Abstract
Recent advances in our understanding of the mechanisms in the cascade of events resulting in retinal cell death in ocular pathologies like glaucoma, diabetic retinopathy and age-related macular degeneration led to the common descriptive term of neurodegenerative diseases of the retina. The final common pathophysiologic pathway of these diseases includes a particular form of metabolic stress, resulting in an insufficient supply of nutrients to the respective target structures (optic nerve head, retina). During metabolic stress, glutamate is released initiating the death of neurones containing ionotropic glutamate (N-methyl-D-aspartat, NMDA) receptors present on ganglion cells and a specific type of amacrine cells. Experimental studies demonstrate that several drugs reduce or prevent the death of retinal neurones deficient of nutrients. These agents generally block NMDA receptors to prevent the action of glutamate or halt the subsequent pathophysiologic cycle resulting in cell death. The major causes for cell death following activation of NMDA receptors are the influx of calcium and sodium into cells, the generation of free radicals linked to the formation of advanced glycation endproducts (AGEs) and/or advanced lipoxidation endproducts (ALEs) as well as defects in the mitochondrial respiratory chain. Substances preventing these cytotoxic events are considered to be potentially neuroprotective.
Key Words: Neurodegeneration, neuroprotection, retina, glaucoma, diabetic retinopathy, age-related macular degeneration, retinal ganglion cells.
INTRODUCTION
Neuroprotection is a topic of a growing number of studies [8, 20, 23, 36, 44, 69, 96, 102, 113, 125 -128, 130 -132, 152, 158, 181] as several ocular pathologies (e.g. special forms of glaucoma [126-128], diabetic retinopathy [75, 120] and age-related macular degeneration (AMD) [107, 127] result in neurodegeneration especially of the retinal ganglion cells (RGCs). Consequently, it seems a promising goal to rescue e.g. RGCs or the retinal pigment epithelium (RPE). Indeed, a functional injury of the RGCs (which, in this respect a very vulnerable cell population) preceeds the onset of a structural damage [39, 126, 127, 130]. Therefore, a recovery would possibly restore their function. However, till now only few substances have been demonstrated to have a neuroprotective capacity and which can be pinpointed to a defined causal mechanism. In addition, such substances should be safe and easily applicable to the eye.
In this review we will concentrate on critical sites of retinal neurodegenerative diseases and possible ways of protection and ways leading to recovery. Critical sites are the RGCs (involved in the progress of glaucoma) the retinal microvessels (additionally damaged in diabetic retinopathy) and the RPE (together with vascular and RGC damages in AMD).
RGCs have a very high metabolic rate - this becomes obvious when considering that the length of an RGC axon would measure about half a mile if the cell body would have the size of an apple. These long axons increase RGC vulnerability to various disorders: during the course of their axons they are likely to encounter metabolic stress like hypoxia, exposure to increased free radicals, mechanical compression (e.g. in the lamina cribrosa - LC) and specifically photooxidative damage by (mainly blue) light passing through the retina and thus potentially damaging the retina and the retinal pigmented epithelium [171].
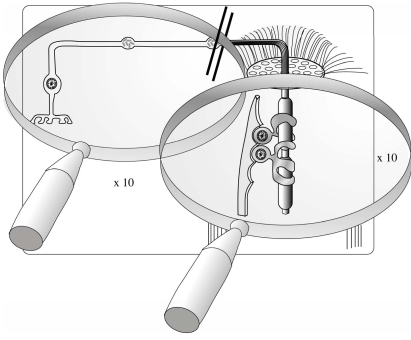
RGCs need to compensate for another unique functional situation when compared to other neurons: their axons are non-myelinated from the retina to the LC and are myelinated after passing this structure (Fig. 1). Normally, large myelinated neurons are in the opposite situation: they lose their myelin sheets only at their very peripheral end. This special RGC feature leads to an “impedance mismatch” [192] which requires a lot of energy. Therefore, RGCs have plenty of protrusions in their axons which are filled with numerous mitochondria [192]. Furthermore, the distribution of the mitochondria (high numbers in retina, in the peripapillar and papillar zone down to the LC and much reduced in the myelinated nerve) reflects the functional requirements of different RGC axon regions [13].
Fig. (1).
Schematic drawing of a retinal ganglion cell (RGC) axon being non-myelinated from the retina to the lamina cribrosa (LC) and being myelinated after passing this structure (Fig. 1).
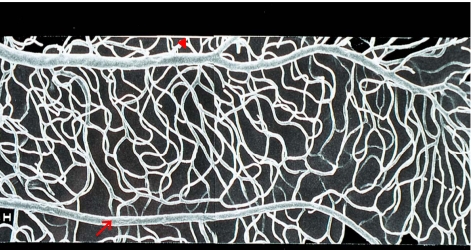
Although RGCs have a high energy demand, the retinal microvasculature cannot be formed as dense as in the brain because the inner retinal layer is required to remain transparent to enable its biological function and let photons pass to the photoreceptors in the outer retinal layer. Thus, nature made a compromise between the metabolic demand of the neurons and a sufficient vascular supply [41]. Indeed, compared to capillaries in other organs, the retinal capillaries are very thin, have a high blood flow velocity, and a relative sparse network [41]. A detailed analysis of the vessel architecture reveals that on the arteriolar side of capillary network (where more oxygen is available) the mesh is wider than on the venular side (Figs. 2a, b). Thus, the minimum of oxygen supply for neurons and glial cells appears to determine the vessel density in the retina.
Fig. (2a).
Retinal microvessels (scanning electron micrograph of a vascular resin cast) showing the capillary meshwork between arteriole (arrow) and venule (arrowhead), being tighter on the venular side and wider on the arteriolar side reflecting different oxygen tension in these vessels and thus altered supply.
Fig. (2b).
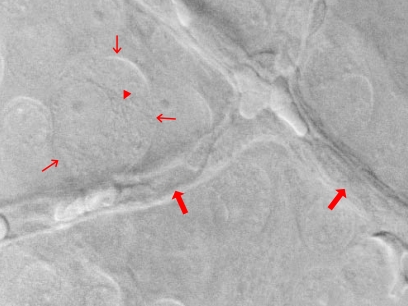
Differential interference contrast microscopy (DIC) of an unstained retina whole mount of a freshly enucleated rat eye. RGCs (thin arrows), even mitochondria are visible: (arrowhead) and capillaries (thick arrows) can be kept well alive up to 9 hours (see 143, 145 and 164).
As other big ganglion cells like the cells in the substantia nigra (which die during the development of M. Parkinson) the RGCs are relatively more prone to accumulation of metabolic end (“waste”) products which cannot be removed from the cell. In phases of increased IOP as in phases of oligemia and hypoxia followed by reperfusion, the production of free radicals and reactive oxygen species (ROS) dramatically increases [113].
As effective antioxidant capacities are generally low in most neurons and nucleic acid repair mechanisms are insufficient (especially in mitochondria) these stressors would induce RGC death. To compensate for these stressors RGCs have a high antioxidant capacity (due to endogenous peroxidases) when compared to other neurons – [85], but RGCs are still more vulnerable than e.g. Müller or vascular cells.
The same is true for the RPE which has the burden of steadily ingesting the damaged (e.g. via radicals by high energetic-, short wavelength light- and chemical – metabolic- “attack”) membrane disks of the photoreceptors (see below).
FACTORS COMPROMISING RETINAL CELL FUNCTION
Advanced Glycation Endproducts (AGEs)
Another chemical “attack” -linked with the production of radicals- is the formation of advanced glycation endproducts (AGEs) and/or advanced lipoxidation endproducts (ALEs). The accumulation of AGEs during the Maillard reaction is associated with the risk of diabetic neuropathy, diabetic retinopathy (the RGCs being the most vulnerable cell population, 44], AMD [77, 172 - 175] and M. Alzheimer [1]. Extracellular effects of the AGEs, like crosslinking of proteins, are known since the 80ties, whereas the effects of AGEs on the cellular function are still under investigation.
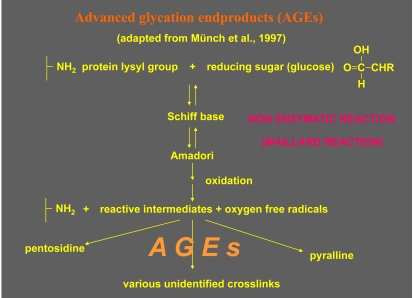
AGEs/ALEs can form on the amino groups of proteins, lipids and DNA through a number of complex pathways including non-enzymatic glycation by glucose and reaction with metabolic intermediates and reactive dicarbonyl intermediates (Fig. 3). These reactions not only modify the structure and function of proteins, but also cause intra-molecular and intermolecular cross-link formation. AGEs/ALEs are known to accumulate in the diabetic retina where they may have important effects on retinal vascular cell function, as determined by a growing number of in vitro and in vivo studies.
Fig. (3).
Schematic drawing indicating the reactions leading to advanced glycation endproducts (AGEs), cross-linking of protein-(lysil-residue) and reducing sugar groups (Maillard reaction). AGEs impair intracellular functional proteins and crosslink extracellular material. The time frame of the reversibility of these reactions depends on the toxicity of respective intermediary metabolic products (e.g. glyoxal).
Since AGEs are constantly forming under physiological conditions, complex receptor systems have evolved to remove senescent, glycation modified molecules and/or degrade existing AGE crosslinksfrom tissues thereby limiting their deleterious effects. Suchreceptors play a critical part in AGE related biology and thepathology associated with diabetes and ageing. Several AGE binding molecules have been described and it has been established that many of the adverse effects caused by advanced glycationare mediated via AGE receptors such as RAGE [151] the AGE receptor complex (AGE-RC) [101, 175] and the type I and II scavenger receptor [63, 174]. Some or all AGE receptors serve to promote or limit AGE mediated cell and tissue dysfunction. AGE receptor binding can initiate important signalling pathways involving activation of protein kinase C [108, 165], tyrosine phosphorylation of Janus kinase (JAK)/signal transducers and activators of transcription (STAT) [66], recruitment of phosphotidylinositol 3' kinase to Ras, [30] and induction of oxidative stress cascades which lead toNFκB and AP-1 transcription [112, 167].
The chronic cellular activation is induced by the AGE receptor (RAGE) [9]. Sustained RAGE-mediated cellular activation has been shown to contribute to disease progression in diabetes, Alzheimer’s disease, rheumatoid arthritis, elastosis, pulmonary fibrosis, and various cancers [18, 82, 135, 180, 188] AGE and other RAGE ligands activate p21ras, MAP ERK1/2 kinases, and NFB nuclear translocation, altering expression of genes involved with cellular stress [172, 188].
AGEs in Retinal Neurodegeneration
Like in other vascular beds AGEs and/or late Amadori products have been localised to retinal vessels and neuroglia of diabetics [15, 54, 117, 150, 175]. In diabetic rats, AGEs are not only localised to vascular basement membranes (BMs), but also appear to accumulate in the retinal pericytes after 8 months of diabetes [174]. Moreover, when non-diabetic animals are infused with preformed AGE albumin, these adducts accumulate around and within the pericytes, co-localise with AGE receptors, induce BM thickening, and cause breakdown of the inner blood-retinal barrier [25, 173, 174]. In clinical studies it has been reported that the levels of serum AGEs, and also the glycoxidation product CML, correlate with the degree of diabetic retinopathy [22, 123]. In hyperglycemic mice, AGEs lead to early inner retinal neuronal dysfunction. Here, AGEs were also localized to the vitreous cavity and internal limiting membrane (ILM) of the retina, where they were intimately associated with the footplates of RAGE-expressing Muller cells. Furthermore, AGE accumulation was increased within the retinal extracellular matrix and attenuation of the RAGE axis with soluble RAGE ameliorated neuronal dysfunction and reduced the development of capillary lesions in these mice [7].
AGE deposits were found in AMD retinas. Furthermore, AGE stimulated RAGE-mediated activation of cultured RPE cells in a dose-dependent manner. Thus, AGE accumulation may induce receptor-mediated activation of RPE/photoreceptor cells, contributing to disease progression in the aging human retinas.
AGEs have been reported to accumulate in aging eyes in Bruch’s membrane, drusen, subfoveal neovascular membranes, and RPE cells [65].
RPE cells are radically influenced by exposure to AGEs in vitro where they express abnormal levels of vascular endothelialgrowth factor (VEGF) and platelet derived growth factor B (PDGF-B) [56, 98]. This may have a bearing on RPE cell function, maintenance of the choriocapillaris, and integrity of the RPE/photoreceptor complex.The accumulation of lipofuscin and reduction of lysosomal degradative capacity in RPE cells may reflect AGE formation and receptor mediated transport of these adducts to the lysosomal compartment. Significantly, intracellular sequestration of these highly reactive adducts can markedly reduce lysosomal enzymatic activity in other epithelial cell types and lead to lipofuscin in RPE cells [11, 175].
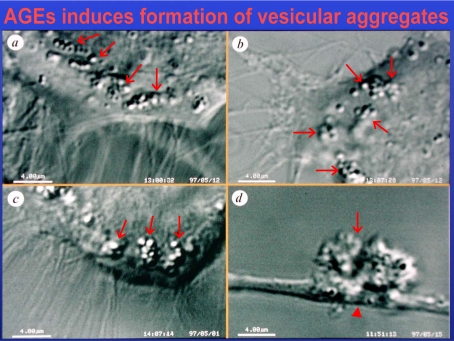
Also a significant reduction of the velocity of intracellular microvesicles was induced by AGEs, the reason for this is unclear [146]. It could be due to disturbed calcium metabolism or caused by microtubuli-changes. The aggregation of intracellular microvesicles could be the result of an altered binding-behaviour of the vesicles or the destruction of the transport apparatus. The proteins dynamin 2 and clathrin, which are involved in transport apparatus, show changes in intracellular distribution indicating a breakdown of the normal cellular distribution system [146].
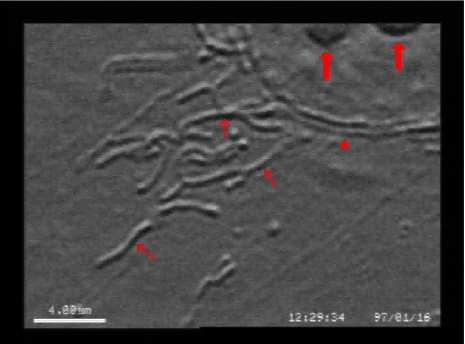
In AGE-loaded RGCs, “breaks” in the tracks of axonal vesicle transport occur which subsequently lead to protrusions of the axons and to accumulation of the transported material. Possibly, AGEs may affect the axonal transport either directly by cross-linking the proteins or by enhanced production of ROS (Fig. 4).
