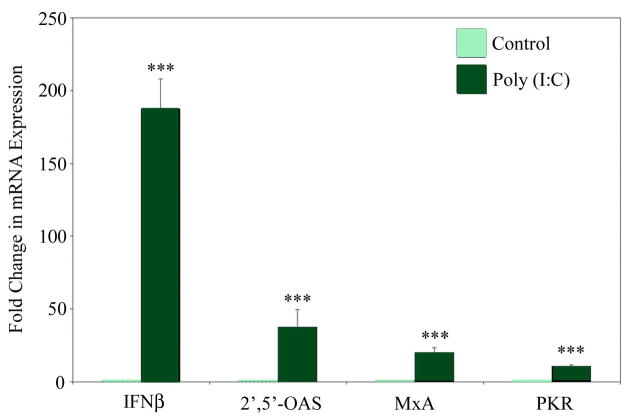
Exposure of FTEC to Poly(I:C) Induces mRNA Expression of IFN-β and Interferon Stimulated Genes MxA, 2′,5′-OAS, and PKR
Because FTEC respond to poly(I:C), we next undertook to determine whether these cells are capable of initiating an antiviral response. FTEC treated apically for 24 h with poly(I:C) were analyzed for the mRNA expression of IFN-β, as well as the IFN-β stimulated genes MxA, 2′,5′-OAS, and PKR, by real-time RT-PCR. As seen in Figure 5, poly(I:C) treatment of FTEC resulted in a dramatic and statistically significant upregulation of mRNA levels for IFN-β, MxA, 2′,-5′-OAS and PKR, indicating that FTEC are indeed capable of initiating an intracellular antiviral innate immune response.
Figure 5. Expression of antiviral genes by FTEC treated with the TLR3 agonist poly(I:C).
FTEC were exposed to poly(I:C) for 24 h. Real-time RT-PCR was used to determine the relative levels of expression of IFN-β, 2′,5′-OAS, MxA, and PKR, normalized against an endogenous control β-actin. The data were further normalized by using values from the control for calibration. The mRNA expression levels for all four genes were found to be significantly upregulated upon poly(I:C) treatment. The p values were calculated using a two-tailed paired t test. ***, p<0.001. Results are a mean of three patient samples.
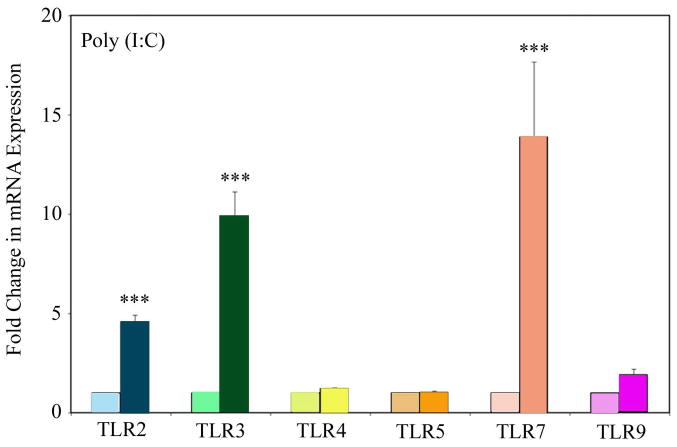
Exposure of FTEC Poly(I:C) Upregulates TLR2, 3, and 7
Epithelial cells of the FRT often encounter multiple pathogens simultaneously which in turn frequently results in co-infections. It is well documented that infection with one pathogen can alter the risks of infection with a second pathogen. For example, a recent study has shown that Herpes simplex virus 2 can induce persistence of Chlamydia trachomatis in human female reproductive tract epithelial cells (57).. To determine whether stimulation of FTEC with TLR3 agonist results in the upregulation of other TLR, we exposed FTEC to apical treatments of poly(I:C) for 24 h and prior to measuring the levels of mRNA expression for TLR2, 3, 4, 5, 7, and 9 by real-time RT-PCR. As shown in Figure 6, poly(I:C) treatment resulted in a significant upregulation of TLR2, 3, and 7. This suggests that in vivo, exposure of FRT epithelial cells to one type of pathogen (e.g. viral dsRNA) might prime the cells to respond to other pathogens (e.g. bacteria).
Figure 6. Upregulation of TLR2, 3, and 7 mRNA following exposure of FTEC to TLR3 ligand.
FTEC were treated apically with poly(I:C) for 24 h after which real-time RT-PCR was used to determine relative expression levels for TLR2, 3, 4, 5, 7, and 9. The data was normalized against endogenous control β-actin and further normalized by using values from the control for calibration. The mRNA expressions of TLR2, 3, and 7 were found to be significantly upregulated upon poly(I:C) treatment. The p values were calculated using a two-tailed paired t test. ***, p<0.001. Results are a mean of two patient samples.
