Abstract
Interaction of the two high-spin hemes in the oxygen reduction site of the bd-type quinol oxidase from Escherichia coli has been studied by femtosecond multicolor transient absorption spectroscopy. The previously unidentified Soret band of ferrous heme b595 was determined to be centered around 440 nm by selective excitation of the fully reduced unliganded or CO-bound cytochrome bd in the α-band of heme b595. The redox state of the b-type hemes strongly affects both the line shape and the kinetics of the absorption changes induced by photodissociation of CO from heme d. In the reduced enzyme, CO photodissociation from heme d perturbs the spectrum of ferrous cytochrome b595 within a few ps, pointing to a direct interaction between hemes b595 and d. Whereas in the reduced enzyme no heme d-CO geminate recombination is observed, in the mixed-valence CO-liganded complex with heme b595 initially oxidized, a significant part of photodissociated CO does not leave the protein and recombines with heme d within a few hundred ps. This caging effect may indicate that ferrous heme b595 provides a transient binding site for carbon monoxide within one of the routes by which the dissociated ligand leaves the protein. Taken together, the data indicate physical proximity of the hemes d and b595 and corroborate the possibility of a functional cooperation between the two hemes in the dioxygen-reducing center of cytochrome bd.
Reduction of molecular oxygen to water is a key step in aerobic energy production in living organisms, catalyzed by terminal oxidases. Although the mitochondrial respiratory chain ends invariably with an aa3-type cytochrome c oxidase, a plethora of terminal oxidases is found within the bacterial kingdom. Most of these different enzymes display a large degree of sequence homology and belong to a superfamily of heme/copper oxidases (1), where a copper ion (CuB) is believed to cooperate with a high-spin heme in the reduction of dioxygen to water and may be directly involved in the redox-linked transmembrane proton pumping by the enzyme (2–8).
A very different class of oxygen-reactive respiratory enzymes is constituted by the bd-type quinol oxidases, which do not display homology to heme/copper oxidases or to any other protein (9, 10). The bd-type oxidases are found in a wide variety of bacteria and are of great physiological significance for survival under low oxygen (“microaerobic”) conditions. The enzyme carries out the same chemical reaction as the heme-copper quinol oxidases, and at least some of the oxygen intermediates formed are analogous to those produced in the catalytic cycle of the heme-copper oxidases (11, 12). However, this process is not coupled to proton pumping (13), and the structure and composition of bd-type oxidases are quite different from those of heme/copper oxidases (reviewed in refs. 10 and 14).
Cytochrome bd, as purified from Escherichia coli or Azotobacter vinelandii, consists of two different subunits, both of which are integral membrane proteins. The three heme groups in the protein complex are located close to the outer surface of the bacterial membrane (9), and no other metals have been found so far. A low-spin heme b (b558) is probably the electron entry site close to the quinol-binding domain (9, 15). High-spin heme d serves as an oxygen anchor and is assumed to be the site where O2 is reduced to water. The functional role of the second high-spin heme, heme b595, has not been well defined. It may act as electron carrier between heme b558 and heme d (16, 17), or it may be a functional analog of CuB in the heme-copper oxidases (18, 19). Arguments in favor of heme d–heme b595 proximity come from experiments demonstrating, at cryogenic temperatures, CO transfer to heme b595 on a time scale of hours after photodissociation from heme d in the membrane-bound cytochrome bd (19).
Ultrafast spectroscopy in the femtosecond regime offers a unique possibility to monitor in real time both structural and electronic dynamics associated with the initially localized perturbation (20), here caused by photodissociation of a ligand. We have undertaken a systematic femtosecond/picosecond study of the bd-type quinol oxidase from E. coli by varying the redox and ligation conditions. The measurements were performed both in the α-band, where the heme absorptions are well separated but weak for heme b595, and in the strong Soret band, where the individual bands of the three hemes have not been resolved so far (14) and the interpretation of absorption changes is controversial. Here we focus on the results relevant to heme b595 (a detailed account of the entire study will be given elsewhere). We have identified the absorption band of this redox center in the Soret and, by monitoring ultrafast absorption changes in this region, have been able to show that heme b595 interacts with heme d and possibly can act as a transient binding site for CO after photodissociation of the ligand from heme d. The data favor proximity of the two hemes and hence their potential cooperation in oxygen reduction.
Materials and Methods
Materials.
N-lauroylsarcosinate was from Fluka; sodium dithionite was from Merck; and N-dodecyl-N,N-dimethylammonio-3-propane-sulfonate (SB-12) was from Sigma. Other basic chemicals and biochemicals were commercial products of high purity from Sigma, Serva, Fluka, Merck, and Air Liquide (Grigny, France).
E. coli cells (strain GO 105/pTK1; ref. 21) overproducing cytochrome bd and deleted in cytochrome bo3, kindly provided by R. Gennis (University of Illinois, Urbana-Champaign) were grown in 10-liter flasks at 37°C in a medium containing 80 mM potassium phosphate, 2.5 mM sodium citrate, 19 mM ammonium sulfate, 1% tryptone, 0.5% yeast extract, 0.5% casamino acids, 0.01% l-tryptophan, 2% glycerol (vol/vol), 0.8 mM MgSO4, 0.18 mM FeSO4⋅7 H2O, 0.1 mM CuSO4⋅5 H2O, 0.005% kanamycin, and 0.01% ampicillin, pH 7.2. Cytochrome bd was isolated from cell membranes as described (22) but the final hydroxylapatite chromatography step was omitted. Its concentration was determined from the dithionite-reduced minus air-oxidized difference absorption spectra by using a value of Δɛ628–607 of 10.8 mM−1⋅cm−1 (23).
