Abstract
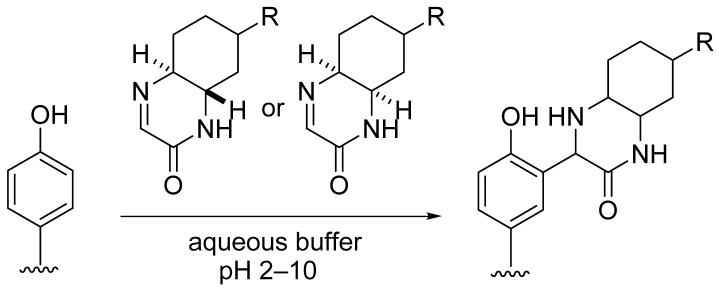
Cyclic imine derivatives that react with phenols, including tyrosine residues of peptides, have been developed. Reactions of the imines with phenols proceeded in water over a wide pH range (pH 2-10) at room temperature to 37 °C and afforded Mannich products without the need of additional catalysts.
Bond-forming chemical transformations that can be performed under biomolecule-compatible conditions (such as in water or aqueous buffers at 4 °C to 40 °C) are necessary for covalent attachment of small molecules to biomolecules and for coupling of small molecule modules in the presence of biomolecules.1-3 Known transformations include reactions of thiol groups with maleimides, reactions of primary amino groups with succinimidyl esters,1a azide-alkyne cycloadditions,1b-d and Staudinger ligations.1e Here we report the development of phenol-reacting molecules, cyclic imine derivatives, that can be used for Mannich-type reactions of phenols in water over a wide pH range at room temperature to 37 °C without the need of additional catalysts.
The phenol functionality is contained in tyrosine, a natural building block of proteins. Tyrosine residues are observed less often on the surface of folded proteins than lysine or carboxylic acid-containing residues, which are commonly used as labeling sites; thus accessible tyrosine residues are attractive covalent labeling sites.2,3 For proteins that do not have tyrosine on the surface, the phenol functionality is orthogonal; these proteins should not be affected by reactions with phenols. Therefore, phenol-reacting molecules in aqueous media can be used for both protein labeling at tyrosine and coupling reactions with phenol-bearing molecules in the presence of biomolecules that are not affected by the phenol-reacting molecules.
Covalent bond-forming reactions at tyrosine residues have been performed with imines prepared from formaldehyde and aniline derivatives in situ;2 however, the optimal pH range for this reaction is 5.5-6.5 and the reaction does not proceed above pH 8.2a Other tyrosine or phenol labeling reactions include Pd-catalyzed-O-alkylation, azonium coupling, and nitration with tetranitromethane.3 Although these were pioneering bond-forming reactions with phenols in water, molecules that react with phenols over a wide pH range without additional catalysts or multi-step conversions after the first reaction step on phenols are needed. When reactions occur over a wide pH range, reaction conditions, including pH, can be chosen depending on proteins present and on functionalities within molecules used as reactants.
We reasoned that imines should become useful phenol-reacting molecules that can be used across a wide pH range when reactivity and stability of the imines are tuned. First, molecules should show some stability in water or aqueous buffers; hydrolysis of imines is often a problem for reactions of imines in water-containing solvents.4 We reasoned that cyclic imines should be more resistant to hydrolysis than acyclic imines or should easily re-form the imines after hydrolysis (if applicable). Second, the cyclic imines should provide enough electrophilicity for reactions with phenols. Nucleophilic addition reactions to imines readily occur when the imines are protonated. Protonation of the imines by the phenolic hydroxyl group of phenols or by water of buffers may be key for the bond-forming reaction of the imines with phenols in some pH ranges.2,5 In addition, imines conjugated with electron-withdrawing groups should be more reactive than imines without electron-withdrawing groups. However, highly reactive imines may react non-specifically and handling of these imine molecules may be difficult. Third, the cyclic imines should not isomerize to enamines, which may cause dimerization and lower the concentration of the imines.6
Based on the considerations described above, we designed and synthesized cyclic imines 1 and 2 as phenol-reacting molecules. Cyclic imine 1 was readily obtained by mixing (±)-trans-1,2-diaminocyclohexane with ethyl glyoxylate (polymer form) in 2-PrOH at room temperature (23 °C) (Scheme 1). The reaction in other solvents, including toluene, ethyl acetate, and water, also afforded 1. Cyclic imine 2 was synthesized using cis-1,2-diaminocyclohexane by the same procedure used for the synthesis of 1, although formation of 2 was slower than formation of 1 (Scheme 1). Note that 1 and 2 were selectively obtained; formation of imidazolidine derivatives7 was not observed.
