Abstract
Objectives
When preterm infants experience heel stick, crying commonly occurs and has adverse physical effects. A reduction in crying is desired. Kangaroo Care, skin contact between mother and infant, reduces pain as measured by the Premature Infant Pain Profile, and may reduce crying time. The purpose of the pilot was to test Kangaroo Care's effect on the preterm infant's crying response to heel-stick.
Methods
A prospective cross-over study with 10 prematures 2-9 days old (30-32 weeks postmenstrual age) was conducted. Infants were randomly assigned to two sequences (Day 1 heel stick in Kangaroo Care [after 30 minutes of prone skin contact upright between maternal breasts] and Day 2 heel stick in incubator [inclined, nested and prone] or the opposite sequence) was conducted. Video tapes of Baseline, Heel Warming, Heel Stick, and Recovery phases were independently scored for audible and inaudible crying times by two research assistants. The audible and inaudible crying times for each subject in each phase were summed and the mean between the scorer's values was analyzed by repeated measures ANOVA.
Results
Subject characteristics did not differ between those in the two sequences. Crying time differed between the study phases on both days (p ≤ 0.001). When in Kangaroo Care as compared to the incubator, crying time was less during the Heel stick (p = 0.001) and Recovery (p = 0.01) phases.
Conclusion
Because Kangaroo Care reduced crying in response to heel stick in medically stable preterm infants who were 2-9 days old, a definitive study is recommended.
INTRODUCTION
When preterm infants experience pain, crying is a common response and is considered to be the most sensitive measure of pain (Franck, 2002; Craig, Gilbert-MacLeod, & Lilley, 2000; Stevens et al., 2005). Crying has many adverse physiological effects such as elevated heart rate (Dinwiddie et al., 1979a), blood pressure (Dinwiddie et al., 1979a), shunting of unoxygenated blood (Dinwiddie et al., 1979b), and arteriovenous spasms causing cerebral blood flow changes (Hirashi et al., 1991). The management of preterm infant pain is a high priority (Breau et al., 2006), and interventions to reduce pain and its responses are needed (Bruce & Franck, 2005). Skin-to-skin contact, also known as Kangaroo Care, may be a useful intervention, but its usefulness is only beginning to be established. The purpose of the proposed study was to test the effect of skin-to-skin contact (SSC) on the preterm infant's crying response to heel stick.
Infants in the NICU are routinely subjected to various diagnostic, surgical or therapeutic procedures which can result in pain (Barker & Rutter, 1995; Evans, McCartney, Lawhon, & Galloway, 2005; Lago et al., 2005; F. L. Porter & Anand, 1998). Premature infants perceive heel stick pain because peripheral and central structures necessary for nociception are present and functional by 12-16 weeks gestation (Wolf, 1999). The cortical capacity to interpret painful stimuli functions as early as 20 weeks of gestation and by 23 weeks gestation the human fetus and preterm infants can mount hormonal stress responses to painful stimuli (Giannakoulopoulos, Sepulveda, Kourtis, Glover, & Fisk, 1994; Gitau et al., 2001) and receive some analgesia through the endogenous endorphin system (Gibbins & Stevens, 2001). By 30 weeks gestation, pain transmission to the brain stem and thalamus known to occur even prior to complete myelinization of the pain pathways (Anand & Hickey, 1992). Therefore, by the end of the second trimester, the fetus posses both the neurochemical and anatomical capabilities of pain perception (Anand & Hickey, 1987; Fitzgerald & Beggs, 2001; Gitau et al., 2001; Kostovic & Rakic, 1990). The cortisol response to pain results in behavioral, autonomic, and hormonal responses (Anand, 1998; Anand & Craig, 1996; Gitau, Fisk, & Glover, 2004). The nerve pathways associated with pain transmission are functional at term birth, but myelination continues throughout infancy. The density of nerve endings or pain receptors in the skin of newborns is similar to or greater than that in adult skin (Anand, 2000). Due to immaturity of the nervous system during infancy, infants may actually have a pain threshold that is 30-50% lower than that of adults and a lower pain tolerance than older children (Broome, Rehwaldt, & Fogg, 1998; Fitzgerald, Millard, & MacIntosh, 1988). Preterm infants are at even higher risk than full-term infants for acquired disease, neurological injury, and adverse developmental outcome as a result of painful experiences during hospitalization (Fitzgerald & Beggs, 2001).
Blood sampling from infants by lancing and squeezing the heel is a routine procedure in maternity and neonatal wards throughout hospitalization (Grunau & Craig, 1987; Lindh, Wiklund, & Hakansson, 1999). Almost every infant undergoes repeated heel puncture to screen for metabolic disorders such as phenylketonuria, and to monitor blood glucose or hemoglobin (Franck & Gilbert, 2002). Premature infants have even more heel sticks with 56% of invasive procedures being heel sticks (Grunau & Craig, 1987). Eighty percent of infants do not have effective pain relief from heel sticks (Choonara, 1992; Walco, Cassidy & SSchecter, 1994). Heel stick has been used as a model noxious stimulus by a number of researchers to investigate the pain response of the fullterm (Gray, Miller, Philipp, & Blass, 2002; Gray, Watt, & Blass, 2000; Grunau, Linhares, Holsti, Oberlander, & Whitfield, 2004) and preterm newborn ( Johnston et al., 2003; Ludington-Hoe, Hosseini, & Torowicz, 2005). Studies have found that infants had more pain response, particularly crying, to heel stick than venipuncture for blood sampling (Eriksson, Gradin, & Schollin, 1999; Larsson, Tannfeldt, Lagercrantz, & Olsson, 1998; Ogawa et al., 2005; Shah, Taddio, Bennett, & Speidel, 1997). However, the use of heel stick as a pain stimulus greatly increases the probability that the resultant crying is due to pain.