Sample Preparation.
All measurements were made at room temperature in a buffer containing 50 mM N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonate)/50 mM 2-(N-cyclohexylamino)-ethanesulfonate (pH 8), 0.5 mM EDTA, and 0.025% sodium N-lauroylsarcosinate in a 1-mm optical pathway cell at 21 μM of cytochrome bd. Experiments have been carried out with four basic stable states: (a) the “as isolated” form, which is mainly an oxycomplex (Oxy, b5583+b5953+d2+-O2); (b) the fully reduced (50 mM dithionite) unliganded state (R, b5582+b5952+d2+); (c) the carbon monoxide complex of the fully reduced state (R-CO, b5582+b5952+d2+-CO, obtained by bubbling with 1 mM CO) and (d) the carbon monoxide complex of the so-called “mixed valence” state (MV-CO, b5583+b5953+d2+-CO, obtained by oxidation of the enzyme in the R-CO state with small quantities of air.
Spectroscopy.
Absolute absorption spectra of the samples were recorded before and after the measurements in a Shimadzu UV-Vis 1601 spectrophotometer. Multicolor femtosecond absorption spectroscopy (24) was performed at a repetition rate of 30 Hz with compressed white light continuum probe pulses, and pump pulses centered at 590 nm [55 fs full width half maximum (fwhm)], 620 nm (70 fs fwhm), or 653 nm (70 fs fwhm). The sample was continuously moved perpendicular to the beams. Global exponential fitting of the data in terms of decay-associated spectra was performed with a procedure described previously (25) involving singular value decomposition and deconvolution with the instrument response function.
Results
The ground state optical absorption spectra of cytochrome bd in different states studied in this work were in general agreement with those published in the literature and did not change significantly during the measurements. The respective Soret regions are represented in Fig. 1A.
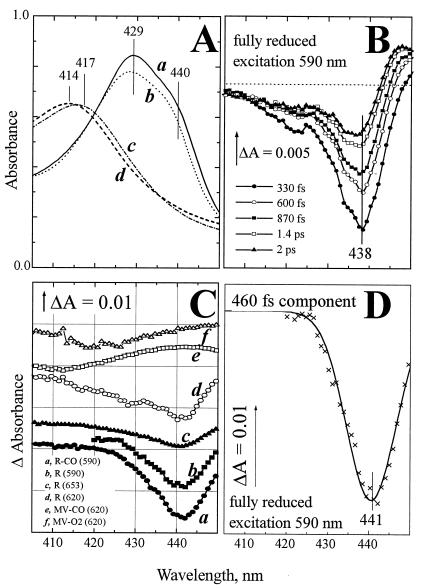
Figure 1.
Absorption characteristics of cytochrome bd in the Soret band. (A) Static absorption spectra of cytochrome bd: (a) fully reduced; (b) CO complex of the fully reduced state; (c) as-prepared oxygenated state; (d) CO complex of the MV state. (B) Evolution of spectral changes induced by femtosecond excitation of the fully reduced cytochrome bd at 590 nm. The difference spectra have been recorded versus the static R state at the indicated time intervals after the photoexcitation. (C) Spectra of the subpicosecond component (0.45–0.9 ps for different states) of the photobleaching induced in various states of cytochrome bd. Experiments have been carried out in the indicated redox and ligand binding states at λexc indicated in brackets. Solid line overlaying spectrum b: approximation with a single Gaussian. (D) γ-Absorption band of ferrous heme b595 resolved by photobleaching of the R state with 590-nm excitation (crosses, redrawn from C, curve b) has been approximated by a single Gaussian (solid line a).
Resolution of Ferrous Heme b595 Soret Band.
Fig. 1B shows the spectral evolution of the Soret band of fully reduced unliganded cytochrome bd (five representative spectra are shown), induced by excitation at 590 nm, close to the α-absorption maximum of ferrous heme b595. During the first ≈0.5 ps, the difference spectra are dominated by a loss of absorbance at 438 nm. This bleaching then partly recovers, and the absorption changes transform to derivative-shaped spectra on the ps time scale, indicating a red-shift of a band centered around 440 nm. Global analysis of the entire spectra set assuming exponential decays resolves two major phases with time constants of 460 fs and 4 ps. Decay components with similar time constants were observed in experiments with other hemeproteins (24). The spectra associated with the major sub-ps decay phase observed in experiments at different redox and ligation states of cytochrome bd are shown in Fig. 1C.
In principle, the decay-associated spectra must include a contribution of excited-state absorption. However, for the fast-decaying excited state (e.g., the 460-fs component in the fully reduced cytochrome bd), presumably analogous to the HbI* state in hemoglobin (26), this absorption is weak and shifted rather far to the red (not shown, cf. ref. 26); therefore its contribution does not significantly distort the line shape of the band associated with bleaching of the ground state. This is not the case for the slower decaying excited state, which clearly has a bandshift-type spectrum (e.g., Fig. 1B, spectra at 1.4 and 2 ps; cf. ref. 26).
