Abstract
Little research has examined the influence of aging or sex on anatomical measures in the basolateral amygdala. We quantified spine density and dendritic material in Golgi-Cox stained tissue of the basolateral nucleus in young adult (3–5 months) and aged (20–24 months) male and female Long-Evans rats. Dendritic branching and spine density were measured in principal neurons. Age, but not sex, influenced the dendritic tree, with aged animals displaying significantly more dendritic material. Previous findings from our laboratory in the same set of subjects indicate an opposite effect of aging on dendritic material in the medial prefrontal cortex and hippocampus. We also report here a sex difference across ages in dendritic spine density, favoring males.
Keywords: aging, basolateral nucleus, dendrites, dendritic spines, Golgi-Cox, sex difference
1. Introduction
Emotion is increasingly recognized to play an essential role in modulating what is attended to, learned, and remembered [4,76], and the basolateral amygdala (BLA) appears to be the major structure critically involved in mediating the effects of emotional arousal on cognition and behavior [66,75]. The BLA is also essential for at least some kinds of emotional learning and memory, both aversive [54,58] and appetitive [27,86]. However, the effects of aging on BLA structure and function are not well characterized. In rats, performance of the BLA-dependent cued fear conditioning task is preserved with age [24,73,91], although an age-related impairment in this task has been reported in mice [39]. In humans, the literature on emotional memory and amygdala function in old age is mixed, with evidence for preservation [23,53,55,62] and, particularly when negative or fearful stimuli are used, impairment [11,29,49,92,101].
Most anatomic studies of the aging human amygdala are volumetric, and have provided evidence both for relative amygdala preservation [38,40,50] and for volumetric decreases [70,72,99]. Unfortunately, in vivo imaging studies of the human amygdala lack the resolution to reliably distinguish among individual amygdalar nuclear groups [108]. However, a handful of aging-related changes in the BLA of rats and mice have been reported [3,95,96,106], with significant implications for its functional connectivity. For example, the ability to prolong hippocampal long-term potentiation by stimulation of the BLA is lost in aged rats [3]. Aging in mice is accompanied by marked decreases in the density of tyrosine hydroxylase-positive fibers in the BLA [95]. Because the modulatory effects of emotional arousal on learning and memory are mediated by catecholaminergic innervation of the BLA [80] these losses may also be expected to significantly impact BLA interactions with other cognitive areas of the brain. These and other [96,106] findings of BLA changes in rodent aging and the mixed literature regarding BLA function in old age suggest the need for further study characterizing aging effects in the cognitively important nuclei of the BLA. One question addressed by the present study relates to the morphology of its principal neurons. Are there age-related losses in the dendritic tree and/or spine density, such as have been described in the aged rat neocortex and hippocampus [41,60,61,90]?
Like aging, sex effects on anatomical measures of the BLA have not been well explored. This is despite some intriguing evidence of functional sex differences in humans [10,12,52] and rats [5,35,104]. In rats, for example, the effects of stress on hippocampal-dependent learning and memory, which are mediated by the BLA [80,81], are sexually dimorphic, impairing performance by rats of one sex – which sex suffers impairment depends on the stress and task paradigms – and enhancing performance by the other [5,15,104]. Similar studies using female rats with and without high ovarian hormone levels show parallel interactions between gonadal steroids and stress effects on learning and memory [6,84,104]. More direct evidence that the BLA may be influenced by sex hormones includes the finding that estradiol dramatically decreases EPSP amplitude in BLA neurons in vitro [103], and that high doses of estradiol to ovariectomized rats impair performance on the BLA-dependent conditioned place preference task [35]. However, the vast majority of studies of anatomical sex differences in the amygdala have been limited to examination of the reproductively important amygdalar nuclei associated with the vomeronasal system, particularly portions of the medial nucleus and extended amygdala and the posteromedial cortical nucleus [e.g., 1,17,18,19,42,43,47,79,94,102].
The present study was therefore designed to investigate the influence of aging, sex, and (in females) ovarian hormones on neuronal morphology in the basolateral nucleus of intact male and female rats. Dendritic material and spine density of principal neurons were quantified in Golgi-Cox stained tissue (figure 2). Ovarian hormone effects were investigated in young adults by comparing brains of females sacrificed in the proestrus phase, when estrogen and progesterone are at their highest levels of the estrous cycle, versus those in the estrus phase, when these hormone levels are at their lowest [87]. Aged females were sacrificed in one of two phases of reproductive senescence: persistent estrus, characterized by moderate levels of circulating estrogens and progesterone, or persistent diestrus, characterized by similar levels of estrogen but elevated progesterone [60].
Figure 2.
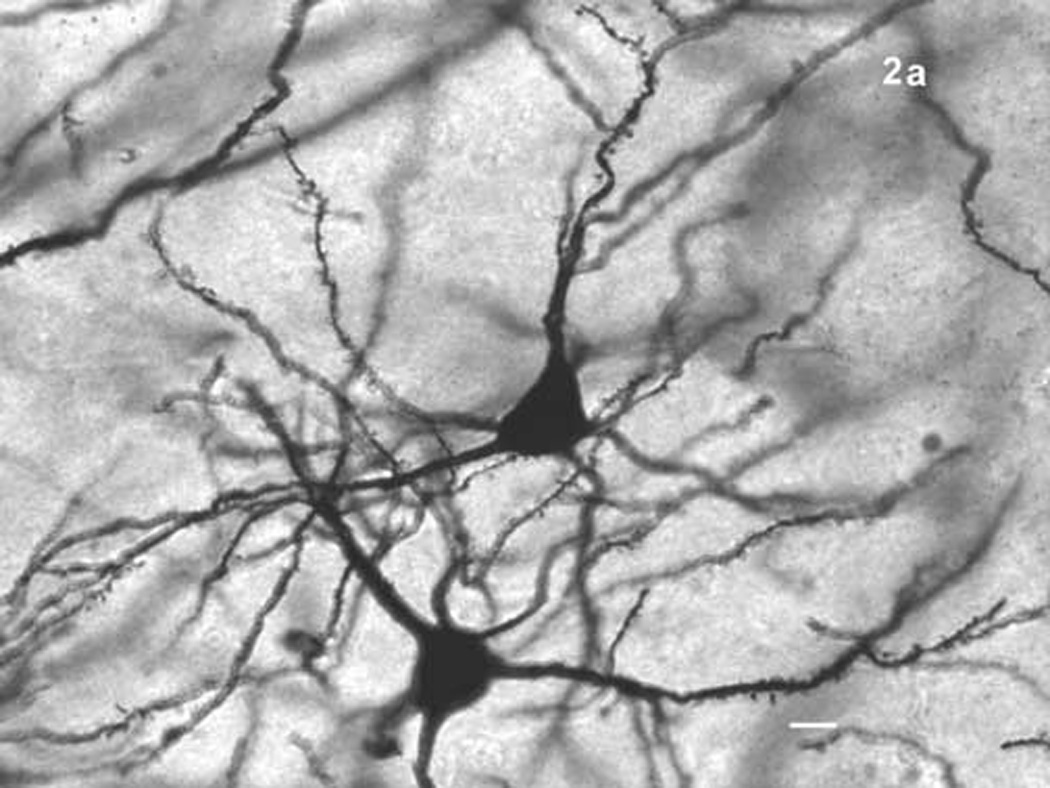
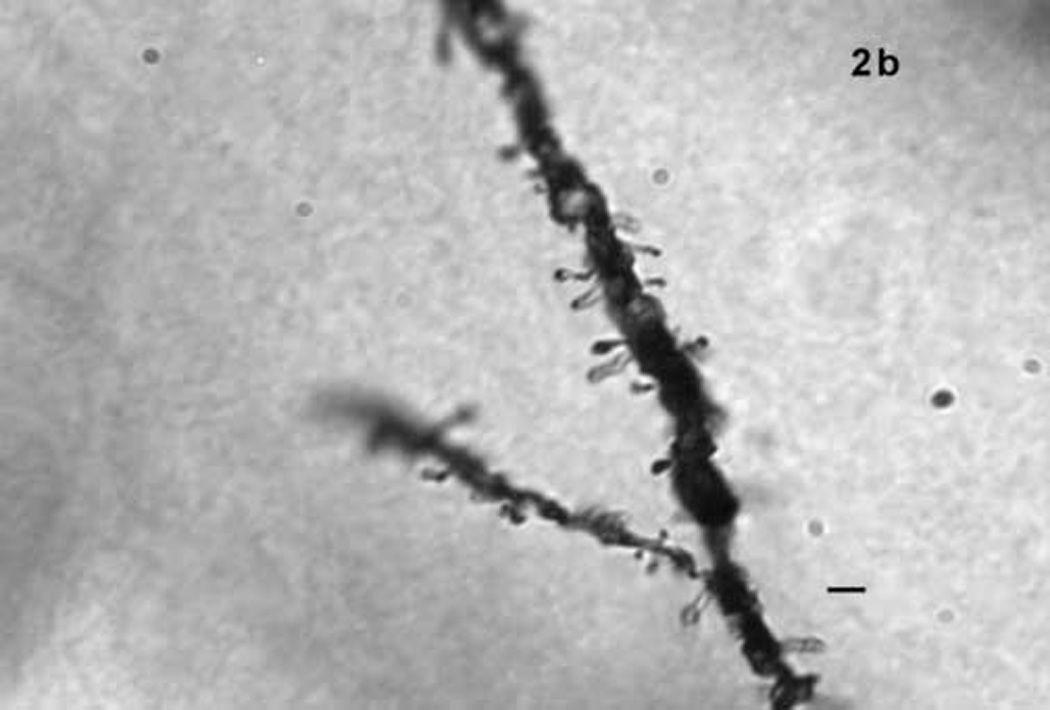
(a) Two principal neurons of the basolateral nucleus visualized with Golgi-Cox. Scale bar = 10 µm. (b) Dendritic spines on a basolateral nucleus principal neuron. Scale bar = 2 µm.