Expression of pain through behavior is the only means by which infants can communicate their pain to observers. Behavioral responses to neonatal pain include vocalization (cry), facial expressions, gross motor movements, and changes in behavioral states and functions (such as sleep-wake changes) during and after each pain stimulus. The cry response is common in preterm and term infants (Brown, 1987; Gibbins & Stevens, 2001b). Crying is the primary method of communication between infant and caregiver and it may signal many different alterations in infant internal state (Fuller, 1991). Cry can be described in terms of its presence or absence the latency to cry, the duration of cry, and the amplitude and pitch of the cry. Infant pain cries are spectrographically distinct in terms of frequency and pitch compared to cries due to other stimuli such as hunger, anger or fear, and fussiness (Fuller, 1991, 1996; Ludington-Hoe, Cong, & Hashemi, 2002; Porter, Porges, & Marshall, 1988). Changes in the patterns of neonatal cries have also been correlated with the intensity of pain experienced during circumcision and the patterns can be accurately differentiated by adult listeners (F. L. Porter, Miller, & Marshall, 1986; Warnock & Sandrin, 2004). Bellieni and colleagues (Bellieni, Sisto, Cordelli, & Buonocore, 2004) found that crying intensity increased with increasing pain in full-term infants undergoing heel stick. The most interesting findings of Bellieni's study were that when DAN pain scores (Douleur Aiguë du Nouveau-né; a behavioral acute pain rating scale for neonates; ranging from 0 to 10) were more than eight a stereotyped cry was produced, and the regularity and repetition of the cry suggested a call for attention help. Other than characteristics determined by spectral analysis and duration and intensity of the cry, no other measures of specificity of the cry are available (Fuller, 2001). Pain cries of preterm and neurologically impaired infants are considerably different from all other cries (Anand, Sippell, & Aynsley-Green, 1987).
Some preterm and acutely ill infants may not cry during heel sticks and other painful procedures, which may be due to depleted energy reserves, or an inability to cry because of the presence of an endotracheal tube (Johnston, Stevens et al., 1999; Ludington-Hoe et al., 2005; McGrath, 1990). In the absence of crying (and with crying), the infant forms a “cry face” that is characteristic of pain and the cry face is called a “silent cry”. Therefore, an audible cry alone may not be a valid or reliable indicator for pain in preterm or acutely ill infants.
An infant's cry is a stress behavior and one of the most potent distress signals that an infant offers (Ludington-Hoe et al., 2002). In premature infants, crying clearly occurs in response to pain. The management of pain is the main nursing responsibility and finding an intervention that reduces crying as a response to pain is needed. Kangaroo care may be a useful intervention. Because KC is a very effective method for preventing crying based on the results from prior studies, KC could be a promising strategy to minimize the crying response to pain.
Kangaroo Care to Relieve Pain
Mother's physical contact with her preterm infant through direct skin-to-skin care provides tactile, olfactory, auditory, thermal, and proprioceptive stimulation in a unique interactive style. Previous investigations have noted in both animals and human infants that interventions providing separate components of the maternal proximity constellation, such as maternal holding, or odor, or voice, and or rocking modulated pain responses.
Though many other non-pahrmacologic interventions have been studied to minimize preterm infant pain (Franck & Lawhon, 1998), none is widely nor routinely used due to side effects. Kangaroo Care has no adverse side effects in infants greater than 28 weeks (Browne, 2004), and mothers are generally available to assist their infants when they visit. Thus, the purpose of the study was to determine the effect of KC on crying response to pain by comparing a heelstick done in KC to a heelstick done incubator.
Materials and Method
Design
A prospective cross-over design with random assignment to having the first heelstick either in KC, called a Kangaroo Care Heelstick (KC H) or in the incubator, called incubator heel stick (IH), was conducted. Infants who had KCH first had the next heel stick in the incubator after 24 hours; infants who had the IH first had the next heel stick in the KCH after 24 hours, all heel sticks were conducted at 11am. Data were collected over four phases, Baseline (10 minutes prior to warming), Heel Warming (5 minutes), Heel Stick and squeezing (2-3 minutes), and Recovery (20minutes). Infants in the study were video taped during the four phases of KCH and IH. Randomization was by permuted block design to ensure highest possible equivalence among infants (Chow & Liu, 1998). The study was approved by the university and hospital IRBs and written informed consent was obtained from both parents
Setting
Infants were tested in a level II 13-bed neonatal intensive care unit NICU in Richland, Washington served by two neonatologists and two neonatal nurse practitioners. Noise and light levels were appropriately reduced at all times. A curtain was drawn around the infant during the study. Routine care to minimize the heelstick pain was nesting; no other pain intervention was used
Sample
Infants reported here were one cohort in a multi-cohort pilot study measuring pain responses (Ludington-Hoe, 2002). Ten infants were in the cohort that received thirty minutes of KC prior to the KCH. Twenty-six subjects were needed to detect moderate difference in crying time but funding permitted only recruitment of ten subjects, thus the data reported here are pilot results. Healthy mothers and infants of singleton birth were recruited if infants met the following criteria: 30-32 weeks gestational age, were within 2-9 days of birth (to control for hyperalgesia due to repeated heel sticks prior to study [(Andrews & Fitzgerald, 1994, 1999; Fitzgerald, Shaw, & MacIntosh, 1988), had no signs of heel tissue inflammation as measured by the Neonatal Skin Condition Score (to control for inflammation induced by tissue damage and inflammation's concomitant repetitive A delta and C fiber inputs activating the central neurons in the dorsal horn of the spinal cord and brainstem causing them to respond to normal inputs in an exaggerated and extended manner [Woolf & Salter, 2000]), either NPO or fed by bolus feed, and were being cared for in an incubator. Infants were not eligible if they had a known congenital anomaly grade III or IV intraventricular hemorrhage, history of surgery, history of drug exposure, and any tissue breakdown or inflammation of either heel. All mothers spoke English and all mothers approached agreed to participate.