The spectrum associated with the 460-fs decay component of photobleaching in the fully reduced enzyme excited at 590 nm is shown separately (Fig. 1D) and represents essentially bleaching of a band centered at ≈441 nm that can be described reasonably well by a Gaussian (solid line). We assign this spectrum to bleaching of the Soret band of ferrous heme b595. First, with excitation of the fully reduced cytochrome bd at 653 nm, far from the α-maximum of ferrous b595, the photobleaching at 441 nm strongly attenuates (compare spectra b and c in Fig. 1C). With excitation at 620 nm (Fig. 1C spectrum d), the bleaching of the 441-nm band was intermediate between excitation at 590 nm and 653 nm, and significant broadening on the blue side was observed, probably associated with a contribution of heme d. Second, virtually the same photobleaching of the 441 band is observed for a CO complex of the fully reduced cytochrome bd (Fig. 1C, spectrum a), indicating that changes in the absorption characteristics of ferrous heme d induced by CO binding do not affect photobleaching of the 441-nm band. Third, this band is missing in the MV-CO and Oxy complexes of the enzyme where heme b595 is oxidized (Fig. 1C, spectra e and f). The Soret band of heme b595 as resolved in this work is centered at about the same wavelength as that of ferrous cytochrome c peroxidase [a high-spin heme b-containing enzyme (27) used earlier as a model for the absorption characteristics of heme b595 in the α,β-region (28)]; however, the peroxidase spectrum is much broader.
Photodissociation of Carbon Monoxide in the R and MV States.
Figs. 2 and 3 compare photodissociation spectra of the CO complexes of the R-CO and MV-CO states. Both the line shape and the dynamics of the spectral changes are strikingly different in the two complexes.
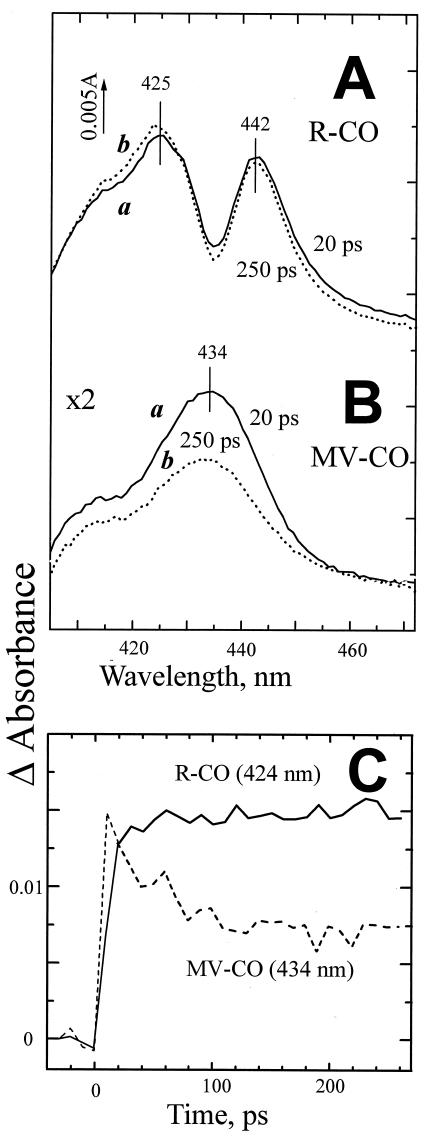
Figure 2.
Photodissociation of CO from the R (A) and MV states (B) of cytochrome bd. The absorption changes observed with the MV-CO complex have been expanded 2-fold. (C) Kinetics of the photoinduced changes in the two states. The responses have been normalized at t = 20 ps.
Figure 3.
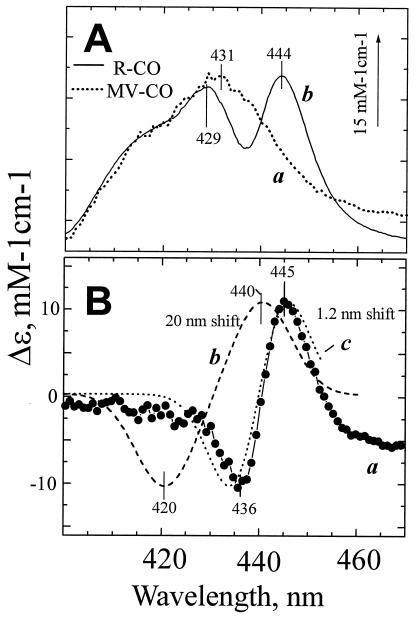
A red shift of ferrous heme b595 absorption band induced by CO dissociation from heme d. (A) (a) Absorption changes induced by CO photodissociation from the MV and (b) static unliganded-CO − liganded spectrum of the R state (difference between curves a and b of Fig. 1A). The curves have been normalized by aligning their blue parts where they have similar line shapes. The scale bar refers to the R-CO state response. (B) Curve a gives the difference between spectra b and a in A. For comparison are shown simulated difference spectra for a 20-nm red shift corresponding to CO dissociation from heme b595 in ≈10% of cytochrome bd (b) and a 1.2-nm red shift of the entire population of b595 (c).
