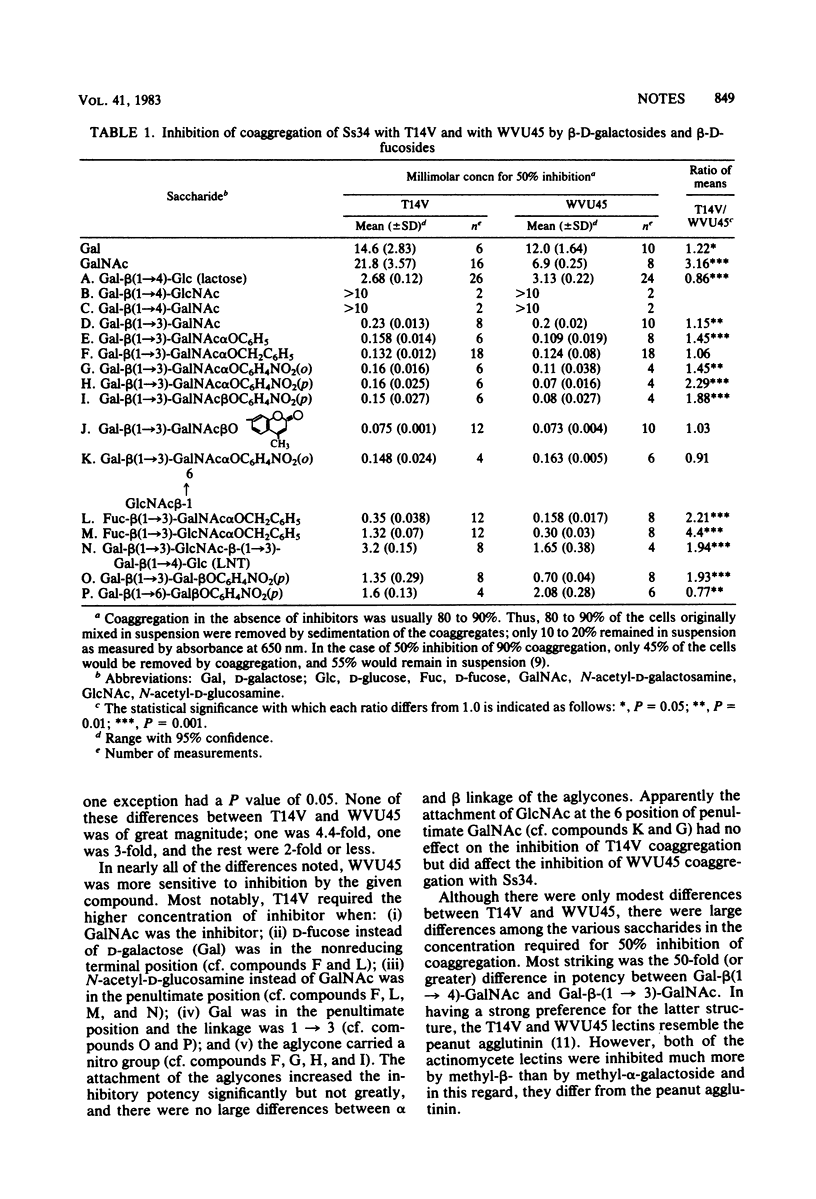
Abstract
Specificities of lectins on Actinomyces viscosus T14V and Actinomyces naeslundii WVU45 were compared by measuring the abilities of D-galactose, N-acetyl-D-galactosamine, 14 beta-D-galacto-oligosaccharides, and 2 beta-D-fuco-oligosaccharides to inhibit coaggregation between Streptococcus sanguis 34 and each actinomycete. Inhibition profiles were similar, but WVU45 was significantly more sensitive to several inhibitors. D-Galactose-beta(1 leads to 3)-N-acetyl-D-galactosamine glycosides were most potent.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cisar J. O., Barsumian E. L., Curl S. H., Vatter A. E., Sandberg A. L., Siraganian R. P. Detection and localization of a lectin on Actinomyces viscosus T14V by monoclonal antibodies. J Immunol. 1981 Oct;127(4):1318–1322. [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Interbacterial aggregation of plaque bacteria. Arch Oral Biol. 1970 Dec;15(12):1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E., Williams B. L. Lactose-reversible coaggregation between oral actinomycetes and Streptococcus sanguis. Infect Immun. 1981 Jul;33(1):95–102. doi: 10.1128/iai.33.1.95-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda N., Ellen R. P., Fillery E. D., Grove D. A. Chemical and immunological comparison of surface fibrils of strains representing six taxonomic groups of Actinomyces viscosus and Actinomyces naeslundii. Infect Immun. 1983 Mar;39(3):1325–1333. doi: 10.1128/iai.39.3.1325-1333.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Crosby L. K., Vatter A. E. Inhibitors of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34: beta-galactosides, related sugars, and anionic amphipathic compounds. Infect Immun. 1982 Apr;36(1):371–378. doi: 10.1128/iai.36.1.371-378.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M. E., Kabat E. A., Lotan R., Sharon N. Immunochemical studies on the specificity of the peanut (Arachis hypogaea) agglutinin. Carbohydr Res. 1976 Oct;51(1):107–118. doi: 10.1016/s0008-6215(00)84040-9. [DOI] [PubMed] [Google Scholar]


