Abstract
INTRODUCTION
Pruritus ani is a common condition with many causes, predominately anorectal pathology. There are some new insights and therapies, but the most recommendations are based on low-level evidence.
PATIENTS AND METHODS
A literature search was carried out using Medline and the internet with the keywords ‘pruritus ani’ from 1950 to 2007.
RESULTS
A review of the evidence is presented and a management plan based on the elimination of irritants and scratching, general control measures and active treatment measures is offered.
CONCLUSIONS
Treatment of primary and secondary pruritus ani has a good prospect of regression of symptoms and skin changes.
Keywords: Pruritus ani, Itch, Capsaicin, Anal tattoo
Pruritus ani is defined as intense chronic itching affecting peri-anal skin. It affects 1–5% of the population, is four times more common in men and is most frequent between the fourth and sixth decades of life.1,2 The symptoms range from mild to intense and depression may result when these are severe and persistent.1
Pruritus ani is classified as idiopathic when no cause can be found. However, as 75% of cases have co-existing pathology, a detailed history and examination are necessary.3 Nearly 100 different causes for pruritus ani have been reported. The majority of co-existing conditions are anorectal, predominately haemorrhoids and fissures,3 hence the number of referrals to coloproctologists. Dermatological conditions are usually not restricted to the peri-anal area, but the morphology of peri-anal skin lesions may be atypical for the disease elsewhere.
Treatment varies dependent upon the physician's interest in this unfashionable condition where patients are embarrassed to present. There are few systematic studies on the management of pruritus ani, so recommendations are based on low-level evidence. Nevertheless, physicians with an interest in pruritus ani have published good results with most people responding well, even though many suffer short-lived relapses.2
The goal of treatment is asymptomatic, intact, dry, clean peri-anal skin with reversal of morphological changes. The aim of this review is to provide a systematic method of diagnosis and management of pruritus ani. This is presented after an overview of how individual aetiologies relate to anal pruritus, the indications and pitfalls of the therapies available.
A literature search was carried out using Medline and the internet with the keywords ‘pruritus ani’ from 1950 to 2007.
Aetiological factors
Perineal faecal contamination
Faecal contamination causing pruritus ani is not simply a matter of prolonged contact with a moist substance or a hygiene issue, nor is it inevitable. Faecal contamination or soiling may be overt or occult. Occult soiling is insufficient for an individual to be aware, but enough to initiate itch and scratching. Peri-anal skin also reacts differently from skin elsewhere, as shown by Caplan.4 Skin-patch testing using autologous faeces produced anal symptoms in a third of patients with pruritus ani and in 53% of asymptomatic individuals.4 Itch mostly occurred within 6 h and was alleviated by washing suggesting irritation rather than an allergy. Only 4% of cases developed irritation with a faecal patch test on the arm.
Any factor which increases occult or overt soiling augments exposure of the peri-anal skin to irritants and is a potential area of therapy. Studies have investigated stool consistency and mucous seepage as an aetiological factor. One found 50% of patients with pruritus ani to have loose stools and this group reported at least weekly faecal soiling in 41%.5 Another study found patients with idiopathic pruritus ani to have a high incidence of loose stools, drink large volumes of fluids and were rarely constipated.6 Exaggerated recto-anal inhibitory reflexes and earlier incontinence were found in anorectal physiology tests in patients with pruritus ani.7–9 Anal sphincter relaxation in reaction to rectal distension may allow occult or overt soiling, as may coffee which lowered anal resting pressures in over 70% of cases.5
Altered anal morphology may lead to faecal soiling and this could be a primary or post surgical problem. These individuals seem to be unable to evacuate their anal canals completely and the retained faecal material escaped later with resultant itch.
Prolonged faecal contact is irritant to peri-anal skin and, as a group, patients with pruritus ani are less able to maintain absolute continence when dealing with loose stools and an anorectum whose physiology and morphology has been altered primarily by food, surgery or, presumably, by medications. Unfortunately, there are no controlled clinical trials regarding the benefit of altered personal hygiene in managing pruritus ani. However, it is widely accepted and recommended by most in the literature and is discussed later. Bulk-forming agents and loperamide are recommended to improve stools consistency.