The difference spectra of CO photodissociation observed in the Soret for the R-CO state of cytochrome bd after completion of the electronic relaxations are shown in Fig. 2A. The line shape of the absorption changes with the two extrema at 425 nm and 442 nm resembles the (mirrored) static difference spectrum induced by CO binding to reduced cytochrome bd with its characteristic (but poorly understood) W-form (e.g., refs. 23 and 29), as well as the difference spectrum of the rapid (microsecond) phase of CO recombination with the fully reduced cytochrome bd from A. vinelandii (30).
In contrast, the response of the MV-CO complex is characterized by a less complex difference spectrum, corresponding to an increased broad absorption band with a maximum in the range of 431 to 434 nm (Figs. 2B and 3A). We attribute this band to transiently generated ligand-free ferrous heme d. This report shows the absorption changes induced by CO in the Soret band of MV cytochrome bd. Transient spectra in the 550- to 660-nm range have been reported on the microsecond/millisecond time scale (15) but did not reveal any difference with respect to the R-CO state (in agreement with our ultrafast measurements in the α-band, data not shown), presumably because of the weak or broad contribution of heme b595 in this region. It must be emphasized that photodissociation of the MV-CO complex is followed by partial dislocation (“backflow”) of electrons from ferrous heme d to ferric hemes b at a rate of 3,000 s−1 (15), which complicates resolution of the genuine photodissociation-linked spectral changes in experiments on a millisecond or microsecond time scale. These secondary changes can be neglected on the picosecond time scale.
A second striking difference between the R-CO and MV-CO states concerns the dynamics of the absorption changes induced by CO photodissociation. In the R-CO complex, the changes attain a stable level after relaxation of the electronic excited states in ≈20 ps so that the difference spectra recorded at 20 and 250 ps after the flash do not differ significantly (Fig. 2A). In contrast, the photoinduced changes observed in the MV-CO complex decrease by about one-half in this time range (Fig. 2 B and C). The line shape of the decay phase (spectrum c in Fig. 2B) is similar to that of the initial photoinduced spectral changes. Within a maximal observation window of 500 ps, a 2-to 3-fold decrease of the photoinduced response was observed in different experiments. The apparent time constant of the decay varied somewhat (≈70–200 ps), and actually the process may be nonexponential. We conclude that in at least half of the photolysed MV-CO complexes, subnanosecond geminate recombination of CO and heme d takes place.
Discussion
Contribution of Heme b595 to the Soret Band of the Reduced Cytochrome bd.
Whereas the absorption characteristics of cytochrome bd in the α-, β-band region have been characterized earlier in detail, and the bands of hemes d, b595, and b558 were identified (28, 31), the individual contributions of the three hemes to the Soret region have not been resolved (14). The absolute absorption spectrum of reduced cytochrome bd in the Soret region (Fig. 1A, curve a) is characterized by an asymmetric band with a maximum at ≈430 nm attributed provisionally to low-spin heme b558 (11, 14) and a shoulder around 440 nm, the origin of which is controversial. The absorption changes around 440 nm induced by binding of CO or NO to reduced cytochrome bd have been assigned earlier either to heme d (29, 30, 32) or heme b595 (11, 23). This work, exploiting the combination of selective heme excitation and ultrafast spectroscopy, provides direct evidence that the Soret shoulder at ≈440 nm corresponds to heme b595. Identification of the heme b595 signature in the γ (Soret) region is essential for interpretation of the intricate spectral changes induced by binding of ligands, such as CO or NO, with the reduced enzyme.
Bandshift of Heme b595 Induced by CO Binding to Heme d.
A major finding of this work is that the redox state of the b-type hemes strongly affects absorption changes induced by photodissociation of CO from heme d. As discussed below, this indicates proximity of hemes d and b595.
The absorption changes induced by CO binding with fully reduced cytochrome bd are rather unusual. In the Q band region, CO brings about a red, rather than a conventional blue, shift of the well-resolved peak of ferrous heme d at about 628 nm, but does not affect significantly the band at 595 nm of heme b595. In the Soret, a peculiar W-shaped trough with two well-resolved minima at ≈ 429 and 444 nm is observed (e.g., see Fig. 3A, curve b). This line shape has been amply discussed in the literature and sometimes is believed to report CO binding with both high-spin hemes d and b595 (refs. 14 and 23 and references therein).
Our data show that the W-shaped absorption changes in the Soret region are only induced by CO when the b hemes are reduced, but not in the MV state of the enzyme. Comparison of the difference spectra associated with CO binding to the R and MV states of cytochrome bd may allow resolving the specific spectral contribution from the reduced b hemes. As the immediate product of CO photodissociation from the fully reduced state shows additional contributions of unrelaxed states discussed below (Fig. 4), we first will compare the line shape of the MV-CO photodissociation spectrum with the conventional spectral changes induced by CO binding with fully reduced bd under static conditions.
