Abstract
Mutations in the kinase WNK4 cause pseudohypoaldosteronism type II (PHAII), a syndrome featuring hypertension and high serum K+ levels (hyperkalemia). WNK4 has distinct functional states that regulate the balance between renal salt reabsorption and K+ secretion by modulating the activities of renal transporters and channels, including the Na-Cl cotransporter NCC and the K+ channel ROMK. WNK4's functions could enable differential responses to intravascular volume depletion (hypovolemia) and hyperkalemia. Because hypovolemia is uniquely associated with high angiotensin II (AngII) levels, AngII signaling might modulate WNK4 activity. We show that AngII signaling in Xenopus oocytes increases NCC activity by abrogating WNK4's inhibition of NCC but does not alter WNK4's inhibition of ROMK. This effect requires AngII, its receptor AT1R, and WNK4, and is prevented by the AT1R inhibitor losartan. NCC activity is also increased by WNK4 harboring mutations found in PHAII, and this activity cannot be further augmented by AngII signaling, consistent with PHAII mutations providing constitutive activation of the signaling pathway between AT1R and NCC. AngII's effect on NCC is also dependent on the kinase SPAK because dominant-negative SPAK or elimination of the SPAK binding motif in NCC prevent activation of NCC by AngII signaling. These effects extend to mammalian cells. AngII increases phosphorylation of specific sites on SPAK and NCC that are necessary for activation of each in mpkDCT cells. These findings place WNK4 in the signaling pathway between AngII and NCC, and provide a mechanism by which hypovolemia maximizes renal salt reabsoprtion without concomitantly increasing K+ secretion.
Keywords: angiotensin II receptor, hypertension, distal convoluted tubule, salt reabsorption, thiazide
Aldosterone is released from the adrenal glomerulosa in 2 different physiologic conditions: intravascular volume depletion and hyperkalemia. In the former, aldosterone promotes maximal renal Na-Cl reabsorption to preserve and restore intravascular volume, whereas in the latter renal K+ secretion is maximized. Classical explanations for these alternative responses have focused on acute changes in solute delivery to the distal nephron. For example, in volume depletion there is enhanced proximal reabsorption of Na+, which reduces the distal electrogenic reabsorption of Na+ via the epithelial sodium channel (ENaC) that is required to establish the electrical gradient necessary for K+ secretion.
The rare autosomal dominant disease pseudohypoaldosteronism type II (PHAII) suggests there must be additional components that regulate the balance between renal salt reabsorption and potassium secretion. Patients with PHAII have chloride-dependent hypertension and hyperkalemia despite otherwise normal renal function and normal aldosterone secretion, suggesting that they constitutively reabsorb Na-Cl at the expense of impaired K+ secretion. Missense mutations in the serine-threonine kinase WNK4 have been shown to cause PHAII (1). Subsequent studies in Xenopus oocytes (2–7), mammalian cells (8) and monolayers (9, 10) have demonstrated that WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion; this is achieved by orchestrating the activities of the Na-Cl cotransporter NCC, the K+ channel ROMK, the Na+ channel ENaC, and the paracellular Cl− flux pathway (11, 12). Wild-type WNK4 inhibits NCC in Xenopus oocytes (2–4), and in mammalian cells [Cos-7 and polarized M1 cells (8)], however, missense mutations that cause PHAII abrogate this inhibition, increasing NCC activity; these effects are mediated at least in part by altering trafficking of NCC to the plasma membrane. Similarly, PHAII mutations allow increased activity of ENaC (6) and selectively increase paracellular Cl− conductance (9), effects that all promote maximal renal NaCl reabsorption. Conversely, although wild-type WNK4 also inhibits the K+ channel ROMK (the major mediator of distal renal K+ secretion), PHAII-mutant WNK shows enhanced, not diminished, inhibition of ROMK (5). This latter effect prevents renal K+ secretion and promotes hyperkalemia. Thus, the kidneys of patients with PHAII behave as although a regulatory switch is stuck in a state that results in constitutive reabsorption of Na-Cl and inhibition of K+ secretion, accounting for the hypertension and hyperkaemia in affected patients.
These in vitro effects of WNK4 are duplicated in mouse models: mice harboring a single additional genomic copy of the wild-type WNK4 locus introduced as a BAC transgene show reduced expression of NCC, lower blood pressure, and predisposition to hypokalemia, whereas BAC transgenes harboring a PHAII mutation induce hypertension and striking hyperkalemia; both traits are reversed by NCC deficiency (13). These findings were confirmed by analysis of a mouse with knockin of a PHAII mutation (14).