DISCUSSION
This study demonstrates that human polarized epithelial cells from the Fallopian tube express TLR2, 3, 4, 5, 7, and 9 and that the TLR3 agonist poly(I:C) stimulates FTEC to secrete proinflammatory cytokines IL-8 and TNF-α. Further, we show that TLR3 stimulation of FTEC induced the secretion of the antimicrobial peptide HBD2 and the mRNA expression of IFN-β and IFN-β stimulated antiviral genes MxA, 2′,5′-OAS, and PKR. Previously we demonstrated that primary human uterine epithelial cells respond to viral dsRNA mimic poly(I:C) by secreting cytokines, chemokines, and antimicrobial factors and that preincubation of these cells with anti-TLR3 antibody abolished the secretion of specific genes suggesting that the effects observed were specific to TLR3 (23, 58, 59). In this study we demonstrate antiviral responses generated by primary human FTEC. These studies also demonstrate that incubation of FTEC with TLR3 agonist poly(I:C) induces the expression of TLR 2, 3, and 7 suggesting that exposure to one pathogen allows the FTEC to be primed to recognize other pathogens.
Once believed to be a sterile environment, the upper reproductive tract is now known to be continuously exposed to organisms colonizing the vagina. The demonstration that macrospheres deposited in the cervix move through the cervix and uterus into the Fallopian tubes within minutes of deposition is supportive evidence that pathogens in the vagina and cervix routinely reach the upper FRT (22). More recent studies in women indicate that uterine peristaltic waves, occurring under the influence of estradiol, enhance the movement of labeled-albumin macrospheres from the vagina into the uterus toward the ovary (60–62). Of particular importance are the findings of Parsons et al. showing that, irrespective of stage of the menstrual cycle or the use of oral contraceptives, radio-opaque dye entered the uterine lumen and Fallopian tubes within 2 h of placement in the vagina of women (63). It is also well-documented that sperm and associated ejaculate can be the carrier for virus thereby exposing the upper reproductive tract to these pathogens (64) (65, 66). Since the uterus and the Fallopian tubes are essential for pregnancy and fertility, the expression of TLR by the uterine and Fallopian tube epithelial cells allows them to recognize and respond to a wide array of pathogens by rapidly initiating innate and adaptive immune responses. Previous studies from our laboratory have shown that human FRT tissues, UEC cell line ECC-1 as well as primary human UEC express TLR and that TLR expression varies throughout the microanatomic compartments of the FRT (23, 24, 67). Here we demonstrate that, similar to uterine epithelial cells, FTEC also express a number of TLR and are able to rapidly recognize and respond to multiple pathogens.
Poly(I:C) is a synthetic mimic of viral dsRNA that binds to and signals through TLR3. Our findings demonstrate TLR3-mediated stimulation of FTEC via poly(I:C) results in enhanced secretion of the proinflammatory cytokines TNF-α and IL-8 as well as antimicrobial peptide HBD2. We have previously demonstrated that primary human UEC express HBD1 and HBD2 and the expression levels are enhanced upon exposure to poly(I:C) (23). Here we demonstrate, for the first time to the best of our knowledge, that HBD2 expression is inducible in primary FTEC upon stimulation with poly(I:C). This is significant because HBD2 is an effective antimicrobial against bacterial (Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus) as well as viral (adenovirus, HSV, rhinovirus, and HIV) agents (68–72). In addition, it has chemotactic functions and can facilitate the recruitment of immune cells, such as memory T lymphocytes, iDC and mast cells (39).
Other investigators have previously reported the antiviral actions of type 1 interferons (IFN α and β) (reviewed in (73)). It is also known that TLR3-mediated immune responses, induced by dsRNA or viral infection, result in the rapid production of IFN-β and IFN-β-stimulated genes, such as MxA and 2′,5′-OAS (74, 75). We have previously reported that poly(I:C) induced UEC to express high levels of IFN-β and IFN-β stimulated genes MxA and 2′,5′-OAS (23). In this study, we observed that poly(I:C) stimulation of FTEC also induces the expression of IFN-β, MxA, 2′,5′-OAS and PKR. To our knowledge, these studies are the first demonstration that human FTEC can express antiviral molecules in response to the viral dsRNA analog poly(I:C), and that this response is mediated in a TLR3-dependent manner. Further, the induction of IFN-β by FTEC may have important effects on leukocytes present at or recruited to the site of viral exposure/infection. For example, IFN-β has been shown to enhance cytotoxic activity and the production of IFN-γ by NK cells, stimulate the maturation of DCs and differentiation of monocytes into DCs, rescue memory T cells from apoptosis, enhance T lymphocyte proliferation and Th1 responses, as well as upregulate iNOS in monocytes and macrophages (76–79). Whether these functions occur in FRT epithelial cells remains to be determined. Our studies suggest that by secreting IFN-β in response to viral challenge, epithelial cells act as sentinels at multiple levels to prevent and/or control viral infection.