Peri-anal infection
Although occurring in the minority, the importance of bacterial and fungal infection should not be underestimated. Fungal infections account for up to 15% of pruritus ani.5,10–12 Dermatophytes should be considered pathogenic and treated.12 Candida albicans is comensal, but becomes pathogenic in a diabetic individual, after steroid and systemic antibiotic use; it should be eradicated in these cases.11,12 Surgery for anal conditions eradicated Candida spp. and dermatophytes in over 85% in one study.13 Candida spp. re-infection may occur via a colonised partner and they too may need therapy.
Threadworms infect multiple family members. Peri-anal viral infections commonly cause itch, but there is no evidence for their causative role in idiopathic pruritus ani.
Sexually transmitted bacterial infections cause pruritus, but chronic symptoms are likely to be caused by other bacteria. β-Haemolytic streptococci,11 Staphylococcus aureus11 and Corynebacterium minutissimum5,11,14 infection can lead to itch lasting over a year. Erythrasma is the cutaneous infection caused by C. minutissimum and frequently infects other sites such as toes and groins;11 it is best diagnosed by Wood's light fluorescence and accounted for 1–18% of pruritus ani in different series.5,11,14 Erythromycin eradicated the infection in most. S. aureus was found in one series and was managed with topical antibiotics.11 β-Haemolytic streptococci may result in erythematous, odematous and eczema-like skin and occur in children in the majority of cases. It is rarely reported in adults and can be treated with oral antibiotics together with a topical steroid combined with an antifungal and antibiotic.15 Detection of bacteria needs specific media and collection to avoid false negatives.16
Allergic contact dermatitis
Contact dermatitis results in erythema, scaly skin and vesicle formation. This can be the result of sensitisation by chemicals found in cleansing and therapeutic preparations including creams, soaps, wet wipes, glyceryl trinitrate, anaesthetic, toilet paper dye and other topical preparations.11,17 Sensitising agents are also found in topical steroid preparations where initial relief results in use over a wider area and growing problem.10 Of pruritus ani patients, 69% were positive in a skin patch test study and 38 of 55 cases were from therapeutic agents.18 For this reason, patients should avoid contact with the irritant and soap. Emollient creams are useful, as perhaps the short-term use of topical steroids, unless secondarily infected. However, most creams and ointments do not sensitise the peri-anal skin in most people. Ointments have fewer preservatives and constituents than creams. They are less likely to sensitise or be washed off.
Food
Foods have been implicated in idiopathic pruritus ani such as caffeinated drinks, alcohol, milk products, peanuts, spices, citrus, grapes, tomato (histamine) and chocolate; some researchers have claimed diminution of itch within 14 days if these were avoided.3,5,6 Gradual re-introduction was then used to calculate acceptable daily levels. However, there are no controlled clinical trials that provide evidence that dietary exclusions improve symptoms. Nevertheless, most of the literature suggests that dietary measures should be tried. Mechanisms by which foods are thought to cause itch are reduction in anal sphincter pressures, exaggerated anal reflexes and undigested foods sensitising the peri-anal skin. In addition, they result in looser stools, quicker transit time and greater stool frequency and, therefore, increased faecal seepage and anal trauma from recurrent wiping.
Colorectal and anal disease
Any co-existing anal conditions can precipitate or exacerbate itch. Up to 52% of patients with pruritus ani have anorectal disease with haemorrhoids being the commonest condition.3,5,11,17 In one series, a quarter of the proctological causes of pruritus ani were anal or colorectal cancer.19 There was no evidence in the literature supporting any one particular therapy for haemorrhoids. Functional bowel disorders may contribute and haemorrhoids may add to sphincter dysfunction and faecal seepage. Many recommend treatment of all colorectal and anal conditions with some excellent results.11,17 Pirone et al.13 suggested that anal surgery contributed to the elimination of peri-anal fungal infection and together reduced pruritus.