2. Methods
2.1. Subjects
Subjects used for the examination of dendritic material were young adult (3–5 months) and aged (20–24 months) male and female Long-Evans hooded rats, first generation descendents from stock obtained from Simonsen Laboratory (Gilroy, CA), bred in the Psychology department at the University of Illinois. The age ranges chosen represent those typically employed in studies of aging in Long-Evans rats [7,83,90]. There were 6 groups: young adult males (n = 7), young adult females in proestrus (n = 4), or estrus (n = 5), aged males (n = 5), and aged females in persistent estrus (n = 5) or persistent diestrus (n = 4). Spine density was evaluated in a largely overlapping group of subjects, in the same groups of the following numbers: young males (n = 6), proestrous females (n = 3), estrous females (n = 3), aged males (n = 4), aged females in persistent estrus (n = 6), and persistent diestrus (n = 5). The aged rats were retired breeders. Hippocampal and prefrontal cortex tissue from subjects in all groups were examined and reported in two previous studies [60,61]. Four additional males were included in the dendritic branching analysis: two young males (sham gonadectomy at least three months prior to sacrifice), and two aged males (one had a sham gonadectomy 9 months prior to sacrifice).
Rats were housed in the vivarium of the Psychology department, in same-sex pairs in clear plexiglass cages, in a temperature-controlled environment, on a 12-hr light-dark cycle (lights off at 1900h). The aged rats were pair-housed following retirement from breeding, at approximately 12 months of age. Rat chow and water were available ad libitum. Animal care and experimental procedures were in accordance with National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee.
2.2. Handling and estrous cycle determination
To minimize the effects of handling stress at sacrifice, all the rats were handled weekly following weaning at postnatal day 25 (young adult rats), or following retirement from breeding (aged rats), until two weeks prior to sacrifice, at which time all the rats were handled daily.
Estrous and estropausal phase were determined from vaginal cells obtained by the lavage method. Young adult females were used only after showing three consecutive, normal 4–5 day estrous cycles. Aged females were used only after they were reliably determined to be in either persistent estrus or persistent diestrus for a minimum of 10 consecutive days.
2.3. Histology
The rats were sacrificed with a lethal dose of sodium pentobarbital between 1600h and 1900h, brains were removed, and the cerebellum was cut away. The brains were immersed in 100 ml of Golgi-Cox solution according to Van der Loos [93] for approximately 18 days [60,61]. Golgi-Cox solution was prepared from 5% solutions of potassium dichromate, mercuric chloride and potassium chromate, in a 5:5:4 volume parts ratio [37]. Tissue was blocked coronally at the optic chiasm, dehydrated, embedded in a celloidin solution, hardened and stored in butanol until sectioning. Before sectioning, the celloidin-embedded tissue was coded so that all quantification was made blind to group. Several blocks of the coded tissue were randomly selected on each day of sectioning and processing, which was completed over a period of months. Brains were sectioned in the coronal plane on a sliding microtome at a thickness of 150 µm. Processing of the sections involved development in ammonium hydroxide, fixation in photographic fixative, dehydration, and clearing in xylene. Sections were mounted on slides with permount and coverslipped.