Conditions
Kangaroo Care was skin-to-skin, chest-to-chest, upright placement of the infant wearing only a diaper between maternal breast with a receiving blanket folded in fourth over the infant's back. Mothers reclined in a lounge chair behind a privacy curtain. Infants began KC thirty minutes before the Baseline phase. After Baseline, the infant's foot was retracted from beneath the blanket for Warming and for the Heel Stick while the infant remained in KC. A band aid was applied and the foot was placed back under the blanket for the twenty-minute Recovery phase which also took place in KC.
Incubator care was placement of the diaper-clad infant prone and nested between rolled blankets on a mattress at a 30 - 40 degree incline in an Ohio IC double-walled incubator. Privacy curtains were drawn around the incubator for 30 minutes prior to warming and heelstick. The incubator heel stick took place in the incubator without changing the baby's position, 20 minutes of recovery occurred in the same position in the incubator. Mothers were not present during the IH. The heelstick was done using the National Association of Neonatal Nursing standardized procedures (NANN, 1995) by the Tenderfoot™ spring-loaded lancet.
Outcome variables
Crying time was defined as the number of mean seconds of inaudible plus audible crying time for each phase. Crying was measured in two ways: summing the total number of seconds of inaudible crying as measured by behavioral observation and the total number of seconds of audible crying as measured by infant vocalization of whimpering, crying, or hard crying. Inaudible crying behaviors have been found to be useful and important pain indicators in preterm infants (Stevens et al., 2006). Inaudible crying was behaviorally scored using the Anderson Behavioral State Scoring System (Gill et al., 1988). The behaviors that characterized crying that occur when no audible sounds are heard are facial grimacing, eye squeezing, brow bulge (ADD ABSSS criteria here). Infants were continuously observed throughout each phase and when behavioral criteria matching states 10 (Fussy), 11 (Crying), or 12 (Hard Crying) were observed in the absence of audible sounds of whimpering/crying/hard crying, stopwatch timing of the number of seconds of inaudible crying began, and stopwatch timing ended when the infant's behaviors ceased to characterize any one of the fussy/crying/hard crying states. Inaudible crying was independently scored from full-face video tapes by two research assistants. Audible crying was measured by stopwatches that began when audible whimpering, crying or hard crying were heard. Data from the stopwatches provided the sum of seconds of all audible crying episodes within each phase. Two research assistants independently viewed the videotapes to score audible and inaudible crying. Inter-rater reliability for crying time was .82-.85 by Kappa coefficient over the two days of study.
Instruments
Infants were videotaped using a Sony video camera (Model TRV38, Tokyo, Japan) and recorder that were focused on the infant's face to record facial actions and auditory vocalization of crying. Stop watches were Seiko Digital Stopwatch Model WO73 that auto-calibrated each time they were turned on and were used in the stop watch mode.
Procedure
At 9:30 a.m. on the first day of study, infants were positioned in either KC or in the incubator depending of the sequence to which each had been randomized. Twenty minutes later, Baseline data collection started and continued for ten minutes. Then the Heel Warming phase, 5 minutes long, took place, followed by a heel stick to collect blood for lab tests required daily. A. 0.5 cc blood sample was needed and generally required 1-2 minutes to obtain. Recovery phase began immediately after the heel stick site had been covered with a band aid and continued for 20 minutes. At the end of the Recovery phase, all data collection ceased and the KC infant was placed back in the incubator. Infants already in the incubator were left there throughout all phases. On the second day, the procedure was the same except that infants who had been in KC on the first day were in the incubator on the second day and first day incubator infants were in KC on the second day.
Statistical Analysis
Each research assistant totaled the number of seconds of inaudible crying plus audible crying for each infant for each phase. The mean of the two research assistant's crying times was calculated for each subject; a mean of the subject means for all subjects in the same condition (KCH or IH on first day; KCH or IH on 2nd day) in each phase on each day was used in a repeated measures ANOVA with one factor being the 4 phases and the second factor being whether KCH was on the first or second day of the study. The repeated measures ANOVA is the best test to analyze the four phase data and all assumptions for the repeated measures ANOVA were met. Further repeated measure ANOVAs then examined interactions and main effects. Data were analyzed using SPSS Version 13.0 (SPSS, 2005). Alpha was set at 0.05. Sample size was sufficient to detect medium main effects (Stevens, 1996).