These effects demonstrated that WNK4 has at last 2 distinct biochemical states and raised the possibility that WNK4 might have a third state that could support K+ secretion without maximizing Na-Cl reabsorption. Phosphorylation of WNK4 in its C terminus by the aldosterone-induced kinase SGK (15) induces such a state; this phosphorylation alleviates inhibition of ENaC and ROMK, which would maximize K+ secretion. Thus, the different states of WNK4 can modulate distal renal activity from a basal state to one that either maximizes salt reabsorption or maximizes K+ secretion.
The different states of WNK4 correspond to the desired alternative responses to intravascular volume depletion and hyperkalemia. This suggests that the PHAII mutations mimic a natural state resulting from volume depletion, and beg the question of what the upstream regulatory signal might be. Signaling of the peptide hormone angiotensin II (AngII) through its G protein coupled receptor AT1R, is an attractive candidate, because AngII levels are markedly elevated by activation of the renin angiotensin system in response to volume depletion, but are not increased by hyperkalemia.
Here, by reconstitution experiments in Xenopus oocytes, we show that AngII signaling acts through WNK4 and the Ste20-type kinase SPAK to increase NCC activity. This effect can be substituted in full by PHAII-mutant WNK4. We further show that AngII signaling increases phosphorylation at key regulatory sites on both SPAK and NCC in mammalian cells. Conversely, we show that AngII signaling does not reverse WNK4's inhibition of ROMK. These findings place WNK4-SPAK in the signaling pathway between AngII and NCC, and reveal a key role for this pathway in the renal response to intravascular volume depletion.
Results
AngII Abrogates WNK4's Inhibition of NCC.
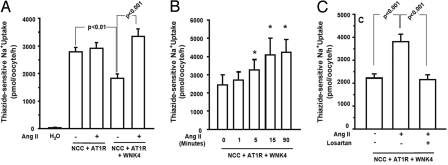
As described in refs. 16 and 17, injection of NCC cRNA into Xenopus laevis oocytes resulted in a marked increase in thiazide-sensitive 22Na+ uptake that is inhibited by coexpression of wild-type (WT) WNK4 (Fig. 1A). Although Xenopus oocytes do not endogenously express the AngII receptor AT1R, injection of AT1R cRNA induces expression and functional receptor signaling, and administration of exogenous AngII results in increased intracellular IP3 and calcium (18). In the absence of WNK4, expression of AT1R with NCC in the presence or absence of AngII had no effect on NCC activity (Fig. 1A). Addition of WT-WNK4 resulted in AngII-regulated NCC activity: WT-WNK4 inhibited NCC activity in the absence of AngII, but this inhibition was completely eliminated by the addition of AngII (P < 0.001) (Fig. 1A). Time-course experiments revealed that, in the presence of WNK4 and AT1R, AngII increased the activity of NCC within 1 to 2 minutes, and its maximal effect was achieved by ≈15 min (Fig. 1B and Fig. S1A). The ability of AngII to increase NCC activity was completely blocked by losartan, a specific antagonist of AT1R (19), demonstrating that AngII's effect is receptor-dependent (Fig. 1C and Fig. S1B). Together, these data show that AngII signaling increases NCC activity by elimination of WNK4's inhibitory activity.
Fig. 1.
AngII relieves WNK4's inhibition of NCC. Xenopus oocytes were injected with water or indicated cRNAs, incubated, and thiazide-sensitive Na+ influx was measured in the presence or absence of AngII and losartan as described in Methods. (A) The results of 8 different experiments are shown. The results demonstrate that WNK4 inhibits NCC activity, that AT1R in the absence of AngII has no effect, but in the presence of AngII, WNK4's inhibition of NCC is abrogated. (B) Time dependence of AngII effect. Groups of oocytes were exposed to AngII for 1, 5, 15, and 90 min. Asterisks denote Na+ influx that is significantly different from uptake observed in the absence of AngII. AngII's effect is rapid, reaching maximal values in ≈15 min. (C) Losartan prevents AngII's effect on NCC. Oocytes were exposed to losartan at 1 μM for 15 min before addition of AngII. Losartan completely blocks the effect of AngII to increase NCC activity.
Insensitivity of PHAII-Mutant WNK4 to Ang II Signaling.
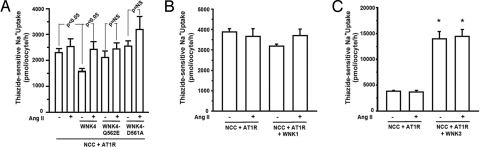
In vivo and in vitro studies have shown that WNK4 harboring missense mutations found in patients with PHAII mutations no longer inhibits NCC activity (2, 13), an effect similar to the observed loss of WT-WNK4's inhibitory activity after activation of AT1R. This suggests that PHAII mutations might be functionally equivalent to the physiologic effect of AngII signaling on WT-WNK4. If this were true, we would expect AngII signaling to have no further stimulatory effect on NCC activity in the presence of PHAII-mutant WNK4. As previously shown, PHAII mutations WNK4-Q562E and WNK4-D561A both abrogate inhibition of NCC activity (2). AngII signaling in the presence of either WNK4-Q562E or WNK4-D561A had no further stimulatory effect on NCC activity (Fig. 2A) (P = 0.24 and P = 0.58, respectively). This result is consistent with PHAII mutant WNK4 constitutively supplying the physiologic effect of activated AT1R in the absence of AT1R signaling.