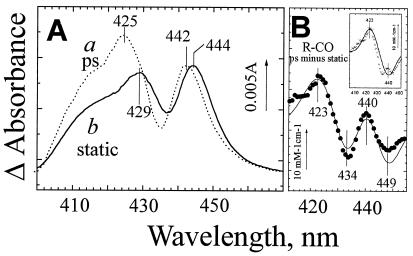
Figure 4.
Evidence for an unrelaxed intermediate state of ferrous heme b595 after CO photodissociation from heme d. (A) Absorption changes induced by CO dissociation from the R-CO state of cytochrome bd shortly after photolysis (a) and under static conditions (b). Curve a corresponds to the difference spectrum resolved by global analysis as a residual constant phase after relaxation of the excited states. Curve b is the difference spectrum (inverted) induced by addition of 20 μM CO to the fully reduced cytochrome bd (the same curve as in Fig. 3A). The spectra have been normalized by the height of the 442-to 444-nm peak. The ΔA scale bar refers to curve a. (B). The experimental difference between spectra a and b in A (●) has been simulated (the line) as narrowing of the b595 440-nm band by 10% plus a 17-nm blue shift of this band in 10% of cytochrome bd. The Gaussian approximation of the band in Fig. 1D has been used for modeling. (Inset) After subtracting the contribution of b595 band narrowing, the difference between the picosecond and static CO photodissociation spectra (crosses) is dominated by the 17-nm blue shift of heme b595 (solid curve).
Fig. 3A shows that in the R-CO difference spectrum, the spectral response of the MV-CO state, assigned provisionally to increased absorption of unliganded heme d at ≈431 nm, is superimposed by a narrow bandshift-type feature centered around 440–441 nm, as illustrated clearly by the difference between the normalized R-CO and MV-CO curves (Fig. 3B, curve a). It is this bandshift feature that confers the characteristic W-like shape to the CO- and NO-induced absorption changes in the reduced cytochrome bd. In principle, this contribution could reflect a perturbation of either low-spin heme b558 or high-spin heme b595. Because the Em values of the two b hemes are very close (14) it is not possible to generate an equilibrium initial state of the enzyme with only one of the two hemes b reduced. However, the low-spin heme b is believed to have an absorption band at 429–430 nm (11, 14), whereas the band of heme b595 is located around 440 nm (this work). Accordingly, the position of the derivative-shaped feature centered at 440–441 nm strongly favors its interpretation as a red shift of ferrous heme b595.
This small red shift may be explained either by CO-photodissociation from heme b595 in a small fraction of the enzyme, or by an indirect perturbation of the heme b595 Soret band induced by CO photodissociation from heme d (cf. ref. 23). The latter possibility is much more likely. First, we found the line shape of the Soret band changes associated with the ligand photodissociation in the R-CO state to be essentially the same for excitation at 653, 620, or 590 nm (data not shown). Were the response caused by CO photodissociation from heme b595, a much lower relative contribution of this spectral perturbation would be expected for excitation at 653 or 620 nm as compared with 590 nm. Second, we have simulated spectrum a in Fig. 3B for different bandshifts, taking the line shape of the heme b595 absorption band from our photobleaching data. We assumed a provisional extinction coefficient of 150 mM−1⋅cm−1 for the absorption maximum of ferrous b595; this is a reasonable value for a high-spin heme γ-band (33) and it fits satisfactorily the absolute spectrum of the reduced cytochrome bd. CO binding to the heme iron is expected to shift the absorption band of a high-spin b-type heme to lower wavelengths by at least 10 nm (33, 34). A difference spectrum simulated for putative photodissociation of a b595-CO complex assuming a 20-nm shift of heme b595 Soret band is shown in Fig. 3B (curve b). It is characterized by a maximum at ≈440 and a minimum at ≈420 nm, which agree reasonably well with the difference spectrum assigned to CO photodissociation from heme b595 at low temperatures (35, 36). Obviously, such a shift does not fit the line shape of the experimental spectrum (neither do smaller, 10- to 15-nm shifts, not shown). On the other hand, a good fit is obtained with respect to both size and line shape for a bandshift of the order of 1 nm (Fig. 3B, curve c).
Therefore we conclude that binding of CO to ferrous heme d in the fully reduced cytochrome bd perturbs the Soret absorption band of heme b595. Such an effect points to an interaction between the two iron-porphyrin groups. A similar (although not identical, see below) shift is seen in the CO photodissociation difference spectrum of the fully reduced cytochrome bd a few ps after photodissociation (e.g., Figs. 2A and 4A). Therefore the heme-heme interactions are unlikely to be mediated by long-range slow conformational changes of the protein. Rather, the two hemes are in close proximity and communicate directly. Such communication could involve (i) excitonic interactions between the hemes and (ii) steric interactions. Concerning the first mechanism, it can be calculated by using the point dipole approximation for excitonic coupling (37) that for two hemes at a distance of 7 Å with heme planes at 55° (38); an 8% change in heme d oscillator strength would be sufficient to alter the interaction with heme b595 by ≈ 60 cm−1 (a shift of ≈1 nm at 440 nm). As to the second mechanism, bound CO could be sandwiched between the two hemes and thus sterically affect the heme b595 iron-macrocycle structure. For example, in the reduced bovine cytochrome c oxidase, the Fea3-CuB distance increases by ≈0.1 Å upon CO binding with heme a3 (39).