RESULTS
Subject Characteristics
Demographic characteristics of the mothers and their infants are presented in Table 1. The majority of the mothers were white (90%), married (80%), delivered by section (80%). Infants were evenly distributed between the sexes. The majority of the infants were delivered by cesarean section (80%), and were 32 weeks gestation (60%). Mean birth weight was 1577 grams. Infants were a mean 6.00 days old (SD = 2.00) on the first day of study and had a mean of 22.00 (SD = 6.00) prior painful experiences. Eighty percent of the subjects had had previous KC experience. No significant differences were found in subject characteristics between infants who had KCH on day 1 and those who had KCH on day 2.
Table 1.
Numbers, Percentage, Means, and Standard deviation of Maternal and Neonatal Characteristics.
| Characteristics | N | % | Mean | SD |
|---|---|---|---|---|
| Maternal Characteristics | ||||
| Marital Status: | ||||
| Married | 7 | 70.00 | ||
| Single | 2 | 20.00 | ||
| Divorced | 1 | 10.00 | ||
| Employment: | ||||
| Full time | 3 | 30.00 | ||
| Part time | 3 | 30.00 | ||
| Homemaker | 2 | 20.00 | ||
| Student | 2 | 20.00 | ||
| Education: | ||||
| Completed graduate school | 1 | 10.00 | ||
| Completed college | 4 | 40.00 | ||
| Some college | 2 | 20.00 | ||
| Completed high school | 3 | 30.00 | ||
| Neonatal Characteristics | ||||
| Gestational age: | 2 | 20 | ||
| 30 weeks | 2 | 20 | ||
| 31 weeks | 6 | 60 | ||
| 32 weeks | ||||
| Gender: | ||||
| Male | 5 | 50 | ||
| Female | 5 | 50 | ||
| Race: | ||||
| White | 9 | 90 | ||
| American Indian | 1 | 10 | ||
| Hispanic background: | ||||
| Yes | 2 | 20.00 | ||
| No | 8 | 80.00 | ||
| Birth type: | ||||
| Vaginal | 2 | 20.00 | ||
| Cesarean section | 8 | 80.00 | ||
| Pre-study KC experience: | ||||
| Yes | 8 | 80.00 | ||
| No | 2 | 20.00 | ||
| Birth weight (gram) | 1577 | 327 | ||
| APGAR Score: | ||||
| 1-minute | 7 | 1 | ||
| 5-minute | 8 | 1 | ||
| Previous pain experiences # | 22 | 6 | ||
| Heel skin condition Score a | ||||
| before KCH | 4 | 1 | ||
| before IH | 4 | 1 |
KCH = kangaroo care heel stick, IH = incubator heel stick,
= by using
Analyses of Audible and Inaudible Crying Time
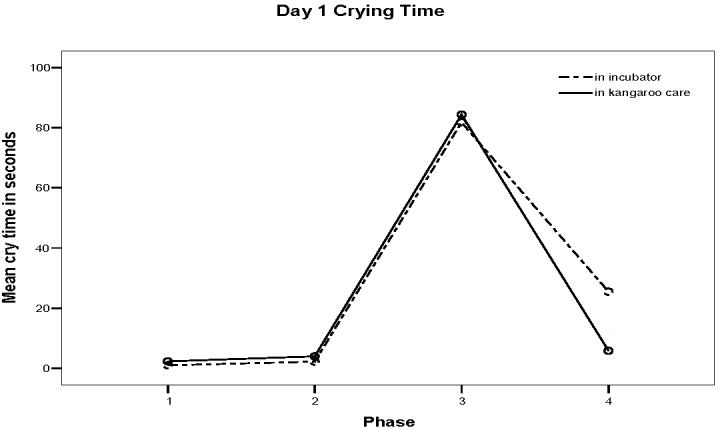
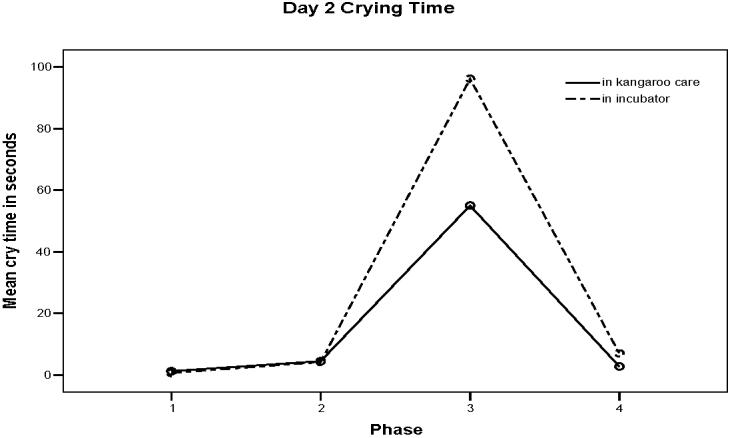
The means and standard deviations for crying time during each phase on the first and second days are presented in Table 2 and clearly show that crying was minimal before Heel Stick, increased during the Heel Stick, and declined during Recovery regardless of day of study. A repeated measures ANOVA revealed a significant difference in crying time between the study phases on both days (F (1, 8) = 10.25, p < 0.001). The differences in crying time between KCH and IH did not occur during Baseline and Heel Warming phases on either day, but did occur during Heel Stick and Recovery phases. On the first day, infants in KCH had less crying during Recovery (M = 5.83 seconds, SD = 7.63) than infants in IH (M = 25.50 seconds, SD = 41.93) (Figure 1). On the second day, KCH infants had significantly less crying time (M= 55.00 seconds, SD = 55.53) during the Heel Stick phase than infants in IH (M = 96.17, SD = 92.42) (F (1,8) = 7.76, p = 0.001) (Figure 2).