An unexpected finding in this study was that, whereas the expression of TLR2 and TLR4 was measurable, we found that FTEC were unresponsive to TLR2 and 4 agonists, zymosan and LPS. One explanation is that these cells have evolved, in a way similar to epithelial cells in the gastrointestinal tract, to be unresponsive to particular microorganisms (3). However, recognizing that the upper FRT is not a sterile environment and that protection is vital to survival of mother and fetus, it is possible that Fallopian tube TLR are functional and that we have as yet to identify the responding immunological parameters. In other studies using uterine epithelial cells in culture, we found that incubation with poly I:C stimulated the synthesis of a spectrum of cytokines and chemokines but that LPS and several other TLR agonists had no effect (23). We reported these cells as being unresponsive. Only recently, when we measured MIF and IL-6 secretion, did we realize that these cells are responsive to PAMPS (Fahey et al, Manuscript in Preparation). Therefore, “unresponsiveness” is possibly limited to the readout system being used. In this manuscript, based on the parameters we were measuring, we found that Fallopian tube epithelial cells appear unresponsive to certain PAMPs. But given our recent uterine epithelial cell findings, we cannot exclude the possibility that there are other immunological parameters that we have not measured and that the Fallopian tube may actually be responding to PAMPs other than poly(I:C) Another possibility might be that the TLR2 and 4 expression that we have detected is intracellular and therefore responsive to intracellular pathogen invasion such as N. gonorrheoe and Chlamydia that are pathogens of the upper FRT. Intracellular expression been shown by Ueta et al (80) in corneal epithelial cells where TLR 2 and 4 were expressed but did not respond to agonists resulting in an immuno-silent environment at the ocular mucosa. Alternatively, if expression of TLR 2 and 4 is exclusively at the cell surface, upregulation of TLR, rather than evoking the release of proinflammatory mediators, might induce signaling inhibitors such as Tollip, IRAK-M, and SOCS-1 (which we have not measured in this study) to promote PAMP tolerance as a mechanism to prevent immune activation. This kind of regulatory control has been demonstrated in intestinal epithelial cells (81). Further studies are needed to more fully understand the unique characteristics of the innate immune system provided by epithelial cells throughout the FRT.
Simultaneous co-infections with multiple pathogens are a common occurrence, particularly in the FRT. It is well documented that infection with one pathogen often enhances (and inhibits) infection with a different pathogen (82). In this study, we demonstrated that FTEC exposed to viral dsRNA mimic poly(I:C) significantly upregulate the expression of TLR2, 3, and 7. This indicates that the FTEC exist in a state of immune preparedness in that when they are exposed to one pathogen, they might be primed to recognize a number of other pathogens.
In contrast to our finding of TLR4 expression in FTEC, a recent study failed to detect TLR4 expression in epithelial cells obtained from Fallopian tubes of women undergoing sterilization or hysterectomy for leiomyomas (83). One explanation for these differences may be in the way the epithelial cells were prepared. Whereas the authors passaged their cells 3 times prior to growing them to confluence on plastic, our FTEC were isolated and placed directly on cell inserts. In contrast to cells grown in culture dishes, cells on inserts are polarized and are therefore more likely to remain functional in ways that reflect their existence within the Fallopian tube. Using mouse uterine epithelial cells, we compared freshly isolated cells with those grown to confluence on cell inserts and found that 5–10 days in culture had minimal effects of TLR expression (84). Whether growth in culture plates and cell passaging affect TLR expression in human FRT epithelial cells, remains to be determined.
Another recent study demonstrated proinflammatory responses by human oviductal epithelial cells upon exposure to poly(I:C) (85). Similar to the Itoh et al study described above, the authors in this paper cultured the oviductal epithelial cells to a confluent monolayer on plastic. In contrast, in our study the Fallopian tube epithelial cells were grown on inserts and allowed to polarize to mimic a more in vivo phenotype. In spite of the differences in the methods of cell isolation, the amounts of proinflammatory cytokine IL-8 secreted in response to poly(I:C) treatment were comparable for both studies. However, in this study, we have further demonstrated antiviral responsiveness of human FTEC by examining the expression of interferon and interferon stimulated genes that are specifically known for their antiviral roles.