Fig. (4).
Differential interference contrast microscopy (DIC) of living astrocytes treated with glyoxal, a reactive intermediate of AGE-production. This leads to accumulation of isolated and aggregated intracellular vesicles (arrows, a-c) in the axons of a neuron and to blockade of the axonal transport (arrowheads, c,d).
On the other hand all other systemic metabolic problems of the organism like diabetes mellitus, hyperlipidemia, hypercholesterolemia, hyperuricemia, high systemic blood pressure and periods of very low perfusion pressure due to vasospasm also affect the microenvironment of the RGCs and RPE – from the capillaries via the extracellular space via the glial cells down to the intracellular “milieu” of these neurons. Furthermore, the above mentioned metabolic stressors often combine (“metabolic syndrome”) and can damage the RGC microenvironment via thickening of the basement membranes, e.g. due to AGEs/ALEs. In this respect it is of considerable interest that some types of M. Alzheimer’s disease (AD) are very similar to dementia caused by arteriosclerosis [186]. Here, it is also unclear why many diabetic damages leave the glia cells of the brain unaffected (despite AGE and lipid peroxide formation) for a long time whereas in certain forms of arteriosclerosis the glial cells become “aggressive” and attack the nerve cells and axons with ROS (e.g. as in “white matter lesions” of the brain).
A study in a mouse model showed that the combination of deleterious factors such as apolipoprotein (APOE) allelic expression, advanced age and diet contribute to atrophic degenerative changes in RGCs and the optic nerve [191]. Another study, also in an animal model demonstrated that the cleavage product of the amyloid precursor protein (APP) (major indicator of AD) is enhanced in ocular hypertension [47] and axonal damage. High IOP, induced hypoxia, lipid peroxides and AGEs per se lead to increased production of free radical species which again initiate periods of cell damage and attempts to regenerate parts of the cell, especially the RGC - axon. Here, the pathogenetic processes in the RGCs are analogue to those in brain neurons during AD:
The repeated stimuli for regeneration of RGCs and neurons in general are characterized by the production of an enormous amount of newly synthesized proteins (like APP) needed for the realignment and restoration of the axon. Therefore, the cell tries to transport all proteins needed to the (peripheral) damaged area (e.g. sAPP which is required during migration and proliferation – 138; and APP as membrane protein which binds kinesin to vesicles which are transported with the fast axonal transport, 74, 166).
On the other hand, APP plays an important physiological role in protecting neurons from the consequences of prolonged endoplasmic reticulum stress which is found particularly in AD [83]. Furthermore, the microtubule associated protein tau inhibits kinesin-dependent transport of peroxisomes, neurofilaments, and Golgi-derived vesicles into neurites (axons). Loss of peroxisomes on the other hand, makes cells vulnerable to oxidative stress and this leads to degeneration. Again, tau inhibits the transport of APP into axons and dendrites, causing its accumulation inthe cell and thus initiates apoptosis - also mediated by caspases 8 and 3 [106, 187]. These factors facilitate degenerative processes as mentioned above.
RADICALS IN OXIDATIVE STRESS AND RPE DYSFUNCTION
Although oxidative stress and RPE dysfunction are generally believed to promote disease progression in AMD, the underlying mechanisms governing these events are poorly understood. The inherently high arterial O2 tension environment, production of radicals in phototransduction, blue light damage, accumulation of photooxidative lipofuscin containing A2E in the RPE, and loss of cellular antioxidant capabilities collectively contribute to oxidative stress in the aging eye [10, 103, 171, 177, 195]. Correspondingly, RPE and choroidal cells alter the expression of genes for cytokines, matrix organization, cell adhesion, and apoptosis [2, 34, 48, 49, 53, 61, 116, 183]. Chronic cellular activation perturbs normal structural and physiological integrity and may induce focal inflammatory responses at the RPE–Bruch’s membrane border [65].
Furthermore, the formation of AGE, such as CML and pentosidine, is accelerated in regions of oxidative stress.
Recent papers also report ROS attack of neurons and a loss of antioxidant capacity within the neurons in the course of some forms of glaucoma via aggressive glial cells [122]. In addition, ROS, induced e.g. by hypoxia are further increased in the presence of AGEs [112].
CELL STRESS AND APOPTOSIS
For early detection of cell stress preceding apoptosis our own group monitored the course of the alteration of pHi and mitochondrial membrane potential in a retinal ganglion cell line [80]. Changes in pH are early events in the progression of cell stress long before the way to apoptosis is irreversible. The efficiency of activation of caspase by cytochrome c has been found to be pH sensitive, with a pH optimum of 6.3-6.8 in vitro [70]. Alterations in cytosolic pHi may be caused by changes in mitochondria (Fig. 5), such as the deleterious opening of the mitochondrial permeability transition pore. Mitochondrial permeability transition is a non-selective increase in the permeability of the inner membrane (presumably involving a multi-protein complex known as the permeability transition pore whose opening commonly occurs during apoptosis) and results in depolarization of mitochondria and loss of the H+ gradient normally present across the inner membrane (Fig. 6). Non-selective entry of ions and water into the solute-rich matrix then leads to an increase in the volume of the mitochondrium [42]. Caspase activation can be a result of mitochondrial-matrix alkalinization and cytosolic acidification [105]. Cytochrome c, normally stored between the inner and outer membranes of mitochondria, is commonly released into the cytosol following exposure of cells to apoptotic stimuli. Once it is in the cytosol, cytochrome c binds to the caspase-activating protein Apaf-1, inducing formation of an oligomeric complex that recruits and proteolytically activates procaspase-9, an activated caspase-9 that then cleaves and activates caspases further downstream, ultimately inducing apoptosis [92].
Fig. (5).
Differential interference contrast microscopy (DIC) of a cultured astrocyte, the mitochondria are clearly outlined (thin arrows). Arrowhead: membrane of the nucleus, thick arrows: nucleoli.
Fig. (6).
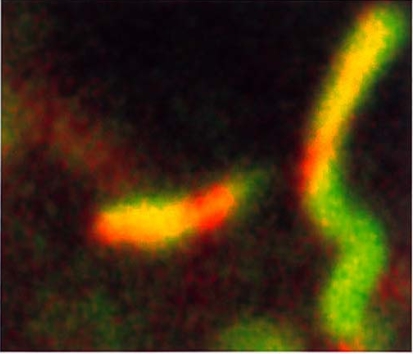
Cross section of single mitochondria (width: 0.5 µm) in an astrocyte: the function of the mitochondrial membrane is clearly indicated by the colour of a dye (JC1) indicating the degree of the membrane potential: red still intact; green compromised.
Mittag et al. 2000 [111] showed that the mitochondrial membrane potential is a good marker for apoptosis of retinal cells in vivo after intravitreal injection of an indicator dye. In the retinal ganglion cell layer of eyes with elevated pressure, mitochondrial membrane potential was reduced by 17.5%. After 3.5 months of elevated IOP the retinas showed cell nuclei at various stages of apoptosis, from the initial DNA condensation to fragmentation.
If glutamate (the major excitatory transmitter in the retina) accumulates as a further consequence of cell stress, uptake of cystine is inhibited which is essential for glutathione (GSH, the most important intracellular antioxidant) biosynthesis, resulting in a depletion of GSH from the cells. This, again, causes an increase of ROS levels [100] which leads to a Ca2+ influx which is mediated by a cobalt sensitive, cyclic guanosine monophosphate (cGMP) – gated Ca2+ channel [92].
All the processes described above are able to reduce cell functions and can lead to apoptosis. In this respect a special form of prolonged apoptosis has recently been found in neurons of the brain. In the RGCs this prolonged apoptosis can last for many years [206].
Summary of the Pathogenetic Processes
For the mentioned neurodegenerative diseases (special forms of glaucoma, diabetic retinopathy and AMD) many findings converge to an age – and metabolic disorder - dependent damage of cellular energy - (mitochondria) and transport processes (e.g. endo – lysosomal pathway and axonal transport). These damages are mostly caused by ROS and all other processes which are linked to radical production: AGEs, ALEs and oxysteroles. A changed metabolic situation can also lead to aggressive glial cells which by themselves produce radicals which vice versa crosslink proteins and lipids to AGEs and ALEs.
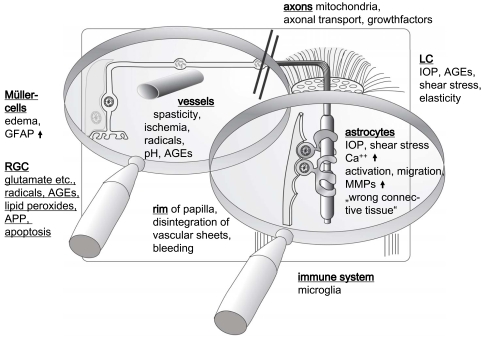
The other hot spot in the pathomechanisms of neuodegenerative diseases (like also AD and PD) is the mitochondrium. Defects in the respiratory chain (like in LHON) are similar in age – and metabolic disorders (electrons deviate from the respiratory chain and represent additional radicals). In addition, other factors like AGEs in the endfeet of Müller cells accumulate and damage the mitochondria which are not only in the cell bodies but also in protrusions of the RGC nerve fibers (Fig. 7) [192].
Fig. (7).
Schematic drawing and localization of the factors leading to neurodegenerative diseases in the retina (GFAP = glial fibrillary protein, APP = amyloid precursor protein, MMPs = matrix metallo-proteinases). Please see text for details.
Therapeutic Strategies
Such strategies should focus on the above mentioned "hot spots" and restore or provide the following features by amelioration of the following parameters:
microcirculation
route from the capillary to the cell and back
metabolic situation within the cell
balance of oxidative and antioxidative parameters
Reduction of AGEs/ALEs and ROS
Prevention or amelioration of AGE mediated cell toxicity has been a key strategy in the prevention of diabetic complicationsand some age related pathology. To date there have been a range of approaches which seek to either prevent AGE formation, reduceAGE effects on cells, or even break established AGE crosslinks.
Amadori product formation is the basis of advanced glycation biochemistry because progression to protein crosslinks requires slow chemical rearrangement to create reactive intermediates before the formation of irreversible AGEs. An important pharmacological strategy for the inhibition of this process utilises the small nucleophilic hydrazine compound aminoguanidine, which is a potent inhibitor of AGE mediated crosslinking [15]. This drug can prevent some diabetic vascular complications in experimental animals, [24, 55, 133] while clinical trials of aminoguanidine were shown to effectively reduce AGE-Hb while leaving HbA1c unaffected [17]. Such optimism has been tempered by the gradual realisation that aminoguanidine also inhibits a range of other important pathways, most notably generation of nitric oxide by eNOS, [72] which may increase non-specific and unwanted side effects of the drug. Other AGE inhibiting drugs have been recently developed, such as the thiazolidine derivative OPB-9195, [120] pyridoxamine, [124] and 2,3 diaminophenazine (2,3 DAP) [170].
Prevention of AGEs interacting with their receptors or other body proteins is a valid therapeutic approach. The use of neutralising antibodies against glycated albumin has been shown to prevent BM thickening in diabetic (db/db) mice despite the fact that the antibodies did not alter the glycaemic status of the animals [25]. Likewise, the use of the AGE binding properties of lysozyme has succeeded in reducing AGE levels in dialysate from diabetic patients with kidney disease [110] and presents a real possibility for reduction of toxic AGE groups in the body fluids of patients with renal failure. Furthermore, elucidation of AGE receptor signal transduction pathways may also offer intracellular strategies to control receptor mediated sequelae.
In the experimental animal, AGE effects could be significantly reversed by the pharmacological AGE inhibitor aminoguanidine [189].
Recently, a novel therapeutic strategy has been to attack the AGE crosslinks formed in biological systems. This is an exciting approach since it would "break" pre-accumulated AGEs and subsequentlyallow clearance via the kidney. Such an AGE crosslink "breaker" prototype has been described to attack dicarbonyl derived crosslinks in vitro [185]. There are now at least two such chemical agents which have the ability to reduce the tissue content of AGEs inexperimental diabetes, [26, 199] reverse hyperglycaemia related arterial distensibility, [120] and ameliorate age related myocardial stiffness [6].
REDUCTION OF APOPTOSIS BY STABILIZING THE INTRACELLULAR PH VIA CARBONIC ANHYDRASE BLOCKERS
While testing the vasodilating capacity of carbonic anhydrase blockers in post vivo whole mounts of the rat retina (the cells were held viable in a special observation chamber [80, 164], the group of Funk [80] found a surprising phenomenon: in the extracellular space (adjoining to the pericytes or smooth muscle cells) a decrease of the pH took place (leading also to vasodilation). However, the intracellular pH (pHi) of the neurons in the retinal whole mounts remained at higher levels in the carbonic anhydrase blocker treated cells compared to the untreated retina cells. This observation was the starting point to the idea that holding the pHi at normal levels would have an anti – apoptotic effect. Due to these results carbonic anhydrase blockers should then have anti – apoptotic properties.: it is known that many cell types (including neurons possess the enzyme carbonic anhydrase and on the other hand, apoptosis is often associated with decreased cytosolic pH. In neurons ischemia or oxidative stress leads to decreased pHi and this renders the cell susceptible to further damage [201, 203].
Reduced pHi promotes apoptosis by favouring caspase activation (pH optimum for caspase-3 is 6.6 – 6.8, 39] and activation of DNase II, [62] but there is a controversial discussion whether low pH leads to enhanced production of free radicals (reactive oxygen species, ROS) [164] or vice versa [107]. There are also studies where acidification of cytosol was inhibited without altering the apoptotic response [44, 126]. Taken these facts together, we conclude that decreased cytosolic pH is permissive of apoptosis but until now it is uncertain whether it plays a role in signalling cell death.
In retinal neurons, advanced glycation endproducts (AGEs) [1, 145] as well as hydrogen peroxide [137] lead to acidification of the cytoplasm, to elevated ROS production and finally to apoptotic cell death [68].