Fig. 2.
Effects of AngII signaling in the presence of PHAII-WNK4, WNK1 and WNK3. (A) PHAII mutant WNK4 phenocopys the effect of AngII. Thiazide-sensitive 22Na+ uptake was measured in oocytes injected with NCC and AT1R alone or together with wild-type WNK4 or WNK4 harboring either the Q562E or D561A PHAII mutation in the presence or absence of AngII. Unlike WT-WNK4, PHAII-WNK4s show no significant inhibition of NCC activity, and AngII signaling imparts no significant further increase in NCC activity. (B and C) AngII signaling does not alter NCC activity in the presence of WNK1 or WNK3. Thiazide-sensitive 22Na+ uptake was measured as in panel A except that WNK1 or WNK3 has been substituted for WNK4. WNK1 has no effect on NCC in the presence or absence of AngII; WNK3 markedly increases NCC activity as previously described, however, AngII signaling does not modulate this effect. *, significantly different from the uptake observed in NCC+AT1R group in the absence of AngII.
Ang II's Stimulation of NCC Specifically Requires WNK4.
The kinase domains of other WNK kinases are highly homologous to WNK4 and have been studied in Xenopus oocytes. Expression of WNK1 with NCC has no effect on NCC activity, whereas WNK3 is a powerful activator of NCC (3, 20). To determine whether AngII signaling can regulate NCC activity in the presence of other WNKs, we measured the effect of activation of AT1R on NCC activity in oocytes expressing either WNK1 or WNK3. AngII signaling had no significant effect on NCC activity in the presence of WNK1 (Fig. 2B). Similarly, AngII signaling had no effect on NCC activity in the presence of WNK3 (Fig. 2C) (as we reported in ref. 20, WNK3 by itself markedly activated NCC). These data demonstrate that AngII's effect on NCC depends on the presence of WNK4, and this effect cannot be substituted by other WNKs in this Xenopus system.
Activated AT1R Does Not Alleviate WNK4's Inhibition of ROMK.
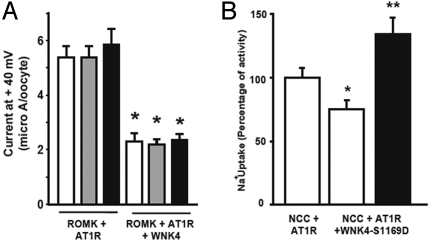
WNK4 also inhibits the potassium channel ROMK and unlike the loss of inhibition of NCC with PHAII mutations, inhibition of ROMK is augmented by PHAII mutations (5). We tested whether AngII signaling is able to modulate the effect of WNK4 on ROMK. In contrast to the loss of WNK4's inhibition of NCC, we found that AngII signaling did not relieve inhibition of ROMK by WNK4 (Fig. 3A). A mutation that mimics phosphorylation of WNK4 at a serum glucocorticoid kinase site (SGK site; WNK4-S1169D) has been shown to release ROMK and ENaC from WNK4 inhibition (15). If this regulatory site is used to help distinguish response to hyperkalemia and volume depletion, we might expect this modification not to alleviate inhibition of NCC. Similar to wild-type WNK4, WNK4-S1169D retained the ability to inhibit NCC. Importantly, AngII retains the ability to alleviate WNK4's inhibition of NCC in the presence of the S1169D mutation (Fig. 3B).
Fig. 3.
Effects of AngII on ROMK and effects of WNK41169D on NCC. (A) No effect of AngII signaling on ROMK activity. Oocytes were injected with ROMK cRNA+AT1R with or without WNK4 cRNA. ROMK activity was assessed in the absence (white bars) or presence of AngII for 5 min (gray bars) or 10 min (black bars). WNK4 significantly inhibited ROMK activity, and this effect was not altered by AngII signaling. Asterisks denote significant differences from ROMK+AT1R in the absence of WNK4 and AngII. (B) Effects of WNK4 S1169D on NCC activity. Oocytes were injected with cRNAs encoding NCC+AT1R with and without WNK4-S1169D cRNA and 22Na+ uptake was assessed. Values were normalized to uptake observed in NCC+AT1R in the absence of AngII. WNK4-S1169D significantly inhibits NCC (white bar) and this inhibition is reversed by AngII signaling (black bar). *, significantly different from uptake observed in NCC+AT1R group in the absence of AngII; **, significantly different from the uptake observed in NCC+AT1R+WNK4-S1169D in the absence of AngII.
