Abstract
Objective
The aim of this study was to assess the clinical role of 18F-FDG PET/CT for the evaluation of lymph node metastasis in periorbital malignancies, compared with CT alone.
Materials and Methods
We analyzed eighteen PET/CT and CT scans in 15 patients with biopsy-proven periorbital malignancies. We compared the diagnostic capabilities of PET/CT and CT with regard to nodal metastasis by level-by-level analysis and by N staging prediction. The reference standards were surgical pathology (n = 7) from dissected lymph node specimens and the results from radiological follow-up (n = 11, mean 20.5 months; range 10-52 months). Moreover, any changes in patient care as prompted by PET/CT were recorded and compared with treatment planning for CT alone.
Results
PET/CT had a sensitivity of 100%, while CT had a sensitivity of 57% (p = 0.03) for nodal metastasis by level-by-level analysis. PET/CT had a specificity of 97%, positive predictive value of 93%, negative predictive value of 100%, and diagnostic accuracy of 98%, while the CT values for these same parameters were 97%, 89%, 82%, and 84%, respectively. PET/CT correctly predicted N staging with an accuracy of 100%, while CT was only 83% accurate (p = 0.01). Regarding the impact on patient care, the extent of surgery for regional lymph nodes and the treatment decision were modified by PET/CT in 39% of patients.
Conclusion
PET/CT could provide useful information in the management of regional lymph node metastases in patients with periorbital malignancies.
Keywords: 18F-FDG, PET/CT, Computed tomography (CT) scans, Lymphatic Metastasis, Eyelid Neoplasm
In periorbital malignancies, regional lymph node metastasis occurs through lymphatic pathways from the upper or lower eyelids. Depending on the site of the primary tumor, draining lymph nodes could be located in the parotid area or cervical region (1, 2). The rate of regional metastasis has been reported to be 10% to 24% in squamous cell carcinomas of the eyelid (3, 4) and 17% to 28% in sebaceous carcinomas (4, 5).
In a previous study, investigators reported the pattern of regional lymph node metastasis in patients with malignant tumors in the periorbital region (6). Four of seven tumors (two squamous cell carcinomas, two sebaceous cell carcinomas) involving the medial half of the periorbital area exhibited lymphatic spread to the lymph nodes around the parotid gland first, and then submandibular and upper cervical lymph node spread. Proper management of regional metastasis is as important as control of local recurrence in obtaining successful treatment outcomes in periorbital malignancies. Surgical treatment for regional metastasis includes cervical lymph node dissection with parotidectomy; therefore precise assessment of the extent of disease is essential to treatment planning.
Contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI) have been the mainstay imaging modalities for the diagnosis and staging of primary lesions, as well as regional lymph node metastasis, in periorbital malignancies. Positron emission tomography with 18F-fluorodeoxyglucose integrated with computed tomography (PET/CT) has been reported to be more accurate than CT or MRI for the detection of malignant tissues, while glucose uptake levels show a good correlation with histological grading for head and neck cancers (7). With regard to head and neck cancers, PET/CT has a sensitivity of 98%, specificity of 92%, and accuracy of 94%, and its accuracy is higher than that seen with PET or CT alone (8). In addition, PET/CT has the clear potential to affect the care of patients with head and neck cancer (9).
Because PET/CT has the potential for helping stage patients with head and neck cancer, identifying responses to non-surgical therapy, and allowing earlier detection of recurrence, specific recommendations for its use should be delineated to determine which patients will get cost-effective benefits from undergoing PET/CT (10). For example, our previous data showed that PET/CT imaging does not provide additional information over laryngoscopic examination or CT at the initial tumor evaluation, nor does it have a major impact on clinical decision-making in patients with glottic cancer (11).
However, little information is available concerning the application of PET/CT in periorbital malignancies (12-16), and the role of PET/CT in the evaluation of regional lymph node metastasis has not been reported in any other study.
The aim of the present study was to assess the diagnostic efficacy of PET/CT for the evaluation of regional lymph node metastasis in patients with periorbital malignancies and to determine the impact of PET/CT on treatment planning.
MATERIALS AND METHODS
Subjects
The Institutional Review Board of the Samsung Medical Center approved this study. Patient informed consent was not required for this retrospective analysis, though radiological examination-related informed consents were obtained from all patients before PET/CT and CT were performed.