Carbonic Anhydrase Blocker Dorzolamide
We studied E1A-NR3 cells which were incubated with varying concentrations of glyoxal, methylglyoxal and H2O2 for different periods of time. To a fraction of the assays dorzolamide was added. Apoptotic changes were determined by measuring cell fluorescence with a cytofluorimeter after incubating the cells with appropriate dyes and antibodies. The following parameters were studied: DNA strand breaks (TUNEL assay), subdiploid DNA content (sub-G1 assay), binding of annexinV, production of reactive oxygen intermediates (ROS), active caspase-3, the glycation product Nє - (carboxymethyl)lysine (CML) and intracellular pH. Dorzolamide proved to reduce the damage, which was inflicted on retinal ganglion cells by agents that induce apoptosis and therefore this carbonic anhydrase blocker can be considered a neuroprotectant as this effect was independent of its IOP-lowering and its positive effect on ocular perfusion [163].
As mentioned above, defects in mitochondrial energy metabolism due to respiratory chain disorders lead to a decrease in mitochondrial membrane potential and induce apoptosis. Since coenzyme Q_{10} (CoQ_{10}) plays a dual role as an antioxidant and bioenergetic agent in the respiratory chain, it has attracted increasing attention concerning the prevention of apoptosis in mitochondrial diseases. In cell studies with rotenone as stressor, pre-treatment with CoQ_{10} (10 or 100 wM) for 48h led to a significant reduction of rotenone-induced loss of mitochondrial membrane potential [109]. These results suggest, that cytoprotection by CoQ_{10} may be mediated by raising cellular resistance against the initiating steps of apoptosis [109].
NEUROPROTECTION IN PRIMARY OPEN ANGLE GLAUCOMA
Studies from POAG patients with IOPs in the normal range (normal tension POAG) demonstrated localized insufficiency in the ocular vasculature [14, 38, 40, 41, 43, 52, 59, 79, 153-157, 160-163]. Vascular risk factors (e.g. vasospasm) and absolutely (high tension POAG) and relatively (normal tension POAG) elevated IOP seem to be connected [38]. An insufficient regulation of blood flow to the optic nerve head in response to IOP compressing the microvasculature appears to increase the vulnerability of RGCs and glial cells and thus increase the risk for POAG while a sufficiently (auto)regulated optic nerve head blood flow may compensate for an increase in IOP and prevent oligemic RGC damage as e.g. in ocular hypertensive patients [156, 160, 163].
Some degree of vascular insufficiency may be tolerated by RGCs but if metabolic stress is added e.g. by increased IOP or reduced systemic perfusion (reduced blood pressure, blood loss), glaucomatous pathology may result (as in high tension POAG), whereas insufficient vascular (auto)regulation may be pathologic even with a “normal” IOP (as in normal tension POAG).
Failure to autoregulate against stressors (e.g. reduced blood pressure, increased IOP) in the vessels supplying the optic nerve head (e.g. in the perioptic short posterior ciliary arteries) or the optic nerve head microvasculature itself (compression theory) could lead to hypoperfusion and focal oligemia initiating autoregulatory mechanisms attempting to restore metabolic homeostasis, a mechanism which could result in repeated transient episodes of oligemia and - over an extended period of time - cause the gradual focal RGC loss of and thinning of the nerve fiber layer seen in POAG patients.
The evidence for a vascular pathology in POAG [14, 38, 40, 41, 43, 52, 59, 79, 153 - 157, 160-163] led to the use of ischemia/reperfusion models to investigate drugs for neuroprotective properties [23, 112, 125, 127, 130-132, 152, 158, 198].
Ischemia/reperfusion results in an energy deficit reflected in a lack of adenosine triphosphate (ATP) in mitochondria, required to satisfy the high energy demand for nerve conduction in unmyelinated neurons [99].
This reduced bioenergetic state is further compromised by an increase in glutamate, the major neurotransmitter for RGCs, to levels toxic to these neurons [23, 112, 125-132, 152, 158, 198]. The prolonged activation of glutamate receptor–coupled ion channels causes prolonged depolarization of the cell via Na+ influx and K+ efflux through the open glutamate receptor-coupled channels, as well as excessive Ca2+ influx into the cell. Among the glutamate receptors, the NMDA and kainate subtypes have increased glutamate affinity, the highest Na+ and Ca2+ conductance, and prolonged open channel times. Each NMDA/kainate receptor has four to five subunits and multiple sites for binding of further ligands. Among these are accessory binding sites for glycine or d-serine acting as a coagonist, a modulatory site binding polyamines and binding sites within the channel for drugs such as MK801 or memantine. Besides these ligand-binding sites, the receptor/channel activity is controlled by cations (Mg2+, Zn2+), by the redox state or nitrosylation of sulfhydryl groups in proteins composing the channel and by phosphorylation/dephosphorylation sites regulated by protein kinase/ phosphoprotein phosphatase enzymes [112].
Progressively accumulating evidence suggests that RGC damage in POAG occurs primarily at the lamina cribrosa reflected in structural alterations due relatively or absolutely elevated IOP which seems to reduce axonal flow and compress the optic nerve head microvessels, reducing a potentially compromised perfusion in this area even further and thus leading to oligemia, a process reducing cellular homeostasis of unmyelinated nerve fibers directly or by causing stress to astrocytes and/or oligodendrocytes resulting in liberation of toxic mediators, e.g. nitric oxide [122].
As a consequence the axonal flow from and to the RGCs and their target neurons in the brain is compromised. A reduction in axoplasmic flow of neurotrophins to RGC somata appears to be a critical step in initiating the cascade of events resulting in apoptosis, the primary cell death mechanism in POAG.
The ischemia-reperfusion, optic nerve cut or crush models are based on these hypotheses, potential target sites for neuroprotective agents acting at the level of the lamina cribrosa could be RGC axons or glial cells (astrocytes, oligodendrocytes).
Based on the etiologic concepts for POAG discussed before, any therapy for POAG protecting RGCs from death, preventing or delaying this process and drugs which save already compromised neurons or which induce regrowth of axonal/dendritic connections and restore function may be termed neuroprotective.
To avoid confusion when discussing neuroprotection in POAG it may be worthwhile to differentiate indirect neuroprotection (reducing risk factors, e.g. reducing IOP, increasing perfusion) from direct neuroprotection, a direct interaction with retinal structures preventing RGC damage.
A direct neuroprotectant would need to reach the retina to exert its pharmacologic effect which can only be proven if this drug is applied directly to the retina, an approach which is currently limited to cell culture and animal experiments (e.g. injection into the vitreous). A neuroprotective effect of a drug applied locally or systemically may be indirect due to an effect on e.g. IOP or perfusion.
A direct neuroprotective substance may reduce upregulated stimulation of ionotrophic receptors e.g. by glutamate, aspartate, NMDA, kainic or domoic acids (excitotoxicity), reduce energy deficieny (lack of ATP), maintain cell membrane ionic balance or axonal function or stop one or several of these cytotoxic processes which reinforce each other [23, 112, 125-152, 152, 158, 198].
Neuroprotective concepts for POAG aim at protecting RGCs from the initiating, extracellular cell death signals, reduce increased Na+ and Ca2+ influx into the cell directly or to block specific NMDA receptors, and on agents which block the intracellular signal cascade for RGC apoptosis (e.g. inhibition of excessive free radical formation) [44, 112, 126, 127, 132, 152, 158].
Na+ and Ca2+
In ischemia-/reperfusion damage Ca2+ overload is preceeded by an intracellular accumulation of Na+ as a result of a blockade of the Na+/K+-exchange, an increase in the Na+/H+ exchange and the Na+-HCO3 cotransport due to lack of ATP and acidosis and the reversal of Na+/Ca2+ exchange. Increased intracellular Ca2+ levels impede the various messenger functions of this ion and lead to a liberation of transmitters, which activate certain receptors (e.g. ionotrophic glutamate receptors) and thus further increase intracellular calcium. Na+ and Ca2+ can increase Ca2+ influx into the cell by activating voltage dependent canals. As a result a further increase in intracellular Ca2+ will disturb cellular homeostasis e.g. by activating cytotoxic enzymes, apoptosis is initiated.
Ca2+ overload and metabolic stress caused by glutamate excitotoxicity e.g. due to ischemia trigger production of further toxic mediators (e.g. nitric oxide - NO, free oxygen radicals) and activate mitochondrial apoptotic signal transduction pathways. Ca2+ acts as a cosignal with PSD-95, a postsynaptic density protein for activation of neuronal NOS and thus couples NMDA receptor activation to NO, which, in excess, is toxic to RGCs [149]. Thus, substances which reduce Na+ and/or Ca2+ influx into the cell in pathologic conditions (i.e. in persistent depolarization) may reduce or even halt cell death.
Several clinically well known drugs e.g. lidocaine, flunarizine, diazepam, betaxolol carbamazepine and phenytoin are Na+ channel blockers and significantly reduce cell damage [112, 126, 127, 152, 158, 198]. Carbamazepine and phenytoin used to treat epilepsy also reduce hypoxic damage in rat optic nerve culture [37]. Ca2+ channel blockers, such as flunarizine [35] and nifidipine [27], vasodilators used in systemic antihypertensive therapy, demonstrated a cytoprotective effect in retinal cells after ischemia/reperfusion.
Furthermore calcium channel blockers (e.g. nimodipine, nifidipine) seem to have a positive effect on the visual field prognosis in normal tension POAG [28, 78, 161].
It is uncertain whether this clinical effect is a result of direct cytoprotection or due to improved microcirculationto the optic nerve head[153, 161, 162] or a combination of both effects.
Following this concept, verapamil, a combined Ca2+ and Na+ channel blocker may have greater neuroprotective potency.
Further studies on the various neural Ca2+ and Na+ channel subtypes and the (state-dependent) binding at multiple sites of potential blockers may help to find channel type(s) specific for POAG and the drug(s) to selectively block these channels to a degree providing neuroprotection for POAG patients without compromising cell function.
THE N-METHYL-D-ASPARTAT (NMDA)-RECEPTOR
Elevated IOP in POAG patients is associated with increased glutamate levels [31], a similar increase of this neurotransmitter was found in the vitreous of rabbits following retinal ischaemia [76] and in the aqueous humour following optic nerve crush in rats [204].
This mechanism seems to be similar in other neural tissues, e.g. the central nervous system where primary ischemic damage is also associated with glutamate induced cell death.
Prolonged NMDA - receptor overactivation by increased levels of glutamate (excitotoxicity) due to e.g. increased IOP and/or oligemia [112, 127, 130, 131, 152, 158], leads to depolarisation - which in excess - initiates Na+ and Ca2+ influx into the cell, the first step in the cascade of events resulting in apoptosis.
So it seems conclusive that NMDA antagonists may delay or halt ganglion cell death. Respective studies demonstrate that NMDA antagonists such as MK801, ifenprodil, and memantine reduce the loss of RGCs in optic nerve crush, ischemia/reperfusion, and chronic high IOP experiments [46, 152, 204].
However it remains unclear whether the small but significant increase of glutamate in the vitreous of POAG patients reflects toxic concentrations of this neurotransmitter at the level of RGCs or whether this is a compensatory mechanism of the glutamate/glutamine metabolism in the process of maintaining normal visual function.
While there is some evidence for the potential role of glutamate in the pathogenesis of POAG, its metabolism needs further investigation.
An excitotoxic increase in glutamate in the extracellular space initiates the death of neurones that express ionotropic glutamate (NMDA) receptors [12, 136], e.g. ganglion cells and a subtype of amacrine cells.
Glutamate could be released from retinal neurones as a reaction to ischemia or arise from a malfunction in the uptake/turnover of this neurotransmitter e.g. in Müller cells.
Glutamate uptake and metabolizing glutamate to glutamine is an energy (ATP) consuming process. ATP is provided by glycolysis. Thus, any impairment of glucose supply or metabolism that reduces ATP in Müller cells, such as oligemia, may reduce glutamate uptake from the extracellular space, thus increase extracellular level of this neurotransmitter, which reduces cellular homeostasis and increases the possibility of RGC apoptosis.
It is clear from a variety of studies that not all RGCs are equally sensitive to a change in cellular homeostasis. As discussed before, several studies suggest that M cells are more sensitive to destruction in POAG than P cells [139, 140], which is in keeping with the finding that the latter ganglion cells are less sensitive to intraocular injection of NMDA or glutamate than M cells [32] and thus supports a role for glutamate in glaucomatous pathology. The variable sensitivity may be explained by the variable profile of excitatory (e.g. glutamate – depolarisation) and inhibitory (e.g. aminobutyric acid, GABA – hyperpolarisation) receptors expressed by a specific subset of RGCs.
Thus the degree of depolarisation (i.e. susceptibility to apoptosis) of any GC is determined by the ratio of excitatory vs. inhibitory receptors expressed by that cell, i.e. a cell that expresses more GABA receptors than glutamate receptors should be more resistant to excitatory damage than a cell with fewer GABA receptors.
The receptor profile of ganglion cells is not limited to GABA and glutamate receptors. Ganglion cell function is further influenced by nicotinic [176], adenosine [90] and α2-adrenergic [73], both hyperpolarizing receptors.
A direct blockade of excitatory receptors to halt ganglion cell death in POAG seems an obvious therapeutic option.
Systemic [88] or intraocular [193] administration of MK-801, an NMDA receptor antagonist can protect against many of the destructive effects of either NMDA or experimental retinal ischaemia in RGCs. Memantine, another NMDA receptor antagonist demonstrated a neuroprotective effect in RGCs when exposed to glutamate in a concentration otherwise toxic to theses cells [190].
Currently, clinical use of NMDA blockers is limited by their side effect profile. As NMDA receptors are widely distributed in the central nervous system and involved in a number of vital functions, a large spectrum of side effects can be expected. E.g. patients who received MK-801 for stroke treatment showed neurotoxic reactions [84].
Interestingly, memantine acts similar to MK-801 but does not display the side effects noted with MK-801 [184], a low affinity for the NMDA receptor and a membrane stabilising effect [134] may explain this effect.
Studies on NMDA receptor subtypes and their specific properties e.g. their ability to influence the activity of a receptor by allosteric change will help to find antagonists for specific NMDA receptors and may increase neuroprotective potency for a specific neurodegenerative disease (e.g. stroke, M. Alzheimer, POAG) and reduce (neuro)toxic side effects. This is crucial for any therapy of POAG, where drugs have to be applied prophylactically for life.