Scheme 1.
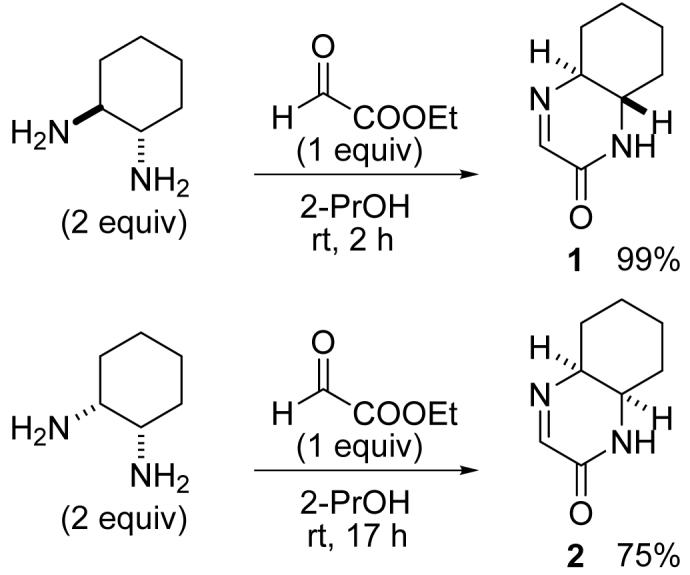
Synthesis of imines 1 and 2.
Imines 1 and 2 were soluble in water at 100 mM. 1H NMR analysis of a solution of 1 in D2O indicated that the imine moiety of 1 was partly hydrated.8 When a solution of 1 or 2 in water was kept at 23 °C for several days and was extracted with CH2Cl2, the imine was recovered quantitatively without sign of decomposition. When acetic acid (1 equiv) was added to a solution of imine 1 in D2O, the area of the signal of the imine proton of 1 decreased. However, when this mixture was neutralized by a solution of NaOH in D2O, the imine proton signal area increased. After extraction of this mixture with CH2Cl2, imine 1 was quantitatively recovered. Imine 1 was also quantitatively recovered after treatment with aqueous NaH2PO4 (pH 5) or Na2HPO4 (pH 9). These results indicate that there is an equilibrium between the imine and the hydrated form in water or aqueous solutions and that re-formation of the imine from the hydrated form occurs readily without hydrolysis to amine and aldehyde.
Reactions of the imines were evaluated using a simple phenol derivative, p-cresol (Tables 1 and 2). First, reactions of imine 1 (95 mM) with p-cresol (25 mM) were performed in aqueous media (pH 5-9) at 23 °C (Table 1, entries 1-4 and 8). Formation of product 3 was analyzed after 24 h. Product 3 was formed without requirement of additional catalysts. Imine 1 and p-cresol were soluble in the aqueous media used for the reactions and the reaction mixtures were initially homogeneous, clear solutions. Neither micelles9 nor biphasic mixtures10 were observed. However, product 3 often precipitated with p-cresol; this may have caused the moderate yield of the product. With lower concentrations of imine 1 and p-cresol, product 3 was also obtained (entries 5-7). Reaction between 1 and p-cresol in water and in other aqueous solutions also afforded product 3 (entries 9-15). Whereas imine 1 reacted with p-cresol at pH range from 2 to 10 as shown in Table 1, no formation of 3 was observed in 5% DMSO/aqueous 10% citric acid or in 5% DMSO/aqueous 1% trifluoroacetic acid (TFA) at 23 °C after 1 day; the imine and p-cresol were recovered. In organic solvents tested, including toluene, CH2Cl2, DMSO, DMF, and 2-PrOH, imine 1 did not react with p-cresol or formation of 3 was very slow (<5% after 1 day) at 23 °C. For the formation of 3 in organic solvents at room temperature, addition of an acid, such as TFA, was required.11
Table 1.