In conclusion, these studies suggest that FTEC are poised to respond to viral infection at several levels. Stimulation of FTEC with dsRNA in a TLR3-dependent manner induces the secretion of proinflammatory cytokines and chemokines that are known to facilitate the recruitment of immune cells to the site of viral infection. At the same time, TLR3 stimulation of FTEC enhances the secretion of the antimicrobial peptide HBD2 that is known to have inhibitory effects on viral entry and replication into susceptible cells. At the intracellular level, IFN-β production by FTEC might induce the expression of IFN-β stimulated genes that have potent antiviral properties. Overall, FTEC appear primed and ready to respond to viral exposure and/or infection of the Fallopian tube in a TLR3-mediated manner, which, in turn, leads to the stimulation of IFN-β and IFN-β stimulated genes that are essential for inhibiting or slowing viral replication until an adaptive response can be mounted. The immune responsiveness of FTEC is especially significant considering that until recently the upper reproductive tract was thought to be a sterile environment. Further studies are needed to extend our understanding of the mechanisms by which the FTEC recognize and respond to pathogenic challenge. Such studies will be important for the development of mucosal vaccines as well as therapeutics targeted against FRT pathogens.
Acknowledgments
The authors thank Vincent Memoli, MD, Section Chief of Anatomical Pathology, for procuring tissues; other members of the Department of Pathology for inspecting and dissecting tissue specimens: Jorge Gonzalez, MD, Alan Schned, MD, Peter Seery, Shannon Schutz, Elizabeth Rizzo, Richard Merrill, Charles-Robert Moultry, Patricia Larkin, Aimee Larson, Jennifer Simonton and Dawn Maddaline; for clinical support and scheduling: Laura Wolfe, Linda Hallock, Kathleen Pilchman, Karen Carter, Kris Ramsey, Tamara Krivit, and Joanne Lavin; surgeons: Barry Smith, Joan Barthold, Jackson Beecham, John Currie, Leslie Demars, Paul Hanissian, John Ketterer, Benjamin Mahlab, Paul Manganiello, Misty Porter, Karen George, William Young, Kris Strohbehn, Roger Young, Stephen Andrews, and Eric Sailer; OR Nurses: Jeanette Sawyer, Tracy Stokes, Fran Reinfrank, Jaclyn Logan.
This work was supported by AI51877 (awarded to Dr. Charles Wira.) from National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Wira CR, Grant-Tschudy KS, Crane-Godreau MA. Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection. Am J Reprod Immunol. 2005;53(2):65–76. doi: 10.1111/j.1600-0897.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- 2.Hillier SL. The vaginal microbial ecosystem and resistance to HIV. AIDS Res Hum Retroviruses. 1998;14(Suppl 1):S17–21. [PubMed] [Google Scholar]
- 3.Quayle AJ. The innate and early immune response to pathogen challenge in the female genital tract and the pivotal role of epithelial cells. J Reprod Immunol. 2002;57(1–2):61–79. doi: 10.1016/s0165-0378(02)00019-0. [DOI] [PubMed] [Google Scholar]
- 4.Kirschning CJ, Schumann RR. TLR2: cellular sensor for microbial and endogenous molecular patterns. Curr Top Microbiol Immunol. 2002;270:121–44. doi: 10.1007/978-3-642-59430-4_8. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410(6832):1099–103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 6.Muroi M, Ohnishi T, Azumi-Mayuzumi S, Tanamoto K. Lipopolysaccharide-mimetic activities of a Toll-like receptor 2-stimulatory substance(s) in enterobacterial lipopolysaccharide preparations. Infect Immun. 2003;71(6):3221–6. doi: 10.1128/IAI.71.6.3221-3226.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith KD, Andersen-Nissen E, Hayashi F, Strobe K, Bergman MA, Barrett SL, et al. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol. 2003;4(12):1247–53. doi: 10.1038/ni1011. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11(4):443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi O, Kawai T, Sanjo H, Copeland NG, Gilbert DJ, Jenkins NA, et al. TLR6: A novel member of an expanding toll-like receptor family. Gene. 1999;231(1–2):59–65. doi: 10.1016/s0378-1119(99)00098-0. [DOI] [PubMed] [Google Scholar]
