Abstract
We discuss a case of saddle embolism with a clinical presentation similar to cauda equina syndrome in a 79-year-old woman with a history of ischaemic heart disease. Saddle embolus is very rare but one of an array of visceral causes for back and leg pain. This case highlights diagnostic difficulties, particularly in patients with multiple disorders. A high index of suspicion for vascular conditions must be exercised in cases of arterial dysfunction presenting with back pain.
Keywords: Cauda equina syndrome, Saddle embolism, Back pain
Back pain is the second most common presenting complaint to medical services in the UK. It is well known that visceral pathologies, such as pancreatitis, can present with back pain. Cauda equina syndrome is an orthopaedic emergency requiring urgent diagnosis (normally with an MRI scan) and surgical intervention.
In the majority of cases, it is easy to distinguish between visceral and musculoskeletal causes of back pain through competent history taking and examination. However, there are cases where there is considerable overlap between the clinical features of different disorders. In the elderly population, there may be multiple problems, further complicating diagnosis.
Case report
A 79-year-old woman, previously independent and fully mobile, presented with sudden-onset, acute, severe, lower back pain radiating down the back of both legs while dismounting a local village bus. She was unable to walk, complaining of altered sensation in both legs. Shortly afterwards, she was incontinent of urine. When reviewed by the orthopaedic team, she was in urinary retention.
Her past medical history revealed a long history of ischaemic heart disease including three previous myocardial infarctions for which she was on medical management. She was also obese, a hypertensive and type II diabetic.
Systemic examination was unremarkable. She complained of altered sensation of both lower limbs from L3 to S1 with bilateral weakness of ankle plantar/dorsiflexion and bilateral weakness of extensor hallucis longus (EHL) measuring 3/5 on the MRC scale. There was an inability to straight-leg raise actively or passively and proximal lower limb power could not be adequately assessed due to the severity of pain. There was no saddle paraesthesia and anal tone was normal.
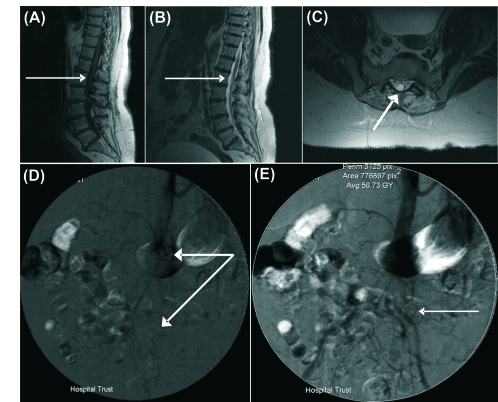
Routine blood tests revealed no abnormalities and lumbar spine radiographs revealed degenerative changes. Magnetic resonance imaging (MRI) showed normal vertebral alignment with a disc protrusion at T11/T12 and disc bulges at all levels from T12 to L5 with the L3/L4 disc bulge abutting the left L4 nerve root and the L4/L5 disc bulge abutting the right L5 nerve root. Despite these disc protrusions, there was no significant spinal cord, cauda equina or nerve root compression at any level (Fig. 1).
Figure 1.
Magnetic resonance images of the lumbar spine and cauda equina. (A) T1-weighted sagittal MRI, (B) T2-weighted sagittal MRI both demonstrating no spinal cord compression (thin white arrows) and (C) T2-weighted coronal MRI showing patent canal and unimpinged spinal cord (thick white arrow). Images from femoral angiogram demonstrate the saddle embolus (D) at the bifurcation of the aorta (thick arrow) showing (E) delayed incomplete blood flow down the common iliacs (thin arrow).
Having excluded cauda equina syndrome radiologically, she was admitted for analgesia and supportive treatment and started on strong opiate analgesia. Her pain was still uncontrolled, so facet joint injections and caudal epidural were required.
Following this, she reported a marked improvement in her back and leg pain. She was able to mobilise with a frame and sensation and power in both legs returned to normal. She was also continent of both bowel and bladder.
Seven days after admission, she began to complain of pain and blue discolouration in the toes of both feet. She still had pain in her lower back and buttocks radiating down her legs bilaterally. Capillary refill was less than two seconds but pulses in her lower extremities were absent on palpation, which was complicated by her obesity; subsequent Doppler scan and an angiogram confirmed the presence of collateral run off only.