Reaction of imine 1 with p-cresol.a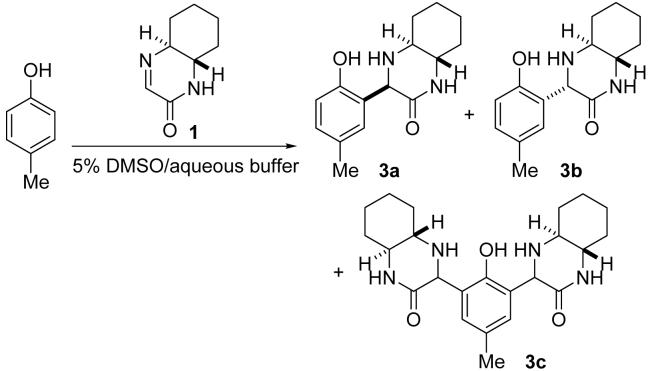
| entry | aqueous buffer (aqueous component) | yieldb (%) | 3a:3bc |
|---|---|---|---|
| 1 | 200 mM NaH2PO4, pH 5 | 55 | 1.3:1 |
| 2 | 200 mM NaH2PO4-Na2HPO4, pH 6.0 | 36 (20) | 1.2:1 |
| 3 | 200 mM NaH2PO4-Na2HPO4, pH 7.0 | 44 | 2.0:1 |
| 4 | 200 mM NaH2PO4-Na2HPO4, pH 8.0 | 46 | 3.3:1 |
| 5d | 200 mM NaH2PO4-Na2HPO4, pH 8.0 | 50 | 2.5:1 |
| 6e | 200 mM NaH2PO4-Na2HPO4, pH 8.0 | 52 | 2.5:1 |
| 7f | 200 mM NaH2PO4-Na2HPO4, pH 8.0 | 53 | 2.8:1 |
| 8 | 200 mM Na2HPO4, pH 9 | 44 | 3.9:1 |
| 9 | PBS, pH 7.4g | 41 | 2.3:1 |
| 10 | 100 mM Tris, pH 8.0g | 41 | 3.2:1 |
| 11 | 25 mM HEPES, pH7.5g | 50 | 2.8:1 |
| 12 | 10% CH3COOH, pH 2 | 24 | 0.8:1 |
| 13 | H2O | 36 | 2.7:1 |
| 14 | 200 mM Na2CO3, pH 10 | 49 | 2.1:1 |
| 15 | 200 mM Et3N, pH 10 | 29 | 2.1:1 |
| 16h | 100 mM NaH2PO4, pH 5 | 23 (60) [143] | 1.3:1 |
| 17h | 100 mM NaH2PO4-Na2HPO4, pH 6 | 31 (48) [127] | 1.5:1 |
| 18h | 100 mM NaH2PO4-Na2HPO4, pH 7 | 39 (27) [93] | 2.0:1 |
| 19h | 100 mM Na2HPO4,pH 9 | [76] | ndi |
| 20j | 100 mM NaH2PO4-Na2HPO4, pH 7 | 32 (45) | 2.0:1 |
Conditions: Imine 1 (95 mM) and p-cresol (25 mM) in 5% DMSO/indicated aqueous buffer (or aqueous component) at 23 °C for 24 h.
Sum of 3a and 3b; yield of 3c is indicated in parenthesis; determined by 1H NMR of the extracted mixture. For entries 1-15, yield of 3c was <10% except where noted. Percentage of modified ortho positions of the phenolic OH of p-cresol is indicated in bracket; complete conversion to 3c = 200% in bracket.
Determined by 1H NMR of the extracted mixture.
Imine 1 (95 mM) and p-cresol (5 mM).
Imine 1 (25 mM) and p-cresol (500 μM).
Imine 1 (5 mM) and p-cresol (500 μM).
PBS = phosphate buffered saline. Tris = tris(hydroxymethyl)aminomethane. HEPES= 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid.
Imine 1 (50 mM) and p-cresol (10 mM) in indicated buffer prepared with D2O (no DMSO was added) at 37 °C for 24 h. Yield was determined by 1H NMR of the reaction mixture.
Not determined.
Imine 1 (50 mM) and p-cresol (10 mM) in the absence of DMSO at 37 °C.
Table 2.
Reaction of imine 2 with p-cresol.a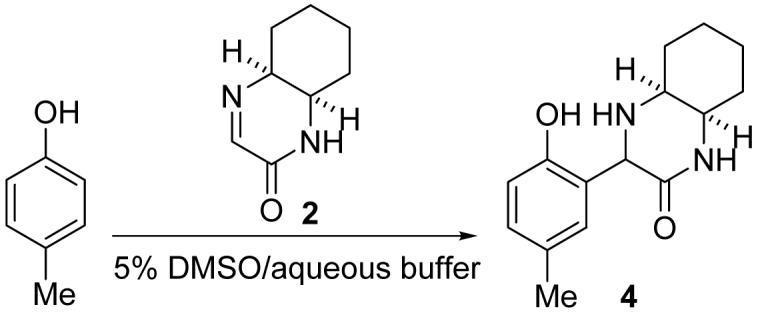
| entry | aqueous buffer | yieldb (%) |
|---|---|---|
| 1 | 200 mM NaH2PO4, pH 5 | 37 |
| 2 | 200 mM NaH2PO4-Na2HPO4, pH 6.0 | 35 |
| 3 | 200 mM NaH2PO4-Na2HPO4, pH 7.0 | 32 |
| 4 | 200 mM NaH2PO4-Na2HPO4, pH 8.0 | 26 |
| 5 | 200 mM Na2HPO4, pH 9 | 22 |
| 6 | 100 mM Tris, pH 8.0 | 25 |
| 7c | 100 mM NaH2PO4-Na2HPO4, pH 7 | [68] |
Imine 2 (95 mM) and p-cresol (25 mM) at 23 °C for 24 h.