The inclusion criteria for this study were as follows: 1) pathologically confirmed malignant tumor in the periorbital region-we defined the periorbital region as the upper and lower eyelid and their appendages, in addition to the lacrimal glands, 2) both PET/CT and CT performed in each patient, with the interval between the two studies being less than one month. Based on these criteria, 15 cases of periorbital malignant tumors were retrieved from the Samsung Medical Center Orbital Cancer Data Registry for the period spanning 1996 to 2007. For these patients, pathological diagnoses were reconfirmed through intra-department consultations.
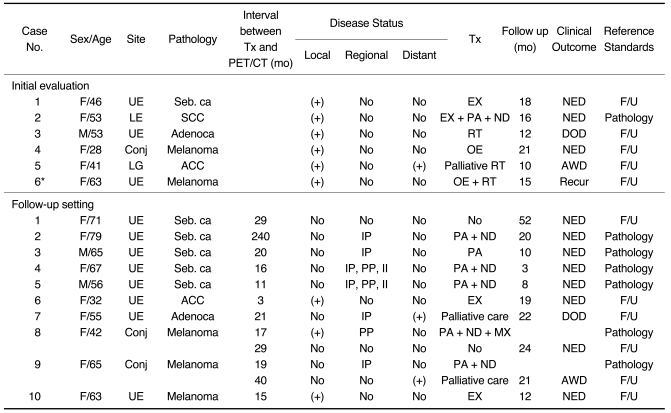
Subject data are presented in Table 1. The mean patient age was 55.3 years (range 28 to 79 years). Three patients were male, and 12 were female. The eyelid was the most commonly involved primary tumor site (11 cases, 73.3%); other sites of involvement included the conjunctiva and lacrimal gland. Pathological diagnoses included six sebaceous carcinomas (40.4%), four malignant melanomas (26.6%), two adenocarcinomas (13.3%), two adenoid cystic carcinomas (13.3%), and one squamous cell carcinoma (6.8%). Surgical treatment alone was performed in 11 cases (73.4%), surgery with adjuvant radiotherapy in two cases (13.3%), and radiotherapy alone in two cases (13.3%). The median follow-up duration was 17.9 months (range 3-52 months). Eleven patients (73.4%) have survived without evidence of disease, whereas two patients are still alive with disease, and two patients have died of disease. A total of 18 PET/CT and CT scans were performed in 15 cases. Most of the scans were performed during the follow-up period (12 scans, 66.7%), and in some cases (6 scans, 33.3%), the scans were done as part of the initial diagnostic work-up. The reference standards for PET/CT and CT were surgical pathology (n = 7) from dissected lymph node specimens and data from radiological follow-up (n = 11, duration 10-52 months, mean 20.5 months). Radiological follow-up included two or more subsequent imaging studies (CT, MRI, or bone scans) for suspected lesions during the follow-up period.
Table 1.
Characteristics of Subjects who had Undergone PET/CT and CT Scans (n = 15)
Note.-UE = upper eyelid, LE = lower eyelid, Conj = conjunctiva, LG = lacrimal gland, Seb. ca = sebaceous carcinoma, SCC = squamous cell carcinoma, Adenoca = adenocarcinoma , ACC = adenoid cystic carcinoma, Tx = treatment, IP = intra-parotid lymph nodes, PP = peri-parotid lymph nodes, II = upper jugular cervical lymph nodes, EX = mass excision, PA = parotidectomy, ND = neck lymph node dissection, RT = radiation therapy, OE = wide excision of tumor with orbital exenteration, MX = maxillectomy, NED = no evidence of disease, DOD = died of disease, AWD = alive with disease, F/U = follow up, *This patient had follow-up PET/CT (number 10 in follow-up setting).
Contrast-Enhanced CT and 18F-FDG PET/CT Scans
CT scans (LightSpeed Ultra or Ultra 16, GE, Milwaukee, WI) were performed using the following parameters: 160 mAs, 120 KeV, section width of 3.75 mm, and table feed of 8.75 mm per rotation. For contrast enhancement, 90 mL of an iodinated contrast agent (Ultravist 300, Schering, Berlin) was injected intravenously at 3 mL/sec using an automated injector. The scan delay time was 30 seconds.