Geminate Recombination of CO in the MV-CO Complex: Does Heme b595 Provide a Transient Binding Site for CO on its Way Out?
The present work shows that at least half of the CO photodissociated from heme d in the MV-CO complex returns to heme d within a few hundred ps, presumably without leaving the heme pocket. In accordance with this finding, we have observed that the yield of CO photodissociation as measured on the millisecond time scale is significantly lower in the MV-CO complex than in the fully reduced CO complex (S. Siletsky, V.B.B., and A.A.K., unpublished work). We note that for no other heme protein, including cytochrome c oxidases aa3 (40) and cbb3 types (G. Lipowski, U.L., and M.H.V., unpublished work), significant geminate CO recombination on this time scale has been reported (in contrast to NO and O2 that often show subnanosecond recombination; ref. 41).
Apparently, the redox state of hemes b (presumably b595) controls the pathway by which CO escapes to the medium. A general possibility of such a control is that reduction of the b heme(s) results in a conformational change that opens an escape pathway or increases the probability/effective lifetime of opening of such a pathway driven by thermal fluctuations of the protein (e.g., see ref. 42). Specific channels conducting small substrates or product molecules like oxygen, hydrogen peroxide, and perhaps water to and from the heme active site have been discerned in many enzymes (ref. 43 and references therein), including cytochrome c oxidase aa3 (39). More specifically, ferrous heme b595 may provide a transient binding site for the ligand on its way from heme d to the medium in analogy to CuB in the heme-copper oxidases (44). Because the ferric heme iron is not expected to be able to bind CO, the pathway will be blocked in the MV-CO state where heme b595 is oxidized.
Indications for transient binding of CO to b595 after ligand photodissociation from heme d in the fully reduced enzyme can be inferred from the spectral characteristics of the photodissociated state shortly after photolysis. Although the difference spectrum of CO photodissociation from reduced cytochrome bd observed on the picosecond time scale reproduces general features of the steady-state CO-dissociation spectrum, close comparison of these difference spectra shows that the line shapes are not identical (Fig. 4A). First, in the picosecond spectrum, the above-discussed b595 bandshift feature is displaced to the blue by ≈2 nm (maximum at 442 nm, minimum at 435 nm) relative to the static spectrum. Second, the picosecond curve reveals increased absorbance around 420 nm relative to the peak around 440 nm. Whereas the various reported static CO-induced difference spectra of cytochrome bd show some variation (23, 29, 45, 46), the long wavelength extreme at 443–445 nm is invariably more intense than the short wavelength one at 425–430 nm (Fig. 4, curve a). The same holds true for the difference spectrum of the rapid phase (k ≈ 108 M−1⋅s−1, τobs ≈ 10−5 s) of bimolecular CO recombination with ferrous cytochrome bd at room temperature (ref. 30; S. Siletsky and V.B.B., unpublished work). Taken together, these results indicate the generation of a transient photodissociated state that is likely to relax within the 10−8- to 10−5-s time gap.
The difference between the picosecond and static difference spectra of CO dissociation from R-CO in Fig. 4A should reflect the difference between the spectra of the unliganded (R) states immediately after CO photodissociation and after relaxation. This difference (Fig. 4B, ●) reveals a rather complex pattern (the details of which depend somewhat on the normalization choice), that can be modeled qualitatively by two overlaying perturbations of heme b595. Notably, the part of the difference spectrum in Fig. 4B above 430 nm with the peak at 440 nm and two minima on both sides of the peak can be modeled reasonably well with the second derivative of the b595 γ-absorption band at 440 nm, indicating that it reflects narrowing of the band. One interpretation of this narrowing is that a relatively well-defined conformation of the environment of heme b595 when CO is still in the heme pocket after photodissociation relaxes to a wider distribution of conformations upon escape of CO to the medium, and hence induces inhomogeneous broadening of its absorption band.
Subtraction of the second derivative-type contribution reveals a residual bandshift with a minimum at ≈440 nm and a maximum at ≈423 nm (Fig. 4B Inset) similar to that assigned to CO binding with ferrous b595 (35, 36). Altogether, the basic features of the experimental absorption difference can be described by a contribution of (i) narrowing of the b595 band by ≈10% and (ii) a 17-nm blue shift in a small fraction (≈10%) of b595. Thus the data are consistent with transient binding of photodissociated CO with heme b595 in a small fraction of the enzyme at room temperature. In combination with the effect of the redox state of heme b595 on rebinding of CO to heme d, an attractive hypothesis is that with reduced heme b595 a quasi-equilibrium between CO-liganded and unliganded heme b595 exists, which keeps CO near a potential escape pathway out of the protein.
CO binding to heme b595 upon photolysis of CO bound to heme d has been observed previously by Fourier transform IR spectroscopy at 20 K (19). At this temperature, CO binding to the heme is very slow (kiloseconds) (ref. 19 and cf. ref. 47). Our analysis indicates that the process also can take place at room temperature, speeding up to the picosecond range.
Concluding Remarks.