Determined by 1H NMR of the extracted mixture. For entries 1-6, yield of di-addition product was <5%. Percentage of modified ortho positions of the phenolic OH of p-cresol is indicated in bracket; complete conversion to the di-addition product = 200% in bracket.
Imine 2 (50 mM) and p-cresol (10 mM) in the buffer prepared with D2O (no DMSO was added) at 37 °C for 24 h.
Next, the reaction was performed at 37 °C in buffer prepared with D2O and the product formation was directly analyzed by 1H NMR (entries 16-19). All compounds including products were soluble under these conditions. Combined yields of 3a, 3b, and 3c after 24 h were 66-83% for the reactions at pH 5-7 (entries 16-18). Yields after extraction (entry 20) were similar to those obtained upon direct analysis of the reaction mixture (entry 18).12
As reactions proceeded, yield of di-addition product 3c increased before complete consumption of p-cresol. Bivalent molecules often have the advantage of binding because of the avidity.13 Use of derivatives of imine 1 in modification of proteins and peptides at a single tyrosine residue under certain conditions may furnish a concise route to useful bivalent molecules.
Imine 2 also reacted with p-cresol and afforded corresponding addition product 4 (Table 2). Although the reaction of 2 was slower than the reaction of 1 under the same conditions, importantly, cyclic imines 1 and 2 both reacted with the phenol derivative in water over a wide pH range.
The C-C bond formed by the reaction was stable even under the conditions where the Mannich-type reaction of p-cresol with the imine did not occur: When 3a was kept in 5% DMSO/200 mM sodium phosphate (pH 8.0), 5% DMSO/200 mM NaH2PO4 (pH 5), 5% DMSO/10% citric acid, or 5% DMSO/1% TFA at 23 °C for 1 day, neither decomposition of 3a (as would be expected from retro-Mannich or retro-Michael14 reaction) nor isomerization of 3a to 3b was detected. The same experiments with 3b (3b:3a >99:1) showed that isomerization of 3b to 3a occurred slowly (3b:3a = 97:3 at pH 5 and 94:6 at pH 8.0 after 1 day), but no decomposition of 3b was detected. Addition product 4 was also stable under the conditions used for the experiments of 3a and 3b.
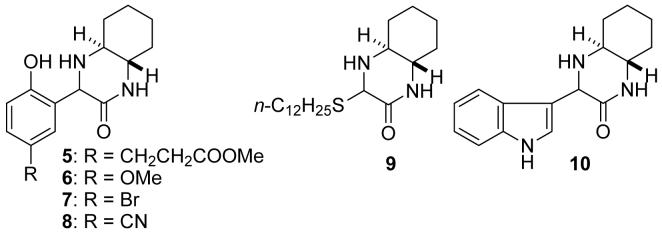
To provide further information about the reactivity of the imines, reactions of imine 1 with a series of phenol derivatives affording 5-8 (Figure 1) in aqueous buffers were examined. Reactions of phenols with electron-withdrawing groups (formation of 7 and 8) were slower than that without (formation of 3, 5, or 6). Mannich products 5, 6, and 7 were obtained in 41%, 41%, and 14% yield, respectively, after 24 h at pH 7.5; formation of 8 was negligible (2% yield) after 24 h under the same conditions.
Figure 1.
Products 5-10 obtained by reactions with 1.
Reactions of imine 1 with potential nucleophiles were also examined. Imine 1 did not react with imidazole, 2-PrOH, n-butylamine, 3-methylindole, or p-anisidine in 5% DMSO/200 mM sodium phosphate (pH 7.5) at 23 °C. Tris, which possesses amine and alcohol functionalities, did not react with imine 1, as the reaction affording 3 occurred in Tris buffer. These results suggest that tyrosine on a protein selectively reacts with imine 1, while histidine, lysine, N-terminal amino group, serine, threonine, and tryptophan do not. An exception was thiol: Reaction of 1-dodecanethoil with imine 1 afforded addition product 9. When both p-cresol and 1-dodecanethoil were present (each at 25 mM) in a reaction mixture with 1 (95 mM) at pH 8.0, both 3 and 9 were formed after 1 day. However, 9 was decomposed in aqueous 10% citric acid or in aqueous 1% TFA at 23 °C and the thiol was recovered (imine 1 was not re-formed by this decomposition and was not recovered). Note that product 3 was stable under these conditions as described above. As an orthogonal functionality to natural proteins, indole (3-non-substituted indole) reacted with imine 1 and formed 10 (87% yield after 24 h) in 5% DMSO/200 mM sodium phosphate (pH 7.5) at 23 °C.