The Stimulatory Effect of AngII on NCC in the Presence of WNK4 Is SPAK-Dependent.
Recent data has shown that WNK1 and WNK4 lie upstream of the Ste20-related serine-threonine proline-alanine rich kinase (SPAK) and oxidative stress response 1 kinase (OSR1) to regulate the bumetanide-sensitive Na+:K+:2Cl− cotransporter NKCC1, a close relative of NCC in the SLC12A gene family (21–25). WNK3 also lies upstream of SPAK to activate the renal specific Na+:K+:2Cl− cotransporter NKCC2 (26). Moreover, SPAK is a key kinase for the activation of NCC by intracellular chloride depletion (27, 28). These observations suggest that the pathway from AT1R to NCC may include SPAK.
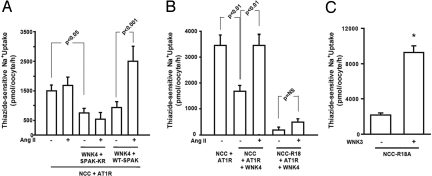
Because oocytes express endogenous SPAK, we analyzed the effect of SPAK harboring the K104R mutation, which is catalytically inactive and functions as a dominant-negative inhibitor of the endogenous SPAK (7, 26). SPAK-K104R prevented AngII's activation of NCC in the presence of WNK4 (Fig. 4A). These data provide evidence that the stimulatory effect of AngII on NCC in the presence of WNK4 is SPAK-dependent. As a control, wild-type SPAK cRNA was added to the injection mixture containing ATR1 and WNK4. In this condition, we observed a significant activation of NCC in the presence of AngII (Fig. 4A).
Fig. 4.
SPAK is required for AngII induced increase of NCC activity. (A) 22Na+ uptake in oocytes injected with combinations of constructs that include wild-type SPAK or the dominant-negative SPAK-K104R (SPAK-KR). SPAK-KR prevents AngII signaling from increasing NCC activity, whereas wild-type SPAK supports a robust increase in NCC activity with AngII signaling. (B) Elimination of the SPAK binding site on NCC drastically reduces NCC activity. NCC-R18A (NCC-R18) mutates the unique SPAK binding site on NCC. Oocytes expressing this mutant NCC show markedly reduced activity that cannot be restored by AngII, suggesting a requirement for SPAK binding. (C) WNK3 can activate NCC-R18A. Oocytes were injected with NCC-R18A cRNA alone or together with WNK3 cRNA. Assessment of Na+ uptake indicates that WNK3 is still capable of activating NCC, showing that WNK3 stimulation of NCC activity does not require the unique SPAK binding site of NCC. The asterisk denotes difference from NCC-R18A control without WNK3.
SPAK/OSR1-possess a Conserved C-Terminal (CCT) domain, which is capable of interacting with RFx(V/I) motifs present in WNK isoforms and substrates such as NCC (29). One such motif is present in the carboxyl terminal domain of WNK4 (994VGRFQVT) and the amino terminal domain of NCC (15CSGRFTIS). To test whether either of these motifs are necessary for AngII's stimulatory effect on NCC in the presence of WNK4, we mutated each (WNK4-F997A; NCC-R18A). Elimination of the SPAK-binding motif in WNK4 did not affect the activation of NCC by activated AT1R (Fig. S2), suggesting that a physical interaction between WNK4 and SPAK via this motif is not required. In contrast, elimination of the SPAK-binding motif in NCC had 2 prominent effects: The basal activity of NCC was virtually eliminated and activation of NCC by AngII in the presence of WNK4 was abrogated (Fig. 4B). These results suggest that the path from AT1R to NCC requires both WNK4 and SPAK. Interestingly, however, NCC-R18A could still be activated by WNK3, suggesting that WNK3's activation of NCC uses an alternative pathway that is not SPAK-dependent (Fig. 4C).
AngII Induces Phosphorylation of SPAK and NCC in Mammalian Cells.