Table 2.
Mean Crying Time for Each Phase on the First and Second Days of Study
| Phase | Crying time 1st day |
Crying time 2nd day |
||
|---|---|---|---|---|
| KCH | IH | KCH | IH | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Baseline |
2.3 ± 3.8 | 1.0 ± 2.0 | 0.67 ± 1.2 | 1.25 ± 2.5 |
| Warming |
4.0 ± 3.7 | 2.3 ± 4.5 | 4.2 ± 5.9 | 4.5 ± 7.1 |
| Heelstick |
48.3 ± 61.9 | 81.8± 97.4 | 96.2 ± 92.4 | 55± 55.5 |
| Recovery | 5.83 ± 7.63 | 25.5 ±41.9 | 7.00 ± 9.9 | 2.75 ± 3.2 |
DISCUSSION
Crying time, a composite of audible and inaudible crying times measured from videotapes of heel sticks conducted when 2-9 day old premature infants were held in Kangaroo Care (skin-to-skin with their mother) on one day and when in an incubator on another day, was less in Kangaroo Care than in the incubator. On both days infants exhibited less crying when in Kangaroo Care as compared to the incubator during and after the acute pain stimulus. The pilot data reported here suggest that Kangaroo Care is effective in reducing the crying response to heel stick pain in medically stable premature infants. A diminished pain response to a Kangaroo Care heel stick is similar to previous studies with preterm infants (Johnston et al., 2003; Ludington-Hoe, Hosseini, & Torowicz, 2005) and fullterm infants (Gray, Watts, & Blass, 2000) examining responses to acute pain experiences. Most of the preterm infants in our study had previously experienced Kangaroo Care, and pain behaviors diminish over repeated Kangaroo Care sessions (Morelius et al., 2005). KC analgesic effects do not seem to endure according to Miles and colleagues (Miles et al., 2006). In Mile's investigation, mothers began daily sessions of KC with preterm infants less than 32 weeks postmenstrual age and continued KC for 4 weeks. Pain responses at 4 and 12 months corrected age were no different between infants who had received KC and those who had not. Nonetheless, for acute procedural pain, KC appears to reduce pain reactivity.
Kangaroo Care's continuous tactile stimulation may serve as a pain inhibitory system by activating endogenous pain modulating systems similarly to the effect of continuous tactile stimulation of non-nutritive sucking. By 23 weeks gestation, the human fetus and preterm infant can mount hormonal stress responses to painful stimuli (Giannakoulopoulos, Sepulveda, Kourtis, Glover, & Fisk, 1994) and receive some analgesia through the endogenous endorphin system (Gibbins & Stevens, 2001b). As little as 20 minutes of KC significantly alters cortisol and beta-endorphin release (Modi & Glover, 1998; Mooncey, Giannakoulopoulos, Glover, Acolet, & Modi, 1997). Or KC might work by minimizing central nerve activation by the painful stimulus. In the rat, non-nutritive sucking reduces nociceptive neuronal activation at the cervical region but not at the lumbar region, suggesting that oro-tactile stimulation analgesia is mediated in the dorsal horn and inhibits stimulation of afferent nociceptive pathways (Ren, Blass, Zhou, & Dubner, 1997). Because maternal holding is more analgesic than a pacifier in infants (Phillips, Chantry, Gallagher et al., 2005), one can speculate that Kangaroo Care analgesia may inhibit stimulation of central pathways too. Future study of infant rats experiencing a heel stick during skin-to-skin contact, which occurs during natural huddling behavior (Farrell & Alberts, 2002), might clarify if Kangaroo Care analgesia is mediated through the dorsal horn and if it inhibits stimulation of central nociception pathways. Resolving the issue of central nociception activation with KC is important because repeated experiences of KC analgesia have the potential of creating associative memory between KC and pain – a memory that is undesirable and may thwart cuddling, closeness, and interaction between mother and infant.
Crying differentiated the heel stick from any other phase, clearly showing a crying response to the acute painful stimulus. (Reference from Xiaomei) No differences were seen in crying time between KCH and IH during Baseline and Heel Warming phases, as one might expect given that infants were undisturbed in their respective environments and crying rarely occurred prior to Heel Stick phase. Kangaroo Care calms infants, decreases their level of stress (Modi & Glover, 1998; Mooncey, Giannakoulopoulos, Glover, Acolet, & Modi, 1997; Morelius, Theodorsson, & Nelson, 2005), decreases behavioral signs of discomfort (Morelius, Theodorsson, & Nelson, 2005), and is associated with very little crying (Ludington, 1990). Thus, the low level of crying before the heel stick was expected.