Dermatological conditions and neoplasia
Psoriasis has been found in 5–55% of patients with pruritus ani.5,11,17 Isolated peri-anal plaques can occur and have a sharp edge; they may not be characteristic as they are altered by repeated scratching10,11 and the histological appearances are altered by topical treatment and trauma.
Peri-anal skin in lichen sclerosis is white, atrophic, wrinkled and biopsy features are typical. Potent topical steroids used for 8 weeks usually results in regression of the condition. Similar to vulval lichen sclerosis, skin should be biopsied in non-responders or recurrent sclerosis as there is a 5% incidence of squamous cell carcinoma.
Half of patients with peri-anal Paget's disease and peri-anal Bowen's disease have associated itch. These skin lesions have well-defined limits. Other skin cancers present with pain and bleeding.
Steroid-induced and other medications
Potent topical steroids are used sparingly as they can cause thinned skin, acute dermatitis and contact dermatitis from sensitisation. There is also the rebound itch after cessation requiring further use which has been described as an addiction.20 Some orally ingested medications such as laxatives, colpermin, colchicine, quinidine, peppermint oil and some antibiotics seem can lead to peri-anal itch.1,2 This could be a direct irritant on the peri-anal skin or indirectly by loosening stools and increasing anal seepage.
Clothing
There is no evidence on the causative effect of clothes, but heat and sweat exacerbated itch. Patients should wear clothes and underwear that allow air circulation and dryness. Seasonal pruritus may be caused by clothes that increase moisture retention or sweating. Residue remaining after the use of biological enzyme based detergents may result in itch and these should be avoided.
Systemic disease and psychological factors
Any illness can lead to pruritus ani most commonly diabetes mellitus, liver disease, leukaemia, lymphoma, renal failure, iron-deficiency anaemia and hyperthyroidism.
Anxiety, stress and certain personality traits may contribute and they should be treated concurrently.5 In fact, pruritus ani may be a manifestation of depression or psychological disturbance.21
Diagnosis
History
Patients may not relate peri-anal itch to skin symptoms elsewhere. Co-existing skin disease, atopy, urticaria and other allergies should be questioned for. Many consider over-the-counter topical therapies to be devoid of side effects or not as a medicine, but these products may have caused the symptoms or alter skin morphology. Direct inquiry of their utilisation is essential, as is questioning of previous patch testing, illness, diarrhoea and treatments such as antibiotics and steroids. Intermittent or seasonal itch may represent recurrent anorectal pathology, different clothes or laundry detergents.
Examination
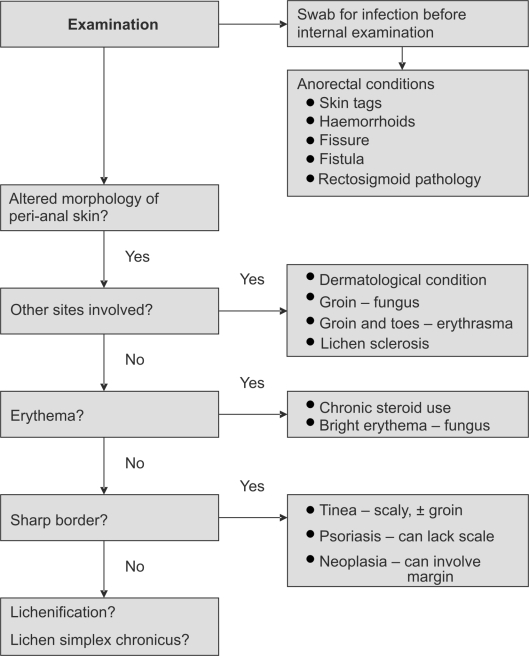
A full examination may reveal other sites of skin disease or infection (Fig. 1). A distinct boundary suggests tinea, psoriasis or neoplasia. The extent of neoplasia extends beyond this sharp border. Erythema is seen with chronic topical steroid use and bright erythema suggests yeast infection. Hyperpigmentation is the result of any chronic inflammation, so infection and chronic discharge should be sought. Lichen sclerosis nearly always involves the labia or perineum. Severe itch with multiple lesions suggests herpes and palpable groin nodes point to neoplasia and sexually transmitted infections. Idiopathic inflammation has indistinct borders and is non-specific visually. In severe cases, the skin becomes lichenified – thickened, leathery, exaggerated skin folds, fissures and erosions.1 Chronic trauma results in lichen simplex chronicus.