FREE RADICALS
Free radicals are formed as part of cellular processes [71]. Oxygen and free oxygen radicals can damage most macromolecular cellular structures, as a consequence an alteration in proteins, lipid peroxidation and destruction of nucleic acids may result [71].
Certain stress conditions (hypoxia, ischemia/reperfusion) lead to excessive free oxygen radical formation which increases e.g. p53 expression neurons [147].
Upregulation of p53 increases expression glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which is the major source of NADH for mitochondrial oxidative phosphorylation. An increase in the cytoplasmic NAD+/NADH ratio liberates the bound form of this proenzyme from its RNA binding site to active GAPDH.
SH oxidation or nitrosylation by NO also free GAPDH, but this process also inactivates GAPDH. Thus GAPDH inhibition by free oxygen radicals leads to a reduction in intracellular NADH and thus may induce apoptosis.
The finding that mice deficient of p53 are resistant to excitotoxic neuronal damage [115] is consistent with this concept.
Cellular protective mechanisms can counteract this damage with the support of specific enzymes (e.g. glutathione peroxidase, glutathione reductase, catalase, superoxid dismutases) and antioxidants (e.g. α-tocopherol, ascorbic acid, glutathion) seem to prevent apoptosis under certain conditions [71].
While the ethiopathogenetic mechanisms of POAG remain unclear it is rather obvious that cell death in this form of progressive optic neuropathy occurs by apoptosis as well as by necrosis, in other words: apoptosis and necrosis could be different forms of the same death process determined by the degree of insult.
Free oxygen radicals and respective free radical scavengers (antioxidants) seem to affect cell death in POAG independent of its mechanism. This oxidative stress may be induced by an elevation of nitric oxide due to oligemia eventually causing oxidative/nitrosative stress and lipid peroxidation of retinal ganglion cells [158]. Studies in rabbits show increased formation of free oxygen radicals after ischemia [119] and reduced retinal function, an effect which could be reversed by antioxidants [119].
Consequently antioxidative capactivity, a marker of free radical formation is increased in the aqueous humour of initial POAG but not in a later stage, which may reflect a local mechanism to compensate for increased oxidative stress in this optic neuropathy exhausted with progression [159].
Also a cytoprotective effect of various antioxidants (vitamin E, 178), katalase [186] and ginkgo biloba [178] was shown in retinal cells following ischemia/reperfusion. Lipidperoxidation, a consequence of free radical formations was reduced by tririlazadmesylate, RGC death due to lack of ATP was reduced in cell culture experiments [91].Osborne et al. [129] demonstrated that light damages isolated mitochondria which correlated to exposure and that light triggers apoptosis of cultured RGCs, an effect exacerbated in nutritionally deprived cells and thus propose that reactive oxygen intermediates (ROI) generated in RGC axon mitochondria due to light (especially short blue wave light - (450 – 490 nm) exposure may further compromise the survival of these neurons in POAG, where RGCs are in an bioenergetically low state (as discussed in detail before) and thus their ability to scavenge ROI is reduced, resulting in toxic ROI concentrations accelerating RGC death in POAG and mitochondrial optic neuropathies.
Antioxidants which counteract cell death independent of the exact mechanism (apoptosis or necrosis) may prove beneficial in the treatment of POAG.
NEUROPROTECTIVE TREATMENT OF POAG
The ideal POAG drug is well tolerated orally, targets specific receptors of specific RGC subpopulations (e.g. M cells) or glial cells to reduce side effects and prevents cell death independent of the cell death mechanism.
As currently there are no drugs available which meet these criteria even in part, considerable side effects limit the use of drugs available for protection of neurons (see inhibition of the NMDA receptor).
As POAG drugs like for any drug that needs to be taken (prophylactically) for life, the side effect profile is an important key to ensure patient compliance.
Our understanding of a current POAG drug is that it can be applied topically, reduces IOP, reaches the back of the eye in sufficient concentration to increase optic nerve head perfusion and to protect RGCs.
Two drugs used clinically, brimonidine an α2-adrenergic receptor agonist and betaxolol a β-adrenergic receptor antagonist were neuroprotective in animal model and cell culture experiments [44, 112, 128, 132, 152, 194, 197, 198].
Clinical evidence that this neuroprotective effect can also be elicited in human POAG and if so is not coupled to IOP reduction and/or altered perfusion (excluded for brimonidine, 155) [60, 64] remains to be established and would include pharmacodynamic experiments which prove that both drugs, when applied topically, reach the back of the eye in sufficient quantity to be pharmacologically active.
The clinical use of neuroprotective agents e.g. NMDA antagonists will largely depend on advances in pharmacokinetics i.e. if a drug with potentially excellent neuroprotective properties but unacceptable side effects when applied systemically or does not reach the optic nerve head when applied topically can be designed to specifically reach their target cells without interacting with other organs, this drug may be used in the treatment of POAG and would increase our treatment options for a still potentially blinding illness.
Based on the fact that studies in humans proving direct neuroprotection without reducing IOP and / or improve optic nerve head microcirculation will be difficult to justify and the disappointingly slow progress in this field despite the enormous efforts in basic science and the pharmaceutical industry in the field of neuroprotection, for the near future, neuroprotective drugs for glaucoma therapy will most probably exert their effect in a combination of indirect and direct effects i.e. a reduction in IOP and / or improvement of optic nerve head microcirculation and direct neuroprotective action (proven in animal model and cell culture experiments). These drugs will be topically active and need to be demonstrated that they reach the optic nerve head in sufficient concentration to exert their neuroprotective effect(s).
REFERENCES
- 1.Ahmed N, Ahmed U, Thornalley PJ, Hager K, Fleischer G, Munch G. Protein Glycation, Oxidation and Nitration Adduct Residues and Free Adducts of Cerebrospinal Fluid in Alzheimer's Disease and Link to Cognitive Impairment. J. Neurochem. 2005;92:255–263. doi: 10.1111/j.1471-4159.2004.02864.x. [DOI] [PubMed] [Google Scholar]
- 2.Alizadeh M, Wada M, Gelfman CM, Handa JT, Hjelmeland LM. Downregulation of Differentiation Specific Gene Expression by Oxidative Stress in ARPE-19 cells. Invest. Ophthalmol. Vis. Sci. 2001;42:2706–2713. [PubMed] [Google Scholar]
- 3.Anderson DR. Glaucoma, Capillaries and Pericytes. 1. Blood Flow Regulation. Ophthalmologica. 1996;210:257–262. doi: 10.1159/000310722. [DOI] [PubMed] [Google Scholar]
- 4.Anderson DR, Davis EB. Glaucoma, Capillaries and Pericytes. 5. Preliminary Evidence that Carbon Dioxide Relaxes Pericyte Contractile Tone. Ophthalmologica. 1996;210:280–284. doi: 10.1159/000310726. [DOI] [PubMed] [Google Scholar]
- 5.Anderson DR, Davis EB. Glaucoma, Capillaries and Pericytes. 2. Identification and Characterization of Retinal Pericytes in Culture. Ophthalmologica. 1996;210:263–268. doi: 10.1159/000310723. [DOI] [PubMed] [Google Scholar]
- 6.Asif M, Egan J, Vasan S, Masurekar MR, Lopez S, Williams C, Torres RL, Wagle D, Ulrich P, Cerami A, Brines M, Regan TJ. An advanced Glycation Endproduct Cross-Link Breaker Can Reverse Age-Related Increases in Myocardial Stiffness. Proc. Natl. Acad. Sci. USA. 2000;97:2809–2813. doi: 10.1073/pnas.040558497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barile GR, Pachydaki SI, Tari SR, Leem SE, Donmoyer CM, Ma W, Rong LL, Buciarelli LG, Wendt T, Horig H, Hudson BI, Qu W, Weinberg AD, Yan SF, Schmidt AM. The RAGE Axis in Early Diabetic Retinopathy. Invest. Ophthalmol. Vis. Sci. 2005;46(8):2916–2924. doi: 10.1167/iovs.04-1409. [DOI] [PubMed] [Google Scholar]
- 8.Barkana Y, Belkin M. Neuroprotection in Ophthalmology: A Review. Brain Res. Bull. 2004;62:447–453. doi: 10.1016/S0361-9230(03)00071-6. [DOI] [PubMed] [Google Scholar]
- 9.Baynes JW, Monnier VM. Progress of Clinical and Biological Research. New York: Alan R Liss; 1989. The Maillard Reaction in Aging, Diabetes, and Nutrition; pp. 1–410. [Google Scholar]
- 10.Beatty S, Koh H, Phil M, Henson D, Boulton M. The Role of Oxidative Stress in the Pathogenesis of Age-Related Macular Degeneration. Surv. Ophthalmol. 2000;45:115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 11.Boulton M, McKechnie NM, Breda J, Bayly M, Marshall J. The Formation of Autofluorescent Granules in Cultured Human RPE. Invest. Ophthalmol. Vis. Sci. 1989;30:82–89. [PubMed] [Google Scholar]
- 12.Brandstatter JH, Hartveit E, Sassoe-Pognetto M, Wassle H. Expression of NMDA and High-Affinity Kainate Receptor Subunit mRNAs in the Adult Rat Retina. Eur. J. Neurosci. 1994;6:1100–1112. doi: 10.1111/j.1460-9568.1994.tb00607.x. [DOI] [PubMed] [Google Scholar]
- 13.Bristow EA, Griffiths PG, Andrews RM, Johnson MA, Turnbull DM. The Distribution of Mitochondrial Activity in Relation to Optic Nerve Structure. Arch. Ophthalmol. 2002;120:791–796. doi: 10.1001/archopht.120.6.791. [DOI] [PubMed] [Google Scholar]
- 14.Brown SM, Jampol LM. New Concepts of Regulation of Retinal Vessel Tone. Arch. Ophthalmol. 1996;114:199–204. doi: 10.1001/archopht.1996.01100130193015. [DOI] [PubMed] [Google Scholar]
- 15.Brownlee M, Cerami A, Vlassara H. Advanced Glycosylation End Products in Tissue and the Biochemical Basis of Diabetic Complications. N. Engl. J. Med. 1988;318:1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 16.Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A. Aminoguanidine Prevents Diabetes-Induced Arterial Wall Protein Cross-Linking. Science. 1986;232:1629–1632. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- 17.Bucala R, Vlassara H. Advanced Glycosylation End Products in Diabetic Renal and Vascular Disease. Am. J. Kidney Dis. 1995;26:875–888. doi: 10.1016/0272-6386(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 18.Cerami A, Stevens VJ, Monnier VM. Role of Nonenzymatic Glycosylation in the Development of the Sequelae of Diabetes Mellitus. Metabolism. 1979;28(Suppl. 1):431–437. doi: 10.1016/0026-0495(79)90051-9. [DOI] [PubMed] [Google Scholar]
- 19.Chakravarthy U, Gardiner TA, Anderson P, Archer DB, Trimble ER. The Effect of Endothelin 1 on the Retinal Microvascular Pericyte. Microvasc. Res. 1992;43:241–254. doi: 10.1016/0026-2862(92)90022-h. [DOI] [PubMed] [Google Scholar]
- 20.Chaum E. Retinal Neuroprotection by Growth Factors: A Mechanistic Perspective. J. Cell. Biochem. 2003;88:57–75. doi: 10.1002/jcb.10354. [DOI] [PubMed] [Google Scholar]
- 21.Chen Q, Anderson DR. Effect of CO2 on Intracellular PH and Contraction of Retinal Capillary Pericytes. Invest. Ophthalmol. Vis. Sci. 1997;38:643–651. [PubMed] [Google Scholar]