Limitations and Future Study
The results should be considered with caution as the data are based on pilot work. Further, all infants in the pilot had no signs of inflammation at the heel stick site, and inflammation may change cry responses. Future study may include a cohort of infants who demonstrate signs of inflammation using the Neonatal Skin Condition Score. Maternal presence per se, as differentiated from Kangaroo Care, may also have influenced pain responses because preterm infants who receive the familiar odor of their mother when having a heel stick performed show no increase in crying as compared to baseline levels (Goubet, Rattaz, Peirrat, Bullingere & Lequien, 2003). Maternal scents reduce post-heel stick crying in fullterm infants (Rattaz, Goubet & Bullinger, 2005), partially due to newborn recognition of maternal scent from the womb (Marlier, Schaal & Soussignan, 1998; Rangel and Leon, 1995). Future study with a cohort of infants who only experience maternal presence rather than the holding, containment, skin contact, heart beat, and rhythmic chest movement elements that comprise Kangaroo Care, may provide data as to the most effective way mothers may contribute to their infant's pain management. Control over prenatal
Clinical Implications
The results reported here support those of earlier studies with preterm and fullterm infants and contribute data on audible and inaudible crying time. A reduction in crying time suggests that less physiologic compromise will occur in response to pain. Less crying may also mean that pain is not perceived as fully, and thus, may be less likely to produce cortical patterns of hyperalgesia and pain memory during childhood. Given that preterm infants learn to anticipate pain when their leg is picked up once they have had two weeks of heel stick experience (Goubet, Clifton, & Shah, 2001), it is important that interventions to reduce pain are in place during painful stimuli. Kangaroo Care has been recommended as a nonpharmacologic pain management strategy (Clifford et al., 2004; Franck & Lawhon, 1998) but the evidence supporting its practice with preterm infant is still limited and definitive studies are needed to assure that Kangaroo Care minimizes pain without creating memories associating mother's holding with painful stimuli. (MOON)
Acknowledgements
All sources, the AWHONN and the NIH grant
References
- Anand KJ. Neonatal analgesia and anesthesia. Introduction. Seminars in Perinatology. 1998;22(5):347–349. doi: 10.1016/s0146-0005(98)80051-7. [DOI] [PubMed] [Google Scholar]
- Anand KJ. Pain, plasticity, and premature birth: a prescription for permanent suffering? Nat Med. 2000;6(9):971–973. doi: 10.1038/79658. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Craig KD. New perspectives on the definition of pain. Pain. 1996;67(1):3–6. doi: 10.1016/0304-3959(96)03135-1. discussion 209-211. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Hickey PR. Pain and its effects in the human neonate and fetus. New England Journal of Medicine. 1987;317(21):1321–9. doi: 10.1056/NEJM198711193172105. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Hickey PR. Halothane-morphine compared with high-dose sufentanil for anesthesia and postoperative analgesia in neonatal cardiac surgery. New England Journal of Medicine. 1992;26(1):1–9. doi: 10.1056/NEJM199201023260101. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Sippell WG, Aynsley-Green A. Pain, anesthesia, and babies. Lancet. 1987;2(8569):1210. doi: 10.1016/s0140-6736(87)91347-x. [DOI] [PubMed] [Google Scholar]
- Barker D, Rutter N. Exposure to invasive procedures in neonatal intensive care unit admissions. Archives of Disease in Childhood. 1995;72:F47. doi: 10.1136/fn.72.1.f47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breau LM, McGrath PJ, Stevens B, Beyene J, Camfield C, Finley GA, Franck L, Gibbins S, Howlett A, McKeever P, O'Briend K, Ohlsson A. Judgments of pain in the neonatal intensive care setting: a survey of direct care staffs' perceptions of pain in infants at risk for neurological impairment. Clinical Journal Pain. 2006;22(2):122–129. doi: 10.1097/01.ajp.0000154045.45402.ec. [DOI] [PubMed] [Google Scholar]
- Broome ME, Rehwaldt M, Fogg L. Relationships between cognitive behavioral techniques, temperament, observed distress, and pain reports in children and adolescents during lumbar puncture. J Pediatr Nurs. 1998;13(1):48–54. doi: 10.1016/S0882-5963(98)80068-7. [DOI] [PubMed] [Google Scholar]
- Brown L. Physiologic responses to cutaneous pain in neonates. Neonatal Netw. 1987;6(3):18–22. [PubMed] [Google Scholar]
- Browne JV. Early relationship environments: physiology of skin-to-skin contact for parents and their preterm infants. Clinical Perinatology. 2004;(2):287–98. doi: 10.1016/j.clp.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Bruce E, Franck L. Using the worldwide web to improve children's pain care. International Nursing Review. 2005;52(3):204–209. doi: 10.1111/j.1466-7657.2005.00424.x. [DOI] [PubMed] [Google Scholar]
- Choonara I. Management of pain in newborn infants. Seminars in Perinatology. 1992;16(1):32–40. [PubMed] [Google Scholar]