2.4. Identification of principal neurons of the basolateral nucleus
The basolateral nucleus (BL) was identified by location, after an examination of Nissl stained tissue through the BL of several rat brains, and by the orientation and morphological characteristics of its neurons, which differ markedly from those of most surrounding nuclei [64]. Figure 1 shows the area of the BL in Nissl (1a) and Golgi-Cox (1b) stains and figure 2 shows representative principal neurons (2a) and dendritic spines (2b). Neurons of the dorsally adjacent lateral nucleus, which are morphologically similar to those of the BL, were distinguishable by location and by their smaller somata [20,64]. Neurons outside of the BL were further avoided with the precaution of restricting analysis to neurons sufficiently far from its borders.
Figure 1.
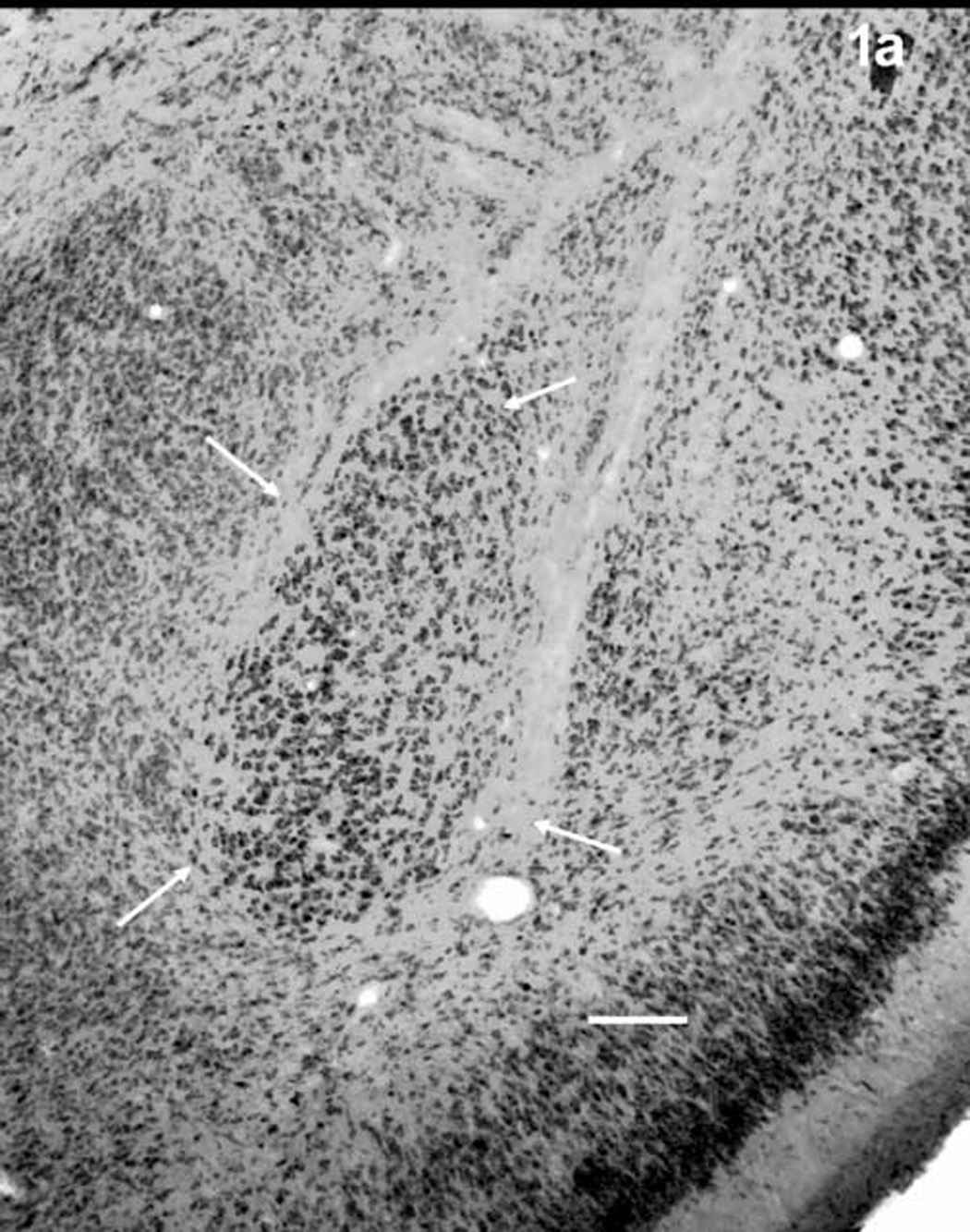
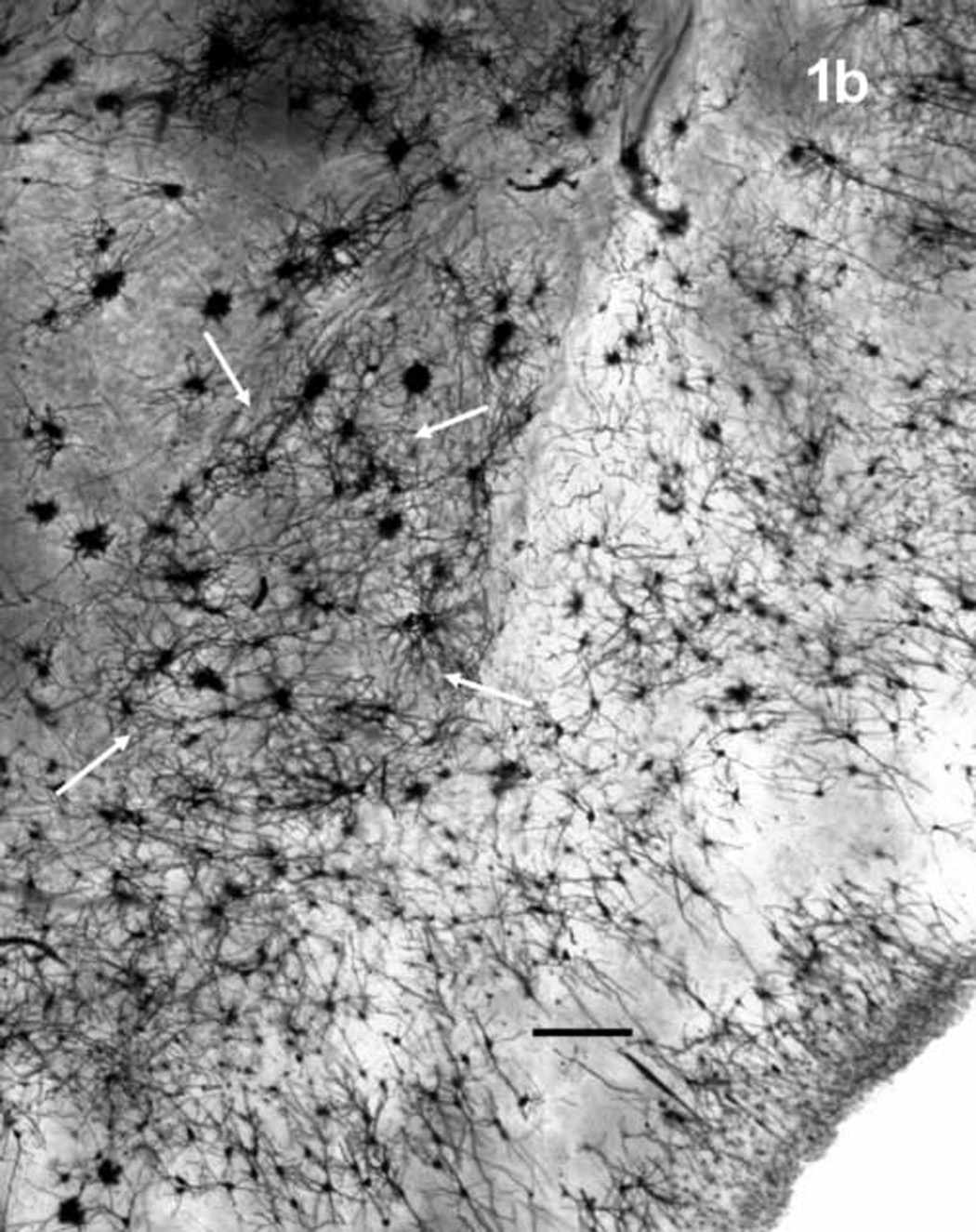
The basolateral nucleus of the amygdala (arrows) in Nissl-stained tissue (a) and Golgi-Cox stained tissue (b). Scale bars = 200 µm.
Principal, also known as class I or pyramidal, neurons were sampled (figure 2a). These represent about 85% of neurons in the BLA [71], are the most frequently impregnated BL neurons in Golgi-Cox stained tissue, and are easily distinguished from spine-sparse interneurons which are also impregnated by the Golgi-Cox stain [64]. Principal neurons of the BL are frequently noted for their similarity to pyramidal neurons of the cerebral cortex [13,63,65]. Though their orientation is irregular, principal neurons of the BL are large, spiny, and frequently exhibit a pyramidal or semi-pyramidal cell body, from which approximately five to seven primary dendrites arise [64]. Some of these neurons display one or two thicker, apical-like dendrites, which arborize more extensively than their thinner, “basal” dendrite-like counterparts. Other class I neurons of the BL are more stellate in shape, with primary dendrites of approximately equal calibers [64].
2.5. Analysis of dendritic material
Dendritic arbors of principal BL neurons were drawn at 500X with the use of a camera lucida. Neurons were selected only if they were unquestionably within the BL, fully impregnated, and dendritic material could be distinguished from that of other neurons. While the great majority of neurons were fully contained within the sections examined, the irregular orientation of dendritic material made it impractical to exclude every neuron containing a truncated branch. Branches that were either cut off or which ran into glia and could not be reliably followed were noted on the drawings. Up to 14 neurons per subject fitting these criteria were drawn, with a minimum of 7 (one case) and an average of 12 neurons per subject. Average number of neurons examined did not differ by group. Total dendritic length was estimated by Sholl ring analysis [88], in which a series of concentric rings were calibrated (20 µm apart in this study), copied onto a transparency, and laid over the drawings, centered on the somata. Figure 3 illustrates Sholl rings laid over camera lucida drawings. The number of intersections made by dendritic material was counted to index the amount of dendritic arborization overall (figure 4) and at each successive ring (figure 5). In addition to Sholl analysis, the number and order of dendritic branches on each neuron were counted.
Figure 3.