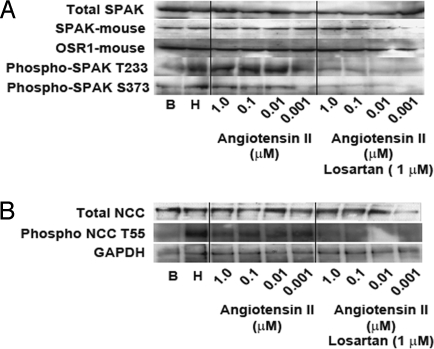
In oocytes (27) and in HEK-293 cells, NCC is activated by intracellular chloride depletion. This activation is accompanied by, and dependent on, phosphorylation of T53, T58, and S71 in rat NCC and homologous threonines T55 and T60 in human NCC (27, 28). In HEK-293 cells or in mpkDCT cells NCC activation is also accompanied by increased phosphorylation of SPAK in the activating T233 and S373 sites (25, 28). We analyzed the effect of AngII in mpkDCT cells on the phosphorylation of SPAK and NCC at these important regulatory residues using previously-characterized antibodies specific for phosphorylation at these sites (28). Phosphorylation at SPAK T233 and S373 was increased by both low chloride hypotonic stress and AngII, and this latter increase was inhibited by losartan (Fig. 5A). Similar effects were seen on NCC at T55 (Fig. 5B). These results extend the effects of AngII signaling on NCC from oocytes to mammalian cells.
Fig. 5.
Increased phosphorylation at regulatory sites of SPAK and NCC in mpkDCT cells with AngII signaling and hypotonic conditions. Representative Western blots analysis of proteins extracted from mpkDCT cells in basal conditions (B), after low chloride hypotonic stress (H), or after exposure to indicated concentrations of AngII with or without losartan at 1 μM. Proteins were resolved in SDS/PAGE and transferred to PDFV membranes and probed with antbodies specific for total SPAK, SPAK phosphorylated at T233 or SPAK phosphorylated at S373 (A) and total NCC or NCC phosphorylated at T55 (B). Phosphorylation at the regulatory sites of SPAK and NCC is increased by hypotonic conditions and AngII signaling, and is blocked by losartan.
Discussion
Prior work in Xenopus oocytes has demonstrated that WNK4 regulates NCC and ROMK. Wild-type WNK4 reduces the activity of both, whereas PHAII mutations have opposite effects on NCC and ROMK, increasing activity of the former, while reducing function of the latter. These findings have provided an explanation for the observed chloride-dependent hypertension and hyperkalemia seen in humans and mice with PHAII-mutations.
These observations together have suggested that WNK4 might be a mediator of AngII signaling, normally contributing to the differential renal response to volume depletion and hyperkalemia. In this model, the PHAII mutations mimic constitutive AngII signaling in renal epithelium. A key part of this model is that AngII signaling should increase NCC activity. Consistent with this proposition, recent work has demonstrated that increased or decreased AngII levels in vivo, respectively increase or decrease expression of NCC at the plasma membrane in the DCT (30).
In the present study, we were able to reconstitute activation of NCC by AngII in the Xenopus oocyte. This reconstitution required exogenous AT1R, NCC and WNK4, and endogenous SPAK. Because all of these gene products are expressed in the native DCT, and because of the demonstrated effects of both AngII and WNK4 function on NCC in vivo, this signaling pathway is highly likely to be relevant in the native DCT. Our findings demonstrate that AngII signaling increases NCC activity through a pathway that requires WNK4 and SPAK. These observations suggest that AngII switches WNK4 from a state that inhibits NCC, to one that allows or promotes NCC activation; the observation that AngII induces no further increase in NCC activity when PHAII-WNK4 is expressed is consistent with PHAII-WNK4 mimicking the state induced by AngII.
Our findings also indicate that the effect of AngII on NCC requires modulation of SPAK activity. It is clear that AngII signaling has similar downstream effects to increase the phosphorylation of SPAK at threonine 233 and serine 373 and NCC at threonine 55 in both oocytes and mammalian cells (Fig. 5 A and B) (31).
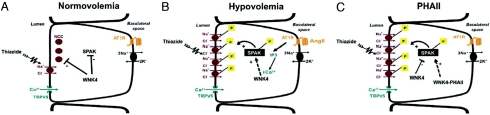
These findings collectively support the physiologic model of AngII and WNK4 activity in the DCT outlined in Fig. 6. In the setting of normal or expanded intravascular volume (Fig. 6A), in which the renin-angiotensin system is suppressed, WNK4 inhibits NCC by reducing the amount of NCC present in plasma membrane (2, 8, 30). In the setting of intravascular volume depletion (Fig. 4B), in which the renin-angiotensin system is activated, AngII signaling alleviates WNK4's inhibition of NCC, resulting in increased NCC activity. This is likely mediated through SPAK and the effect on NCC is likely increased trafficking to the plasma membrane (30). WNK1 has also been shown to be capable of activating SPAK in vitro (32); whether WNK1 fails to activate NCC in oocytes because a factor necessary for its activation is missing or because an inhibitory component is present is unknown. The model depicts AT1R in the basolateral membrane, however, the detection of renin and angiotensin-converting enzyme within the distal nephron (33, 34) and the demonstration of an apical AT1R in the collecting duct (35) open the possibility that AngII in the distal tubular fluid may influence distal tubule transport.
Fig. 6.
