Abstract
INTRODUCTION
C-reactive protein (CRP) is an acute-phase protein used clinically to diagnose infectious and inflammatory disease and monitor response to treatment. CRP measurement in the peri-operative period was audited and patterns of change analysed for elective general surgical patients.
PATIENTS AND METHODS
General surgical patients (201) admitted for elective general surgery over a 3-month period were considered for the study. CRP results pre- and postoperatively were recorded, and data on co-morbid conditions and surgical procedure were noted.
RESULTS
CRP was requested pre-operatively on 84% of patients. A high CRP was more likely to be found in patients with co-morbidity. Postoperatively, CRP was requested during the first 3 days on 69% of patients. CRP peaked at postoperative days two or three, and then fell. In patients who had a high pre-operative CRP, the peak CRP was higher and occurred later, than those who had a normal pre-operative CRP.
CONCLUSIONS
CRP requesting pre-operatively is common, but is not recommended in NICE guidelines. Postoperatively, CRP levels rise; as a result, its use as a tool to screen for infection is limited. CRP has a role in diagnosis of infection after the first three postoperative days and in monitoring response to treatment. Therefore, routine use of CRP measurements pre-operatively and in the first 2 or 3 days post-operatively is not recommended. A peri-operative CRP should only be requested if there is a clear clinical indication.
Keywords: C-reactive protein, Peri-operative management, Audit, Elective surgery
C-reactive protein (CRP) is an acute-phase protein with a half-life of 19 h.1 It is an acute-phase reactant synthesised and secreted by the liver, largely in response to interleukin-6 and other pro-inflammatory cytokines. CRP binds to various ligands including phospholipids exposed on bacterial surfaces, damaged and dying eukaryotic cells, and cellular nuclear debris. Once bound, CRP activates the classical complement cascade, binds Fcγ receptors and stimulates phagocytosis (for review, see Black et al.2). A rise in CRP concentrations in serum may be seen with infection, inflammation, trauma, malignancy and tissue infarction.3 It is, therefore, not specific for a particular disease. A rise in CRP may be seen earlier in a disease process than other non-specific markers (e.g. fever), and falls rapidly on resolution of inflammation.3 CRP may, therefore, be useful as a screening test to detect an inflammatory response early in its course, and also for monitoring disease activity and response to therapy in conditions where CRP is raised.
The National Institute for Health and Clinical Excellence (NICE) has recently published guidelines on pre-operative testing for elective surgery.4 These guidelines do not recommend routine CRP testing on patients admitted for elective surgery, although this was not explicit since CRP was not considered as part of the panel of pre-operative tests examined. No guidelines on post-operative testing have been published to date. The aims of this study were to: (i) examine the practice of pre-operative CRP requesting in a UK teaching hospital and compare this with the NICE guidelines; and (ii) analyse the practice of postoperative CRP testing and review the existing literature such that guidelines could be set.
Patients and Methods
All patients admitted to the University Hospital of Wales (UHW) for elective surgery under general surgeons during October, November and December 2005 were considered for the study retrospectively. The study was approved by the Department of Clinical Audit at UHW. Patient details were provided by the Department of Clinical Audit and data on CRP requesting and co-morbidity were collected using Cardiff and Vale NHS Trust Clinical Portal and examining patients' clinical notes. CRP results were collected on patients pre-operatively (pre-operative admission clinic or following admission and before surgery) and on the first 5 days postoperatively, where requested by attending clinicians. Patients who had prolonged pre-operative admissions (≥ 5 days) were excluded from the study. CRP was measured in the Department of Medical Biochemistry and Immunology at UHW using a turbidometric immunoprecipitation assay on an Abbot Aeroset c8000 analyser. Statistical analysis was carried out using the unpaired t-test. Statistically significant differences were identified as P < 0.05.