Imine 1 covalently reacted with tyrosine-containing peptides as shown in Table 3. 1H NMR analyses of the reaction mixtures showed that bond-formation occurred at tyrosine. Formation of the Mannich products was also confirmed by high-resolution mass analysis. A 24-mer peptide, YKLLKELLAKLKWLLRKLLGPTSL,15 also covalently reacted with 1 in sodium phosphate (pH 7.0), in Tris (pH 8.0), and in HEPES (pH 7.5) as confirmed by mass analysis. Imine 1 also reacted with lysozyme, which has tyrosine on the folded surface,2,16 as confirmed by mass analysis. Myoglobin, which lacks surface-accessible tyrosine,2,3a,14 did not react with imine 1 under the conditions used for the reactions of lysozyme with 1.
Table 3.
Addition reactions of tyrosine-containing peptides to imines 1 and 11.a
| entry | imine | peptide | reaction pH | yieldb (%) |
|---|---|---|---|---|
| 1 | 1 | GlyTyr | pH 5 | 25c (54)d [133]e |
| 2 | 1 | GlyTyr | pH 7 | 34c (38)d [110]e |
| 3 | 1 | GlyTyr | pH 8.5 | [91]e |
| 4 | 1 | AlaTyrAla | pH 7 | [109]e |
| 5 | 11 | GlyTyr | pH 7 | [91]e |
| 6 | 11 | AlaTyrAla | pH 7 | [74]e [99]e,f |
| 7g | 11 | AlaTyrAla | pH 6 | [58]e [96]e,f |
Conditions: Imine (50 mM) and peptide (10 mM) in 100 mM NaH2PO4-Na2HPO4 (pH 7), 100 mM NaH2PO4 (for pH 5), or 100 mM Na2HPO4 (for pH 8.5) prepared with D2O at 37 °C for 24 h.
Determined by 1H NMR of the reaction mixture.
Mono-addition product.
Di-addition product (di-addition at tyrosine).
Percentage of modified ortho positions of the tyrosine phenolic OH; complete conversion to the di-addition product = 200%.
Data after 48 h.
Reaction in D2O.
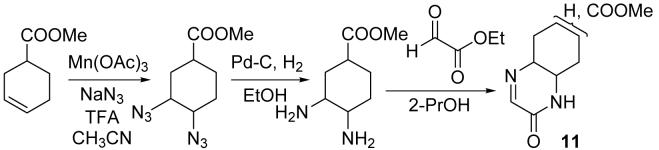
To use the imines for protein labeling and module coupling, the imines must be conjugated to labeling molecules (such as fluorescent molecule or biotin) or modules. The imine conjugates may be prepared using an ester or amide linkage on the cyclohexane ring of the imines. To demonstrate the feasibility of reactions of such imine conjugates, imine derivative 11 was synthesized as a mixture of the regio- and diastereo-isomers (Scheme 2)17 and reactions of 11 with tyrosine-containing peptides were evaluated (Table 3). Formation of addition products was observed, suggesting that derivatives of imines 1 and 2 bearing other moieties at the cyclohexane ring may also react with phenols.
Scheme 2.
Synthesis of imine 11.
We have developed cyclic imine derivatives that can covalently modify phenols, including tyrosine residues, in water across a wide pH range at room temperature to 37 °C without the need of additional catalysts. These imines were relatively stable but efficiently reacted with phenols. Derivatives of these imines bearing fluorescent or other moieties should be useful for modification of peptide and proteins at tyrosine and for module coupling reactions in aqueous media.