As for PET/CT scans, all patients fasted for at least six hours prior to the examinations, which were performed using a Discovery LS PET/CT scanner (GE Healthcare, Milwaukee, WI). Whole body CT scanning was performed using a continuous spiral technique with an 8-slice helical CT with a gantry rotation speed of 0.8 sec. The CT scan data were collected using the following parameters: 80 mAs, 140 KeV, section width of 5 mm, and table feed of 5 mm per rotation. No intravenous or oral contrast agents were used. Following the CT scan, and after the intravenous injection of 370 MBq 18F-FDG, an emission scan was performed from the thigh to the head at 5 minutes per frame, for a total of 45 minutes. The uptake phase duration was 45 minutes. The attenuation-corrected 18F-FDG PET images from the CT data were reconstructed with an ordered subset expectation maximization algorithm (28 subsets, 2 iterations). The images were displayed in a 128×128 matrix (pixel size = 4.29×4.29 mm, with a slice thickness of 4.25 mm). The separate CT and PET scan data were accurately co-registered using commercial software (eNTEGRA, Elgems, Haifa, Israel). The standardized uptake values (SUVs) were acquired using the attenuation-corrected images, the amount of injected 18F-FDG, the body weight of each patient, and the cross-calibration factors between the 18F-FDG PET and the dose calibrator.
Data Analysis
For analysis, regional lymph nodes were divided into lymph node levels (intra-parotid and peri-parotid lymph node groups, levels I, IIa, IIb, III) based on the AJCC staging manual (6th edition, 2002). The peri-parotid lymph node group was defined to encompass the pre-auricular lymph nodes and the infra-parotid lymph nodes. Pathological data were available in 23 dissected node levels, and radiological follow-up examination was used in 20 node levels. As a result, a total of 43 node levels were assessed with CT and PET/CT scans in the present study. A radiologist specializing in head and neck imaging reviewed the CT scans, and a nuclear medicine physician reviewed the PET/CT scans. Both of them were blinded to the clinical and pathological information for the patients.
The criteria for abnormal lymph nodes on CT included spherical or conglomerated shape, enlarged size (> 1.5 cm at levels I and II, > 1 cm at level III), or enhancement pattern of contrast media. Regarding the PET/CT scans, the nuclear medicine physician first reviewed the images to evaluate for any abnormal FDG uptake in the salivary glands and neck nodes by using the maximal uptake values with intensity higher than that of surrounding tissues. The interpretation was then revised based on the anatomical information provided by the combined PET/CT images.
Treatment planning was addressed in the multidisciplinary head and neck tumor conference along with the results of imaging studies. To compare the diagnostic accuracy of both imaging studies for regional metastasis, pathological data in the cases of surgery (n = 7) or the follow-up results of radiological evaluation (n = 11) were used as a reference standard. Image findings were confirmed by radiological follow-up alone for eleven scans, with a mean follow-up duration of 20.5 months (range 10-52 months).
We compared the sensitivity (SN), specificity (SP), positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy (DA) in predicting regional lymph node metastasis on level-by-level analysis and N staging. Statistical differences between the imaging modalities were analyzed employing the McNemar test, and 95% confidence levels were determined using Wilson's method. Two-tailed p-values less than 0.05 were considered statistically significant. The impact of PET/CT on patient care was also assessed with regard to changes in the extent of surgery or in treatment planning.
RESULTS
Diagnostic Values for Regional Lymph Node Metastasis
Primary tumor evaluation information was only available in five of 18 scans; the tumors were diagnosed correctly using PET/CT scans for the initial work-up in four cases and in one recurrent case. SUVs in the primary tumors ranged from 2.9 to 7.5 (median 4). In our series, the maximum SUV ranged from 2.2 to 8.1 (mean 5.3) for lymph node metastases from sebaceous carcinoma, and from 2.0 to 38.7 from malignant melanoma. One patient with adenocarcinoma lymphatic metastasis showed FDG uptake in lymph nodes with maximum SUV = 18.7.