Figure 1.
Examination algorithm for pruritis ani.
Investigations
The concurrent rate of infection is significant; however, as microbiological investigations are frequently incorrectly performed, false negatives occur resulting in treatment failure. Fungal and bacterial specimens should be placed in bacterial transport medium and refrigerated, anaerobic samples need specific anaerobic medium and storage at room temperature, vesicles should be unroofed and exudates placed on a slide or viral culture medium. Streptococcus spp., Staphylococcus spp., C. minutissimum and fungus should be sought in all. Water-soluble lubricant has bactericidal activity, so swabs should be taken before internal examination. Skin scarping can be sent for fungal culture and microscopy.
Biopsies should include neighbouring normal skin as well as affected skin. In persistent cases, skin-patch testing should be carried out.19
Treatment
Dermatological conditions should be treated by an appropriate specialist. The patient should be informed about the chronic nature of the condition, not just to reduce the expectation of immediate cure, but also to improve compliance with advice given. At any stage, if the response is poor, one must reconsider the diagnosis. An information pamphlet should be provided, either prepared in-house or available online.22
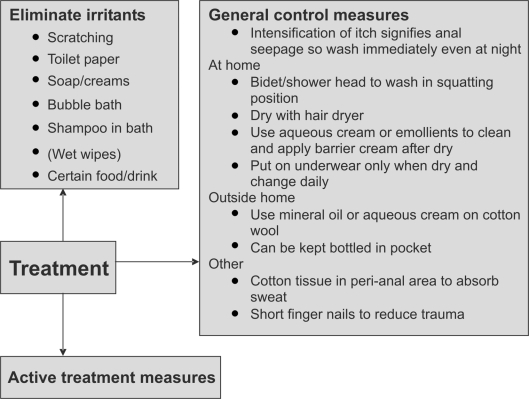
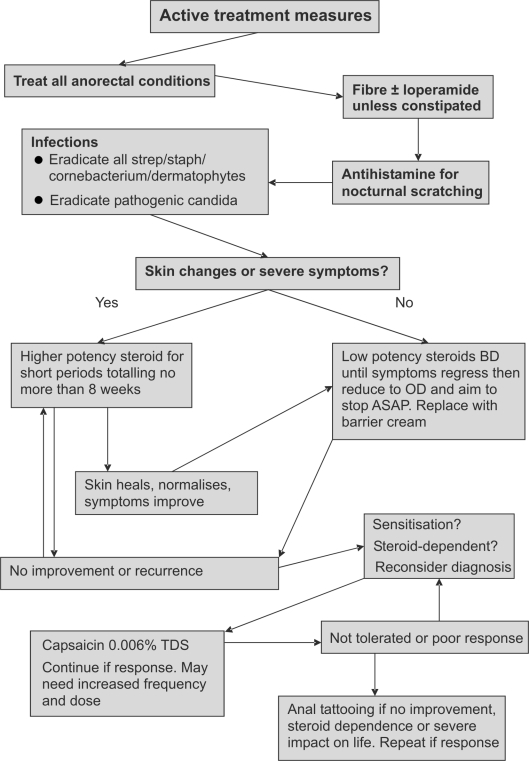
Management has three components which function in parallel – elimination of irritants and scratching, general control measures and active treatment measures (Figs 2 and 3).
Figure 2.
Treatment options for pruritis ani: eliminate irritants and general control measures.