- 10.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303(5663):1529–31. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 11.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303(5663):1526–9. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 12.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 13.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3(2):196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 14.Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H, et al. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol. 2002;3(6):499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 15.Lee J, Chuang TH, Redecke V, She L, Pitha PM, Carson DA, et al. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc Natl Acad Sci U S A. 2003;100(11):6646–51. doi: 10.1073/pnas.0631696100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexopoulou L, Thomas V, Schnare M, Lobet Y, Anguita J, Schoen RT, et al. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat Med. 2002;8(8):878–84. doi: 10.1038/nm732. [DOI] [PubMed] [Google Scholar]
- 17.Kariko K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279(13):12542–50. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- 18.Chuang T, Ulevitch RJ. Identification of hTLR10: a novel human Toll-like receptor preferentially expressed in immune cells. Biochim Biophys Acta. 2001;1518(1–2):157–61. doi: 10.1016/s0167-4781(00)00289-x. [DOI] [PubMed] [Google Scholar]
- 19.Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, et al. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303(5663):1522–6. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- 20.Fichorova RN, Cronin AO, Lien E, Anderson DJ, Ingalls RR. Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of toll-like receptor 4-mediated signaling. J Immunol. 2002;168(5):2424–32. doi: 10.4049/jimmunol.168.5.2424. [DOI] [PubMed] [Google Scholar]
- 21.Fazeli A, Bruce C, Anumba DO. Characterization of Toll-like receptors in the female reproductive tract in humans. Hum Reprod. 2005;20(5):1372–8. doi: 10.1093/humrep/deh775. [DOI] [PubMed] [Google Scholar]
- 22.Kunz G, Beil D, Deininger H, Wildt L, Leyendecker G. The dynamics of rapid sperm transport through the female genital tract: evidence from vaginal sonography of uterine peristalsis and hysterosalpingoscintigraphy. Hum Reprod. 1996;11(3):627–32. doi: 10.1093/humrep/11.3.627. [DOI] [PubMed] [Google Scholar]
- 23.Schaefer TM, Fahey JV, Wright JA, Wira CR. Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist poly(I:C) J Immunol. 2005;174(2):992–1002. doi: 10.4049/jimmunol.174.2.992. [DOI] [PubMed] [Google Scholar]
- 24.Schaefer TM, Desouza K, Fahey JV, Beagley KW, Wira CR. Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology. 2004;112(3):428–36. doi: 10.1111/j.1365-2567.2004.01898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soboll G, Shen L, Wira CR. Expression of Toll-like receptors (TLR) and responsiveness to TLR agonists by polarized mouse uterine epithelial cells in culture. Biol Reprod. 2006;75(1):131–9. doi: 10.1095/biolreprod.106.050690. [DOI] [PubMed] [Google Scholar]
- 26.Birchler T, Seibl R, Buchner K, Loeliger S, Seger R, Hossle JP, et al. Human Toll-like receptor 2 mediates induction of the antimicrobial peptide human beta-defensin 2 in response to bacterial lipoprotein. Eur J Immunol. 2001;31(11):3131–7. doi: 10.1002/1521-4141(200111)31:11<3131::aid-immu3131>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 27.Fisette PL, Ram S, Andersen JM, Guo W, Ingalls RR. The Lip lipoprotein from Neisseria gonorrhoeae stimulates cytokine release and NF-kappaB activation in epithelial cells in a Toll-like receptor 2-dependent manner. J Biol Chem. 2003;278(47):46252–60. doi: 10.1074/jbc.M306587200. [DOI] [PubMed] [Google Scholar]
- 28.Akhtar M, Watson JL, Nazli A, McKay DM. Bacterial DNA evokes epithelial IL-8 production by a MAPK-dependent, NF-kappaB-independent pathway. Faseb J. 2003;17(10):1319–21. doi: 10.1096/fj.03-0950fje. [DOI] [PubMed] [Google Scholar]
- 29.Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–28. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 30.Lehrer RI, Ganz T. Defensins of vertebrate animals. Curr Opin Immunol. 2002;14(1):96–102. doi: 10.1016/s0952-7915(01)00303-x. [DOI] [PubMed] [Google Scholar]