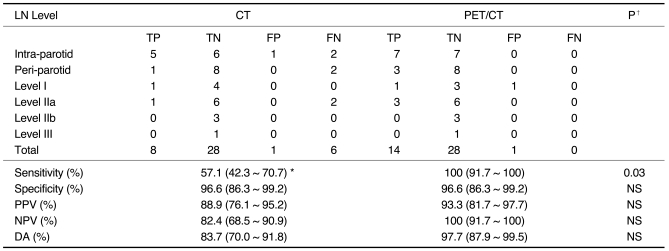
On the lymph node level-by-level analysis, a total of 43 node levels were assessed with CT and PET/CT (Table 2). There were 14 malignant node levels, with seven intra-parotid lymph nodes, three peri-parotid lymph nodes, one level I lymph node group, and three level IIa lymph node groups. Interestingly, metastasis to the cervical node levels (I, II, III) was always coincident with metastasis to peri- or intra-parotid nodes. CT had an SN of 57%, SP of 97%, PPV of 89%, NPV of 82%, and DA of 84%. For PET/CT, corresponding values were 100%, 97%, 93%, 100%, and 98%, respectively. PET/CT had a value of 100% for SN and NPV. Of note, the difference in the sensitivity value between CT and PET/CT was statistically significant, with a p value of 0.03.
Table 2.
Diagnostic Values for Contrast-Enhanced CT and PET/CT with Regard to Regional Lymph Node Metastasis in Patients with Periorbital Malignancies on Lymph Node Level-by-Level Analysis (n = 43 node levels)
Note.-LN level = lymph node level, TP = true positive, TN = true negative, FP = false positive, FN = false negative, PPV = positive predictive value, NPV = negative predictive value, DA = diagnostic accuracy, NS = not statistically significant *95% CI = 95% confidence interval using Wilson's method. ‡ Comparison of diagnostic values between CT and PET/CT using McNemar test.
In the prediction of N staging according to the AJCC staging manual (6th edition, 2002), PET/CT showed 100% accuracy (95% confidence interval: 82.4-100%), while CT showed 83% accuracy (95% confidence interval: 60.7-94.1%, p = 0.01).
Impact of PET/CT on Patient Care
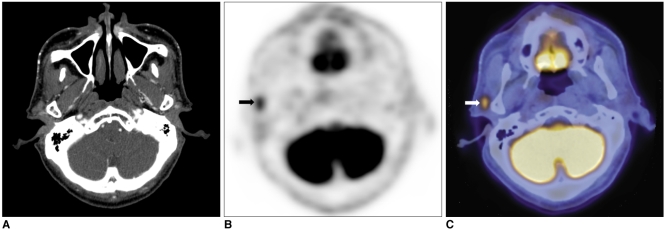
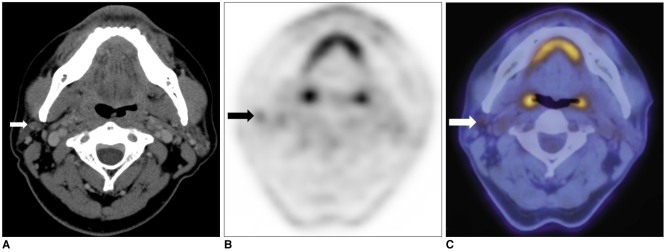
The extent of the surgical field for regional lymph nodes was changed in the case of three PET/CT scans (3/18, 16.6%). In two of the three cases, intra-parotid metastatic lymph nodes were detected on PET/CT, but not on CT. A 65-year-old male patient underwent eyelid and orbit exenteration secondary to biopsy-proven sebaceous carcinoma in the upper eyelid extending to the levator muscle and bulbar conjunctiva. After 16 months, a small node was detected in the ipsilateral parotid area during a follow-up visit. Contrast-enhanced CT scan showed no abnormal mass lesion in the parotid area (Fig. 1). On the contrary, PET/CT revealed a high glucose uptake lesion with an SUV of 4.7 at the same site; lymph node metastasis was strongly suspected. A parotidectomy was performed, and metastatic sebaceous carcinoma was diagnosed in an intra-parotid lymph node. Another 67-year-old female patient was suspected of having recurrent sebaceous carcinoma in the parotid area. Intra-parotid and peri-parotid metastatic lymph nodes were equally detected on CT and PET/CT, but the level II lymph node was diagnosed as containing malignancy only on PET/CT. As a result, the surgical extent was expanded into the level II area; a metastatic lymph node was demonstrated pathologically. Another 56-year-old male patient who had undergone orbital exenteration for sebaceous carcinoma in the upper eyelid was suspected of having regional recurrence in the ipsilateral parotid area (Fig. 2). On CT, a 0.8 cm sized lymph node was detected just behind the angle of the mandible, but the contrast enhancement was not definite, and the lymph node maintained an oval shape, so a radiologist diagnosed it as an insignificant lymph node. However, PET/CT showed an asymmetrical lesion with an SUV of 2.2, suggesting metastasis. As a result, the extent of surgery was changed to include the above lymph node group, which proved to have metastasis on pathology.