Proposed model for AngII modulation of WNK4-SPAK-NCC interaction in physiological conditions and pathophysiological conditions. Epithelial cells of the DCT are shown. (A) In normovolemia, AngII levels are low, and WNK4 inhibits activation of NCC via inhibition of phosphorylation of SPAK and NCC. (B) In hypovolemia, AngII levels and AT1R signaling are increased, WNK4 inhibition of SPAK/NCC is prevented, and NCC activity increases via increased trafficking of the phosphorylated cotransporter to the plasma membrane. (C) PHAII-WNK4 alleviates WNK4 inhibition of SPAK, leading to SPAK phosphorylation and increased delivery of NCC to the plasma membrane.
Thus, PHAII-mutant WNK4 has many features of constitutive AngII signaling in the DCT, resulting in unrestrained salt reabsorption by NCC and hypertension. Consistent with the proposal that AngII signaling promotes salt reabsorption without K+ secretion, AngII had no effect on WNK4's inhibition of ROMK (Fig. 3A) and WNK4 harboring mutations that mimic SGK phosphorylation maintained inhibition of NCC but was still capable of responding to AngII (Fig. 3B).
The molecular mechanism by which AngII modulates WNK4's regulation of NCC remains to be elucidated. AT1R is a typical heptahelical G protein-coupled receptor that, on AngII binding, elicits multiple cellular responses, predominantly via coupling to Gq/11 proteins (36). Gq/11-mediated inositol phosphate/Ca2+ signaling is the primary transduction mechanism initiated by AngII in its major physiological target tissues, including DCT cells (37, 38). PHAII mutations in WNK4 cluster in a negatively charged segment that bears some similarity to EF hand domains, raising the possibility that increased intracellular Ca2+ concentrations could be a direct signal to change WNK4 activity by binding to this segment. There is presently, however, no direct evidence that this is the case. Further studies will be required to determine the molecular mechanism by which AngII signals to WNK4, and how this signal alters the downstream effects of WNK4.
Methods
Clones and Mutagenesis.
The cDNAs used in this study are described in refs. 2, 7, 17, and 20, except for the AT1R that was obtained for Origine. The full length cDNA was sequenced and sublconed in to the pgh19 vector. Site-directed mutations (QuikChange; Stratagene) were performed to substitute phenylalanine 997 for alanine in WNK4 and arginine 18 for alanine in NCC. DNA sequencing was used to confirm all mutations. All primers were custom made (Sigma).
Assessment of the Na-Cl Cotransporter and ROMK Function.
rNCC activity was assessed by functional expression in Xenopus laevis oocytes as described in refs. 17, 20, 39, and 40. Oocytes were injected with water or 10 ng cRNA per oocyte of NCC and different combinations of other cRNAs expressing AT1R and wild-type or mutant WNK4, WNK1, WNK3, and SPAK, as indicated in each experiment. Three to 4 days after injection, NCC activity was assessed by measuring tracer 22Na+ uptake in the presence or absence of metolazone following a protocol that includes a 30-min preincubation in Cl− free solution and a 60-min incubation in uptake solution. Unless otherwise indicated, AngII stimulation was performed by including 100 pM AngII (Sigma) in the preincubation solution for 15 min before measurement of uptake. Inhibition of AT1R was performed with Losartan (1 μM), which was added during the 30-min preincubation period. Mean Na+ uptake in the presence of matolazone was substracted from values obtained in its absence to yield the thaizide-sensitive Na+ uptake attributable to NCC activity. ROMK activity was assessed as Ba2+- sensitive whole-cell K+ currents measured by a 2-electrode voltage clamp as described in refs. 5 and 15. Oocytes were injected with ROMK and AT1R cRNA with or without WNK4 cRNA and incubated for 2–3 days. Reported K+ currents refer to Ba2+- sensitive currents at + 40 mV.
Phosphorylation at Regulatory Sites of NCC and SPAK.
Immunoblots for NCC were performed using rabbit polyclonal antibody Chemicon AB3553 as described in ref. 28. In brief, 20 μg of mpkDCT protein extract was fractionated on 3–8% SDS Page gels and transferred to a nitrocellulose membrane. After blocking with TBS-T 5% milk, blots were incubated overnight in the presence of the primary antibody, washed in TBS-T, and then incubated with the secondary Ab and washed again. Phosphorylation at specific sites on NCC and SPAK was measured by blotting with antibodies specific for phosphorylation at NCC T55 and SPAK T233 or S373 as described in ref. 28.
Data Analysis.
All results presented are based on a minimum of 3 different experiments with at least 10 oocytes per group in each experiment. Statistical significance is defined as 2-tailed, with P < 0.05 considered significant, and the results are presented as mean ± SEM. The significance of the differences between groups was tested by 1-way ANOVA with multiple comparisons using Bonferroni's correction.