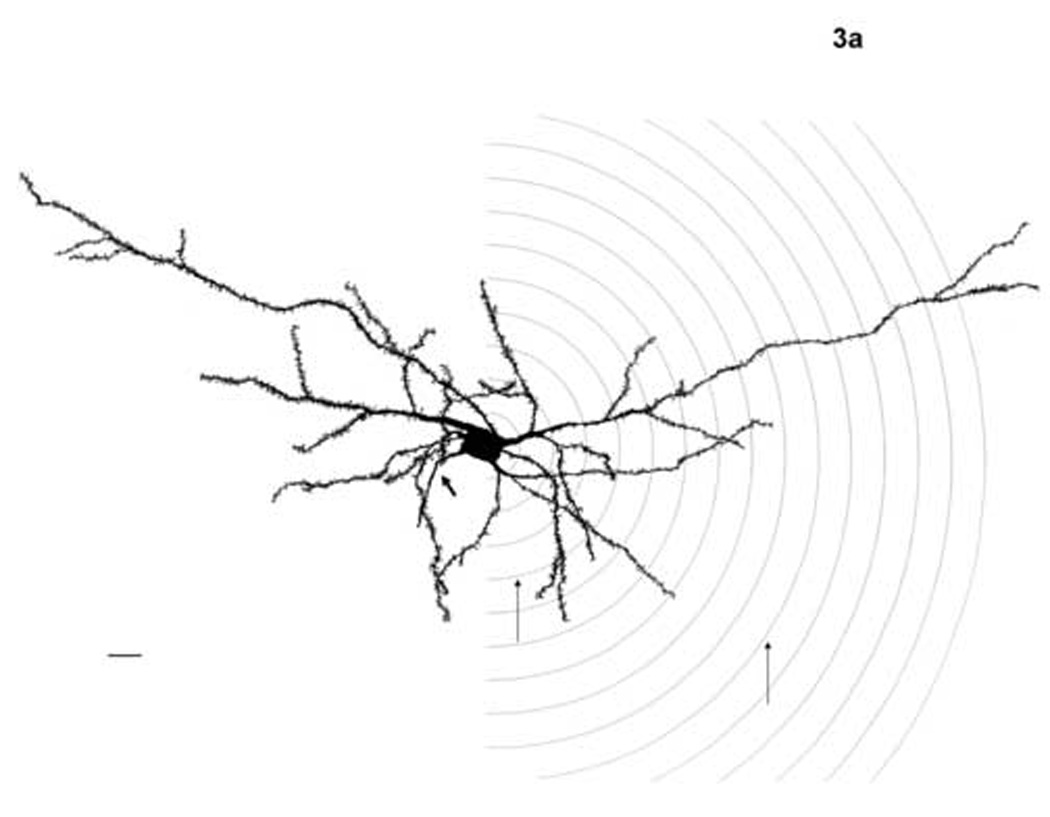
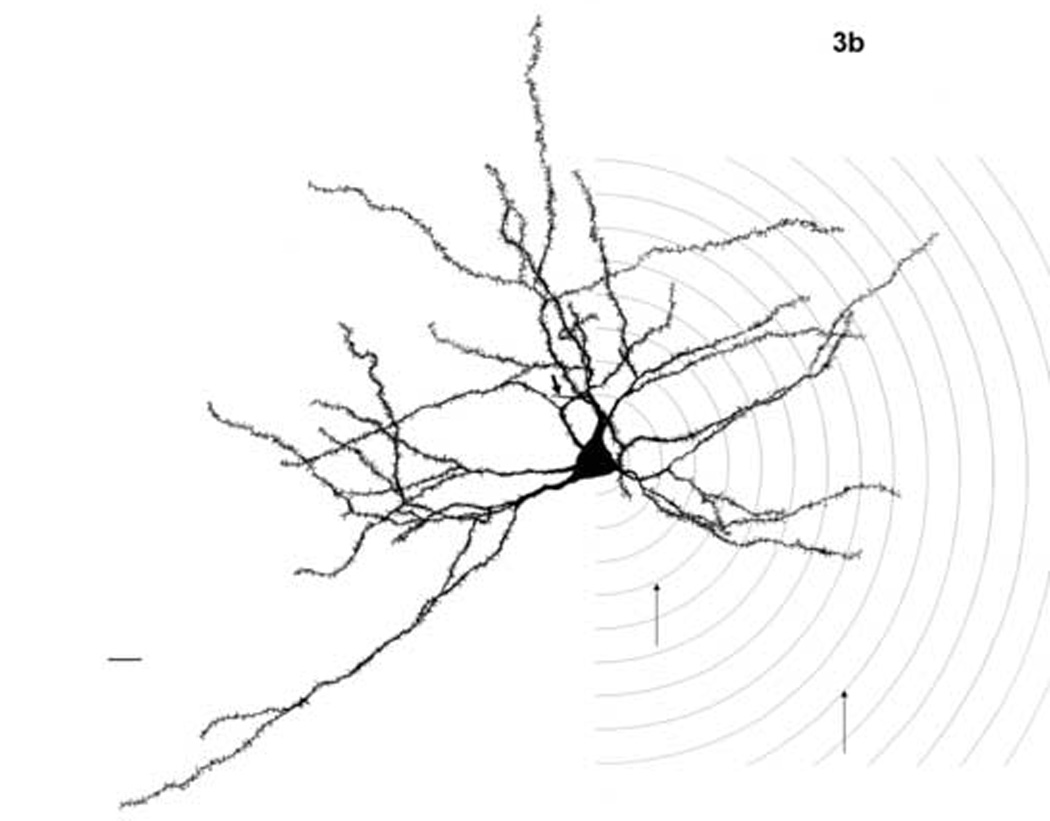
Camera lucida drawings of basolateral nucleus principal neurons from young adult (a) and aged (b) subjects, both female. Thick arrow indicates axon in both figures. Sholl rings are illustrated on the right half of the illustrations. Thin arrows indicate rings 4 and 10, boundaries of the 80–200 µm range from the soma center where age differences were found. Scale bars = 20 µm.
Figure 4.
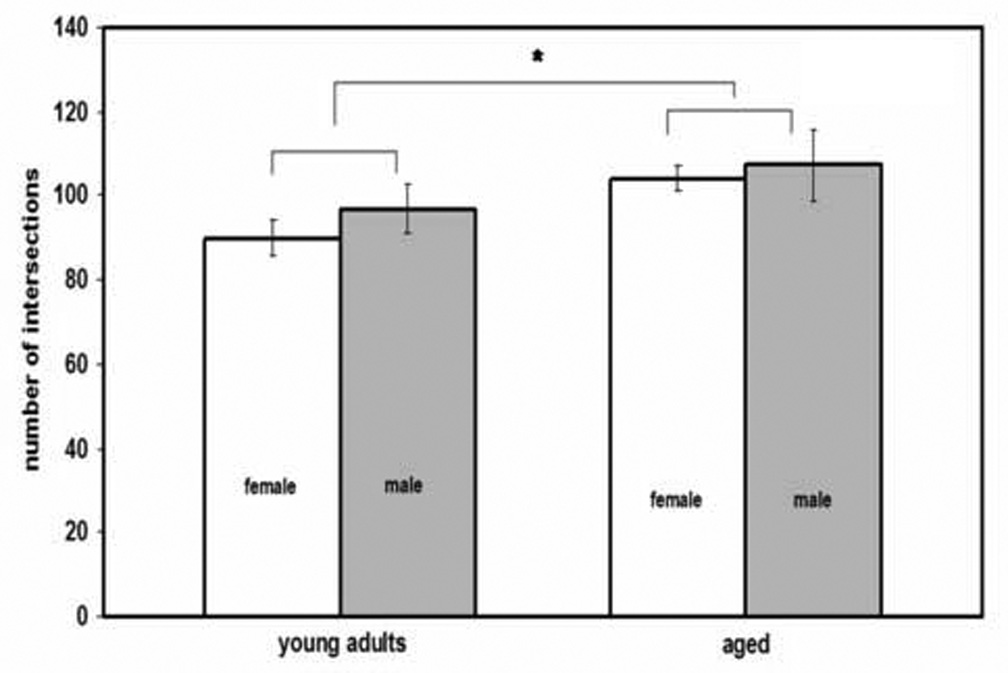
Age-related increase in dendritic material of principal neurons of the basolateral nucleus as measured by intersections with Sholl rings. Error bars, +/− SEM. *P = 0.005 for age.
Figure 5.
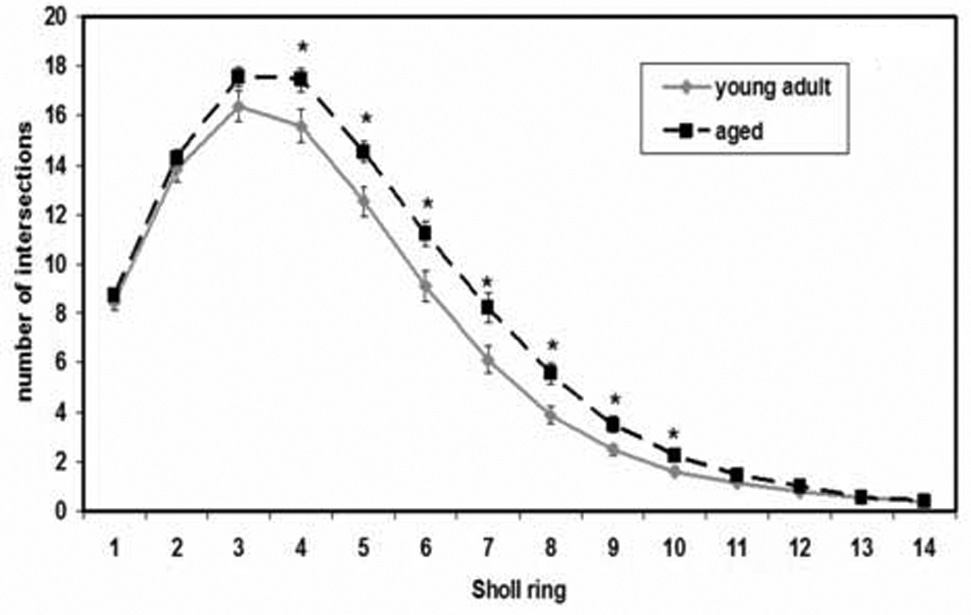
Average number of intersections for young adult and aged rats, collapsed across sex, at successive Sholl rings. Error bars, +/− SEM. *P < 0.04.