Figure 3.
Treatment options for pruritis ani: active treatment measures.
Elimination step
Eliminate irritants such as creams, soaps, bubble baths, toilet paper, scratching, certain foods (Table 1) and drinks.1,2 If a shampoo or any other cleanser is used whilst in the bath, the perineum should be rinsed free of residue after with a shower head. A list of foods that have been associated with itching should be provided. An elimination diet may be attempted and symptom diary kept. Clothes should be washed in non-perfumed detergent. Patients must be warned that, if itch worsens after the use of wet wipes, their use they must be stopped immediately in case of sensitisation.
Table 1.
Foods associated with pruritus ani
| Most common | Coffee (caffeinated and decaffeinated) |
| Tea | |
| Cola | |
| Other caffeinated drinks | |
| Alcohol, especially beer and wine | |
| Chocolate | |
| Tomato including ketchup (histamine) | |
| Others | Milk products |
| Peanuts and nuts | |
| Spices | |
| Citrus fruits | |
| Grapes | |
| Popcorn | |
| Spicy foods | |
| Prunes | |
| Figs |
General control measures
A bidet or shower head should be used to wash the perineum, without soap, and dried with a hair dryer. The perineal cleansing should ideally be in the squatting positions so the anal canal is washed of retained faeces. Aqueous cream or emollients should be used instead of soap and barrier creams, such as zinc oxide; alternatively, petroleum ointment can be applied after washing.1,2 The perineum should be dry before underwear is worn which should be changed daily. Acute itch is a marker of faecal seepage and immediate cleansing is the most effective remedy, especially for nocturnal itch. Patients should be given advice on how to cleanse when outside their homes. Small containers of oil-based preparations (balneol) or aqueous cream tubes take up little space and the contents are squeezed onto cotton wool to clean the perineum. Cotton tissue can be placed peri-analy to absorb moisture in those who sweat excessively. Patient groups advocate short finger nails to reduce trauma from inadvertent nocturnal scratching. Unfortunately, there have been no randomised, controlled, clinical studies evaluating the impact of creams or ointments in pruritus ani, but individual series using the above advice achieve impressive results.
Active treatment
All dermatophyte infections should be treated in all with a topical imidazole or terbinafine. Candida spp. should be treated with a topical imidazole if thought to be pathogenic or in non-responders; in practice, this equates to the majority. β-Haemolytic streptococci, S. aureus and C. minutissimum should be eliminated with topical antibiotics such as fusidic acid or mupirocin, and oral antibiotics may be necessary especially if the itch has a long history (1 year) or there are skin changes present. Other sites may also need to be treated.
All anorectal conditions should be sought and treated, as even small skin tags may hide faecal residue or trap moisture perpetuating the condition.
If not constipated, seepage may be reduced by adding fibre to the diet and loperamide.1,2
Systemic antihistamines may reduce nocturnal scratching; however, as this is probably a marker of anal seepage, the patient should be advised to wash the area immediately and apply a barrier cream. There have been no randomised trials exploring the usefulness of antihistamines in pruritus ani, but some series have reported them to have some effect against peri-anal itch.23 Sedating antihistamines may be effective by aiding sleep rather than local inhibition. Topical antihistamines are not potent enough and sensitise the skin.