- 31.Garcia JR, Jaumann F, Schulz S, Krause A, Rodriguez-Jimenez J, Forssmann U, et al. Identification of a novel, multifunctional beta-defensin (human beta-defensin 3) with specific antimicrobial activity. Its interaction with plasma membranes of Xenopus oocytes and the induction of macrophage chemoattraction. Cell Tissue Res. 2001;306(2):257–64. doi: 10.1007/s004410100433. [DOI] [PubMed] [Google Scholar]
- 32.Garcia JR, Krause A, Schulz S, Rodriguez-Jimenez FJ, Kluver E, Adermann K, et al. Human beta-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. Faseb J. 2001;15(10):1819–21. [PubMed] [Google Scholar]
- 33.King AE, Fleming DC, Critchley HO, Kelly RW. Regulation of natural antibiotic expression by inflammatory mediators and mimics of infection in human endometrial epithelial cells. Mol Hum Reprod. 2002;8(4):341–9. doi: 10.1093/molehr/8.4.341. [DOI] [PubMed] [Google Scholar]
- 34.Valore EV, Park CH, Quayle AJ, Wiles KR, McCray PB, Jr, Ganz T. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Invest. 1998;101(8):1633–42. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang D, Chertov O, Oppenheim JJ. Participation of mammalian defensins and cathelicidins in anti-microbial immunity: receptors and activities of human defensins and cathelicidin (LL-37) J Leukoc Biol. 2001;69(5):691–7. [PubMed] [Google Scholar]
- 36.Schutte BC, McCray PB., Jr [beta]-defensins in lung host defense. Annu Rev Physiol. 2002;64:709–48. doi: 10.1146/annurev.physiol.64.081501.134340. [DOI] [PubMed] [Google Scholar]
- 37.Ganz T. Immunology. Versatile defensins. Science. 2002;298(5595):977–9. doi: 10.1126/science.1078708. [DOI] [PubMed] [Google Scholar]
- 38.Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276(8):5707–13. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 39.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286(5439):525–8. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 40.Le Bon A, Tough DF. Links between innate and adaptive immunity via type I interferon. Curr Opin Immunol. 2002;14(4):432–6. doi: 10.1016/s0952-7915(02)00354-0. [DOI] [PubMed] [Google Scholar]
- 41.Williams BR. PKR; a sentinel kinase for cellular stress. Oncogene. 1999;18(45):6112–20. doi: 10.1038/sj.onc.1203127. [DOI] [PubMed] [Google Scholar]
- 42.Silverman RH. Fascination with 2-5A-dependent RNase: a unique enzyme that functions in interferon action. J Interferon Res. 1994;14(3):101–4. doi: 10.1089/jir.1994.14.101. [DOI] [PubMed] [Google Scholar]
- 43.Haller O, Kochs G. Interferon-induced mx proteins: dynamin-like GTPases with antiviral activity. Traffic. 2002;3(10):710–7. doi: 10.1034/j.1600-0854.2002.31003.x. [DOI] [PubMed] [Google Scholar]
- 44.Zhou A, Paranjape J, Brown TL, Nie H, Naik S, Dong B, et al. Interferon action and apoptosis are defective in mice devoid of 2′,5′-oligoadenylate-dependent RNase L. Embo J. 1997;16(21):6355–63. doi: 10.1093/emboj/16.21.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou A, Hassel BA, Silverman RH. Expression cloning of 2-5A-dependent RNAase: a uniquely regulated mediator of interferon action. Cell. 1993;72(5):753–65. doi: 10.1016/0092-8674(93)90403-d. [DOI] [PubMed] [Google Scholar]
- 46.Clemens MJ, Vaquero CM. Inhibition of protein synthesis by double-stranded RNA in reticulocyte lysates: evidence for activation of an endoribonuclease. Biochem Biophys Res Commun. 1978;83(1):59–68. doi: 10.1016/0006-291x(78)90397-2. [DOI] [PubMed] [Google Scholar]
- 47.Sledz CA, Williams BR. RNA interference and double-stranded-RNA-activated pathways. Biochem Soc Trans. 2004;32(Pt 6):952–6. doi: 10.1042/BST0320952. [DOI] [PubMed] [Google Scholar]
- 48.Burgert HG, Ruzsics Z, Obermeier S, Hilgendorf A, Windheim M, Elsing A. Subversion of host defense mechanisms by adenoviruses. Curr Top Microbiol Immunol. 2002;269:273–318. doi: 10.1007/978-3-642-59421-2_16. [DOI] [PubMed] [Google Scholar]
- 49.Leib DA. Counteraction of interferon-induced antiviral responses by herpes simplex viruses. Curr Top Microbiol Immunol. 2002;269:171–85. doi: 10.1007/978-3-642-59421-2_11. [DOI] [PubMed] [Google Scholar]
- 50.Gatignol A, Laine S, Clerzius G. Dual role of TRBP in HIV replication and RNA interference: viral diversion of a cellular pathway or evasion from antiviral immunity? Retrovirology. 2005;2:65. doi: 10.1186/1742-4690-2-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou A, Paranjape JM, Der SD, Williams BR, Silverman RH. Interferon action in triply deficient mice reveals the existence of alternative antiviral pathways. Virology. 1999;258(2):435–40. doi: 10.1006/viro.1999.9738. [DOI] [PubMed] [Google Scholar]