- Clifford PA, Stringer M, Christensen H, Mountain D. Pain assessment and intervention for term newborns. J Midwifery Womens Health. 2004;49(6):514–519. doi: 10.1016/j.jmwh.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Craig KD, Gilbert –MacLeod CA, Lilley CM. Crying as an indicator of pain in infants. In: Barr RG, Hopkins B, Green J, editors. Crying As A Sign, A Symptom, and a Signal. Cambridge University Press; London: 2000. pp. 23–40. [Google Scholar]
- Dinwiddie R, et al. The effects of crying on arterial oxygen tension in infants recovering from respiratory distress. Critical Care Medicine. 1979 b;7(2):50–53. doi: 10.1097/00003246-197902000-00004. [DOI] [PubMed] [Google Scholar]
- Dinwiddie, et al. Cardiopulmonary changes in the crying neonate. Pediatric Research. 1979 a;13(8):900–903. doi: 10.1203/00006450-197908000-00006. [DOI] [PubMed] [Google Scholar]
- Evans JC, McCartney EM, Lawhon G, Galloway J. Longitudinal comparison of preterm pain responses to repeated heelsticks. Pediatric Nursing. 2005;31(3):216–21. [PubMed] [Google Scholar]
- Eriksson M, Gradin M, Schollin J. Oral glucose and venepuncture reduce blood sampling pain in newborns. Early Hum Dev. 1999;55(3):211–218. doi: 10.1016/s0378-3782(99)00018-3. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Beggs S. The neurobiology of pain: developmental aspects. Neuroscientist. 2001;7(3):246–257. doi: 10.1177/107385840100700309. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Millard C, MacIntosh N. Hyperalgesia in premature infants. Lancet. 1988;1(8580):292. doi: 10.1016/s0140-6736(88)90365-0. [DOI] [PubMed] [Google Scholar]
- Franck LS. Some pain, some gain: reflections on the past two decades of neonatal pain research and treatment. Neonatal Network. 2002;21(5):37–41. doi: 10.1891/0730-0832.21.5.37. [DOI] [PubMed] [Google Scholar]
- Franck LS, Gilbert R. Reducing pain during blood sampling in infants. In: Barton S, editor. Clinical Evidence. British Medical Journal Publishing; London: 2002. pp. 352–366. [PubMed] [Google Scholar]
- Franck LS, Lawhon G. Environmental and behavioral strategies to prevent and manage neonatal pain. Seminars in Perinatology. 1998;22(5):434–443. doi: 10.1016/s0146-0005(98)80059-1. [DOI] [PubMed] [Google Scholar]
- Fuller BF. Acoustic discrimination of three types of infant cries. Nursing Research. 1991;40(3):156–160. [PubMed] [Google Scholar]
- Fuller BF. Meanings of discomfort and fussy-irritable in infant pain assessment. Journal of Pediatric Health Care. 1996;10(6):255–263. doi: 10.1016/s0891-5245(96)90051-6. [DOI] [PubMed] [Google Scholar]
- Fuller BF. Infant behaviors as indicators of established acute pain. J Soc Pediatric Nursing. 2001;6(3):109–115. doi: 10.1111/j.1744-6155.2001.tb00132.x. [DOI] [PubMed] [Google Scholar]
- Giannakoulopoulos X, Sepulveda W, Kourtis P, Glover V, Fisk NM. Fetal plasma cortisol and beta-endorphin response to intrauterine needling. Lancet. 1994;344(8915):77–81. doi: 10.1016/s0140-6736(94)91279-3. [DOI] [PubMed] [Google Scholar]
- Gibbins S, Stevens B. State of the arts: Pain assessment and management in high risk infants. Newborn and Infant Nursing Reviews. 2001;1(2):85–96. [Google Scholar]
- Gill N, Behnke M, Conlon M, McNeely J, Anderson CG. Effect of nonnutritive sucking on behavioral state in preterm infants before feeding. Nursing Research. 1988;37:347–350. [PubMed] [Google Scholar]
- Gitau R, Fisk NM, Glover V. Human fetal and maternal corticotrophin releasing hormone responses to acute stress. Arch Dis Child Fetal Neonatal Ed. 2004;89(1):F29–32. doi: 10.1136/fn.89.1.F29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitau R, Fisk NM, Teixeira JM, Cameron A, Glover V. Fetal hypothalamic-pituitary-adrenal stress responses to invasive procedures are independent of maternal responses. J Clin Endocrinol Metab. 2001;86(1):104–109. doi: 10.1210/jcem.86.1.7090. [DOI] [PubMed] [Google Scholar]
- Goubet N, Clefton RK, Shah B. Learning about pain in preterm newborns. J. Developmental & Behavioral Pediatrics. 2001;22(6):418–424. doi: 10.1097/00004703-200112000-00009. [DOI] [PubMed] [Google Scholar]
- Goubet N, Rattaz C, Pierrat V, Bullinger A, Lequien P. Olfactory Experience mediates response to pain in preterm newborns. Developmental Psychobiology. 2003;42(2):171–180. doi: 10.1002/dev.10085. [DOI] [PubMed] [Google Scholar]
- Gray L, Miller LW, Philipp BL, Blass EM. Breastfeeding is analgesic in healthy newborns. Pediatrics. 2002;109(4):590–593. doi: 10.1542/peds.109.4.590. [DOI] [PubMed] [Google Scholar]
- Gray L, Watt L, Blass EM. Skin-to-skin contact is analgesic in healthy newborns. Pediatrics. 2000;105(1):e14. doi: 10.1542/peds.105.1.e14. [DOI] [PubMed] [Google Scholar]
- Grunau RVE, Craig KD. Pain expression in neonates: facial action and cry. Pain. 1987;28:395–405. doi: 10.1016/0304-3959(87)90073-X. [DOI] [PubMed] [Google Scholar]