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health Grant DK-64635 (to G.G.), El Consejo Nacional de Ciencia y Tecnología (CONACYT-Mexico) Grant 59992 (to G.G.), the Foundation Leducq Transatlantic Network on Hypertension (to R.P.L. and GG), the Howard Hughes Medical Institute (R.P.L.), the U.K. Medical research Council (to D.R.A), a scholarship from CONACYT-Mexico (to P.S.-C.), a special Fellowship from Dundee Camper Down Lodge and the Medical Research Council (to P.S.-C.), the Yale Medical Scientist Training Program (to K.T.K.), and a Goldwater Scholarship (A.M.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813238106/DCSupplemental.
References
- 1.Wilson FH, et al. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 2.Wilson FH, et al. Molecular pathogenesis of inherited hypertension with hyperkalemia: The Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc Natl Acad Sci USA. 2003;100:680–684. doi: 10.1073/pnas.242735399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest. 2003;111:1039–1045. doi: 10.1172/JCI17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golbang AP, et al. Regulation of the Expression of the Na/Cl cotransporter (NCCT) by WNK4 and WNK1: Evidence that accelerated dynamin-dependent endocytosis is not involved. Am J Physiol Renal Physiol. 2006;291:F1369–F1376. doi: 10.1152/ajprenal.00468.2005. [DOI] [PubMed] [Google Scholar]
- 5.Kahle KT, et al. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet. 2003;35:372–376. doi: 10.1038/ng1271. [DOI] [PubMed] [Google Scholar]
- 6.Ring AM, et al. WNK4 regulates activity of the epithelial Na+ channel in vitro and in vivo. Proc Natl Acad Sci USA. 2007;104:4020–4024. doi: 10.1073/pnas.0611727104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garzon-Muvdi T, et al. WNK4 kinase is a negative regulator of K+-Cl− cotransporters. Am J Physiol Renal Physiol. 2007;292:F1197–F1207. doi: 10.1152/ajprenal.00335.2006. [DOI] [PubMed] [Google Scholar]
- 8.Cai H, et al. WNK4 kinase regulates surface expression of the human sodium chloride cotransporter in mammalian cells. Kidney Int. 2006;69:2162–2170. doi: 10.1038/sj.ki.5000333. [DOI] [PubMed] [Google Scholar]
- 9.Kahle KT, et al. Paracellular Cl− permeability is regulated by WNK4 kinase: Insight into normal physiology and hypertension. Proc Natl Acad Sci USA. 101:14877–14882. doi: 10.1073/pnas.0406172101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamauchi K, et al. Disease-causing mutant WNK4 increases paracellular chloride permeability and phosphorylates claudins. Proc Natl Acad Sci USA. 2004;101:4690–4694. doi: 10.1073/pnas.0306924101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.San-Cristobal P, De-Los-Heros P, Ponce-Coria J, Moreno E, Gamba G. WNK kinases, renal ion transport and hypertension. Am J Nephrol. 2008;28:860–870. doi: 10.1159/000139639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahle KT, Ring AM, Lifton RP. Molecular physiology of the WNK kinases. Annu Rev Physiol. 2008;70:329–355. doi: 10.1146/annurev.physiol.70.113006.100651. [DOI] [PubMed] [Google Scholar]
- 13.Lalioti MD, et al. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet. 2006;38:1124–1132. doi: 10.1038/ng1877. [DOI] [PubMed] [Google Scholar]
- 14.Yang SS, et al. Molecular pathogenesis of pseudohypoaldosteronism type II: Generation and analysis of a Wnk4(D561A+) knockin mouse model. Cell Metab. 2007;5:331–344. doi: 10.1016/j.cmet.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Ring AM, et al. An SGK1 site in WNK4 regulates Na+ channel and K+ channel activity and has implications for aldosterone signaling and K+ homeostasis. Proc Natl Acad Sci USA. 2007;104:4025–4029. doi: 10.1073/pnas.0611728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gamba G, et al. Primary structure and functional expression of a cDNA encoding the thiazide-sensitive, electroneutral sodium-chloride cotransporter. Proc Natl Acad Sci USA. 1993;90:2749–2753. doi: 10.1073/pnas.90.7.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gamba G, et al. Molecular cloning, primary structure and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J Biol Chem. 1994;269:17713–17722. [PubMed] [Google Scholar]
- 18.Sandberg K, et al. Angiotensin II-induced calcium mobilization in oocytes by signal transfer through gap junctions. Science. 1990;249:298–301. doi: 10.1126/science.2374929. [DOI] [PubMed] [Google Scholar]