Fig. 1.
65-year-old male patient with sebaceous carcinoma in upper eyelid.
A. On contrast-enhanced CT, no definite lesion is detected around parotid gland.
B, C. On PET/CT, malignant lesion with high glucose uptake of SUV 4.7 is diagnosed at same site (arrows). According to fused image, malignant lesion is located around parotid gland.
Fig. 2.
56-year-old male patient with sebaceous carcinoma in upper eyelid.
A. On contrast-enhanced CT, upper jugular lymph node behind angle of mandible is considered benign, with pattern of low enhancement and oval shape (arrow).
B, C. On PET/CT, same lesion showed asymmetrical glucose uptake with SUV 2.2 and is diagnosed as metastatic lymph node (arrows). As result, surgical fields were extended to include specific lymph node, which was pathologically proven to have metastatic lesion.
Three patients in this study had distant metastases that were first detected by PET/CT. The pathology of the primary tumors included an adenocarcinoma in the upper eyelid, a melanoma in the conjunctiva, and an adenoid cystic carcinoma in the upper eyelid. PET/CT during the follow-up period detected distant metastases in the brain, pancreas, liver, stomach, or cervical spine, so treatment plans were changed to palliative care, including palliative chemotherapy or radiotherapy.
In one case (Case No. 1 in Table 1), a malignant lymph node was suspected in the peri-parotid region on CT, contradictory to the PET/CT findings, which suggested a benign lymph node. Surgical treatment was withheld until after confirmation using fine needle aspiration biopsy, and the patient still shows no evidence of disease in that area after 18 months of follow-up.
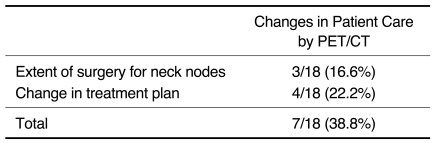
As shown in Table 3, PET/CT affected treatment by correctly modifying the surgical extent or treatment decision in seven cases (38.8%).
Table 3.
Impact on Patient Care: Contrast-enhanced CT versus PET/CT (n = 18 CT and PET/CT scans)
DISCUSSION
Basal cell carcinoma and squamous cell carcinoma are the most common malignancies arising in the periorbital region, with malignant melanoma and sebaceous carcinoma occurring less frequently (2). As in most solid tumors, the presence of regional lymph node metastasis affects treatment outcomes and prognosis profoundly. Regional metastases from periorbital malignancies spread through the lymphatic system, so careful evaluation of the regional lymph nodes is important. In this study, we included the three conjunctival melanomas for analysis, because they showed frequent metastasis to regional lymph nodes and distant sites, similar to other periorbital cancers.
In a previous case study (6), our group observed no direct tumor drainage to the submandibular lymph nodes-even from primary tumors located on the medial portion of the eyelid-without initial metastasis to the lymph nodes around the parotid gland (first echelon lymph node); this is concordant with the results of the present study.
CT, with its high sensitivity (93%) (17), has been the mainstay imaging modality for diagnosing periorbital malignancies. However, the sensitivity of CT in the present study was as low as 57%. This is attributable to false negative lesions at peri-parotid and intra-parotid sites. Some of these lesions were interpreted as inflammatory hyperplasia or infected cysts due to the absence of distinct features of malignant parotid tumors, such as lobular or irregular contour of the mass or ill-defined tumor margin. According to recent reports (18, 19), the higher sensitivity and diagnostic accuracy of PET/CT endows it with many advantages over CT alone in managing malignancies around the salivary gland, especially high-grade types. The present study also showed that PET/CT was effective in detecting lymphatic spread to the parotid region and cervical nodes in the setting of periorbital malignancies.
Among the 15 subjects enrolled in this study, seven had true regional lymph node metastasis (46.7%). Interestingly, all seven had recurrent metastasis in regional lymph nodes. The duration between the treatment of the primary tumor and the detection of regional recurrence ranged from 11 months to 240 months. On standard follow-up, PET/CT accurately diagnosed seven cases of regional recurrence. However, CT alone misdiagnosed two cases as negative for recurrence.