- 22.Chiarelli F, Catino M, Tumini S, Cipollonem F, Mezzettim A, Vanelli M, Verrotti A. Advanced Glycation End Products in Adolescents and Young Adults with Diabetic Angiopathy. Pediatr. Nephrol. 2000;14(8-9):841–846. doi: 10.1007/pl00013443. [DOI] [PubMed] [Google Scholar]
- 23.Chidlow G, Schmidt KG, Wood JPM, Osborne NN. Lipoic Acid Protects the Retina Against Ischaemia/Reperfusion. Neuropharmacology. 2002;43:15–25. doi: 10.1016/s0028-3908(02)00129-6. [DOI] [PubMed] [Google Scholar]
- 24.Cho HK, Kozu H, Peyman GA, Parry GJ, Khoobehi B. The Effect of Aminoguanidine on the Blood Retinal Barrier in Streptozotocine Induced Diabetic Rats. Ophthalmic Surg. 1991;22:44–47. [PubMed] [Google Scholar]
- 25.Clements RS Jr, Robison WG Jr, Cohen MP. Anti-Glycate Albumin Therapy Ameliorates Early Retinal Microvascular Pathology in Db/Db mice. J. Diabetes Complications. 1998;12:28–33. doi: 10.1016/s1056-8727(97)00051-2. [DOI] [PubMed] [Google Scholar]
- 26.Cooper ME, Thallas V, Forbes J, Scalbert E, Sastra S, Darby I, Soulis T. The Cross-Link Breaker, N-Phenacylthiazolium Bromide Prevents Vascular Advanced Glycation End-Product Accumulation. Diabetologia. 2000;43:660–664. doi: 10.1007/s001250051355. [DOI] [PubMed] [Google Scholar]
- 27.Crosson CE, DeBenedetto R, Gidday JM. Functional Evidence for Retinal Adenosine Receptors. J. Ocul. Pharmacol. 1994;10:499–507. doi: 10.1089/jop.1994.10.499. [DOI] [PubMed] [Google Scholar]
- 28.Crosson CE, Willis JA, Potter DE. Effect of the Calcium Antagonist, Nifedipine, on Ischemic Retinal Dysfunction. J. Ocul. Pharmacol. 1990;6:293–299. doi: 10.1089/jop.1990.6.293. [DOI] [PubMed] [Google Scholar]
- 29.Danser AH, Derkx FH, Admiraal PJ, Deinum J, de Jong PT, Schalekamp MA. Angiotensin Levels in the Eye. Invest. Ophthalmol. Vis. Sci. 1994;35:1008–1018. [PubMed] [Google Scholar]
- 30.Deora AA, Win T, Vanhaesebroeck B, Lander HM. A Redox-Triggered Ras-Effector Interaction. Recruitment of Phosphatidylinositol 3'-Kinase to Ras by Redox Stress. J. Biol. Chem. 1998;273:29923–29928. doi: 10.1074/jbc.273.45.29923. [DOI] [PubMed] [Google Scholar]
- 31.Dodge AB, Hechtman HB, Shepro D. Microvascular Endothelial-Derived Autacoids Regulate Pericyte Contractility. Cell Motil. Cytoskeleton. 1991;8:18880–188815. doi: 10.1002/cm.970180304. [DOI] [PubMed] [Google Scholar]
- 32.Dreyer EB, Zurakowski D, Schumer RA, Podos SM, Lipton SA. Elevated Glutamate Levels in the Vitreous Body of Humans and Monkeys with Glaucoma. Arch. Ophthalmol. 1996;114:299–305. doi: 10.1001/archopht.1996.01100130295012. [DOI] [PubMed] [Google Scholar]
- 33.Edelstein D, Brownlee M. Mechanistic Studies of Advanced Glycosylation Endproduct Inhibition by Aminoguanidine. Diabetes. 1992;41:26–28. doi: 10.2337/diab.41.1.26. [DOI] [PubMed] [Google Scholar]
- 34.Eichler W, Friedrichs U, Thies A, Tratz C, Wiedemann P. Modulation of Matrix Metalloproteinase and TIMP-1 Expression by Cytokines in Human RPE Cells. Invest. Ophthalmol. Vis. Sci. 2002;43:2767–2773. [PubMed] [Google Scholar]
- 35.Eschweiler GW, Bahr M. Flunarizine Enhances Rat Retinal Ganglion Cell Survival After Axotomy. J. Neurol. Sci. 1993;116:34–40. doi: 10.1016/0022-510x(93)90086-e. [DOI] [PubMed] [Google Scholar]
- 36.Farkas RH, Grosskreutz CL. Apoptosis, Neuroprotection, and Retinal Ganglion Cell Death: An overview. Int. Ophthalmol. Clin. 2001;41:111–130. doi: 10.1097/00004397-200101000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Fern R, Ransom BR, Stys PK, Waxman SG. Pharmacological protection of CNS white matter during anoxia: Actions of Phenytoin, Carbamazepine and Diazepam. J. Pharmacol. Exp. Ther. 1993;266:1549–1555. [PubMed] [Google Scholar]
- 38.Flammer J, Haefliger IO, Orgül S, Resink T. Vascular Dysregulation: A Principal Risk Factor for Glaucoma. J. Glaucoma. 1999;8:212–219. [PubMed] [Google Scholar]
- 39.Fortune B, Bui BV, Morrison JC, Johnson EC, Dong J, Cepurna WO, Jia L, Barber S, Cioffi GA. Selective Ganglion Cell Functional Loss in Rats with Experimental Glaucoma. Invest. Ophthalmol. Vis. Sci. 2004;45:1854–1862. doi: 10.1167/iovs.03-1411. [DOI] [PubMed] [Google Scholar]
- 40.Funk R. Studies on the Functional Morphology of Rat Ocular Vessels with Scanning Electron Microscopy. Acta Anat. 1986;125(4):252–257. doi: 10.1159/000146172. [DOI] [PubMed] [Google Scholar]
- 41.Funk RH. Blood Supply of the Retina. Ophthalmic Res. 1997;29:320–325. doi: 10.1159/000268030. [DOI] [PubMed] [Google Scholar]
- 42.Funk RH, Nagel F, Wonka F, Krinke HE, Gölfert F, Hofer A. Effects of Heat Shock on the Functional Morphology of Cell Organelles Observed by Video-Enhanced Microscopy. Anat. Rec. 1999;255:458–464. doi: 10.1002/(SICI)1097-0185(19990801)255:4<458::AID-AR11>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 43.Funk R, Rohen JW. Comparative Morphological Studies on Blood Vessels in Eyes of Normotensive and Spontaneously Hypertensive Rats. Exp. Eye Res. 1985;40:191–203. doi: 10.1016/0014-4835(85)90004-1. [DOI] [PubMed] [Google Scholar]
- 44.Funk RH, Schmidt KG. Characteristic Features of Optic Nerve Ganglion Cells and Approaches for Neuroprotection From Intracellular to Capillary Processes and Therapeutic Considerations. Ophthalmologe. 2004;101:1062–1070. doi: 10.1007/s00347-004-1116-z. [DOI] [PubMed] [Google Scholar]
- 45.Gasser P, Flammer J. Short- and Long-Term Effect of Nifedipine on the Visual Field in Patients with Presumed Vasospasm. Eur. J. Med. Res. 1990;18:334–339. doi: 10.1177/030006059001800411. [DOI] [PubMed] [Google Scholar]
- 46.Goebel DJ, Poosch MS. Transient Down-regulation of NMDA Receptor Subunit Gene Expression in the Rat Retina Following NMDA-Induced Neurotoxicity is Attenuated in the Presence of the Non-Competitive NMDA Receptor Antagonist MK-801. Exp. Eye Res. 2001;72(5):547–558. doi: 10.1006/exer.2001.0981. [DOI] [PubMed] [Google Scholar]
- 47.Goldblum D, Kauer A, McKinnon SJ, Kasmala L, Frueh BE. Distribution of Amyloid Precursor Protein and ß-Amyloid in Ocular Hypertensive C57BL/6 Mouse Eyes. Invest. Ophthalmol. Vis. Sci. 2005;46:1266–1271. [Google Scholar]
- 48.Grossniklaus HE, Ling JX, Wallace TM, Dithmar S, Lawson DH, Cohen C, Elner VM, Elner SG, Sternbergm P Jr. Macrophage and Retinal Pigment Epithelium Expression of Angiogenic Cytokines in Choroidal Neovascularization. Mol. Vis. 2002;8:119–126. [PubMed] [Google Scholar]
- 49.Guidry C, Medeiros NE, Curcio CA. Phenotypic Variation of Retinal Pigment Epithelium in Age-Related Macular Degeneration. Invest. Ophthalmol. Vis. Sci. 2002;43:267–273. [PubMed] [Google Scholar]
- 50.Haefliger IO, Anderson DR. Oxygen Modulation of Guanylate Cyclase-Mediated Retinal Pericyte Relaxations with 3-Morpholino-Sydnonimine and Atrial Natriuretic Peptide. Invest. Ophthalmol. Vis. Sci. 1997;38(8):1563–1568. [PubMed] [Google Scholar]
- 51.Haefliger IO, Chen Q, Anderson DR. Effect of Oxygen on Relaxation of Retinal Pericytes by Sodium Nitroprusside. Graefes Arch. Clin. Exp. Ophthalmol. 1997;235:388–392. doi: 10.1007/BF00937289. [DOI] [PubMed] [Google Scholar]
- 52.Haefliger IO, Meyer P, Flammer J, Luscher TF. The Vascular Endothelium as a Regulator of the Ocular Circulation: A New Concept in Ophthalmology? Surv. Ophthalmol. 1994;39:123–132. doi: 10.1016/0039-6257(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 53.Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An Integrated Hypothesis that Considers Drusen as Biomarkers of Immune-Mediated Processes at the RPE-Bruch’s Membrane Interface in Aging and Age-Related Macular Degeneration. Prog. Retin. Eye Res. 2001;20:705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 54.Hammes HP, Alt A, Niwa T, Clausen JT, Bretzel RG, Brownlee M, Schleicher ED. Differential Accumulation of Advanced Glycation end Products in the Course of Diabetic Retinopathy. Diabetologia. 1999;42:728–736. doi: 10.1007/s001250051221. [DOI] [PubMed] [Google Scholar]
- 55.Hammes HP, Martin S, Federlin K, Geisen K, Brownlee M. Aminoguanidine Treatment Inhibits the Development of Experimental Diabetic Retinopathy. Proc. Natl. Acad. Sci. U.S.A. 1991;88:11555–11558. doi: 10.1073/pnas.88.24.11555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Handa JT, Reiser KM, Matsunaga H, Hjelmeland LM. The Advanced Glycation Endproduct Pentosidine Induces the Expression of PDGF-B in Human Retinal Pigment Epithelial Cells. Exp. Eye Res. 1998;66:411–419. doi: 10.1006/exer.1997.0442. [DOI] [PubMed] [Google Scholar]
- 57.Hayashi H, Miyata H, Noda N, Kobayashi A, Hirano M, Kawai T, Yamazaki N. Intracellular Ca2+ Concentration and PHi During Metabolic Inhibition. Am. J. Physiol. 1992;262:C628–C634. doi: 10.1152/ajpcell.1992.262.3.C628. [DOI] [PubMed] [Google Scholar]
- 58.Hernandez MR, Pena JD, Selvidge JA, Salvador-Silva M, Yang P. Hydrostatic Pressure Stimulates Synthesis of Elastin in Cultured Optic Nerve Head Astrocytes. Glia. 2000;32(2):122–136. doi: 10.1002/1098-1136(200011)32:2<122::aid-glia20>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 59.Hessemer V, Schmidt KG. Influence of the Vasodilator Drug Isosorbide Dinitrate on Ocular Circulation. Arch. Ophthalmol. 1997;115:324–327. doi: 10.1001/archopht.1997.01100150326003. [DOI] [PubMed] [Google Scholar]
- 60.Hester RK, Chen Z, Becker EJ, McLaughlin M, DeSantis L. The Direct Vascular Relaxing Action of Betaxolol, Carteolol and Timolol in Porcine Long Posterior Ciliary Artery. Surv. Ophthalmol. 1994;38(Suppl):S125–134. doi: 10.1016/0039-6257(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 61.Higgins GT, Wang JH, Dockery P, Cleary PE, Redmond HP. Induction of Angiogenic Cytokine Expression in Cultured RPE by Ingestion of Oxidized Photoreceptor Outer Segments. Invest. Ophthalmol. Vis. Sci. 2003;44:1775–1782. doi: 10.1167/iovs.02-0742. [DOI] [PubMed] [Google Scholar]
- 62.Hofer A, Nagel F, Wonka F, Krinke HE, Gölfert F, Funk RH. A New Perfusion Cell Chamber System for Determination of Heat Shock Effects by Means of Video-Enhanced Microscopy. Med. Biol. Eng. Comput. 1999;37:667–669. doi: 10.1007/BF02513364. [DOI] [PubMed] [Google Scholar]
- 63.Horiuchi S, Higashi T, Ikeda K, Saishoji T, Jinnouchi Y, Sano H, Shibayama R, Sakamoto T, Araki N. Advanced Glycation End Products and Their Recognition by Macrophage and Macrophage-Derived cells. Diabetes. 1996;45(Suppl.3):S73–S76. doi: 10.2337/diab.45.3.s73. [DOI] [PubMed] [Google Scholar]
- 64.Hoste AM, Sys SU. The Relaxant Action of Betaxolol on Isolated Bovine Retinal Microarteries. Curr. Eye Res. 1994;3(7):483–487. doi: 10.3109/02713689408999879. [DOI] [PubMed] [Google Scholar]
- 65.Howes KA, Liu Y, Dunaief JL, Milam A, Frederick JM, Marks A, Baehr W. Receptor for Advanced Glycation End Products and Age-Related Macular Degeneration. Invest. Ophthalmol. Vis. Sci. 2004;45:3713–3120. doi: 10.1167/iovs.04-0404. [DOI] [PubMed] [Google Scholar]
- 66.Huang JS, Guh JY, Hung WC, Yang ML, Lai YH, Chen HC, Chuang LY. Role of the Janus Kinase (JAK)/Signal Transducters and Activators of Transcription (STAT) Cascade in Advanced Glycation End-Product-Induced Cellular Mitogenesis in NRK-49F Cells. Biochem. J. 1999;342:231–238. [PMC free article] [PubMed] [Google Scholar]
- 67.Ido Y, Chang K, Ostrow E. Aminoguanidine Prevents Regional Blood Flow Increases in Streptozotocine-Diabetic Rats. Invest. Ophthalmol. Vis. Sci. 1990;45(10):3713–3720. [Google Scholar]
- 68.Ishaque A, Al-Rubeai M. Use of Intracellular PH and Annexin-V Flow Cytometric Assays to Monitor Apoptosis and its Suppression by Bcl-2 Over-Expression in Hybridoma Cell Culture. J. Immunol. Methods. 1998;221:43–57. doi: 10.1016/s0022-1759(98)00166-5. [DOI] [PubMed] [Google Scholar]