Experimental Section
Synthesis of imine 1
To a solution of trans-1,2-diaminocyclohexane (228 mg, 2.00 mmol) in 2-PrOH (3.0 mL), a solution of ethyl glyoxylate polymer form (45-50% in toluene, 0.21 mL, 1.00 mmol) in 2-PrOH (3.0 mL) was added dropwise over 15 min at room temperature (23 °C). The mixture was stirred for 2 h, concentrated under reduced pressure, and purified by flash column chromatography (AcOEt) to afford 1 (151 mg, 99%) as a colorless solid. 1H NMR (500 MHz, CDCl3): δ 1.28-1.37 (m, 1H), 1.39-1.49 (m, 3H), 1.80-1.84 (m, 1H), 1.88-1.92 (m, 1H), 1.94-1.98 (m, 1H), 2.35-2.41 (m, 1H), 3.06-3.12 (m, 1H), 3.17 (dt, J = 3.9 Hz, 11.6 Hz, 1H), 7.07 (brs, 1H), 7.72 (t, J = 2.8 Hz, 1H). 13C NMR (125 MHz, CDCl3): δ 23.6, 25.2, 31.0, 31.5, 54.1, 63.0, 156.3, 158.0. HRMS: calcd for C8H13N2O (MH+) 153.1022, found 153.1019.
Synthesis of imine 2
To a solution of cis-1,2-diaminocyclohexane (228 mg, 2.00 mmol) in 2-PrOH (3.0 mL), a solution of ethyl glyoxylate polymer form (45-50% in toluene, 0.21 mL, 1.00 mmol) in 2-PrOH (3.0 mL) was added dropwise over 15 min at rt. The mixture was stirred for 17 h, concentrated under reduced pressure, and purified by flash column chromatography (AcOEt) to afford 2 (114 mg, 75%) as a colorless solid. 1H NMR (500 MHz, CDCl3): δ 1.37-1.45 (m, 1H), 1.46-1.55 (m, 3H), 1.62-1.68 (m, 1H), 1.70-1.77 (m, 2H), 1.86-1.92 (m, 1H), 3.70 (m, 1H), 3.85-3.87 (m, 1H), 5.79 (brs, 1H), 7.75 (t, J = 2.3 Hz, 1H). 13C NMR (125 MHz, CDCl3): δ 20.7, 22.8, 28.2, 28.8, 48.5, 57.7, 155.9, 157.8. HRMS: calcd for C8H13N2O (MH+) 153.1022, found 153.1026.
Reactions of imine 1 with p-cresol (Table 1, entries 1-4 and 8-15)
A 100 mM solution of imine 1 in 200 mM sodium phosphate buffer (or indicated aqueous component) was prepared immediately before starting reaction. To the 100 mM solution of 1 (950 μL), a 500 mM solution of p-cresol in DMSO (50 μL) was added at rt and the mixture was stirred for 24 h. After pH of the reaction mixture was adjusted to pH 7~8 with 200 mM Na2HPO4 or with 200 mM NaH2PO4, the mixture was extracted with CH2Cl2. Organic layers were combined, washed with brine, dried over Na2SO4, and concentrated under reduced pressure. The crude mixture was dissolved in CDCl3 and was analyzed by 1H NMR to determine the yield.
Compound 3a
1H NMR (500 MHz, CDCl3-CD3OD): δ 1.30-1.47 (m, 4H), 1.81-1.86 (m, 2H), 1.89-1.92 (m, 2H), 2.25 (s, 3H, CH3), 2.65 (ddd, J = 3.5 Hz, 9.5 Hz, 11.0 Hz, 1H), 3.31-3.26 (m, 1H), 4.59 (s, 1H), 6.71 (d, J = 8.0 Hz, 1H), 6.98 (dd, J = 2.0 Hz, 8.0 Hz, 1H), 7.04 (d, J = 2.0 Hz, 1H). 13C NMR (125 MHz, CDCl3-CD3OD): δ 19.8, 23.4, 24.2, 30.0, 30.7, 57.2, 57.7, 62.3, 115.9, 123.1, 128.5, 129.6, 131.0, 152.7, 170.9. HRMS: calcd for C15H21N2O2 (MH+) 261.1597, found 261.1606.
Compound 3b
1H NMR (500 MHz, CDCl3): δ 1.18-1.36 (m, 4H), 1.75 (m 1H), 1.79-1.86 (m, 2H), 1.96 (dd, J = 2.8, 12.6 Hz, 1H), 2.26 (s, 3H), 2.64 (ddd, J = 3.5 Hz, 9.5 Hz, 11.5 Hz, 1H), 3.08 (dt, J = 4.0 Hz, 10.5 Hz, 1H), 4.96 (s, 1H), 6.76 (brs, 1H), 6.80 (d, J = 8.0 Hz, 1H), 7.00 (dd, J = 2.0 Hz, 8.0 Hz, 1H), 7.06 (s, 1H) 10.8 (br, 1H). 13C NMR (125 MHz, CDCl3): δ 20.8, 23.7, 24.8, 30.6, 31.6, 53.4, 58.9, 59.4, 117.5, 122.1, 127.3, 128.7, 129.7, 154.7, 170.6. HRMS: calcd for C15H21N2 (MH+) 261.1597, found 261.1595.