Angiogram revealed a saddle embolism at the bifurcation of the aorta showing complete occlusion of both common iliac arteries with stenosis of the distal aorta. The common femoral arteries were shown to be patent bilaterally but had poor flow and the popliteal arteries were normal on both sides.
Investigation of the embolic source was undertaken using transthoracic echocardiography. The embolic source was revealed as a large left ventricular mural thrombus most likely from a recent silent non ST elevation myocardial infarction (troponin T was increased by 0.02 µg/l). This patient was discharged from hospital on warfarin with full mobility despite some unresolved sensory loss and recovering discolouration of her feet.
Discussion
This patient presented with sudden onset back pain which retrospectively can be attributed to a vascular cause. She had an abdominal aortic saddle embolus secondary to a ventricular mural thrombus that masqueraded as a possible cauda equina syndrome with back pain, bilateral leg pain, altered sensation, leg weakness and bowel and bladder symptoms.
A saddle embolism is characterised by an embolus that extends across the bifurcation of a vessel for example the main pulmonary artery, the distal aorta, or even a patent foramen ovale.1 Serial studies have reported cardiac disease in 90% of patients with peripheral arterial embolism or saddle embolus, particularly ischaemic heart disease, valvular disorders or atrial fibrillation.2
The cauda equina, and rarely the conus medullaris, are supplied by the lower lumbar, iliolumbar and lateral sacral arteries.3 In 85% of patients, the greater radicular artery actually originates on the left side of the aorta from branches of the intercostal arteries at the level of T7–T12; in 60% of patients, it originates via branches of the intercostal or lumbar arteries at the level of T8–L4.3 Very rarely does this arise at the level of L3 (1.4%) or L4–L5 (0.2%) and a low origin may be the reason for paralysis or cauda equina like symptoms in patients if thromboembolic disease is present as in this patient here.3
Thus, a patient with reduced blood flow in the lower lumbar, iliolumbar and/or lateral sacral arteries, which could be consistent with an embolic event, may have transient ischaemia to the cauda equina, mimicking compression. It is documented that approximately 50% of cases have no peripheral vascular symptoms or delayed symptoms making diagnosis of saddle embolism difficult in the early stages.3 This case was further complicated by the presence of degenerative lumbar spine pathology and disc herniation and her symptoms being ameliorated by caudal epidural.
The recommended treatment for saddle embolism includes anticoagulation with a bolus dose of 5000–20,000 units of heparin followed by removal of the embolism via a catheter or open surgery.4,5 Anticoagulation with warfarin was considered the treatment of choice in our patient due to her extensive cardiac history. Her symptoms have largely resolved and she is now mobile.
Conclusions
In patients presenting with suspected cauda equina syndrome without confirmatory radiological evidence, a high index of suspicion for vascular diagnoses is required, particularly in known cases of arterial dysfunction.
References
- 1.Enzweiler CN, Wiese TH, Lembcke AE, Taupitz M, Rogalla P, Kivelitz DE, et al. Electron beam tomography of interpulmonary saddle embolism: extent and vascular distribution. J Comput Assist Tomogr. 2002;26:26–32. doi: 10.1097/00004728-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Busuttil RW, Keehn G, Milliken J, Paredero VM, Baker JD, Machleder HI, et al. Aortic saddle embolus. A twenty-year experience. Ann Surg. 1983;197:698–706. doi: 10.1097/00000658-198306000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olearchyk AS. Saddle embolism of the aorta with sudden paraplegia. Can J Surg. 2004;47:472–3. [PMC free article] [PubMed] [Google Scholar]
- 4.Ross SA, Hood JM, Barros D'Sa AA. Immediate heparinisation and surgery in the management of saddle embolism. Eur J Vasc Surg. 1990;4:191–4. doi: 10.1016/s0950-821x(05)80436-0. [DOI] [PubMed] [Google Scholar]
- 5.Schwickert HC, Schweden F, Schild HH, Piepenburg R, Düber C, Kauczor HU, et al. Pulmonary arteries and lung parenchyma in chronic pulmonary embolism: preoperative and postoperative CT findings. Radiology. 1994;191:351–7. doi: 10.1148/radiology.191.2.8153305. [DOI] [PubMed] [Google Scholar]