2.6. Spine density analysis
Spine density was quantified at 1250X under oil on dendritic branches of principal neurons. Eleven to 15 camera lucida drawings per subject were made of short (range 12–30 µm) lengths of dendritic segments, on which the dendritic diameter was measured and the number of spines was counted. Typically no more than one or two dendritic segments were used from the same neuron, though occasionally three segments, originating from different primary branches, were used. Dendritic segments were 0.8–1.6 µm in diameter, with average diameter 1.08 µm. Because principal neurons of the BL do not exhibit regular branching as in neocortex, quantification was not limited to a particular branch order. Instead, dendritic segments were selected from a set range (40–100 µm) of distance from somata. To further control variability only segments at least 50 µm from their terminal ends were used. Only dendritic segments oriented parallel to the plane of the section were selected. Parallel orientation was necessary to maximize spine visibility and avoid foreshortened estimates of segment lengths. Spine density was calculated as average number of spines per 10 µm of dendritic length per subject.
3. Results
3.1. Dendritic material
Total dendritic length was estimated by the number of Sholl ring intersections. Initial, two-tailed t-tests were made comparing the average total number of intersections in the proestrous vs. estrous females and the persistent estrous vs. persistent diestrous aged females, to determine whether either of the two female age groups should be combined for ANOVA analysis. These comparisons revealed no effect of estrous phase (t(7) = 0.34, ns) or estropausal phase (t(7) = 0.78, ns) on Sholl ring intersections; accordingly, the young adult females were combined into a single group (n = 9), as were the aged females (n = 9).
A two-way ANOVA with one repeated measure (sex, age, and Sholl ring as the repeated measure) revealed a significant effect of age on number of intersections (F(1,26) = 9.34; P = 0.005), due to an age-related increase in dendritic material of 14% (figure 4). Sex did not significantly influence dendritic material (F(1,26) = 2.26; P = 0.144), nor did sex interact with age (F(1,26) = 0.002, ns; figure 4), indicating the age-related increase in dendritic material occurred in both sexes.
The within-subject repeated measure revealed a significant effect of Sholl ring (i.e., distance from soma) on dendritic material (F(13,338) = 645.87; P < 0.001), as well as a significant age x ring interaction (F(13,338) = 2.85; P = 0.001), indicating that distance from soma affected dendritic material differently in the young vs. the aged rats. Sex did not interact with Sholl ring (F(13,338) = 0.78; P = 0.682). The age x Sholl ring interaction was explored with post hoc comparisons of the number of intersections in aged vs. young adult rats at each ring. Aged rats had significantly greater numbers of Sholl ring intersections at each point quantified between 80 (Sholl ring 4) and 200 µm (ring 10) from the soma (Sholl ring 4 (P = 0.038), 5 (P = 0.015), 6 (P = 0.016), 7 (P = 0.015), 8 (P = 0.005), 9 (P = 0.013), 10 (P = 0.028); figure 5). There were no age effects at either the most proximal points measured (20 through 60 µm from the soma; rings 1, 2 and 3) or the most distal (220 through 280 µm; rings 11 through 14), suggesting age did not influence the number of primary dendrites emerging from the soma (see next paragraph) or the overall reach of the distal dendritic field. Figure 3 shows camera lucida drawings of principal neurons from female adult (3a) and aged (3b) subjects, with the area of Sholl rings 4 and 10 marked.
Quantification of the total number of dendritic branches at each order revealed an average of 5.5 primary branches per neuron, and an average total of 59.4 branches. There was no effect of age (F(1,26) = 0.96; P = 0.337) or sex (F(1,26) = 0.38; P = 0.545) on the total number of branches or in the number of branches at any order.
Analysis of the number of branches either cut off by the section or unresolvable (average = 0.36 per neuron) revealed no effect of age (F(1,26) = 0.34; P = 0.855) or sex (F(1,26) = 1.21; P = 0.281) on this measure, or in the ratio of cut-off/irresolvable terminals to all terminals (for age: F(1,26) = 0.10; P = 0.756); for sex: F(1,26) = 1.33; P = 0.259), indicating no group bias in the analysis due to these factors.
3.2. Spine Density
Spine density was measured as average number of spines per 10 µm per group. Initial, two-tailed t-tests comparing the young adult females in proestrus vs. estrus revealed that spine density did not significantly change with estrous phase (t(4) = 2.04; P = 0.112), although the near-trend (favoring estrous over proestrous females) suggests that an effect of cycle, which could not be detected with the small number of subjects examined (n = 3 per group), may exist. A t-test comparing spine density in the two aged female groups showed no effect of estropausal phase (t(9) = 0.86; P > 0.41). Accordingly, aged females were grouped for overall analysis (n = 11), as were the young adult females (n = 6).
A two-way ANOVA (sex, age) revealed a significant effect of sex on spine density (F(1,23) = 8.60; P = 0.007), with males having 12% greater spine density than females (figure 6). Age did not influence spine density (F (1,23) =1.25; P = 0.274), nor did sex interact with age in influencing this variable (F (1,23) = 0.053, ns), indicating the sex difference occurred across ages.
Figure 6.
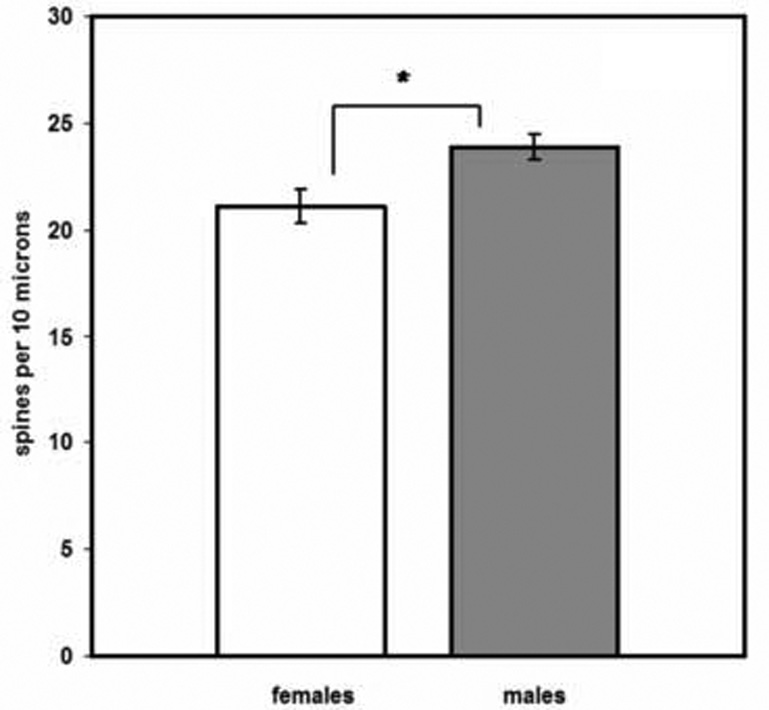
Sexually dimorphic spine density in principal neurons of the rat basolateral nucleus. Data are shown collapsed across age. *P = 0.007. Error bars, +/− SEM.
Branch order on which spines were counted (average = 3rd-order) did not differ between groups (F (1,23) < 1.0, ns for sex, age, and sex × age interaction). Analysis of the diameter of dendritic segments on which spines were counted revealed no significant sex difference in this measure (F(1,23) = 1.38; P = 0.252). However, dendritic segments on which spines were counted were slightly thicker in the aged than in the young subjects (F(1,23) = 5.09; P = 0.034), with average dendrite thickness of 1.1 µm for aged, vs. 1.05 µm for young adult subjects. However, there were no differences in spine head diameters or spine lengths between groups and the dendritic segment thickness difference represented less than 5% of average spine length. This minimal difference is well within the normal variability of dendrites from the same population [48], such that correction for hidden spines [28] would not meaningfully affect the relative number of spines between groups and is therefore considered unnecessary [48].
4. Discussion
These results demonstrate that aging and sex have significant, and distinct, effects on the morphology of principal neurons of the basolateral nucleus (BL) of the rat: dendritic arborization increases with age and is not significantly affected by sex, while spine density is sexually dimorphic, and unaffected by age.
We found an age-related increase in dendritic material of principal neurons, of approximately 14%. This is the first study, to our knowledge, to examine changes in dendritic morphology of neurons of the BLA in aged rats. Whether there is continual growth of the BL dendritic field or a non-linear pattern is not yet clear. Previous studies in human hippocampus [31] and rat hypothalamus [32] have described dendritic growth followed by regression in older age. If this is also the case in the BL, future studies including both intermediate and very old ages [14] can help to pinpoint whether dendritic losses occur after the age (20–24 months) examined here, or perhaps peak before this age and begin to regress thereafter.