- 69.Ishii Y, Kwong JM, Caprioli J. Retinal Ganglion Cell Protection with Geranylgeranylacetone, a Heat Shock Protein Inducer, in a Rat Glaucoma Model. Invest. Ophthalmol. Vis. Sci. 2003;44:1982–1992. [PubMed] [Google Scholar]
- 70.Ishisaka R, Utsumi T, Kanno T, Arita K, Katunuma N, Akiyama J, Utsumi K. Participation of a Cathepsin L-type Protease in the Activation of Caspase-3. Cell. Struct. Funct. 1999;24:465–470. doi: 10.1247/csf.24.465. [DOI] [PubMed] [Google Scholar]
- 71.Jacobson MD. Reactive Oxygen Species and Programmed Cell Death. Trends Biochem. Sci. 1996;21:83–86. [PubMed] [Google Scholar]
- 72.Jianmongkol S, Vuletich JL, Bender AT, Moeller M. Aminoguanidine-Mediated Inactivation and Alteration of Neuronal Nitric-Oxide Synthase. J. Biol. Chem. 2000;275:13370–13376. doi: 10.1074/jbc.275.18.13370. [DOI] [PubMed] [Google Scholar]
- 73.Kalapesi FB, Coroneo MT, Hill MA. Human Ganglion Cells Express the Alpha-2 Adrenergic Receptor: Relevance to Neuroprotection. Br. J. Ophthalmol. 2005;89(6):758–763. doi: 10.1136/bjo.2004.053025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kamal A, Almenar-Queralt A, LeBlanc JF, Roberts EA, Goldstein LS. Kinesin-Mediated Axonal Transport of a Membrane Compartment Containing Beta-Secretase Ans Preseni-lin-1 Requires APP. Nature. 2001;6864:643–648. doi: 10.1038/414643a. [DOI] [PubMed] [Google Scholar]
- 75.Kanamori A, Nakamura M, Mukuno H, Maeda H, Negi A. Diabetes Has an Additive Effect on Neural Apoptosis in Rat Retina with Chronically Elevated Intraocular Pressure. Curr. Eye Res. 2004;28(1):47–54. doi: 10.1076/ceyr.28.1.47.23487. [DOI] [PubMed] [Google Scholar]
- 76.Kim TW, Kang KB, Choung HK, Park KH, Kim DM. Elevated Glutamate Levels in the Vitreous Body of an In Vivo Model of Optic Nerve Ischemia. Arch. Ophthalmol. 2000;118(4):533–536. doi: 10.1001/archopht.118.4.533. [DOI] [PubMed] [Google Scholar]
- 77.King A, Gottlieb E, Brooks DG, Murphy MP, Dunaief JL. Mitochondria-Derived Reactive Oxygen Species Mediate Blue Light-Induced Death of Retinal Pigment Epithelial Cells. Photochem. Photobiol. 2004;79:470–475. doi: 10.1562/le-03-17.1. [DOI] [PubMed] [Google Scholar]
- 78.Kitazawa Y, Shirai H, Go FJ. The Effect of Ca2(+) -Antagonist on Visual Field in low-Tension Glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 1989;227:408–412. doi: 10.1007/BF02172889. [DOI] [PubMed] [Google Scholar]
- 79.Klingmüller V, Schmidt KG, v Rückmann A, Gumbrecht S, Stein A, Koch B. Farb- und Spektral-Doppler-sonographische Perfusionsmessungen in den kurzen hinteren Ziliararterien bei gesunden Probanden. In: Schmidt KG, Pillunat LE, editors. Fortbildung Glaukom, Stuttgart, Enke. Vol. 3. 2000. pp. 29–38. [Google Scholar]
- 80.Kniep EM, Roehlecke C, Ozkucur N, Steinberg A, Reber F, Knels L, Funk RH. Inhibition of Apoptosis and Reduction of Intracellular PH Decrease in Retinal Neural Cell Cultures by a Blocker of Carbonic Anhydrase. Invest. Ophthalmol. Vis. Sci. 2006;47(3):1185–11892. doi: 10.1167/iovs.05-0555. [DOI] [PubMed] [Google Scholar]
- 81.Koch T, Funk RH. Cellular Dysfunction in the Pathogenesis of Organ Failure. New Insights From Molecular and Cell Biology. Anaesthesist. 2001;50(10):742–749. doi: 10.1007/s001010100201. [DOI] [PubMed] [Google Scholar]
- 82.Koenig RJ, Blobstein SH, Cerami A. Structure of Carbohydrate of Hemoglobin A1c. J. Biol. Chem. 1977;252:2992–2997. [PubMed] [Google Scholar]
- 83.Kogel D, Reimertz C, Dussmann H, Mech P, Scheidtmann KH, Prehn JH. The Death Associated Protein (DAP) Kinase Homologue Dlk/ZIP Kinase Induces p19ARF- and p53-Independent Apoptosis. Eur. J. Cancer. 2003;39:249–256. doi: 10.1016/s0959-8049(02)00477-x. [DOI] [PubMed] [Google Scholar]
- 84.Kornhuber J, Weller M. Psychotogenicity and N-Methyl-D-Aspartate Receptor Antagonism: Implications for Neuroprotective Pharmacotherapy. Biol. Psychiatr. 1997;4:135–144. doi: 10.1016/S0006-3223(96)00047-9. [DOI] [PubMed] [Google Scholar]
- 85.Kortuem K, Geiger LK, Levin LA. Differential Susceptibility of Retinal Ganglion Cells to Reactive Oxygen Species. Invest. Ophthalmol. Vis. Sci. 2000;41:3176–3182. [PubMed] [Google Scholar]
- 86.Korzyn AD. The Underdiagnosis of the Vascular Contribution to Dementia. J. Neurol. Sci. 2005;15:229–230. doi: 10.1016/j.jns.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 87.Kumagai N, Yuda K, Kadota T, Goris RC, Kishida R. Substance P-like Immunoreactivity in the Central Retinal Artery of the Rabbit. Exp. Eye Res. 1988;46:591–596. doi: 10.1016/s0014-4835(88)80015-0. [DOI] [PubMed] [Google Scholar]
- 88.Lam TT, Siew E, Chu R, Tso MO. Ameliorative Effect of MK-801 on Retinal Ischemia. J. Ocul. Pharmacol. Ther. 1997;13:129–137. doi: 10.1089/jop.1997.13.129. [DOI] [PubMed] [Google Scholar]
- 89.Lander HM, Tauras JM, Ogiste JS, Hori O, Moss RA, Schmidt AM. Activation of the Receptor for Advanced Glycation End Products Triggers a p21(ras)-Dependent Mitogen-Activated Protein Kinase Pathway Regulated by Oxidant Stress. J. Biol. Chem. 1997;272:17810–17814. doi: 10.1074/jbc.272.28.17810. [DOI] [PubMed] [Google Scholar]
- 90.Larsen AK, Osborne NN. Involvement of Adenosine in Retinal Ischemia Studies on the Rat. Invest. Ophthalmol. Vis. Sci. 1996;37:2603–2611. [PubMed] [Google Scholar]
- 91.Levin LA, Clark JA, Johns LK. Effect of Lipid Peroxidation Inhibition on Retinal Ganglion Cell Death. Invest. Ophthalmol. Vis. Sci. 1996;37:2744–2749. [PubMed] [Google Scholar]
- 92.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-Dependent Formation of Apaf-1/Caspase-9 Complex Initiates an Apoptotic Protease Cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 93.Li W, Yanoff M, Liu X, Ye X. Retinal Capillary Pericyte Apoptosis in Early Human Diabetic Retinopathy. Chin. Med. J. 1997;110:659–663. [PubMed] [Google Scholar]
- 94.Li YM, Mitsuhashi T, Wojciehowicz D, Shimizu N, Li J, Stitt A, He C, Banerjee D, Vlassara H. Molecular Identity and Cellular Distribution of Advanced Glycation Endproduct Receptors Relationship of p60 to OST-48 and 80K-H membrane proteins. Proc. Natl. Acad. Sci. USA. 1996;93:1047–11052. doi: 10.1073/pnas.93.20.11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lieberman MF, Maumenee AE, Green WR. Histologic Studies of the Vasculature of the Anterior Optic Nerve. Am. J. Ophthalmol. 1976;82(3):405–423. doi: 10.1016/0002-9394(76)90489-x. [DOI] [PubMed] [Google Scholar]
- 96.Lipton SA. Retinal Ganglion Cells, Glaucoma and Neuroprotection. Prog. Brain Res. 2001;1131:712–718. [PubMed] [Google Scholar]
- 97.Lu M, Kuroki M, Amano S, Tolentino M, Keough K, Kim I, Bucala R, Adamis AP. Advanced Glycation End Products Increase Retinal Vascular Endothelial Growth Factor Expression. J. Clin. Invest. 1998;101:1219–1224. doi: 10.1172/JCI1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luksch A, Polak K, Beier C, Polska E, Wolzt M, Dorner GT, Eichler HG, Schmetterer L. Effects of Systemic NO Synthase Inhibition on Choroidal and Optic Nerve Head Blood Flow in Healthy Subjects. Invest. Ophthalmol. Vis. Sci. 2000;41(10):3080–3084. [PubMed] [Google Scholar]
- 99.Lynch DR, Farmer J. Practical Approaches to Neurogenetic Disease. J. Neuroophthalmol. 2002;22:297–304. doi: 10.1097/00041327-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 100.Maher P, Hanneken A. The Molecular Basis of Oxidative Stress-Induced Cell Death in an Immortalized Retinal Ganglion Cell Line. Invest. Ophthalmol. Vis. Sci. 2005;46:749–757. doi: 10.1167/iovs.04-0883. [DOI] [PubMed] [Google Scholar]
- 101.Maillard LC. Action des Acides Amines sur Les Sucres: Formation Des Melanoides par Voie Methodique. Acad. Sci. 1912;154:66–68. [Google Scholar]
- 102.Marcic TS, Belyea DA, Katz B. Neuroprotection in Glaucoma: A Model for Neuroprotection in Optic Neuropathies. Curr. Opin. Ophthalmol. 2003;14:353–356. doi: 10.1097/00055735-200312000-00006. [DOI] [PubMed] [Google Scholar]
- 103.Mata NL, Tzekov RT, Liu X, Weng J, Birch DG, Travis GH. Delayed Dark-Adaptation and Lipofuscin Accumulation in Abcr+/- Mice: Implications for Involvement of ABCR in Age-Related Macular Degeneration. Invest. Ophthalmol. Vis. Sci. 2001;42:1685–1690. [PubMed] [Google Scholar]
- 104.Matsugi T, Chen Q, Anderson DR. Adenosine-Induced Relaxation of Cultured Bovine Retinal Pericytes. Invest. Ophthalmol. Vis. Sci. 1997;38:2695–2701. [PubMed] [Google Scholar]
- 105.Matsuyama S, Llopis J, Deveraux QL, Tsien RY, Reed JC. Changes in Intramitochondrial and Cytosolic PH: Early Events That Modulate Caspase Activation During Apoptosis. Nat. Cell. Biol. 2000;2:318–325. doi: 10.1038/35014006. [DOI] [PubMed] [Google Scholar]
- 106.McKinnon SJ, Lehman DM, Kerrigan-Baumrind LA, Merges CA, Pease ME, Kerrigan DF, Ransom NL, Tahzib NG, Reitsamer HA, Levkovitch-Verbin H, Quigley HA, Zack DJ. Caspase Activation and Amyloid Precursor Protein Cleavage in Rat Ocular Hypertension. Invest. Ophthalmol. Vis. Sci. 2002;43:1077–1087. [PubMed] [Google Scholar]
- 107.Medeiros NE, Curcio CA. Preservation of Ganglion Cell Layer Neurons in Age-Related Macular Degeneration. Invest. Ophthalmol. Vis. Sci. 2001;42:795–803. [PubMed] [Google Scholar]
- 108.Mene P, Pascale C, Teti A, Bernardini S, Cinotti GA, Pugliese F. Effects of Advanced Glycation End Products on Cytosolic Ca2+ Signaling of Cultured Human Mesangial Cells. J. Am. Soc. Nephrol. 1999;10:1478–1486. doi: 10.1681/ASN.V1071478. [DOI] [PubMed] [Google Scholar]
- 109.Menke T, Gille G, Reber F, Janetzky B, Andler W, Funk RH, Reichmann H. Coenzyme Q10 Reduces the Toxicity of Rotenone in Neuronal Cultures by Preserving the Mitochondrial Membrane Potential. Biofactors. 2003;18(1-4):65–72. doi: 10.1002/biof.5520180208. [DOI] [PubMed] [Google Scholar]
- 110.Mitsuhashi T, Li YM, Fishbane S, Vlassara H. Depletion of Reactive Advanced Glycation Endproducts from Diabetic Uremic Sera Using a Lysozyme-Linked matrix. J. Clin. Invest. 1997;100:847–854. doi: 10.1172/JCI119600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mittag TW, Danias J, Pohorenec G, Yuan HM, Burakgazi E, Chalmers-Redman R, Podos SM, Tatton WG. Retinal Damage After 3 to 4 Months of Elevated Intraocular Pressure in a Rat Glaucoma Model. Invest. Ophthalmol. Vis. Sci. 2000;41:3451–3459. [PubMed] [Google Scholar]
- 112.Mittag T, Schmidt KG. Mechanismen der Neuroprotektion bei Glaukomen. Ophthalmologe. 2004;101:1076–1086. doi: 10.1007/s00347-004-1130-1. [DOI] [PubMed] [Google Scholar]
- 113.Moreno MC, Campanelli J, Sane P, Sanez DA, Sarmiento K, Rosenstein RE. Retinal Oxidative Stress Induced by High Intraocular Pressure. Free Radic. Biol. Med. 2004;37:803–812. doi: 10.1016/j.freeradbiomed.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 114.Mori H, Mishina M. Structure and Function of the NMDA Receptor Channel. Neuropharmacology. 1995;34:1219–1237. doi: 10.1016/0028-3908(95)00109-j. [DOI] [PubMed] [Google Scholar]
- 115.Morrison JC, Johnson EC, Cepurnam WO, Funk RH. Microvasculature of the Rat Optic Nerve Head. Invest. Ophthalmol. Vis. Sci. 1999;40(8):1702–1709. [PubMed] [Google Scholar]
- 116.Mousa SA, Lorelli W, Campochiaro PA. Role of Hypoxia and Extracellular Matrix-Integrin Binding in the Modulation of Angiogenic Growth Factors Secretion by Retinal Pigmented Epithelial Cells. J. Cell. Biochem. 1999;74:135–143. [PubMed] [Google Scholar]