Results
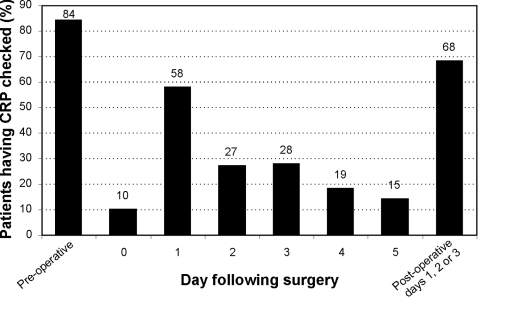
A total of 201 patients were admitted from the waiting list for general surgery during October, November and December 2005, under 12 general surgeons. Nine patients were subsequently excluded from the analysis for prolonged pre-operative admission. Results showed 84% of patients had their CRP checked pre-operatively (Fig. 1). Table 1 demonstrates that 56% of patients who had CRP checked had CRP in the normal range (< 6 mg/l). 5% had a CRP ≥ 30 mg/l. The remainder had mild elevations in CRP (40%). Table 2 shows that where patients had no recorded co-morbidity, CRP measurements were lower than where co-morbidity was recorded (P < 0.001; unpaired t-test). When co-morbidity groups were analysed (Table 2), patients with cancer, diabetes and renal disease showed most patients with high CRP (≥ 30 mg/l) on admission, although there were only a small number of patients with renal disease.
Figure 1.
Peri-operative CRP requests for elective surgery.
Table 1.
Cumulative CRP results on 164 pre-operative elective surgery patients
| CRP (mg/l) | Patients (%) |
|---|---|
| < 6 | 56 |
| < 10 | 74 |
| < 20 | 91 |
| < 30 | 95 |
Table 2.
Pre-operative CRP results (where measured) according presence or absence of co-morbidity
| No co-morbidity | Co-morbidity | CA | DM | CVS | RS | Renal | |
|---|---|---|---|---|---|---|---|
| No. of patients | 37 | 127 | 53 | 21 | 53 | 29 | 8 |
| CRP < 6 mg/l (%) | 70 | 52 | 42 | 43 | 51 | 38 | 25 |
| CRP < 10 mg/l (%) | 92 | 69 | 58 | 62 | 66 | 55 | 50 |
| CRP < 20 mg/l (%) | 100 | 88 | 83 | 81 | 89 | 83 | 62 |
| CRP < 30 mg/l (%) | 100 | 93 | 89 | 86 | 96 | 97 | 75 |
Patients may have more than one co-morbid condition. Conditions grouped according to category. Cumulative results shown.
Co-morbidities: CA, cancer; DM, diabetes mellitus; CVS, cardiovascular system; RS, respiratory system.
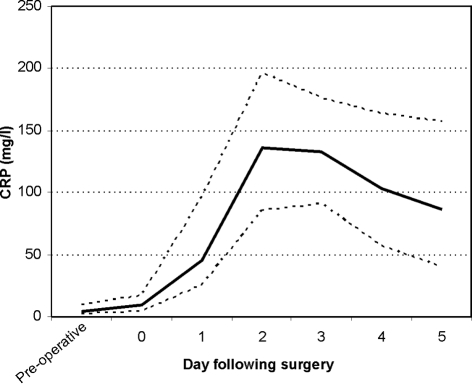
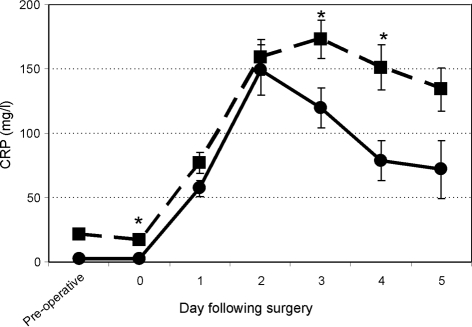
A total of 402 CRP requests were made pre-operatively and during the first 3 days postoperatively during the study period. Postoperatively, 69% of patients had CRP checked one or more times in the first 3 days; the majority had it checked on day 1 (Fig. 1). After the third postoperative day, less than 20% of patients had their CRP checked. Figure 2 shows that, where tested, CRP was raised in the first 3 days following surgery, and that this peaked at day 2. CRP levels subsequently fell over the following 2 days. The postoperative CRP profile was also compared between those patients with a normal pre-operative CRP and those with a high pre-operative CRP (Fig. 3). Those with a high pre-operative CRP peaked postoperatively at day 3, compared to those with a normal CRP, who peaked at day 2. The high CRP group also had postoperative CRP levels that remained elevated for longer. The difference between the two groups was statistically significant (P < 0.05) on days 0, 3 and 4 postoperatively. The correlation coefficient r between surgical grade and pre-operative CRP was 0.032, indicating no correlation between the two variables.