Distant metastases occurred in three cases. The metastatic sites were diverse, including brain, cervical spine, pancreas, and lung. Distant metastasis was detected in the cervical spine in one patient using PET/CT as an initial evaluation, and palliative treatment instead of curative surgery was implemented as a result.
The higher sensitivity of PET/CT vindicated it as a useful screening method in the evaluation of regional lymph node metastasis, particularly in the follow-up setting. PET/CT had a higher NPV (100%) than did CT alone (82.4%), although this difference was not statistically significant. Prediction of N stage was also more reliable with PET/CT than with CT alone. Therefore, PET/CT can provide more accurate information about prognosis through revision of N staging of periorbital malignancies.
In practice, how much diagnostic methods affect treatment decision-making is of great concern for clinicians. In this study, PET/CT had a positive impact on patient care by correctly modifying the treatment plan in approximately 40% of the patients.
Errors in interpretation of abnormal lymph node FDG uptake may be instigated in cases of larygopharyngeal inflammation. However, several diagnostic clues help differentiate lymph nodes metastasis from inflammation. First, asymmetrical abnormal lymph node uptake strongly suggests metastasis, because inflammation frequently causes bilateral FDG uptake. Malignant tumors in the periorbital area have lymph nodes around the parotid area as the first-echelon nodal group (6). Thus, without abnormal uptake in the lymph nodes around the parotid area, the increase of FDG uptake in the upper cervical lymph nodes, which commonly occurs in laryngo-pharyngitis, may be demarcated from the lymph node metastasis of periorbital malignancy. In addition, an SUV of 2.0 in lymph nodes can be used as a cut-off value in determining the presence of metastasis, based on our results.
Our study has some limitations. First, this was a retrospective analysis with a small number of cases. Prospective study with a larger number of cases is needed to fully assess the role of PET/CT in the management of lymph node metastasis in the setting of periorbital malignancies. Second, heterogeneity of the neoplasms in the present study prompted the question of the radiographic equivalence of different tumors on PET/CT. Characteristics of FDG uptake may vary among different pathologies, and it would be premature to claim that PET/CT is equally effective in all periorbital malignancies. Third, our data does not answer the question of when to perform PET/CT in the setting of periorbital malignancies, though our results showed that PET/CT provided more accurate information about nodal status when lymphatic metastases were suspected. These questions demand a further study enrolling a large number of patients.
Nevertheless, this study showed that PET/CT could provide more accurate diagnostic information regarding lymph node status. Furthermore, it was more reliable in predicting N staging in the setting of periorbital malignancies than was CT alone. PET/CT also had a significant impact on therapeutic decision-making.
Footnotes
This work was supported by the Samsung Medical Center Clinical Research Development Program, Grant # CRS 107-54-2.
References
- 1.Limawararut V, Leibovitch I, Sullivan T, Selva D. Periocular squamous cell carcinoma. Clin Experiment Ophthalmol. 2007;35:174–185. doi: 10.1111/j.1442-9071.2006.01411.x. [DOI] [PubMed] [Google Scholar]
- 2.Cook BE, Jr, Bartley GB. Treatment options and future prospects for the management of eyelid malignancies: an evidence-based update. Ophthalmology. 2001;108:2088–2098. doi: 10.1016/s0161-6420(01)00796-5. [DOI] [PubMed] [Google Scholar]
- 3.Donaldson MJ, Sullivan TJ, Whitehead KJ, Williamson RM. Squamous cell carcinoma of the eyelids. Br J Ophthalmol. 2002;86:1161–1165. doi: 10.1136/bjo.86.10.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reifler DM, Hornblass A. Squamous cell carcinoma of the eyelid. Surv Ophthalmol. 1986;30:349–365. doi: 10.1016/0039-6257(86)90089-5. [DOI] [PubMed] [Google Scholar]