- 117.Murata T, Nagai R, Ishibashi T, Inomuta H, Ikeda K, Horiuchi S. The Relationship Between Accumulation of Advanced Glycation End Products and Expression of Vascular Endothelial Growth Factor in Human Diabetic Retinas. Diabetologia. 1997;40:764–769. doi: 10.1007/s001250050747. [DOI] [PubMed] [Google Scholar]
- 118.Müller M, Albrecht S, Gölfert F, Hofer A, Funk RH, Magdolen V, Flössel C, Luther T. Localization of Tissue Factor in Actin-Filament-Rich Membrane Areas of Epithelial Cells. Exp. Cell Res. 1999;248:136–147. doi: 10.1006/excr.1999.4395. [DOI] [PubMed] [Google Scholar]
- 119.Müller A, Pietri S, Villain M, Frejaville C, Bonne C, Culcas M. Free Radicals in Rabbit Retina under Ocular Hyperpressure and Functional Consequences. Exp. Eye Res. 1997;64:637–643. doi: 10.1006/exer.1996.0277. [DOI] [PubMed] [Google Scholar]
- 120.Nakamura S, Makita Z, Ishikawa S, Yasumura K, Fujii W, Yanagisawa K, Kawata T, Koike T. Progression of Nephropathy in Spontaneous Diabetic Rats is Prevented by OPB-9195, a Novel Inhibitor of Advanced Glycation. Diabetes. 1997;46:895–899. doi: 10.2337/diab.46.5.895. [DOI] [PubMed] [Google Scholar]
- 121.Neufeld AH, Hernandez MR, Gonzalez M. Nitric Oxide Synthase in the Human Glaucomatous Optic Nerve Head. Arch. Ophthalmol. 1997;115:497–503. doi: 10.1001/archopht.1997.01100150499009. [DOI] [PubMed] [Google Scholar]
- 122.Neufeld AH, Liu B. Glaucomatous Optic Neuropathy: When Glia Misbehave. Neuroscientist. 2003;9:485–495. doi: 10.1177/1073858403253460. [DOI] [PubMed] [Google Scholar]
- 123.Ono Y, Aoki S, Ohnishi K, Yasuda T, Kawano K, Tsukada Y. Increased Serum Levels of Advanced Glycation End-Products and Diabetic Complications. Diabetes Res. Clin. Pract. 1998;41(2):131–137. doi: 10.1016/s0168-8227(98)00074-6. [DOI] [PubMed] [Google Scholar]
- 124.Onorato JM, Jenkins AJ, Thorpe SR, Baynes JW. Pyridoxamine, an Inhibitor of Advanced Glycation Reactions, Also Inhibits Advanced Lipoxidation Reactions: Mechanism of Action of Pyridoxamine. J. Biol. Chem. 2000;275:13370–13376. doi: 10.1074/jbc.M003263200. [DOI] [PubMed] [Google Scholar]
- 125.Osborne NN. Memantine Reduces Alterations to the Mammalian Retina, in Situ, Induced by Ischaemia. Vision Res. 1999;16:45–52. doi: 10.1017/s0952523899161017. [DOI] [PubMed] [Google Scholar]
- 126.Osborne NN, Chidlow G, Layton CJ, Wood JP, Casson RJ, Melena J. Optic Nerve and Neuroprotection Strategies. Eye. 2004;18:1075–1084. doi: 10.1038/sj.eye.6701588. [DOI] [PubMed] [Google Scholar]
- 127.Osborne NN, Chidlow G, Wood JPM, Schmidt KG, Casson R, Melena J. Expectations in the Treatment of Retinal Diseases: Neuroprotection. Curr. Eye Res. 2001;22:321–332. doi: 10.1076/ceyr.22.5.321.5496. [DOI] [PubMed] [Google Scholar]
- 128.Osborne NN, DeSantis L, Bae JH, Ugarte M, Wood JP, Nash MS, Chidlow G. Topically Applied Betaxolol Attenuates NMDA-Induced Toxicity to Ganglion Cells and the Effects of Ischaemia to the Retina. Exp. Eye Res. 1999;69:331–342. doi: 10.1006/exer.1999.0706. [DOI] [PubMed] [Google Scholar]
- 129.Osborne NN, Lascaratos G, Bron AJ, Chidlow G, Wood JPM. A Hypothesis to Suggest that Light is a Risk Factor in Glaucoma and the Mitochondrial Optic Neuropathies. Br. J. Ophthalmol. 2006;90:237–241. doi: 10.1136/bjo.2005.082230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Osborne NN, Schmidt KG. Neuroprotektion bei Glaukom bleibt ein Konzept. Ophthalmologe. 2004;101:1087–1092. doi: 10.1007/s00347-004-1129-7. [DOI] [PubMed] [Google Scholar]
- 131.Osborne NN, Ugarte M, Chao M, Chidlow G, Bae JH, Wood JP, Nash MS. Neuroprotection in Relation to Retinal Ischaemia and Relevance to Glaucoma. Surv. Ophthalmol. 1999;43(Suppl.):S102–S129. doi: 10.1016/s0039-6257(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 132.Osborne NN, Wood JPM, Chidlow G, Bae JH, Melena J, Nash MS. Ganglion Cell Death in Glaucoma: What Do We Really Know? Br. J. Ophthalmol. 1999;83:980–986. doi: 10.1136/bjo.83.8.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Panagiotopoulos S, O'Brien RC, Bucala R, Cooper ME, Jerums G. Aminoguanidine has an Anti-Atherogenic Effect in the Cholesterol-Fed Rabbit. Atherosclerosis. 1998;136:125–131. doi: 10.1016/s0021-9150(97)00192-5. [DOI] [PubMed] [Google Scholar]
- 134.Parsons CG, Gruner R, Rozental J, Millar J, Lodge D. Patch Clamp Studies on the Kinetics and Selectivity of N-Methyl-D-Aspartate Receptor Antagonism by Memantine (1-amino-3,5-dimethyladamantan) Neuropharmacology. 1993;32:1337–1350. doi: 10.1016/0028-3908(93)90029-3. [DOI] [PubMed] [Google Scholar]
- 135.Patton AR, Hill EG. Inactivation of Nutrients by Heating with Glucose. Science. 1948;107:68–69. doi: 10.1126/science.107.2768.68. [DOI] [PubMed] [Google Scholar]
- 136.Peng YW, Blackstone CD, Huganir RL, Yau KW. Distribution of glutamate receptor subtypes in the vertebrate retina. Neuroscience. 1995;66:483–497. doi: 10.1016/0306-4522(94)00569-q. [DOI] [PubMed] [Google Scholar]
- 137.Pervaiz S, Clement MV. Hydrogen Peroxide-Induced Apoptosis: Oxidative or Reductive Stress? Methods Enzymol. 2002;352:150–159. doi: 10.1016/s0076-6879(02)52015-2. [DOI] [PubMed] [Google Scholar]
- 138.Quast T, Wehner S, Kirfel G, Jaeger K, De Luca M, Herzog V. sAPP as a Regulator of Dendrite Motility and Melanin Release in Epidermal Melanocytes and Melanoma Cells. Exp. Biol. 2003;17(12):1739–1741. doi: 10.1096/fj.02-1059fje. [DOI] [PubMed] [Google Scholar]
- 139.Quigley HA, Dunkelberger GR, Green WR. Retinal Ganglion Cell Atrophy Correlated with Automated Perimetry in Human Eyes with Glaucoma. Am. J. Ophthalmol. 1989;107:453–464. doi: 10.1016/0002-9394(89)90488-1. [DOI] [PubMed] [Google Scholar]
- 140.Quigley HA, Dunkelberger GR, Green WR. Chronic Human Glaucoma Causing Selectively Greater Loss of Large Optic Nerve Fibers. Ophthalmology. 1998;95:357–363. doi: 10.1016/s0161-6420(88)33176-3. [DOI] [PubMed] [Google Scholar]
- 141.Quigley HA, Nickells RW, Kerigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal Cell Ganglion Cell Death in Experimental Glaucoma and After Axotomy Occurs by Apoptosis. Invest. Ophthalmol. Vis. Sci. 1995;36:774–786. [PubMed] [Google Scholar]
- 142.Quigley HA, Sanchez RM, Dunkelberger GR, Baginski TA. Chronic Glaucoma Selectively Damages Large Optic Nerve Fibers. Invest. Ophthalmol. Vis. Sci. 1987;28:913–920. [PubMed] [Google Scholar]
- 143.Reber F, Geffarth R, Kasper M, Reichenbach A, Schleicher ED, Siegner A, Funk RH. Graded Sensitiveness of the Various Retinal Neuron Populations on the Glyoxal-Mediated Formation of Advanced Glycation End Products and Ways of Protection. Graefes Arch. Clin. Exp. Ophthalmol. 2003;241(3):213–225. doi: 10.1007/s00417-002-0528-1. [DOI] [PubMed] [Google Scholar]
- 144.Reber F, Gersch U, Funk RW. Blockers of Carbonic Anhydrase Can Cause Increase of Retinal Capillary Diameter, Decrease of Extracellular and Increase of Intracellular PH in Rat Retinal Organ Culture. Graefes Arch. Clin. Exp. Ophthalmol. 2003;241:140–148. doi: 10.1007/s00417-002-0560-1. [DOI] [PubMed] [Google Scholar]
- 145.Reber F, Kasper M, Siegner A, Kniep E, Seigel G, Funk RH. Alteration of the Intracellular PH and Apoptosis Induction in a Retinal Cell Line by the AGE-Inducing Agent Glyoxal. Graefes Arch. Clin. Exp. Ophthalmol. 2002;240:1022–1032. doi: 10.1007/s00417-002-0588-2. [DOI] [PubMed] [Google Scholar]
- 146.Reber F, Reber U, Funk RH. Intracellular Changes in Astrocytes and NG 108-15 Neuroblastoma X Glioma Cells Induced by Advanced Glycation End Products. J. Neural. Transm. 2003;110(10):1103–1118. doi: 10.1007/s00702-003-0031-9. [DOI] [PubMed] [Google Scholar]
- 147.Rosenbaum DM, Rosenbaum PS, Gupta H, Singh M, Aggarwal A, Hall DH, Roth S, Kessler JA. The Role of the p53 Protein in the Selective Vulnerability of the Inner Retina to Transient Ischemia. Invest. Ophthalmol. Vis. Sci. 1998;39:2132–2139. [PubMed] [Google Scholar]
- 148.Sakagami K, Wu DM, Puro DG. Physiology of Rat Retinal Pericytes: Modulation of Ion Channel Activity by Serum-Derived Molecules. J. Physiol. 1999;521(Pt 3):637–650. doi: 10.1111/j.1469-7793.1999.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M. Specific Coupling of NMDA Receptor Activation to Nitric Oxide Neurotoxicity by PSD-95 Protein. Science. 1999;284:1845–1848. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- 150.Schalkwijk CG, Ligtvoet N, Twaalfhoven H, Jager A, Blaauwgeers HG, Schlingemann RO, Tarnow L, Parving HH, Stehouwer CD, van Hinsbergh VW. Amadori Albumin in Type 1 Diabetic Patients: Correlation with Markers of Endothelial Function, Association with Diabetic Nephropathy, and Localization in Retinal Capillaries. Diabetes. 1999;48:2446–2453. doi: 10.2337/diabetes.48.12.2446. [DOI] [PubMed] [Google Scholar]
- 151.Schmidt AM, Hori O, Brett J, Yan SD, Wautier JL, Stern D. Cellular Receptors for Advanced Glycation End Products Implications for Induction of Oxidant Stress and Cellular Dysfunction in the Pathogenesis of Vascular Lesions. Arterioscler. Thromb. Vasc. Biol. 1994;14:1521–1528. doi: 10.1161/01.atv.14.10.1521. [DOI] [PubMed] [Google Scholar]
- 152.Schmidt KG. Neurodegeneration und Neuroprotektion - Aktueller Forschungsstand. Ophthalmologe. 2004;101:1059–1061. doi: 10.1007/s00347-004-1104-3. [DOI] [PubMed] [Google Scholar]
- 153.Schmidt KG. Antiglaukomatosa und choroidale Perfusion bei primärem und experimentell induziertem Offenwinkelglaukom. In: Schmidt KG, Pillunat LE, editors. Fortbildung Glaukom, Vol. 1, Perfusion und Pharmakologie. Stuttgart Enke. 1999. pp. 39–50. [Google Scholar]
- 154.Schmidt KG, Geyer O, Mittag TW. Adenylyl and Guanylyl Cyclase Activity in the Choroid. Exp. Eye Res. 2004;78:901–907. doi: 10.1016/j.exer.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 155.Schmidt KG, Klingmüller V, Gouveia SM, Osborne NN, Pillunat LE. Short Posterior Ciliary Artery, Central Retinal Artery, and Choroidal Hemodynamics in Brimonidine-Treated Primary Open-Angle Glaucoma Patients. Am. J. Ophthalmol. 2003;136(6):1038–1048. doi: 10.1016/s0002-9394(03)00631-7. [DOI] [PubMed] [Google Scholar]
- 156.Schmidt KG, Klingmüller V, v Rückmann A, Koch B. Retrobulbäre und chorioidale Hämodynamik bei Hochdruck- und Normaldruckglaukom. In: Schmidt KG, Pillunat LE, editors. Fortbildung Glaukom, Vol. 3, Perfusion und Pharmakologie. Stuttgart,Enke. 2000. pp. 103–114. [Google Scholar]
- 157.Schmidt KG, Mittag TW, Pavlovic S, Hessemer V. Influence of Physical Exercise and Nifedipine on Ocular Pulse Amplitude. Graefes Arch. Clin. Exp. Ophthalmol. 1996;234:527–532. doi: 10.1007/BF00184863. [DOI] [PubMed] [Google Scholar]
- 158.Schmidt KG, Pillunat LE, Osborne NN. Ischämie und Hypoxie - Ein Erklärungsversuch zur unterschiedlichen Absterberate retinaler Ganglienzellen bei Glaukom. Ophthalmologe. 2004;101:1071–1075. doi: 10.1007/s00347-004-1131-0. [DOI] [PubMed] [Google Scholar]
- 159.Schmidt KG, Raddatz H, Jahn C, Matthé E, Kohlhaas M, Pillunat LE. Oxidative Status in the Aqueous Humour and Serum of Primary Open Angle Glaucoma Patients. Invest. Ophthalmol. Vis. Sci. 2006;47(4):2700. [Google Scholar]
- 160.Schmidt KG, Stegmann DY, Serle JB, Garrett DT, Camras CB, Mittag TW, Podos SM. Ocular Pulse Amplitude (OPA) in Primary Open Angle Glaucoma, Low Tension Glaucoma, and in Ocular Hypertensive Patients Before and After Topical Drug Treatment. Invest. Ophthalmol. Vis. Sci. 1991;32(4):943. [Google Scholar]