Figure 2.
CRP concentrations following elective surgery. Data shown as median (solid line) and interquartile range (dashed lines).
Figure 3.
Postoperative CRP responses comparing high pre-operative CRP (dashed line; n = 73) and low pre-operative CRP (solid line; n = 90). Data are shown as mean ± SEM. *P < 0.05.
Discussion
This study has demonstrated that CRP testing is a common practice in pre-operative assessment of elective general surgery patients, and that the majority of patients also have CRP measured postoperatively. Pre-operatively, most patients with no co-morbidity had a normal or only slightly elevated CRP. Where co-morbidity was present, CRP results were more likely to be abnormal. Current NICE guidelines do not recommend routine CRP testing in pre-operative assessment of elective surgery patients.4
Pre-operative measurement of CRP has been shown to be a prognostic indicator for both oesophageal and colorectal carcinoma.5–7 This is due, in part, to the secretion of CRP by the tumour8 and was shown to be independent of tumour stage. There is some evidence that patients with a raised pre-operative CRP who undergo oesophagectomy are more likely to develop postoperative complications,5 although this may be confounded by other variables and does not relate specifically to infection. In patients undergoing cardiac surgery, a raised pre-operative CRP has been shown to be a risk factor for postoperative infection and predicts higher inhospital mortality.9,10 It has, therefore, been suggested that pre-operative CRP measurement may be useful to risk-stratify cardiac surgery patients. However, it is unclear how patient management would be altered as a consequence of finding a high CRP, and there are no data to indicate that clinical decisions taken on this basis influence the rate of infection or other surgical complications. We suggest that further studies are needed to assess whether reducing inflammatory status and, therefore, CRP in patients with chronically activated immune systems prior to surgery makes a difference to outcome. We also suggest that, if CRP is to be measured prior to surgery, it should be done early enough to influence pre-operative and surgical management without delaying the scheduled operation. It should, therefore, not be assessed in the immediate pre-operative period, where the focus is on assessing the patient for likely anaesthetic and surgical complications. In addition, the non-specific nature of CRP measurements suggests results should be interpreted with caution when used to evaluate prognosis and the likelihood of postoperative complications.
A rise in CRP is known to occur as a result of surgical trauma, and peaks at 48 h postoperatively.11,12 However, the CRP response in individual patients is highly variable,11 and in some patients may be incomplete or even absent.13 Some authors have noted a link between the peak postoperative CRP response and degree of surgical trauma,14 but we did not find such a correlation when assessing CRP response to different grade surgical procedures. However, our study was not designed to identify such a link, and most studies indicate that lower CRP responses are seen in laparoscopic procedures compared to the equivalent open procedure,15,16 although not all studies agree.14 It was noted that CRP was measured postoperatively in the majority of patients we studied; therefore, we analysed these results further. This showed that CRP, where measured, was raised in the postoperative period, peaked at days 2–3 and then fell. This confirmed that a raised CRP is to be expected postoperatively in a wide range of elective surgical procedures and agrees with previous studies. We also noted that patients with a high CRP pre-operatively had, on average, a higher and later CRP peak postoperatively than did those that had a normal CRP response, a finding that has also been noted in cardiac surgery patients.10 This did not appear to correlate with surgical operation grade, and may be a consequence of inflammatory co-morbidity in addition to surgical trauma. Importantly, it is these patients who have been shown to be at increased risk of infection postoperatively; the increased CRP response noted, therefore, makes the use of CRP as a screening tool for infection even more problematic in this situation.