Though aging effects have not previously been examined in the dendritic tree of the rat BLA, age-related changes on this measure have been described in neocortical and hippocampal pyramidal neurons, which bear a close resemblance to principal neurons of the BL [13,63,65], and that literature is decidedly mixed. For example, in rat neocortex, age-related dendritic regression [41,60,90] and growth [67,105] have both been described. There are likewise reports of dendritic regression [61] and growth [78] in area CA1 of the rat hippocampus. In humans, normal aging is accompanied by dendritic increases in pyramidal cells of the parahippocampal gyrus [8], and no changes in pyramidal cell dendrites of the subiculum [30] or the CA fields [33,44]. Some age-related dendritic regression has been described in pyramidal neurons of superior temporal cortex in macaques [26]. Thus, the finding here of increased dendritic material in aged subjects is not unique. It was nevertheless somewhat unexpected, in that age-related losses of dendritic material were found in the same tissue examined here, in both the medial prefrontal cortex (mPFC) [60] and, among the males, in area CA1 of the hippocampus [61].
This internal control eliminates variability from subject variables (age, strain) and housing and animal handling protocols that may differ across laboratories. The circumstance of examining the BL in tissue where two other brain areas have already been studied also creates an advantage in interpreting the data despite some caveats regarding quantitative analysis of Golgi-stained tissue. For example, any age-related differences in impregnation (though we have no evidence of this) would bias aging data; but since these data reflect an opposite aging effect in the BL vs. both the mPFC and hippocampus, any potential aging effect decreasing or increasing impregnation in the tissue would have been overcome by the relative difference. Two-dimensional analysis of dendritic material does underestimate the length of dendrites. However, there is no reason to assume this bias would differ in young adult vs. aged rats, and the lack of any group differences in the number of branches cut off by the section supports this. A more definitive estimate of true length can be obtained with three-dimensional analysis of neurons with intracellular fills that can be followed through sections. However, in contrast to intracellular fills, Golgi stains provide the advantage that many neurons from a given animal can be quantified.
The present finding of dendritic hypertrophy in aged rats demonstrates differential effects of aging on hippocampal [61] and mPFC [60] pyramidal neurons vs. principal neurons of the basolateral nucleus of the amygdala. This result fits a pattern of contrasting roles of the amygdala with each of these brain areas. In the case of the hippocampus, the same variable (here, aging) often affects that structure differently than it affects the BLA [2,97,98]. For example, estradiol has excitatory effects on hippocampal electrophysiology [100], but inhibitory effects on BLA electrophysiology [103]. And, in parallel to our findings in this tissue, chronic stress induces dendritic atrophy in hippocampal CA3 pyramidal neurons, and dendritic hypertrophy in principal neurons of the BLA [98]. The mPFC likewise undergoes dendritic atrophy following chronic stress [16], and the amygdala also can have opposite effects from both the hippocampus and mPFC in regulating the hypothalamic-pituitary-adrenal axis, as both mPFC and hippocampal efferents lead to net inhibition, and amygdala efferents to net excitation, of neurosecretory cells of the paraventricular nucleus [45,46]. The mPFC and BLA have a number of other opposing roles, and in some cases mutually inhibitory interactions [36,56,82,89]. For example, stimulation of the mPFC has been shown to suppress responses to conditioned stimuli in the lateral nucleus of the BLA [82], and a recent tract-tracing and electron microscopy study confirmed that basolateral nucleus efferents monosynaptically innervate inhibitory local circuit neurons of the mPFC [34]. Indeed, a disrupted ability of the mPFC to regulate affective responses via its interactions with the amygdala is widely thought to contribute to the pathophysiology of depression and post-traumatic stress disorder [25,56].
We also found a sexual dimorphism on the measure of spine density, with male rats exhibiting 12% greater spine density than females on principal cell dendrites (figure 6). The greater spine density observed in males reflects the possibility for more excitatory inputs from any number of afferent populations. Excitatory projection neurons providing inputs to principal BL neurons include those originating from the hippocampus (both the ventral subiculum and area CA1), thalamus, and cerebral cortex [63,71]. Other excitatory inputs come from the lateral nucleus of the BLA and other amygdala divisions, and from collaterals of axons of BL principal neurons themselves [71]. While the cause of this sex difference is not known, the current literature suggests a possible role for estrogen. Estrogen applied to BLA neurons in vitro decreases EPSP amplitude by a rather astonishing 77% [103], and high doses of estradiol also impair performance of the BLA-dependent conditioned place preference task [35].
The functional implications of this sex difference are nevertheless not obvious. For example, data on sex differences in performance of the BLA-dependent fear conditioning task are mixed [59,77]. Sex differences in the stress response are well documented [51], and both chronic and acute stress have been shown to increase spine density in the BL, at least among male rats [69]. However, if the observed sex difference in spine density were due to basal sex differences in response to normal stressors, one would expect an alteration with age, because of changes in both the stress response and sex differences therein with age [51]; instead, we observed no main effect of age and no change in the sex difference in spine density with age. However, both sex [5,15,57,104] and ovarian hormones [6,84,104] have been repeatedly shown to affect the BLA-dependent modulatory effects of stress on hippocampal-dependent learning and memory. It is possible that the sex difference seen here is related to that phenomenon, perhaps for instance reflecting more efficient BLA-hippocampal interactions in males.
The functional implication of age-related dendritic growth is not known. The observed increases of dendritic material in the 80–200 µm range coupled with the lack of differences in the number of dendritic branches suggest an age-related growth of branch length in this middle range. It has been posited that dendritic hypertrophy in aging may occur as a compensatory response to neuronal losses or to decreased afferentation [8,31,105] and some evidence exists for dendritic increases accompanied by neuronal loss [74]. Another possibility is that dendritic growth in the BL, which occurs in response to chronic stress [98] as well as aging, is related to age-associated changes in the stress response [21,68,107], including decreases in corticotrophin-releasing factor (CRF)-binding protein in the BLA of aged rats [106]. For instance, a decreased capacity to regulate CRF levels might render the aged BLA more vulnerable to stress-induced dendritic hypertrophy. On the other hand, given the very different findings of hippocampal and mPFC vs. BLA dendritic changes with aging seen here within the same group of rats, it is perhaps noteworthy that another contrast between the BLA and both the hippocampus and mPFC is that cognitive processes supported by the latter areas are widely known to suffer age-related declines [reviewed in 9,22], whereas there is at least some evidence to support preserved amygdala function with age in rats [24,73,85,91] and in humans [23,53,55,62]. We are currently quantifying neuron and glial cell number in BLA nuclei of male and female young and aged rats, which will provide a better context for interpreting the present findings, particularly in the case of the aged rats.
Acknowledgements
The authors thank Dr. Julie Markham and Lori Scardina for their contributions to this study. This work was supported by NIH AG 18046, AG 022499 and NSF IBN 0136468. M.J.R. was supported by NIH HD07333. Portions of this study were presented at the International Behavioral Neuroscience Society and Society for Neuroscience annual conferences in 2005.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors have no conflicts of interest to disclose.
Treatment of animal subjects in this study and all experimental procedures were in accordance with National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee at the University of Illinois.