We have exploited femtosecond optical spectroscopy to identify the spectral contribution of heme b595 in the Soret region and to study its role in ligand routing in cytochrome bd. The finding that heme b595 interacts with heme d on a picosecond time scale favors a di-heme oxygen reducing site in the bd-type oxidase; such an arrangement may facilitate trapping the oxygen and routing it toward the active site.
Acknowledgments
We are indebted to Dr. R. Gennis for the strain of E. coli GO 105/pTK1 and Dr. N. Yu. Safronova for growing the cells. The work was supported by an Action Spécifique avec les Pays d'Europe Centrale et Orientale grant from the Institut National de la Santé et de la Recherche Médicale, Grants 97–04-49765, 98–04-48847, and 99–04-48095 from the Russian Fund for Basic Research, and INTAS-Russian Fund for Basic Research Grant 95–1259. M.H.V. is supported by the Centre National de la Recherche Scientifique.
Abbreviations
- R, fully reduced
MV, mixed valence.
Note Added in Proof
The differences between the picosecond and static CO photodissociation spectra (Fig. 4) also could originate in the parallel polarization of the pump and probe pulses used in this work, as compared to unpolarized static absorption spectra. We recently performed polarized photoselection experiments, which indeed show that at least part of the spectral changes considered as evidence for transient CO binding with heme b595 can be accounted for by polarization effects. A preliminary analysis further strengthens the main assessment of the paper; i.e., a change in interaction between hemes d and b595 upon CO dissociation.
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.030528197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.030528197
References
- 1.Ferguson-Miller S, Babcock G T. Chem Rev. 1996;96:2889–2908. doi: 10.1021/cr950051s. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P. FEBS Lett. 1987;222:235–245. doi: 10.1016/0014-5793(87)80378-2. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell P. Ann NY Acad Sci. 1988;550:185–198. doi: 10.1111/j.1749-6632.1988.tb35335.x. [DOI] [PubMed] [Google Scholar]
- 4.Morgan J E, Verkhovsky M I, Wikström M. J Bioenerg Biomembr. 1994;26:599–608. doi: 10.1007/BF00831534. [DOI] [PubMed] [Google Scholar]
- 5.Rich P R. Biosci Rep. 1991;11:539–571. doi: 10.1007/BF01130217. [DOI] [PubMed] [Google Scholar]
- 6.Wikström M. Biochim Biophys Acta. 1998;1365:185–192. doi: 10.1016/s0005-2728(98)00095-4. [DOI] [PubMed] [Google Scholar]
- 7.Iwata S, Ostermeier C, Ludwig B, Michel H. Nature (London) 1995;376:660–669. doi: 10.1038/376660a0. [DOI] [PubMed] [Google Scholar]
- 8.Michel H. Proc Natl Acad Sci USA. 1998;95:12819–12824. doi: 10.1073/pnas.95.22.12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osborne J P, Gennis R B. Biochim Biophys Acta. 1999;1410:32–50. doi: 10.1016/s0005-2728(98)00171-6. [DOI] [PubMed] [Google Scholar]
- 10.Mogi T, Tsubaki M, Hori H, Miyoshi H, Nakamura H, Anraku Y. J Biochem Mol Biol Biophys. 1998;2:79–110. [Google Scholar]
- 11.Poole R K. In: Bacterial Energy Transduction. Anthony C, editor. London: Academic; 1988. pp. 231–291. [Google Scholar]
- 12.Hill B C. J Bioenerg Biomemb. 1993;25:115–120. doi: 10.1007/BF00762853. [DOI] [PubMed] [Google Scholar]
- 13.Puustinen A, Finel M, Haltia T, Gennis R B, Wikström M. Biochemistry. 1991;30:3936–3942. doi: 10.1021/bi00230a019. [DOI] [PubMed] [Google Scholar]
- 14.Junemann S. Biochim Biophys Acta. 1997;1321:107–127. doi: 10.1016/s0005-2728(97)00046-7. [DOI] [PubMed] [Google Scholar]
- 15.Junemann S, Wrigglesworth J M, Rich P R. Biochemistry. 1997;36:9323–9331. doi: 10.1021/bi970055m. [DOI] [PubMed] [Google Scholar]
- 16.Poole R K, Williams H D. FEBS Lett. 1987;217:49–52. doi: 10.1016/0014-5793(87)81240-1. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi K, Tagawa S, Mogi T. Biochemistry. 1999;38:5913–5917. doi: 10.1021/bi982088n. [DOI] [PubMed] [Google Scholar]
- 18.Krasnoselskaya I, Arutjunjan A M, Smirnova I, Gennis R, Konstantinov A A. FEBS Lett. 1993;327:279–283. doi: 10.1016/0014-5793(93)81004-j. [DOI] [PubMed] [Google Scholar]