We did not see any correlation between CRP level and occurrence of infection in the first 3 days; indeed, other studies have confirmed that CRP is not a good indicator of the presence of early postoperative infection.17 However, a rising CRP after the second or third postoperative day may indicate infection,12,18–20 and CRP has a clear role in monitoring clinical response to treatment when infection is diagnosed.21 Some authors have advocated developing reference ranges for postoperative CRP levels22 and the use of two or more markers in detecting infection after the third post-operative day;23 at present, it is not clear if such an approach would be of general use since studies so far have looked at specific types of operation in determining reference ranges.
Conclusions
Routine use of CRP measurements pre-operatively and in the first 2–3 days postoperatively is not recommended. The trauma of surgery confounds results and makes interpretation difficult. If CRP is to be used for screening for infection in high-risk patients, levels should be checked on the 3rd and 5th postoperative days. Further investigations should be instituted if a rise in CRP is seen. In line with general recommendations in the NICE guidelines, a peri-operative CRP should be requested only where there is a specific clinical reason for doing so, i.e. the result is likely to alter patient management, or is required to monitor response to treatment.
References
- 1.Vigushin DM, Pepys MB, Hawkins PN. Metabolic and scintigraphic studies of radioiodinated human C-reactive protein in health and disease. J Clin Invest. 1993;91:1351–7. doi: 10.1172/JCI116336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black S, Kushner I, Samols D. C-reactive protein. J Biol Chem. 2004;279:48487–90. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- 3.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institute for Health and Clinical Excellence. Guideline development group Pre-operative tests. The use of pre-operative tests for elective surgery: evidence, methods and guidance. London: NICE; 2003. [Google Scholar]
- 5.Gockel I, Dirksen K, Messow CM, Junginger T. Significance of preoperative C-reactive protein as a parameter of the perioperative course and long-term prognosis in squamous cell carcinoma and adenocarcinoma of the oesophagus. World J Gastroenterol. 2006;12:3746–50. doi: 10.3748/wjg.v12.i23.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nozoe T, Matsumata T, Kitamura M, Sugimachi K. Significance of preoperative elevation of serum C-reactive protein as an indicator for prognosis in colorectal cancer. Am J Surg. 1998;176:335–8. doi: 10.1016/s0002-9610(98)00204-9. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen HJ, Christensen IJ, Sørensen S, Moesgaard F, Brünner N. Preoperative plasma plasminogen activator inhibitor type-1 and serum C-reactive protein levels in patients with colorectal cancer. The RANX05 Colorectal Cancer Study Group. Ann Surg Oncol. 2000;7:617–23. doi: 10.1007/BF02725342. [DOI] [PubMed] [Google Scholar]
- 8.Nozoe T, Korenaga D, Futatsugi M, Saeki H, Maehara Y, Sugimachi K. Immunohistochemical expression of C-reactive protein in squamous cell carcinoma of the esophagus – significance as a tumor marker. Cancer Lett. 2003;192:89–95. doi: 10.1016/s0304-3835(02)00630-4. [DOI] [PubMed] [Google Scholar]
- 9.Fransen EJ, Maessen JG, Elenbaas TWO, van Aamhem EEHL, Stobberingh E, Visschers H, et al. Enhanced preoperative C-reactive protein plasma levels as a risk factor for postoperative infections after cardiac surgery. Ann Thorac Surg. 1999;67:134–8. doi: 10.1016/s0003-4975(98)00973-4. [DOI] [PubMed] [Google Scholar]