- Grunau RE, Linhares MB, Holsti L, Oberlander TF, Whitfield MF. Does prone or supine position influence pain responses in preterm infants at 32 weeks gestational age? Clin J Pain. 2004;20(2):76–82. doi: 10.1097/00002508-200403000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashi S, et al. Inter-atrial shunt flow profiles in newborn infants: a colour flow and pulsed Doppler echocardiographic study. British Heart Journal. 1991;65(1):41–45. doi: 10.1136/hrt.65.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CC, Stevens BJ, Franck LS, Jack A, Stremler R, Platt R. Factors explaining lack of response to heel stick in preterm newborns. J Obstet Gynecol Neonatal Nurs. 1999;28(6):587–594. doi: 10.1111/j.1552-6909.1999.tb02167.x. [DOI] [PubMed] [Google Scholar]
- Johnston CC, Stevens B, Pinelli J, Gibbins S, Filion F, Jack A, et al. Kangaroo care is effective in diminishing pain response in preterm neonates. Arch Pediatr Adolesc Med. 2003;157(11):1084–1088. doi: 10.1001/archpedi.157.11.1084. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 1990;297(3):441–470. doi: 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- Lago P, Guadagni A, Merazzi D, Ancora G, Bellieni CV, Cavazza A, The Pain Study Group of the Italian Society of Neonatology Pain management in the neonatal intensive care unit: a national survey in Italy. Pediatric Anesthesia. 2005;15(11):925–31. doi: 10.1111/j.1460-9592.2005.01688.x. [DOI] [PubMed] [Google Scholar]
- Larsson BA, Tannfeldt G, Lagercrantz H, Olsson GL. Venipuncture is more effective and less painful than heel lancing for blood tests in neonates. Pediatrics. 1998;101(5):882–886. doi: 10.1542/peds.101.5.882. [DOI] [PubMed] [Google Scholar]
- Lindt V, Wiklund U, Hakansson S. Heel lancing in term newborn infants: an evaluation of pain by frequency domain analysis of heart rate variability. Pain. 1999;80(12):143–148. doi: 10.1016/s0304-3959(98)00215-2. [DOI] [PubMed] [Google Scholar]
- Ludington-Hoe S, Cong X, Hashemi F. Infant crying: nature, physiologic consequences, and select interventions. Neonatal Netw. 2002;21(2):29–36. doi: 10.1891/0730-0832.21.2.29. [DOI] [PubMed] [Google Scholar]
- Ludington-Hoe S, Hosseini R, Torowicz DL. Skin-to-skin contact (Kangaroo Care) analgesia for preterm infant heel stick. AACN Clinical Issues. 2005;16(3):373–387. doi: 10.1097/00044067-200507000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlier L, Schaal R, Soussignan R. Neonatal responsiveness to the odor of the amniotic and lacteal fluids: a test of perinatal chemosensory continuity. Child Development. 1998;69(3):611–623. [PubMed] [Google Scholar]
- McGrath P. Pain in Children. Guilford Press; New York: 1990. [Google Scholar]
- Miles R, Cowan F, Glover V, Stevenson J, Modi N. A controlled trial of skin-to-skin contact in extremely preterm infants. Early Hum Dev. 2006 Jan 31; doi: 10.1016/j.earlhumdev.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Ogihara T, Fujiwara E, Ito K, Nakano M, Nakayama S, et al. Venepuncture is preferable to heel lance for blood sampling in term neonates. Arch Dis Child Fetal Neonatal Ed. 2005;90(5):F432–436. doi: 10.1136/adc.2004.069328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RM, Chantry CJ, Gallagher MP. Analgesic effects of breast-feeding or pacifier use with maternal holding in term infants. Ambul Pediatr. 2005;5(6):359–364. doi: 10.1367/A04-189R.1. [DOI] [PubMed] [Google Scholar]
- Porter FL, Anand KJS. Epidemiology of pain in neonates. Research and Clinical Forums. 1998;20:9–18. [Google Scholar]
- Porter FL, Miller RH, Marshall RE. Neonatal pain cries: effect of circumcision on acoustic features and perceived urgency. Child Development. 1986;57(3):790–802. [PubMed] [Google Scholar]
- Rangel S, Leon M. Early odor preference training increases olfactory bulb Norepinephrine. Brain Research and Developmental Brain Research. 85(2):187–191. doi: 10.1016/0165-3806(94)00211-h. [DOI] [PubMed] [Google Scholar]
- Rattaz C, Goubeet N, Bullinger A. The calming effect of a familiar odor On full-term newborns. J. Dev. Behav Pediatr. 2005;26(2):86–92. doi: 10.1097/00004703-200504000-00003. [DOI] [PubMed] [Google Scholar]
- Shah V, Taddio A, Bennett S, Speidel BD. Neonatal pain response to heel stick vs venepuncture for routine blood sampling. Arch Dis Child Fetal Neonatal Ed. 1997;77(2):F143–144. doi: 10.1136/fn.77.2.f143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPSS Inc. 2005. SPSS 13.0. Chicago, IL: SPSS Inc
- Stevens J. 3rd Lawrence Erlbaum Assoc; Mahwah, NJ: 1996. Applied multivariate statistics for the social sciences. [Google Scholar]
- Stevens BJ, McGrath P, Yamada J, Gibbins S, Beyene J, Breau L, Camfield C, Finley A, Franck L, Howlett A, Johnston C, McKeever P, O'brien K, Ohlsson A. Identification of pain indicators for infants at risk for neurological impairment: A Delphi consensus study. BMC Pediatr. 2006;26(1) doi: 10.1186/1471-2431-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walco GA, Cassidy R, Schecter N. Pain, hurt, and harm. The ethics of pain control in infants and children. New England Journal of Medicine. 1994;331(8):541–543. doi: 10.1056/NEJM199408253310812. [DOI] [PubMed] [Google Scholar]
- Warnock F, Sandrin D. Comprehensive description of newborn distress behavior in response to acute pain (newborn male circumcision) Pain. 2004;107(3):242–255. doi: 10.1016/j.pain.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Wolf Pain, nociception and the developing infant. Pediatric Anesthesia. 1999;9(1):7–17. [PubMed] [Google Scholar]