References
- 1.Akhmadeev AV, Kalimullina LB. Dendroarchitectonics of neurons in the posterior cortical nucleus of the amygdaloid body of the rat brain as influenced by gender and neonatal androgenization. Neurosci Behav Physiol. 2005;35(4):393–397. doi: 10.1007/s11055-005-0039-8. [DOI] [PubMed] [Google Scholar]
- 2.Akirav I, Sandi C, Richter-Levin G. Differential activation of hippocampus and amygdala following spatial learning under stress. Eur J Neurosci. 2001;14(4):719–725. doi: 10.1046/j.0953-816x.2001.01687.x. [DOI] [PubMed] [Google Scholar]
- 3.Almaguer W, Estupinan B, Uwe Frey J, Bergado JA. Aging impairs amygdala-hippocampus interactions involved in hippocampal LTP. Neurobiol Aging. 2002;23(2):319–324. doi: 10.1016/s0197-4580(01)00278-0. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard C, Blanchard R, Fellous JM, Guimaraes FS, Irwin W, LeDoux JE, McGaugh JL, Rosen JB, Schenberg LC, Volchan E, Da Cunha C. The brain decade in debate: III. Neurobiology of emotion. Braz J Med Biol Res. 2001;34(3):283–293. doi: 10.1590/s0100-879x2001000300001. [DOI] [PubMed] [Google Scholar]
- 5.Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Horm Behav. 2003;43(1):48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- 6.Bowman RE, Ferguson D, Luine VN. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002;113(2):401–410. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan JB, Peloso E, Satinoff E. Influence of ambient temperature on peripherally induced interleukin-1 beta fever in young and old rats. Physiol Behav. 2006;88(4–5):453–458. doi: 10.1016/j.physbeh.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Buell SJ, Coleman PD. Quantitative evidence for selective dendritic growth in normal human aging but not in senile dementia. Brain Res. 1981;214(1):23–41. doi: 10.1016/0006-8993(81)90436-4. [DOI] [PubMed] [Google Scholar]
- 9.Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7(1):30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- 10.Cahill L. Sex- and hemisphere-related influences on the neurobiology of emotionally influenced memory. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(8):1235–1241. doi: 10.1016/j.pnpbp.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Calder AJ, Keane J, Manly T, Sprengelmeyer R, Scott S, Nimmo-Smith I, Young AW. Facial expression recognition across the adult life span. Neuropsychologia. 2003;41(2):195–202. doi: 10.1016/s0028-3932(02)00149-5. [DOI] [PubMed] [Google Scholar]
- 12.Canli T, Desmond JE, Zhao Z, Gabrieli JD. Sex differences in the neural basis of emotional memories. Proc Natl Acad Sci U S A. 2002;99(16):10789–10794. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlsen J, Heimer L. The basolateral amygdaloid complex as a cortical-like structure. Brain Res. 1988;441(1–2):377–380. doi: 10.1016/0006-8993(88)91418-7. [DOI] [PubMed] [Google Scholar]
- 14.Coleman PD. How old is old? Neurobiol Aging. 2004;25(1):1. doi: 10.1016/j.neurobiolaging.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman JS, Wright RL, Korol DL. Acute stress impairs spatial memory in male but not female rats: influence of estrous cycle. Pharmacol Biochem Behav. 2004;78(3):569–579. doi: 10.1016/j.pbb.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 16.Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60(2):236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- 17.Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proc Natl Acad Sci U S A. 1999;96(13):7538–7540. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol. 2005;64(1):34–46. doi: 10.1002/neu.20143. [DOI] [PubMed] [Google Scholar]
- 19.Cooke BM, Woolley CS. Sexually dimorphic synaptic organization of the medial amygdala. J Neurosci. 2005;25(46):10759–10767. doi: 10.1523/JNEUROSCI.2919-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Olmos JS, Beltramino CA, Alheid G. Amygdala and extended amygdala of the rat: a cytoarchitectonical, fibroarchitectonical, and chemoarchitectonical survey. In: Paxinos G, editor. The rat nervous system. 3rd Edition. Sidney: Elsevier Academic Press; 2004. pp. 509–603. [Google Scholar]
- 21.Del Arco A, Segovia G, Mora F. Dopamine release during stress in the prefrontal cortex of the rat decreases with age. Neuroreport. 2001;12(18):4019–4022. doi: 10.1097/00001756-200112210-00033. [DOI] [PubMed] [Google Scholar]
- 22.Della-Maggiore V, Grady CL, McIntosh AR. Dissecting the effect of aging on the neural substrates of memory: deterioration, preservation or functional reorganization? Rev Neurosci. 2002;13(2):167–181. doi: 10.1515/revneuro.2002.13.2.167. [DOI] [PubMed] [Google Scholar]
- 23.Denburg NL, Buchanan TW, Tranel D, Adolphs R. Evidence for preserved emotional memory in normal older persons. Emotion. 2003;3(3):239–253. doi: 10.1037/1528-3542.3.3.239. [DOI] [PubMed] [Google Scholar]
- 24.Doyere V, Gisquet-Verrier P, de Marsanich B, Ammassari-Teule M. Age-related modifications of contextual information processing in rats: role of emotional reactivity, arousal and testing procedure. Behav Brain Res. 2000;114(1–2):153–165. doi: 10.1016/s0166-4328(00)00223-0. [DOI] [PubMed] [Google Scholar]
- 25.Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- 26.Duan H, Wearne SL, Rocher AB, Macedo A, Morrison JH, Hof PR. Age-related dendritic and spine changes in corticocortically projecting neurons in macaque monkeys. Cereb Cortex. 2003;13(9):950–961. doi: 10.1093/cercor/13.9.950. [DOI] [PubMed] [Google Scholar]
- 27.Everitt BJ, Morris KA, O’Brien A, Robbins TW. The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience. 1991;42(1):1–18. doi: 10.1016/0306-4522(91)90145-e. [DOI] [PubMed] [Google Scholar]
- 28.Feldman ML, Peters A. A technique for estimating total spine numbers on Golgi-impregnated dendrites. J Comp Neurol. 1979;188(4):527–542. doi: 10.1002/cne.901880403. [DOI] [PubMed] [Google Scholar]
- 29.Fischer H, Sandblom J, Gavazzeni J, Fransson P, Wright CI, Bäckman L. Age-differential patterns of brain activation during perception of angry faces. Neurosci Lett. 2005;386(2):99–104. doi: 10.1016/j.neulet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Flood DG. Region-specific stability of dendritic extent in normal human aging and regression in Alzheimer’s disease. II. Subiculum. Brain Res. 1991;540(1–2):83–95. doi: 10.1016/0006-8993(91)90494-g. [DOI] [PubMed] [Google Scholar]
- 31.Flood DG, Buell SJ, Horwitz GJ, Coleman PD. Dendritic extent in human dentate gyrus granule cells in normal aging and senile dementia. Brain Res. 1987;402(2):205–216. doi: 10.1016/0006-8993(87)90027-8. [DOI] [PubMed] [Google Scholar]
- 32.Flood DG, Coleman PD. Dendritic regression dissociated from neuronal death but associated with partial deafferentation in aging rat supraoptic nucleus. Neurobiol Aging. 1992;14(6):575–587. doi: 10.1016/0197-4580(93)90042-a. [DOI] [PubMed] [Google Scholar]
- 33.Flood DG, Guarnaccia M, Coleman PD. Dendritic extent in human CA2-3 hippocampal pyramidal neurons in normal aging and senile dementia. Brain Res. 1987;409(1):88–96. doi: 10.1016/0006-8993(87)90744-x. [DOI] [PubMed] [Google Scholar]
- 34.Gabbott PL, Warner TA, Busby SJ. Amygdala input monosynaptically innervates parvalbumin immunoreactive local circuit neurons in rat medial prefrontal cortex. Neuroscience. 2006;139(3):1039–1048. doi: 10.1016/j.neuroscience.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 35.Galea LA, Wide JK, Paine TA, Holmes MM, Ormerod BK, Floresco SB. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behav Brain Res. 2001;126(1–2):115–126. doi: 10.1016/s0166-4328(01)00255-8. [DOI] [PubMed] [Google Scholar]
- 36.Garcia R, Vouimba RM, Baudry M, Thompson RF. The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature. 1999;402(6759):294–296. doi: 10.1038/46286. [DOI] [PubMed] [Google Scholar]
- 37.Glaser EM, Van der Loos H. Analysis of thick brain sections by obverse-reverse computer microscopy: application of a new, high clarity Golgi-Nissl stain. J Neurosci Methods. 1981;4(2):117–125. doi: 10.1016/0165-0270(81)90045-5. [DOI] [PubMed] [Google Scholar]
- 38.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 39.Gould TJ, Feiro OR. Age-related deficits in the retention of memories for cued fear conditioning are reversed by galantamine treatment. Behav Brain Res. 2005;165(2):160–171. doi: 10.1016/j.bbr.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 40.Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E. Preservation of limbic and paralimbic structures in aging. Hum Brain Mapp. 2005;25(4):391–401. doi: 10.1002/hbm.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grill JD, Riddle DR. Age-related and laminar-specific dendritic changes in the medial frontal cortex of the rat. Brain Res. 2002;937(1–2):8–21. doi: 10.1016/s0006-8993(02)02457-5. [DOI] [PubMed] [Google Scholar]