Mild-to-moderate symptoms with minimal skin changes can be treated with a weak topical steroid such as 1% hydrocortisone applied morning and night after washing and can be combined with antibacterials or antifungals.1,2 Once symptoms regress, application can be reduced while substituting in a barrier cream. Patients should be informed of side effects and the need to limit treatment duration. Severe symptoms and skin changes require stronger topical steroids, but for a definite period of time of no more than 8 weeks unless directed by a specialist. Once the skin heals or normalises, switch to a lower potency topical steroid. Side effects occur much less frequently with 1% hydrocortisone than potent topical steroids, but the maximum safe duration has not been described. It would seem reasonable to allow weak steroids to be used for significantly longer than the 8 weeks, but not indefinitely. There is a 1000-fold difference in potency between the weak and the most potent steroid preparations. Three preparations should be agreed upon with the pharmacy to provide low-, medium- and high-potency treatments. Ointments are less prone to skin atrophy when compared to creams with the same constituents,24 as are double-ester, non-fluorinated steroids.24,25 Some have advocated the use of topical immune modulators (such tacrolimus), which avoid skin atrophy and have antifungal activity,26 but no studies have yet investigated this. Interestingly, a solitary study found cleansing with a liquid cleanser to be as effective as topical corticosteroid in symptom control. However, like much of the literature in pruritus ani, the study's conclusion should be treated with caution as allocation to treatment arms was not randomised.27 Systemic corticosteroids should not be used, but there is one case report of the benefit of intralesional corticosteroid injection with no relapse after 1 year.28
The short, intense, burning sensation produced by topical capsaicin produces an inhibitory feedback which may eliminate to need to scratch. A randomised, cross-over study showed topical 0.006% capsaicin cream applied three times a day to be superior to placebo (1% methanol) in those who had pruritus ani for greater than 3 months. Overall, 31 of 44 (70%) patients had a response to capsaicin, 8 had no response, 1 had response equally with capsaicin and methanol and 4 withdrew because of side effects. Response was seen within 3 days, most within 1 day. Capsaicin caused a mild peri-anal burning sensation. Three of the four who dropped out of the study had moderate-to-severe burning and the fourth case developed urticaria. It must be noted that methanol also caused peri-anal burning, but to a lesser degree. The burning sensation caused by capsaicin decreased with time and two others with moderate burning completed the trial. Of the 31 patients whose symptoms improved with capsaicin and wished to continue therapy, 29 required an average of a once daily application to keep symptoms at bay when followed up for a mean of 10.9 months.29 Some needed increased frequency of application and two increased the dose to 0.012%, but this still remains much lower than doses used in other pain studies (0.025–0.075%). Currently in the UK, only a 0.025% preparation is available which can be diluted by a pharmacy.
Anal tattooing should be considered in those who have failed other treatment measures, become steroid dependent or in whom symptoms severely impact on quality of life. The original series reported complete relief in 13 of 26 patients, partial in 8 more and no response in 9%. Unfortunately, 3 of the 26 cases had skin necrosis.30 The technique was modified and the next 11 patients had good results with no ulcers.31 The modified technique involves several intradermal and subcutaneous injections of 10 ml 1% methylene blue + 5 ml normal saline + 7.5 ml 0.25% bupivocaine with adrenaline (1/200,000) + 7.5 ml 0.5% lignocaine in prone jack-knife under sedation or general anaesthesia in the anodermal and peri-analy. Others have also reported good results (76–88% at 1 year), some requiring repeat injections up to 5 years after32–34 without the universal use of antibiotic prophylaxis. However, further modifications to the mixture were made in these studies such as adding hydrocortisone. As well as managing symptoms, the skin changes resolve. Most have some hypo-aesthesia, which may take a year to recover. This lead to the suggestion that nerve endings had been destroyed by the methylene blue,30 which is supported by an electron microscopy study.35 The tattoo should be visible for 2–6 weeks, any less suggests placement of the solution too deeply.32
There is no place for the topical use of anaesthetics as they do not alter the disease, provide relief for only a few hours and sensitise the skin. Hypnosis has been used, but there is insufficient evidence available for its recommendation.
Conclusions
Pruritus ani has many causes mostly dermatological or anorectal, treatment of which results in regression of symptoms and skin changes. If after a rigorous history, examination and investigations a cause cannot be found, pruritus ani is described as idiopathic. These patients can still be managed with great success with the elimination of irritants and scratching, general control measures and active treatment measures.