- 161.Schmidt KG, v Rückmann A, Geyer O, Mittag TW. Einfluß des Nifedipins auf die okuläre Pulsamplitude bei Normaldruckglaukom. Klin. Monatsbl. Augenheilkd. 1997;210:355–359. doi: 10.1055/s-2008-1035074. [DOI] [PubMed] [Google Scholar]
- 162.Schmidt KG, v Rückmann A, Mittag TW, Hessemer V, Pillunat LE. Reduced Ocular Pulse Amplitude in Low-Tension Glaucoma is Independent of Vasospasm. Eye. 1997;11:485–488. doi: 10.1038/eye.1997.131. [DOI] [PubMed] [Google Scholar]
- 163.Schmidt KG, V Rückmann A, Pillunat LE. Dorzolamide Increases Ocular Pulse Amplitude in High Tension Primary Open Angle Glaucoma. Br. J. Ophthalmol. 1998;82:758–762. doi: 10.1136/bjo.82.7.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schönfelder U, Hofer A, Paul M, Funk RH. In Situ Observation of Living Pericytes in Rat Retinal Capillaries. Microvasc. Res. 1998;56:22–29. doi: 10.1006/mvre.1998.2086. [DOI] [PubMed] [Google Scholar]
- 165.Scivittaro V, Ganz MB, Weiss MF. AGEs Induce Oxidative Stress and Activate Protein Kinase C-beta(II) in Neonatal Mesangial Cells. Am. J. Physiol. Ren. Physiol. 2000;278:F676–F683. doi: 10.1152/ajprenal.2000.278.4.F676. [DOI] [PubMed] [Google Scholar]
- 166.Sennvik K, Bogdanovic N, Volkmann I, Fastbom J, Benedikz E. Beta-Secretase-Cleaved Amyloid Precursor Protein in Alzheimer Brain: A Morphologic Study. J. Cell. Mol. Med. 2004;8:127–134. doi: 10.1111/j.1582-4934.2004.tb00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Simm A, Münch G, Seif F, Schenk O, Heidland A, Richter H, Vamvakas S, Schinzel R. Advanced Glycation End-products Stimulate the MAP-Kinase Pathway in Tubulus Cell Line LLC-PK1. FEBS Lett. 1997;410:481–484. doi: 10.1016/s0014-5793(97)00644-3. [DOI] [PubMed] [Google Scholar]
- 168.Sims DE. Recent Advances in Pericyte Biology - Implications for Health and Disease. Can. J. Cardiol. 1991;7:431–443. [PubMed] [Google Scholar]
- 169.Soulis Liparota T, Cooper M, Papazoglou D, Clarke B, Jerums G. Retardation by Aminoguanidine of Development of Albuminuria, Mesangial Expansion, and Tissue Fluorescence in Streptozotocine Induced Rat. Diabetes. 1991;40:1328–1334. doi: 10.2337/diab.40.10.1328. [DOI] [PubMed] [Google Scholar]
- 170.Soulis T, Sastra S, Thallas V, Mortensen SB, Wilken M, Clausen JT, Bjerrum OJ, Petersen H, Lau J, Jerums G, Boel E, Cooper ME. A Novel Inhibitor of Advanced Glycation End-Product Formation Inhibits Mesenteric Vascular Hypertrophy in Experimental Diabetes. Diabetologia. 1999;42:472–479. doi: 10.1007/s001250051181. [DOI] [PubMed] [Google Scholar]
- 171.Sparrow JR, Zhou J, Ben Shabat S, Vollmer H, Itagaki Y, Nakanishi K. Involvement of Oxidative Mechanisms in Blue-Light-Induced Damage to A2E-Laden RPE. Invest. Ophthalmol. Vis. Sci. 2002;43:1222–1227. [PubMed] [Google Scholar]
- 172.Stitt AW. The Maillard Reaction in Eye Diseases. Ann. N. Y. Acad. Sci. 2005;1043:582–597. doi: 10.1196/annals.1338.066. [DOI] [PubMed] [Google Scholar]
- 173.Stitt AW, Bhaduri T, McMullen CB, Gardiner TA, Archer DB. Advanced Glycation End Products Induce Blood-Retinal Barrier Dysfunction in Normoglycemic Rats. Biochem. Biophys. Res. Commun. 2000;3:380–388. doi: 10.1006/mcbr.2000.0243. [DOI] [PubMed] [Google Scholar]
- 174.Stitt AW, Curtis TM. Advanced Glycation and Retinal Pathology During Diabetes. Pharmacol. Rep. 2005;57(Suppl):156–168. [PubMed] [Google Scholar]
- 175.Stitt AW, He CJ, Vlassara H. Characterization of the Advanced Glycation Endproduct Receptor Complex in Human Vascular Endothelial Cells. Biochem. Biophys. Res. Commun. 1999;256:549–556. doi: 10.1006/bbrc.1999.0291. [DOI] [PubMed] [Google Scholar]
- 176.Strang CE, Andison ME, Amthor FR, Keyser KT. Rabbit Retinal Ganglion Cells Express Functional {alpha}7 nAChRs. Cell Physiol. 2005;289(3):C644–C655. doi: 10.1152/ajpcell.00633.2004. [DOI] [PubMed] [Google Scholar]
- 177.Sun H, Nathans J. ABCR the ATP-Binding Cassette Transporter Responsible for Stargardt Macular Dystrophy, is an Efficient Target of All-Trans-Retinal-Mediated Photooxidative Damage in Vitro Implications for Retinal Disease. J. Biol. Chem. 2001;276:11766–11774. doi: 10.1074/jbc.M010152200. [DOI] [PubMed] [Google Scholar]
- 178.Szabo ME, Droy S, Lefaix MT, Doly M, Braquet P. Ischaemia- and Reperfusion-Induced Na+, K+, Ca2+ and Mg2+ Shifts in Rat Retina: Effects of Two Free Radical Scavengers, SOD and EGB 761. Exp. Eye Res. 1992;55:39–45. doi: 10.1016/0014-4835(92)90089-b. [DOI] [PubMed] [Google Scholar]
- 179.Takahashi K, Lam TT, Edward DP, Buchi ER, Tso MO. Protective Effects of Flunarizine on Ischemic Injury in the Rat Retina. Arch. Ophthalmol. 1992;110:862–870. doi: 10.1001/archopht.1992.01080180134041. [DOI] [PubMed] [Google Scholar]
- 180.Takagi Y, Kashiwagi A, Tanaka Y, Asahina T, Kikkawa R, Shigeta Y. Significance of Fructose-Induced Protein Oxidation and Formation of Advanced Glycation Endproduct. J. Diabetes Complicat. 1995;9:87–91. doi: 10.1016/1056-8727(94)00022-g. [DOI] [PubMed] [Google Scholar]
- 181.Tezel G, Yang X. Caspase-Independent Component of Retinal Ganglion Cell Death, In Vitro.Invest. Ophthalmol. Vis. Sci. 2004;45:4049–4059. doi: 10.1167/iovs.04-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Toda N, Kitamura Y, Okamura T. Functional Role of Nerve-Derived Nitric Oxide in Isolated Dog Ophthalmic Arteries. Invest. Ophthalmol. Vis. Sci. 1995;36:563–570. [PubMed] [Google Scholar]
- 183.Uetama T, Ohno-Matsui K, Nakahama K, Morita I, Mochizuki M. Phenotypic Change Regulates Monocyte Chemoattractant Protein-1 (MCP-1) Gene Expression in Human Retinal Pigment Epithelial Cells. J. Cell Physiol. 2003;197:77–85. doi: 10.1002/jcp.10342. [DOI] [PubMed] [Google Scholar]
- 184.Urena J, Fernandez-Chacon R, Benot AR, Alvarez de Toledo GA, Lopez-Barneo J. Hypoxia Induces Voltage-Dependent Ca2+ Entry and Quantal Dopamine Secretion in Carotid Body Glomus Cells. Proc. Natl. Acad. Sci. USA. 1994;91:10208–10211. doi: 10.1073/pnas.91.21.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vasan S, Zhang X, Zhang X, Kapurniotu A, Bernhagen J, Teichberg S, Basgen J, Wagle D, Shih D, Terlecky I, Bucala R, Cerami A, Egan J, Ulrich P. An Agent Cleaving Glucose-Derived Protein Crosslinks in Vitro and In Vivo. Nature. 1996;382:275–278. doi: 10.1038/382275a0. [DOI] [PubMed] [Google Scholar]
- 186.Veriac S, Tissie G, Bonne C. Oxygen Free Radicals Adversely Affect the Regulation of Vascular Tone by Nitric Oxide in the Rabbit Retina Under High Intraocular Pressure. Exp. Eye Res. 1993;56:85–88. doi: 10.1006/exer.1993.1012. [DOI] [PubMed] [Google Scholar]
- 187.Vickers JC. The Cellular Mechanism Underlying Neuronal Degeneration In Glaucoma: Parallels with Alzheimer’s Disease. Aust. N. Z. J. Ophthalmol. 1997;25:105–109. doi: 10.1111/j.1442-9071.1997.tb01290.x. [DOI] [PubMed] [Google Scholar]
- 188.Vishwanath V, Frank KE, Elmets CA, Dauchot PJ, Monnier VM. Glycation of Skin Collagen in Type I Diabetes Mellitus Correlation with Long-Term Complications. Diabetes. 1986;35:916–921. doi: 10.2337/diab.35.8.916. [DOI] [PubMed] [Google Scholar]
- 189.Vlassara H, Fuhm H, Makitam Z, Krungkrai S, Cerami A, Bucala R. Exogenous Advanced Glycosylation End Products Induce Complex Vascular Dysfunction in Normal Animals: A Model for Diabetic and Ageing Complications. Proc. Natl. Acad. Sci. U.S.A. 1992;89:12043–12047. doi: 10.1073/pnas.89.24.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vorwerk CK, Lipton SA, Zurakowski D, Hyman BT, Sabel BA, Dreyer EB. Chronic Low-Dose Glutamate is Toxic to Retinal Ganglion Cells Toxicity Blocked by Memantine. Invest. Ophthalmol. Vis. Sci. 1996;37:1618–1624. [PubMed] [Google Scholar]
- 191.Walsh DT, Bresciani L, Saunders D, Manca MF, Jen A, Gentleman SM, Jen LS. Amyloid Beta Peptide Causes Chronic Glial Cell Activation and Neuro-Degeneration After Intravitreal Injection. Neuropathol. Appl. Neurobiol. 2005;31(5):491–502. doi: 10.1111/j.1365-2990.2005.00666.x. [DOI] [PubMed] [Google Scholar]
- 192.Wang L, Dong J, Cull G, Fortune B, Cioffi GA. Varicosities of Intraretinal Ganglion Cell Axons in Human and Nonhuman Primates. Invest. Ophthalmol. Vis. Sci. 2003;44(1):2–9. doi: 10.1167/iovs.02-0333. [DOI] [PubMed] [Google Scholar]
- 193.Weber M, Bonaventure N, Sahel JA. Protective Role of Excitatory Amino Acid Antagonists in Experimental Retinal Ischemia. Graefes Arch. Clin. Exp. Ophthalmol. 1995;233:360–365. doi: 10.1007/BF00200485. [DOI] [PubMed] [Google Scholar]
- 194.Wheeler L, WoldeMussie E, Lai R. Role of Alpha-2 Agonists in Neuroprotection. Surv. Ophthalmol. 2003;48(Suppl. 1):S47–S51. doi: 10.1016/s0039-6257(03)00004-3. [DOI] [PubMed] [Google Scholar]
- 195.Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative Damage and Age-Related Macular Degeneration. Mol. Vis. 1999;5:32–39. [PMC free article] [PubMed] [Google Scholar]
- 196.Winter M, Eberhardt W, Scholz C, Reichenbach A. Failure of Potassium Siphoning By Müller Cells: A New Hypothesis of Perfluorocarbon Liquid-Induced Retinopathy. Invest. Ophthalmol. Vis. Sci. 2000;41:256–261. [PubMed] [Google Scholar]
- 197.WoldeMussie E, Ruiz G, Wijono M, Wheeler LA. Neuroprotection of retinal ganglion cells by brimonidine in rats with laser-induced chronic ocular hypertension. Invest. Ophthalmol. Vis. Sci. 2001;42(12):2849–2855. [PubMed] [Google Scholar]
- 198.Wood JPM, Schmidt KG, Melena J, Chidlow G, Allmeier H, Osborne NN. The β-Adrenoceptor Antagonists Metipranolol and Timolol are Retinal Neuroprotectants Comparison with Betaxolol. Exp. Eye Res. 2003;76:505–516. doi: 10.1016/s0014-4835(02)00335-4. [DOI] [PubMed] [Google Scholar]
- 199.Wolffenbuttel BH, Boulanger CM, Crijns FR, Huijberts MS, Poitevin P, Swennen GN, Vasan S, Egan JJ, Ulrich P, Cerami A, Lévy BI. Breakers of Advanced Glycation End Products Restore Large Artery Properties in Experimental Diabetes. Proc. Natl. Acad. Sci. USA. 1998;95:4630–4634. doi: 10.1073/pnas.95.8.4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yamamoto T, Kitazawa Y. Vascular Pathogenesis of Normal-Tension Glaucoma: A Possible Pathogenic Factor, Other Than Intraocular Pressure, of Glaucomatous Optic Neuropathy. Prog. Retin. Eye Res. 1998;17:127–143. doi: 10.1016/s1350-9462(97)00009-8. [DOI] [PubMed] [Google Scholar]
- 201.Yao H, Haddad GG. Calcium and pH homeostasis in neurons during hypoxia and ischemia. Cell Calcium. 2004;36(3-4):247–255. doi: 10.1016/j.ceca.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 202.Ye XD, Laties AM, Stone RA. Peptidergic Innervation of the Retinal Vasculature and Optic Nerve Head. Invest. Ophthalmol. Vis. Sci. 1990;31:1731–1737. [PubMed] [Google Scholar]
- 203.Ying W, Han SK, Miller JW, Swanson RA. Acidosis Potentiates Oxidative Neuronal Death by Multiple Mechanisms. J. Neurochem. 1999;73(4):1549–1556. doi: 10.1046/j.1471-4159.1999.0731549.x. [DOI] [PubMed] [Google Scholar]
- 204.Yoles E, Muller S, Schwartz M. NMDA-Receptor Antagonist Protects Neurons from Secondary Degeneration After Partial Optic Nerve Crush. J. Neurotrauma. 1997;14(9):665–675. doi: 10.1089/neu.1997.14.665. [DOI] [PubMed] [Google Scholar]
- 205.Yoon YH, Marmor MF. Dextromethorphan Protects Retina Against Ischemic Injury In Vivo. Arch. Ophthalmol. 1989;107:409–411. doi: 10.1001/archopht.1989.01070010419037. [DOI] [PubMed] [Google Scholar]
- 206.Zhang F, Lu C, Severin C, Sretavan DW. GAP-43 Mediates Retinal Axon Interaction with Lateral Diencephalon Cells During Optic Tract Formation. Development. 2000;127(5):969–980. doi: 10.1242/dev.127.5.969. [DOI] [PubMed] [Google Scholar]