- 10.Cappabianca G, Paparella D, Visicchio G, Capone G, Lionetti G, Numis F, et al. Preoperative C-reactive protein predicts mid-term outcome after cardiac surgery. Ann Thorac Surg. 2006;82:2170–8. doi: 10.1016/j.athoracsur.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 11.Colley CM, Fleck A, Goode AW, Muller BR, Myers MA. Early time course of the acute phase protein response in man. J Clin Pathol. 1983;36:203–7. doi: 10.1136/jcp.36.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White J, Kelly M, Dunsmuir R. C-reactive protein level after total hip and total knee replacement. J Bone Joint Surg Br. 1998;80:909–11. doi: 10.1302/0301-620x.80b5.8708. [DOI] [PubMed] [Google Scholar]
- 13.Bourguignat A, Férard G, Jenny JY, Gaudias J. Incomplete or absent acute phase response in some postoperative patients. Clin Chim Acta. 1997;264:27–35. doi: 10.1016/s0009-8981(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 14.Brewster N, Guthrie C, McBirnie J. CRP levels as a measure of surgical trauma: a comparison of different general surgical procedures. J R Coll Surg Edinb. 1994;39:86–8. [PubMed] [Google Scholar]
- 15.Grande M, Tucci GF, Adorisio O, Barini A, Rulli F, Neri A, et al. Systemic acutephase response after laparoscopic and open cholecystectomy. Surg Endosc. 2002;16:313–6. doi: 10.1007/s00464-001-9042-5. [DOI] [PubMed] [Google Scholar]
- 16.Hildebrandt U, Kessler K, Plusczyk T, Pistorius G, Vollmar B, Menger MD. Comparison of surgical stress between laparoscopic and open colonic resections. Surg Endosc. 2003;17:242–6. doi: 10.1007/s00464-001-9148-9. [DOI] [PubMed] [Google Scholar]
- 17.Giannoudis PV, Smith MR, Evans RT, Bellamy MC, Guillou PJ. Serum CRP and IL-6 levels after trauma. Not predictive of septic complications in 31 patients. Acta Orthop Scand. 1998;69:184–8. doi: 10.3109/17453679809117625. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi J, Ebara S, Kamimura M, Kinoshita T, Itoh H, Yuzawa Y, et al. Early-phase enhanced inflammatory reaction after spinal instrumentation surgery. Spine. 2001;26:1698–704. doi: 10.1097/00007632-200108010-00014. [DOI] [PubMed] [Google Scholar]
- 19.Mustard RA, Jr, Bohnen JM, Haseeb S, Kasina R. C-reactive protein levels predict postoperative septic complications. Arch Surg. 1987;122:69–73. doi: 10.1001/archsurg.1987.01400130075011. [DOI] [PubMed] [Google Scholar]
- 20.Bianchi RA, Silva NA, Natal ML, Romero MC. Utility of base deficit, lactic acid, microalbuminuria, and C-reactive protein in the early detection of complications in the immediate postoperative evolution. Clin Biochem. 2004;37:404–7. doi: 10.1016/j.clinbiochem.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Khan MH, Smith PN, Rao N, Donaldson WF. Serum C-reactive protein levels correlate with clinical response in patients treated with antibiotics for wound infections after spinal surgery. Spine J. 2006;6:311–5. doi: 10.1016/j.spinee.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Lindberg M, Hole A, Johnsen H, Asberg A, Rydning A, Myrvold HE, et al. Reference intervals for procalcitonin and C-reactive protein after major abdominal surgery. Scand J Clin Lab Invest. 2002;62:189–94. doi: 10.1080/003655102317475443. [DOI] [PubMed] [Google Scholar]
- 23.Férard G, Gaudias J, Bourguignat A, Ingenbleek Y. C-reactive protein to transthyretin ratio for the early diagnosis and follow-up of postoperative infection. Clin Chem Lab Med. 2002;40:1334–8. doi: 10.1515/CCLM.2002.230. [DOI] [PubMed] [Google Scholar]