Compound 3c
1H NMR (500 MHz, CDCl3): δ 1.24-1.39 (m, 8H), 1.76-1.85 (m, 8H), 2.25 (s, 3H), 2.55-2.64 (m, 2H), 3.15-3.21 (m, 2H), 4.54 (s, 1H), 4.75 (s, 1H), 6.23 (s, 1H), 6.33 (s, 1H), 7.02 (s, 1H), 7.07 (s, 1H). 13C NMR (125 MHz, CDCl3): δ 20.5, 20.6, 23.8, 24.6, 24.7, 30.50, 30.53, 31.3, 31.4, 57.8, 58.2, 58.4, 61.4, 62.9, 124.7, 125.2, 128.3, 128.7, 130.5, 131.6, 151.7, 170.6, 170.7. HRMS: calcd for C23H33N4O3 (MH+) 413.2547, found 413.2544.
Compound 4
1H NMR (500 MHz, CDCl3): δ 1.33-1.39 (m, 1H), 1.40-1.46 (m, 1H), 1.55-1.59 (m, 3H), 1.63-1.69 (m, 1H), 1.72-1.78 (m, 1H), 1.88-1.95 (m, 1H), 2.25 (s, 3H), 3.26 (dt, J = 7.5 Hz, 3.5 Hz, 1H), 3.64 (brd, J = 3.5, 1H), 4.81 (s, 1H), 6.57 (brs, 1H), 6.77 (d, J = 8.0 Hz, 1H), 6.99 (dd, J = 2.0 Hz, 8.0 Hz, 1H), 7.06 (d, J = 2.0 Hz, 1H). 13C NMR (125 MHz, CDCl3): δ 20.6, 21.3, 22.0, 27.4, 30.5, 48.6, 51.8, 58.1, 117.3, 122.1, 128.7, 129.0, 129.8, 154.5, 169.9. HRMS: calcd for C15H21N2O2S8 (MH+) 261.1597, found 261.1594.
Supplementary Material
Acknowledgment
This study was supported by NIH R21 GM078447.
Footnotes
Supporting Information Available Additional experimental procedures, synthesis and characterization of compounds, and NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Antos JM, Francis MB. Curr. Opin. Chem. Biol. 2006;10:253. doi: 10.1016/j.cbpa.2006.04.009..Lewis WG, Green LG, Grynszpan F, Radic Z, Carlier PR, Taylor P, Finn MG, Sharpless KB. Angew. Chem., Int. Ed. 2002;41:1053. doi: 10.1002/1521-3773(20020315)41:6<1053::aid-anie1053>3.0.co;2-4..Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. J. Am. Chem. Soc. 2003;125:3192. doi: 10.1021/ja021381e..Agard NJ, Prescher JA, Bertozzi CR. J. Am. Chem. Soc. 2004;126:15046. doi: 10.1021/ja044996f..Saxon E, Bertozzi CR. Science. 2000;287:2007. doi: 10.1126/science.287.5460.2007..Dirksen A, Hackeng TM, Dawson PE. Angew. Chem., Int. Ed. 2006;45:7581. doi: 10.1002/anie.200602877..Crich D, Banerjee A. J. Am. Chem. Soc. 2007;129:10064. doi: 10.1021/ja072804l..Tanaka K, Masuyama T, Hasegawa K, Tahara T, Mizuma H, Wada Y, Watanabe Y, Fukase K. Angew. Chem., Int. Ed. 2008;47:102. doi: 10.1002/anie.200702989..Prescher JA, Bertozzi CR. Nat. Chem. Biol. 2005;1:13. doi: 10.1038/nchembio0605-13.. See also Maruoka K, Tayama E, Ooi T. Proc. Natl. Acad. Sci. U.S.A. 2004;101:5824. doi: 10.1073/pnas.0307725101.Hadley EB, Witek AM, Freire F, Peoples AJ, Gellman SH. Angew. Chem., Int. Ed. 2007;46:7056. doi: 10.1002/anie.200702449..