- 19.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: From physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 20.Rinehart J, et al. WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl− cotransporters required for normal blood pressure homeostasis. Proc Natl Acad Sci USA. 2005;102:16777–16782. doi: 10.1073/pnas.0508303102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gagnon KB, England R, Delpire E. Volume sensitivity of cation-Cl− cotransporters is modulated by the interaction of two kinases: Ste20-related proline-alanine-rich kinase and WNK4. Am J Physiol Cell Physiol. 2006;290:C134–C142. doi: 10.1152/ajpcell.00037.2005. [DOI] [PubMed] [Google Scholar]
- 22.Vitari AC, Deak M, Morrice NA, Alessi DR. The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome, phosphorylate and active SPAK and OSR1 protein kinases. Biochem J. 2005;391:17–24. doi: 10.1042/BJ20051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vitari AC, et al. Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem J. 2006;397:223–231. doi: 10.1042/BJ20060220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moriguchi T, et al. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem. 2005;280:42685–42693. doi: 10.1074/jbc.M510042200. [DOI] [PubMed] [Google Scholar]
- 25.Richardson C, Alessi DR. The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signalling pathway. J Cell Sci. 2008;121:3293–3304. doi: 10.1242/jcs.029223. [DOI] [PubMed] [Google Scholar]
- 26.Ponce-Coria J, et al. Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci USA. 2008;105:8458–8463. doi: 10.1073/pnas.0802966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pacheco-Alvarez D, et al. The Na-Cl cotransporter is activated and phosphorylated at the amino terminal domain upon intracellular chloride depletion. J Biol Chem. 2006;281:28755–28763. doi: 10.1074/jbc.M603773200. [DOI] [PubMed] [Google Scholar]
- 28.Richardson C, et al. Activation of the thiazide-sensitive Na+-Cl− cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci. 2008;121:675–684. doi: 10.1242/jcs.025312. [DOI] [PubMed] [Google Scholar]
- 29.Piechotta K, Lu J, Delpire E. Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1) J Biol Chem. 2002;277:50812–50819. doi: 10.1074/jbc.M208108200. [DOI] [PubMed] [Google Scholar]
- 30.Sandberg MB, Riquier AD, Pihakaski-Maunsbach K, McDonough AA, Maunsbach AB. Angiotensin II provokes acute trafficking of distal tubule NaCl cotransporter (NCC) to apical membrane. Am J Physiol Renal Physiol. 2007;293:F662–F669. doi: 10.1152/ajprenal.00064.2007. [DOI] [PubMed] [Google Scholar]
- 31.Chiga M, et al. Dietary salt regulates the phosphorylation of OSR1/SPAK kinases and the sodium chloride cotransporter through aldosterone. Kidney Int. 2008;74:1403–1409. doi: 10.1038/ki.2008.451. [DOI] [PubMed] [Google Scholar]
- 32.Anselmo AN, et al. WNK1 and OSR1 regulate the Na+, K+, 2Cl− cotransporter in HeLa cells. Proc Natl Acad Sci USA. 2006;103:10883–10888. doi: 10.1073/pnas.0604607103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rohrwasser A, et al. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 34.Komlosi P, et al. Angiotensin I conversion to angiotensin II stimulates cortical collecting duct sodium transport. Hypertension. 2003;42:195–199. doi: 10.1161/01.HYP.0000081221.36703.01. [DOI] [PubMed] [Google Scholar]
- 35.Wei Y, Zavilowitz B, Satlin LM, Wang WH. Angiotensin II inhibits the ROMK-like small conductance K channel in renal cortical collecting duct during dietary potassium restriction. J Biol Chem. 2007;282:6455–6462. doi: 10.1074/jbc.M607477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol. 2006;20:953–970. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- 37.Harrison-Bernard LM, Navar LG, Ho MM, Vinson GP, el Dahr SS. Immunohistochemical localization of ANG II AT1 receptor in adult rat kidney using a monoclonal antibody. Am J Physiol. 1997;273:F170–F177. doi: 10.1152/ajprenal.1997.273.1.F170. [DOI] [PubMed] [Google Scholar]
- 38.Miyata N, Park F, Li XF, Cowley AW., Jr Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am J Physiol. 1999;277:F437–F446. doi: 10.1152/ajprenal.1999.277.3.F437. [DOI] [PubMed] [Google Scholar]
- 39.Monroy A, Plata C, Hebert SC, Gamba G. Characterization of the thiazide-sensitive Na+-Cl− cotransporter: A new model for ions and diuretics interaction. Am J Physiol Renal Physiol. 2000;279:F161–F169. doi: 10.1152/ajprenal.2000.279.1.F161. [DOI] [PubMed] [Google Scholar]
- 40.Moreno E, et al. Affinity defining domains in the Na-Cl cotransporter: Different location for Cl− and thiazide binding. J Biol Chem. 2006;281:17266–17275. doi: 10.1074/jbc.M602614200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.