References
- 1.Chaudhry V, Bastawrous A. Idiopathic pruritus ani. Semin Colon Rectal Surg. 2003;14:196–202. [Google Scholar]
- 2.Hanno R, Murphy P. Pruritus ani. Classification and management. Dermatol Clin. 1987;5:811–16. [PubMed] [Google Scholar]
- 3.Daniel GL, Longo WE, Vernava AM., III Pruritus ani. Causes and concerns. Dis Colon Rectum. 1994;37:670–4. doi: 10.1007/BF02054410. [DOI] [PubMed] [Google Scholar]
- 4.Caplan RM. The irritant role of feces in the genesis of perianal itch. Gastroenterology. 1966;50:19–23. [PubMed] [Google Scholar]
- 5.Smith LE, Henrichs D, McCullah RD. Prospective studies on the etiology and treatment of pruritus ani. Dis Colon Rectum. 1982;25:358–63. doi: 10.1007/BF02553616. [DOI] [PubMed] [Google Scholar]
- 6.Friend WG. The cause and treatment of idiopathic pruritus ani. Dis Colon Rectum. 1977;20:40–2. doi: 10.1007/BF02587451. [DOI] [PubMed] [Google Scholar]
- 7.Allan A, Ambrose NS, Silverman S, Keighley MR. Physiological study of pruritus ani. Br J Surg. 1987;74:576–9. doi: 10.1002/bjs.1800740710. [DOI] [PubMed] [Google Scholar]
- 8.Eyers AA, Thomson JP. Pruritus ani: is anal sphincter dysfunction important in aetiology? BMJ. 1979;2:1549–51. doi: 10.1136/bmj.2.6204.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farouk R, Duthie GS, Pryde A, Bartolo DC. Abnormal transient internal sphincter relaxation in idiopathic pruritus ani: physiological evidence from ambulatory monitoring. Br J Surg. 1994;81:603–6. doi: 10.1002/bjs.1800810442. [DOI] [PubMed] [Google Scholar]
- 10.Alexander S. Dermatological aspects of anorectal disease. Clin Gastroenterol. 1975;4:651–7. [PubMed] [Google Scholar]
- 11.Bowyer A, McColl I. A study of 200 patients with pruritus ani. Proc R Soc Med. 1970;63(Suppl):96–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Dodi G, Pirone E, Bettin A, Veller C, Infantino A, Pianon P, et al. The mycotic flora in proctological patients with and without pruritus ani. Br J Surg. 1985;72:967–9. doi: 10.1002/bjs.1800721210. [DOI] [PubMed] [Google Scholar]
- 13.Pirone E, Infantino A, Masin A, Melega F, Pianon P, Dodi G, et al. Can proctological procedures resolve perianal pruritus and mycosis? A prospective study of 23 cases. Int J Colorectal Dis. 1992;7:18–20. doi: 10.1007/BF01647655. [DOI] [PubMed] [Google Scholar]
- 14.Bowyer A, McColl I. Erythrasma and pruritus ani. Acta Dermatol Venereol. 1971;51:444–7. [PubMed] [Google Scholar]
- 15.Mahendran R, Highet AS. Acute perianal streptococcal infection in an adult. Clin Exp Dermatol. 2002;27:69. doi: 10.1046/j.0307-6938.2001.00936.x. [DOI] [PubMed] [Google Scholar]
- 16.McClatchey KD. Clinical Laboratory Medicine. 2nd edn. Philadelphia, PA: Lippincott Williams & Williams; 2002. [Google Scholar]
- 17.Dasan S, Neill SM, Donaldson DR, Scott HJ. Treatment of persistent pruritus ani in a combined colorectal and dermatological clinic. Br J Surg. 1999;86:1337–40. doi: 10.1046/j.1365-2168.1999.01231.x. [DOI] [PubMed] [Google Scholar]
- 18.Harrington CI, Lewis FM, McDonagh AJ, Gawkrodger DJ. Dermatological causes of pruritus ani. BMJ. 1992;305:955. doi: 10.1136/bmj.305.6859.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuccati G, Lotti T, Mastrolorenzo A, Rapaccini A, Tiradritti L. Pruritus ani. Dermatol Ther. 2005;18:355–62. doi: 10.1111/j.1529-8019.2005.00031.x. [DOI] [PubMed] [Google Scholar]