- 5.Kass LG, Hornblass A. Sebaceous carcinoma of the ocular adnexa. Surv Ophthalmol. 1989;33:477–490. doi: 10.1016/0039-6257(89)90049-0. [DOI] [PubMed] [Google Scholar]
- 6.Jeong HS, Son YI, Baek CH. The pattern of lymphatic metastasis of malignant tumors in the periorbital area. Am J Otolaryngol. 2006;27:5–8. doi: 10.1016/j.amjoto.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Nowak B, Di Martino E, Jänicke S, Cremerius U, Adam G, Zimny M, et al. Diagnostic evaluation of malignant head and neck cancer by F-18-FDG PET compared to CT/MRI. Nuklearmedizin. 1999;38:312–318. [PubMed] [Google Scholar]
- 8.Branstetter BF, 4th, Blodgett TM, Zimmer LA, Snyderman CH, Johnson JT, Raman S, et al. Head and neck malignancy: is PET/CT more accurate than PET or CT alone? Radiology. 2005;235:580–586. doi: 10.1148/radiol.2352040134. [DOI] [PubMed] [Google Scholar]
- 9.Schoder H, Yeung HW, Gonen M, Kraus D, Larson SM. Head and neck cancer: clinical usefulness and accuracy of PET/CT image fusion. Radiology. 2004;231:65–72. doi: 10.1148/radiol.2311030271. [DOI] [PubMed] [Google Scholar]
- 10.Zimmer LA, Branstetter BF, Nayak JV, Johnson JT. Current use of 18F-fluorodeoxyglucose positron emission tomography and combined positron emission tomography and computed tomography in squamous cell carcinoma of the head and neck. Laryngoscope. 2005;115:2029–2034. doi: 10.1097/01.MLG.0000181495.94611.A6. [DOI] [PubMed] [Google Scholar]
- 11.Jeong HS, Chung MK, Baek CH, Choi JY, Son YI, Kim HJ, et al. Combined 18F-FDG PET/CT imaging for the initial evaluation of glottic cancer. Clin Exp Otorhinolaryngol. 2008;1:35–40. doi: 10.3342/ceo.2008.1.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donaldson MJ, Pulido JS, Mullan BP, Inwards DJ, Cantrill H, Johnson MR, et al. Combined positron emission tomography/computed tomography for evaluation of presumed choroidal metastases. Clin Experiment Ophthalmol. 2006;34:846–851. doi: 10.1111/j.1442-9071.2006.01364.x. [DOI] [PubMed] [Google Scholar]
- 13.Lane KA, Bilyk JR. Preliminary study of positron emission tomography in the detection and management of orbital malignancy. Ophthal Plast Reconstr Surg. 2006;22:361–365. doi: 10.1097/01.iop.0000235493.82052.37. [DOI] [PubMed] [Google Scholar]
- 14.Roe RH, Finger PT, Kurli M, Tena LB, Iacob CE. Whole-body positron emission tomography/computed tomography imaging and staging of orbital lymphoma. Ophthalmology. 2006;113:1854–1858. doi: 10.1016/j.ophtha.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 15.Valenzuela AA, Allen C, Grimes D, Wong D, Sullivan TJ. Positron emission tomography in the detection and staging of ocular adnexal lymphoproliferative disease. Ophthalmology. 2006;113:2331–2337. doi: 10.1016/j.ophtha.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 16.Wild D, Eyrich GK, Ciernik IF, Stoeckli SJ, Schuknecht B, Goerres GW. In-line (18)F-fluorodeoxyglucose positron emission tomography with computed tomography (PET/CT) in patients with carcinoma of the sinus/nasal area and orbit. J Craniomaxillofac Surg. 2006;34:9–16. doi: 10.1016/j.jcms.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Kim KH, Sung MW, Yun JB, Han MH, Baek CH, Chu KC, et al. The significance of CT scan or MRI in the evaluation of salivary gland tumors. Auris Nasus Larynx. 1998;25:397–402. doi: 10.1016/s0385-8146(98)00012-1. [DOI] [PubMed] [Google Scholar]
- 18.Jeong HS, Chung MK, Son YI, Choi JY, Kim HJ, Ko YH, et al. Role of 18F-FDG PET/CT in management of high-grade salivary gland malignancies. J Nucl Med. 2007;48:1237–1244. doi: 10.2967/jnumed.107.041350. [DOI] [PubMed] [Google Scholar]
- 19.Roh JL, Ryu CH, Choi SH, Kim JS, Lee JH, Cho KJ, et al. Clinical utility of 18F-FDG PET for patients with salivary gland malignancies. J Nucl Med. 2007;48:240–246. [PubMed] [Google Scholar]