- 52.Fahey JV, Prabhala RH, Guyre PM, Wira CR. Antigen-presenting cells in the human female reproductive tract: analysis of antigen presentation in pre- and post-menopausal women. Am J Reprod Immunol. 1999;42(1):49–57. doi: 10.1111/j.1600-0897.1999.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 53.Fahey JV, Humphrey SL, Stern JE, Wira CR. Secretory component production by polarized epithelial cells from the human female reproductive tract. Immunol Invest. 1998;27(3):167–80. doi: 10.3109/08820139809089454. [DOI] [PubMed] [Google Scholar]
- 54.Meter RA, Wira CR, Fahey JV. Secretion of monocyte chemotactic protein-1 by human uterine epithelium directs monocyte migration in culture. Fertil Steril. 2005;84(1):191–201. doi: 10.1016/j.fertnstert.2005.01.104. [DOI] [PubMed] [Google Scholar]
- 55.Richardson JM, Wira CR. Secretory component production by rat uterine epithelial cells in culture. Adv Exp Med Biol. 1995;371A:383–6. doi: 10.1007/978-1-4615-1941-6_79. [DOI] [PubMed] [Google Scholar]
- 56.Godfrey TE, Kim SH, Chavira M, Ruff DW, Warren RS, Gray JW, et al. Quantitative mRNA expression analysis from formalin-fixed, paraffin-embedded tissues using 5′ nuclease quantitative reverse transcription-polymerase chain reaction. J Mol Diagn. 2000;2(2):84–91. doi: 10.1016/S1525-1578(10)60621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deka S, Vanover J, Sun J, Kintner J, Whittimore J, Schoborg RV. An early event in the herpes simplex virus type-2 replication cycle is sufficient to induce Chlamydia trachomatis persistence. Cell Microbiol. 2007;9(3):725–37. doi: 10.1111/j.1462-5822.2006.00823.x. [DOI] [PubMed] [Google Scholar]
- 58.Schaefer TM, Fahey JV, Wright JA, Wira CR. Migration inhibitory factor secretion by polarized uterine epithelial cells is enhanced in response to the TLR3 agonist poly (I:C) Am J Reprod Immunol. 2005;54(4):193–202. doi: 10.1111/j.1600-0897.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 59.Fahey JV, Schaefer TM, Channon JY, Wira CR. Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Hum Reprod. 2005;20(6):1439–46. doi: 10.1093/humrep/deh806. [DOI] [PubMed] [Google Scholar]
- 60.Kunz G, Herbertz M, Noe M, Leyendecker G. Sonographic evidence for the involvement of the utero-ovarian counter-current system in the ovarian control of directed uterine sperm transport. Hum Reprod Update. 1998;4(5):667–72. doi: 10.1093/humupd/4.5.667. [DOI] [PubMed] [Google Scholar]
- 61.Kunz G, Noe M, Herbertz M, Leyendecker G. Uterine peristalsis during the follicular phase of the menstrual cycle: effects of oestrogen, antioestrogen and oxytocin. Hum Reprod Update. 1998;4(5):647–54. doi: 10.1093/humupd/4.5.647. [DOI] [PubMed] [Google Scholar]
- 62.Kunz G, Beil D, Deiniger H, Einspanier A, Mall G, Leyendecker G. The uterine peristaltic pump. Normal and impeded sperm transport within the female genital tract. Adv Exp Med Biol. 1997;424:267–77. [PubMed] [Google Scholar]
- 63.Anna K, Parsons JLB, Londano L Jorge, Thomas R Moench, Richard A Cone. Method for measurement of peristaltic transport of vaginal fluids to the uterine cavity: Implications for subclinical infection and anti-microbial intervention. Am J Reprod Immunol. 2003;49(6):355–6. [Google Scholar]
- 64.Hadchouel M, Scotto J, Huret JL, Molinie C, Villa E, Degos F, et al. Presence of HBV DNA in spermatozoa: a possible vertical transmission of HBV via the germ line. J Med Virol. 1985;16(1):61–6. doi: 10.1002/jmv.1890160109. [DOI] [PubMed] [Google Scholar]
- 65.Kotronias D, Kapranos N. Detection of herpes simplex virus DNA in human spermatozoa by in situ hybridization technique. In Vivo. 1998;12(4):391–4. [PubMed] [Google Scholar]
- 66.Piomboni P, Baccetti B. Spermatozoon as a vehicle for HIV-1 and other viruses: a review. Mol Reprod Dev. 2000;56(2 Suppl):238–42. doi: 10.1002/(SICI)1098-2795(200006)56:2+<238::AID-MRD5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 67.Pioli PA, Amiel E, Schaefer TM, Connolly JE, Wira CR, Guyre PM. Differential expression of Toll-like receptors 2 and 4 in tissues of the human female reproductive tract. Infect Immun. 2004;72(10):5799–806. doi: 10.1128/IAI.72.10.5799-5806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duits LA, Nibbering PH, van Strijen E, Vos JB, Mannesse-Lazeroms SP, van Sterkenburg MA, et al. Rhinovirus increases human beta-defensin-2 and -3 mRNA expression in cultured bronchial epithelial cells. FEMS Immunol Med Microbiol. 2003;38(1):59–64. doi: 10.1016/S0928-8244(03)00106-8. [DOI] [PubMed] [Google Scholar]