- 20.Kligman AM, Frosch PJ. Steroid addiction. Int J Dermatol. 1979;18:23–31. doi: 10.1111/j.1365-4362.1979.tb01905.x. [DOI] [PubMed] [Google Scholar]
- 21.Doucet P. Pruritus ani. Int J Psychoanal. 1988;69:409–17. [PubMed] [Google Scholar]
- 22.<www.prodigy.nhs.uk/PILs/index.asp>
- 23.Bernhard JD. Itch: mechanisms and management of pruritus. New York: McGraw-Hill; 1994. [Google Scholar]
- 24.Kerscher MJ, Korting HC. Comparative atrophogenicity potential of medium and highly potent topical glucocorticoids in cream and ointment according to ultra-sound analysis. Skin Pharmacol. 1992;5:77–80. doi: 10.1159/000211022. [DOI] [PubMed] [Google Scholar]
- 25.Kerscher MJ, Hart H, Korting HC, Stalleicken D. In vivo assessment of the atrophogenic potency of mometasone furoate, a newly developed chlorinated potent topical glucocorticoid as compared to other topical glucocorticoids old and new. Int J Clin Pharmacol Ther. 1995;33:187–9. [PubMed] [Google Scholar]
- 26.Robinson N, Singri P, Gordon KB. Safety of the new macrolide immunomodulators. Semin Cutan Med Surg. 2001;20:242–9. doi: 10.1053/sder.2001.29063. [DOI] [PubMed] [Google Scholar]
- 27.Oztas MO, Oztas P, Onder M. Idiopathic perianal pruritus: washing compared with topical corticosteroids. Postgrad Med J. 2004;80:295–7. doi: 10.1136/pgmj.2003.013045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tunuguntla A, Sullivan MJ. A new concept for the treatment of intractable pruritus ani. South Med J. 2004;97:710. doi: 10.1097/00007611-200407000-00022. [DOI] [PubMed] [Google Scholar]
- 29.Lysy J, Sistiery-Ittah M, Israelit Y, Shmueli A, Strauss-Liviatan N, Mindrul V, et al. Topical capsaicin – a novel and effective treatment for idiopathic intractable pruritus ani: a randomised, placebo controlled, crossover study. Gut. 2003;52:1323–6. doi: 10.1136/gut.52.9.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eusebio EB, Graham J, Mody N. Treatment of intractable pruritus ani. Dis Colon Rectum. 1990;33:770–2. doi: 10.1007/BF02052324. [DOI] [PubMed] [Google Scholar]
- 31.Eusebio EB. New treatment of intractable pruritus ani. Dis Colon Rectum. 1991;34:289. doi: 10.1007/BF02090176. [DOI] [PubMed] [Google Scholar]
- 32.Botterill ID, Sagar PM. Intra-dermal methylene blue, hydrocortisone and ligno-caine for chronic, intractable pruritus ani. Colorectal Dis. 2002;4:144–6. doi: 10.1046/j.1463-1318.2002.00329.x. [DOI] [PubMed] [Google Scholar]
- 33.Farouk R, Lee PW. Intradermal methylene blue injection for the treatment of intractable idiopathic pruritus ani. Br J Surg. 1997;84:670. [PubMed] [Google Scholar]
- 34.Mentes BB, Akin M, Leventoglu S, Gultekin FA, Oguz M. Intradermal methylene blue injection for the treatment of intractable idiopathic pruritus ani: results of 30 cases. Tech Coloproctol. 2004;8:11–4. doi: 10.1007/s10151-004-0043-y. [DOI] [PubMed] [Google Scholar]
- 35.Wolloch Y, Dintsman M. A simple and effective method of treatment for intractable pruritus ani. Am J Proctol Gastroenterol Colon Rectal Surg. 1979;30:34–6. [PubMed] [Google Scholar]