- 19.Hill J J, Alben J O, Gennis R B. Proc Natl Acad Sci USA. 1993;90:5863–5867. doi: 10.1073/pnas.90.12.5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vos M H, Martin J-L. Biochim Biophys Acta. 1999;1411:1–20. doi: 10.1016/s0005-2728(99)00035-3. [DOI] [PubMed] [Google Scholar]
- 21.Kaysser T M, Ghaim J B, Georgiou C, Gennis R B. Biochemistry. 1995;34:13491–13501. doi: 10.1021/bi00041a029. [DOI] [PubMed] [Google Scholar]
- 22.Miller M J, Gennis R B. Methods Enzymol. 1986;126:87–94. doi: 10.1016/s0076-6879(86)26011-5. [DOI] [PubMed] [Google Scholar]
- 23.Borisov V, Arutyunyan A M, Osborne J P, Gennis R B, Konstantinov A A. Biochemistry. 1999;38:740–750. doi: 10.1021/bi981908t. [DOI] [PubMed] [Google Scholar]
- 24.Martin J-L, Vos M H. Methods Enzymol. 1994;232:416–430. doi: 10.1016/0076-6879(94)32057-8. [DOI] [PubMed] [Google Scholar]
- 25.Liebl U, Lambry J-C, Leibl W, Breton J, Martin J-L, Vos M H. Biochemistry. 1996;35:9925–9934. doi: 10.1021/bi960462i. [DOI] [PubMed] [Google Scholar]
- 26.Petrich J W, Poyart C, Martin J-L. Biochemistry. 1988;27:4049–4060. doi: 10.1021/bi00411a022. [DOI] [PubMed] [Google Scholar]
- 27.Yonetani T, Ray G S. J Biol Chem. 1965;240:4503–4508. [PubMed] [Google Scholar]
- 28.Lorence R M, Koland J G, Gennis R B. Biochemistry. 1986;25:2314–2321. doi: 10.1021/bi00357a003. [DOI] [PubMed] [Google Scholar]
- 29.Junemann S, Wrigglesworth J M. J Biol Chem. 1995;270:16213–16220. doi: 10.1074/jbc.270.27.16213. [DOI] [PubMed] [Google Scholar]
- 30.Junemann S, Rich P R, Wrigglesworth J M. Biochem Soc Trans. 1995;23:157S. doi: 10.1042/bst023157s. [DOI] [PubMed] [Google Scholar]
- 31.Koland J G, Miller M J, Gennis R B. Biochemistry. 1984;23:1051–1056. doi: 10.1021/bi00298a008. [DOI] [PubMed] [Google Scholar]
- 32.Kauffman H F, van Gelder B F, DerVartanian D V. J Bioenerg Biomembr. 1980;12:265–276. doi: 10.1007/BF00744688. [DOI] [PubMed] [Google Scholar]
- 33.Wood P M. Biochim Biophys Acta. 1984;768:293–317. doi: 10.1016/0304-4173(84)90020-x. [DOI] [PubMed] [Google Scholar]
- 34.Poole R K. In: Chemical Methods in Prokaryotic Systematics. Goodfellow M, O'Donnell A G, editors. New York: Wiley; 1994. pp. 311–344. [Google Scholar]
- 35.D'mello R, Palmer S, Hill S, Poole R K. FEMS Microbiol Lett. 1994;121:115–120. [Google Scholar]
- 36.D'mello R, Hill S, Poole R K. Microbiology. 1996;142:755–763. doi: 10.1099/00221287-142-4-755. [DOI] [PubMed] [Google Scholar]
- 37.Pearlstein R M. In: Photosynthesis. Amesz J, editor. Amsterdam: Elsevier; 1987. pp. 299–317. [Google Scholar]
- 38.Ingledew W J, Rothery R A, Gennis R B, Salerno J C. Biochem J. 1992;282:255–259. doi: 10.1042/bj2820255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshikawa S, Shinzawa-Itoh K, Nakashima R, Yaono R, Yamashita E, Inoue N, Yao M, Fei M J, Libeu C P, Mizushima T, et al. Science. 1998;280:1723–1729. doi: 10.1126/science.280.5370.1723. [DOI] [PubMed] [Google Scholar]
- 40.Stoutland P O, Lambry J-C, Martin J-L, Woodruff W H. J Phys Chem. 1991;95:6406–6408. [Google Scholar]
- 41.Martin J-L, Vos M H. Annu Rev Biophys Biomol Struct. 1992;21:199–222. doi: 10.1146/annurev.bb.21.060192.001215. [DOI] [PubMed] [Google Scholar]
- 42.Perutz M F, Mathews F S. J Mol Biol. 1966;21:199–202. doi: 10.1016/0022-2836(66)90088-x. [DOI] [PubMed] [Google Scholar]
- 43.Bravo J, Mate M J, Schneider T, Switala J, Wilson K, Loewen P C, Fita I. Proteins. 1999;34:155–166. doi: 10.1002/(sici)1097-0134(19990201)34:2<155::aid-prot1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 44.Einarsdóttir O. Biochim Biophys Acta. 1995;1229:129–147. doi: 10.1016/0005-2728(94)00196-c. [DOI] [PubMed] [Google Scholar]
- 45.Miller M J, Gennis R B. J Biol Chem. 1983;258:9159–9165. [PubMed] [Google Scholar]
- 46.Kita K, Konishi K, Anraku Y. J Biol Chem. 1984;259:3375–3381. [PubMed] [Google Scholar]
- 47.Frauenfelder H, Sligar S G, Wolynes P G. Science. 1991;254:1598–1603. doi: 10.1126/science.1749933. [DOI] [PubMed] [Google Scholar]