- 69.Ganz T. Defensins and host defense. Science. 1999;286(5439):420–1. doi: 10.1126/science.286.5439.420. [DOI] [PubMed] [Google Scholar]
- 70.Gropp R, Frye M, Wagner TO, Bargon J. Epithelial defensins impair adenoviral infection: implication for adenovirus-mediated gene therapy. Hum Gene Ther. 1999;10(6):957–64. doi: 10.1089/10430349950018355. [DOI] [PubMed] [Google Scholar]
- 71.Quinones-Mateu ME, Lederman MM, Feng Z, Chakraborty B, Weber J, Rangel HR, et al. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. Aids. 2003;17(16):F39–48. doi: 10.1097/00002030-200311070-00001. [DOI] [PubMed] [Google Scholar]
- 72.Wehkamp K, Schwichtenberg L, Schroder JM, Harder J. Pseudomonas aeruginosa- and IL-1beta-mediated induction of human beta-defensin-2 in keratinocytes is controlled by NF-kappaB and AP-1. J Invest Dermatol. 2006;126(1):121–7. doi: 10.1038/sj.jid.5700020. [DOI] [PubMed] [Google Scholar]
- 73.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14(4):778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tanabe M, Kurita-Taniguchi M, Takeuchi K, Takeda M, Ayata M, Ogura H, et al. Mechanism of up-regulation of human Toll-like receptor 3 secondary to infection of measles virus-attenuated strains. Biochem Biophys Res Commun. 2003;311(1):39–48. doi: 10.1016/j.bbrc.2003.09.159. [DOI] [PubMed] [Google Scholar]
- 75.Ronni T, Matikainen S, Sareneva T, Melen K, Pirhonen J, Keskinen P, et al. Regulation of IFN-alpha/beta, MxA, 2′,5′-oligoadenylate synthetase, and HLA gene expression in influenza A-infected human lung epithelial cells. J Immunol. 1997;158(5):2363–74. [PubMed] [Google Scholar]
- 76.Dalod M, Hamilton T, Salomon R, Salazar-Mather TP, Henry SC, Hamilton JD, et al. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon alpha/beta. J Exp Med. 2003;197(7):885–98. doi: 10.1084/jem.20021522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Biron CA, Brossay L. NK cells and NKT cells in innate defense against viral infections. Curr Opin Immunol. 2001;13(4):458–64. doi: 10.1016/s0952-7915(00)00241-7. [DOI] [PubMed] [Google Scholar]
- 78.Bogdan C, Rollinghoff M, Diefenbach A. The role of nitric oxide in innate immunity. Immunol Rev. 2000;173:17–26. doi: 10.1034/j.1600-065x.2000.917307.x. [DOI] [PubMed] [Google Scholar]
- 79.Santini SM, Lapenta C, Logozzi M, Parlato S, Spada M, Di Pucchio T, et al. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191(10):1777–88. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ueta M, Nochi T, Jang MH, Park EJ, Igarashi O, Hino A, et al. Intracellularly expressed TLR2s and TLR4s contribution to an immunosilent environment at the ocular mucosal epithelium. J Immunol. 2004;173(5):3337–47. doi: 10.4049/jimmunol.173.5.3337. [DOI] [PubMed] [Google Scholar]
- 81.Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, Arditi M. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol. 2001;167(3):1609–16. doi: 10.4049/jimmunol.167.3.1609. [DOI] [PubMed] [Google Scholar]
- 82.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. Aids. 2006;20(1):73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 83.Itoh H, Nasu K, Nishida M, Matsumoto H, Yuge A, Narahara H. Human oviductal stromal fibroblasts, but not oviductal epithelial cells, express Toll-like receptor 4: the site-specific mucosal immunity of the human fallopian tube against bacterial infection. Am J Reprod Immunol. 2006;56(2):91–101. doi: 10.1111/j.1600-0897.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- 84.Soboll G, Schaefer TM, Wira CR. Effect of toll-like receptor (TLR) agonists on TLR and microbicide expression in uterine and vaginal tissues of the mouse. Am J Reprod Immunol. 2006;55(6):434–46. doi: 10.1111/j.1600-0897.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- 85.Nasu K, Itoh H, Yuge A, Nishida M, Narahara H. Human oviductal epithelial cells express Toll-like receptor 3 and respond to double-stranded RNA: Fallopian tube-specific mucosal immunity against viral infection. Hum Reprod. 2007;22(2):356–61. doi: 10.1093/humrep/del385. [DOI] [PubMed] [Google Scholar]