- 2.(a) Joshi NS, Whitaker LR, Francis MBA. J. Am. Chem. Soc. 2004;126:15942. doi: 10.1021/ja0439017. [DOI] [PubMed] [Google Scholar]; (b) McFarland JM, Joshi NS, Francis MB. J. Am. Chem. Soc. 2008;130:7639. doi: 10.1021/ja710927q. [DOI] [PubMed] [Google Scholar]
- 3.(a) Tilley SD, Francis MB. J. Am. Chem. Soc. 2006;128:1080. doi: 10.1021/ja057106k. [DOI] [PubMed] [Google Scholar]; (b) Hooker JM, Kovacs EW, Francis MB. J. Am. Chem. Soc. 2004;126:3718. doi: 10.1021/ja031790q. [DOI] [PubMed] [Google Scholar]; (c) Kenner RA, Neurath H. Biochemistry. 1971;10:551. [PubMed] [Google Scholar]; (d) Hass JA, Frederick MA, Fox BG. Protein Expr. Purif. 2000;20:274. doi: 10.1006/prep.2000.1293. [DOI] [PubMed] [Google Scholar]; (e) Koshi Y, Nakata E, Miyagawa M, Tsukiji S, Ogawa T, Hamachi I. J. Am. Chem. Soc. 2008;130:245. doi: 10.1021/ja075684q. [DOI] [PubMed] [Google Scholar]; (f) Guo H-M, Minakawa M, Tanaka F. J. Org. Chem. 2008;73:3964. doi: 10.1021/jo8003293. [DOI] [PubMed] [Google Scholar]
- 4.(a) Kobayashi S, Hamada T, Manabe K. J. Am. Chem. Soc. 2002;124:5640. doi: 10.1021/ja026094p. [DOI] [PubMed] [Google Scholar]; (b) Hamada T, Manabe K, Kobayashi S. Chem. Eur. J. 2006;12:1205. doi: 10.1002/chem.200500673. [DOI] [PubMed] [Google Scholar]
- 5.Miyano S, Abe N. Tetrahedron Lett. 1970;11:1909. [Google Scholar]
- 6.(a) Gravel E, Poupon E, Hocquemiller R. Org. Lett. 2005;7:2497. doi: 10.1021/ol050849q. [DOI] [PubMed] [Google Scholar]; (b) Wanner M, Koomen G-J. J. Org. Chem. 1995;60:5634. [Google Scholar]
- 7.Halland N, Hazell RG, Jorgensen KA. J. Org. Chem. 2002;67:8331. doi: 10.1021/jo0261449. [DOI] [PubMed] [Google Scholar]
- 8.(a) Struve C, Christophersen C. Heterocycles. 2003;60:1907. [Google Scholar]; (b) Calveras J, Bujons J, Parella T, Crehuet R, Espelt L, Joglar J, Clapes P. Tetrahedron. 2006;62:2648. [Google Scholar]
- 9.Itoh J, Fuchibe K, Akiyama T. Synthesis. 2006:4075. [Google Scholar]
- 10.Narayan S, Muldoon J, Finn MG, Fokin VV, Kolb HC, Sharpless B. Angew. Chem., Int. Ed. 2005;44:3275. doi: 10.1002/anie.200462883. [DOI] [PubMed] [Google Scholar]
- 11.(a) Tohma S, Rikimaru K, Endo A, Shimamoto K, Kan T, Fukuyama T. Synthesis. 2004:909. [Google Scholar]; (b) Chen Y-J, Lei F, Liu L, Wang D. Tetrahedron. 2003;59:7609. [Google Scholar]; (c) Rondot C, Zhu J. Org. Lett. 2005;7:1641. doi: 10.1021/ol050334z. [DOI] [PubMed] [Google Scholar]
- 12.Reactions in buffer prepared with D2O were slightly slower than the reactions in buffer prepared with H2O under identical conditions; this may be due to the solvent isotope effect.
- 13.Tanaka F, Kinoshita K, Tanimura R, Fujii I. J. Am. Chem. Soc. 1996;118:2332. [Google Scholar]
- 14.Wang P, Liu R, Wu X, Ma H, Cao X, Zhou P, Zhang J, Weng X, Zhang X-L, Qi J, Zho X, Weng L. J. Am. Chem. Soc. 2003;125:1116. doi: 10.1021/ja029040o. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka F, Fuller R, Barbas CF., III Biochemistry. 2005;44:7583. doi: 10.1021/bi050216j. [DOI] [PubMed] [Google Scholar]
- 16.Ly T, Julian RR. J. Am. Chem. Soc. 2008;130:351. doi: 10.1021/ja076535a. [DOI] [PubMed] [Google Scholar]
- 17.Azides are potentially hazardous. For safety in handling of azides, see: Brase S, Gil C, Knepper K, Zimmermann V. Angew. Chem., Int. Ed. 2005;44:5188. doi: 10.1002/anie.200400657..
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.