- 42.Gu G, Cornea A, Simerly RB. Sexual differentiation of projections from the principal nucleus of the bed nuclei of the stria terminalis. J Comp Neurol. 2003;460(4):542–562. doi: 10.1002/cne.10677. [DOI] [PubMed] [Google Scholar]
- 43.Guillamón A, Segovia S. Sex differences in the vomeronasal system. Brain Res Bull. 1997;44(4):377–382. doi: 10.1016/s0361-9230(97)00217-7. [DOI] [PubMed] [Google Scholar]
- 44.Hanks SD, Flood DG. Region-specific stability of dendritic extent in normal human aging and regression in Alzheimer’s disease. I. CA1 of hippocampus. Brain Res. 1991;540(1–2):63–82. doi: 10.1016/0006-8993(91)90493-f. [DOI] [PubMed] [Google Scholar]
- 45.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20(2):78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 46.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24(3):151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Hines M, Allen LS, Gorski RA. Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Res. 1992;579(2):321–326. doi: 10.1016/0006-8993(92)90068-k. [DOI] [PubMed] [Google Scholar]
- 48.Horner CH, Arbuthnott E. Methods of estimation of spine density-are spines evenly distributed throughout the dendritic field? J Anat. 1991;177:179–184. [PMC free article] [PubMed] [Google Scholar]
- 49.Iidaka T, Okada T, Murata T, Omori M, Kosaka H, Sadato N, Yonekura Y. Age-related differences in the medial temporal lobe responses to emotional faces as revealed by fMRI. Hippocampus. 2002;12(3):352–362. doi: 10.1002/hipo.1113. [DOI] [PubMed] [Google Scholar]
- 50.Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;22(4):581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- 51.Juraska JM, Rubinow MJ. Hormones and memory. In: Byrne J, editor; Eichenbaum H, editor. Learning and Memory: A Comprehensive Reference. 3. Memory Systems. London, Elsevier: Elsevier; In press. [Google Scholar]
- 52.Kilpatrick L, Zald DH, Parvo JV, Cahill LF. Sex-related differences in amygdala functional connectivity during resting conditions. Neuroimage. 2006;30(2):452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 53.LaBar KS, Cook CA, Torpey DC, Welsh-Bohmer KA. Impact of healthy aging on awareness and fear conditioning. Behav Neurosci. 2004;118(5):905–915. doi: 10.1037/0735-7044.118.5.905. [DOI] [PubMed] [Google Scholar]
- 54.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23(4–5):727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leigland LA, Schulz LE, Janowsky JS. Age related changes in emotional memory. Neurobiol Aging. 2004;25(8):1117–1124. doi: 10.1016/j.neurobiolaging.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 56.Likhtik E, Pelletier JG, Paz R, Paré D. Prefrontal control of the amygdala. J Neurosci. 2005;25(32):7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luine V. Sex differences in chronic stress effects on memory in rats. Stress. 2002;5(3):205–216. doi: 10.1080/1025389021000010549. [DOI] [PubMed] [Google Scholar]
- 58.Maren S. Long-term potentiation in the amygdala: a mechanism for emotional learning and memory. Trends Neurosci. 1999;22(12):561–567. doi: 10.1016/s0166-2236(99)01465-4. [DOI] [PubMed] [Google Scholar]
- 59.Maren S, De Oca B, Fanselow MS. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res. 1994;661(1–2):25–34. doi: 10.1016/0006-8993(94)91176-2. [DOI] [PubMed] [Google Scholar]
- 60.Markham JA, Juraska JM. Aging and sex influence the anatomy of the rat anterior cingulate cortex. Neurobiol Aging. 2002;23(4):579–588. doi: 10.1016/s0197-4580(02)00004-0. [DOI] [PubMed] [Google Scholar]
- 61.Markham JA, McKian KP, Stroup TS, Juraska JM. Sexually dimorphic aging of dendritic morphology in CA1 of hippocampus. Hippocampus. 2005;15(1):97–103. doi: 10.1002/hipo.20034. [DOI] [PubMed] [Google Scholar]
- 62.Mather M, Canli T, English T, Whitfield S, Wais P, Ochsner K, Gabrieli JD, Carstensen LL. Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychol Sci. 2004;15(4):259–263. doi: 10.1111/j.0956-7976.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- 63.McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55(3):257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 64.McDonald AJ. Neurons of the lateral and basolateral amygdaloid nuclei: a Golgi study in the rat. J Comp Neurol. 1982;212(3):293–312. doi: 10.1002/cne.902120307. [DOI] [PubMed] [Google Scholar]
- 65.McDonald AJ, Mascagni F, Muller JF. Immunocytological localization of GABABR1 receptor subunits in the basolateral amygdala. Brain Res. 2004;1018(2):147–158. doi: 10.1016/j.brainres.2004.05.053. [DOI] [PubMed] [Google Scholar]
- 66.McIntyre CK, Power AE, Roozendaal B, McGaugh JL. Role of the basolateral amygdala in memory consolidation. Ann N Y Acad Sci. 2003;985:273–293. doi: 10.1111/j.1749-6632.2003.tb07088.x. [DOI] [PubMed] [Google Scholar]
- 67.Mervis RF, Pope D, Lewis R, Dvorak RM, Williams LR. Exogenous nerve growth factor reverses age-related structural changes in neocortical neurons in the aging rat. A quantitative Golgi study. Ann N Y Acad Sci. 1991;640:95–101. doi: 10.1111/j.1749-6632.1991.tb00198.x. [DOI] [PubMed] [Google Scholar]
- 68.Miller DB, O’Callaghan JP. Aging, stress, and the hippocampus. Ageing Res Rev. 2005;4(2):123–140. doi: 10.1016/j.arr.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 69.Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2005;102(26):9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mu Q, Xie J, Wen Z, Weng Y, Shuyun Z. A quantitative MR study of the hippocampal formation, the amygdala, and the temporal horn of the lateral ventricle in healthy subjects 40 to 90 years of age. AJNR Am J Neuroradiol. 1999;20(2):207–211. [PMC free article] [PubMed] [Google Scholar]
- 71.Muller JF, Mascagni F, McDonald AJ. Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin-immunoreactive interneurons. J Comp Neurol. 2006;494(4):635–650. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murphy DG, DeCarli C, McIntosh AR, Daly E, Mentis MJ, Pietrini P, Szczepanik J, Schapiro MB, Grady CL, Horwitz B, Rapoport SI. Sex differences in human brain morphometry and metabolism: an in vivo magnetic resonance imaging and positron emission tomography study on the effect of aging. Arch Gen Psychiatry. 1996;53(7):585–594. doi: 10.1001/archpsyc.1996.01830070031007. [DOI] [PubMed] [Google Scholar]
- 73.Oler JA, Markus EJ. Age-related deficits on the radial maze and in fear conditioning: hippocampal processing and consolidation. Hippocampus. 1998;8(4):402–415. doi: 10.1002/(SICI)1098-1063(1998)8:4<402::AID-HIPO8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 74.Ouda L, Nwabueze-Ogbo FC, Druga R, Syka J. NADPH-diaphorase-positive neurons in the auditory cortex of young and old rats. Neuroreport. 2003;14(3):363–366. doi: 10.1097/00001756-200303030-00013. [DOI] [PubMed] [Google Scholar]
- 75.Paré D. Role of the basolateral amygdala in memory consolidation. Prog Neurobiol. 2003;70(5):409–420. doi: 10.1016/s0301-0082(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 76.Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 77.Pryce CR, Lehmann J, Feldon J. Effect of sex on fear conditioning is similar for context and discrete CS in Wistar, Lewis and Fischer rat strains. Pharmacol Biochem Behav. 1999;64(4):753–759. doi: 10.1016/s0091-3057(99)00147-1. [DOI] [PubMed] [Google Scholar]
- 78.Pyapali GK, Turner DA. Increased dendritic extent in hippocampal CA1 neurons from aged F344 rats. Neurobiol Aging. 1996;17(4):601–611. doi: 10.1016/0197-4580(96)00034-6. [DOI] [PubMed] [Google Scholar]
- 79.Rasia-Filho AA, Fabian C, Rigoti KM, Achaval M. Influence of sex, estrous cycle and motherhood on dendritic spine density in the rat medial amygdala revealed by the Golgi method. Neuroscience. 2004;126(4):839–847. doi: 10.1016/j.neuroscience.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 80.Roozendaal B. Systems mediating acute glucocorticoid effects on memory consolidation and retrieval. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(8):1213–1223. doi: 10.1016/j.pnpbp.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 81.Roozendaal B, Griffith QK, Buranday J, De Quervain DJ, McGaugh JL. The hippocampus mediates glucocorticoid-induced impairment of spatial memory retrieval: dependence on the basolateral amygdala. Proc Natl Acad Sci U S A. 2003;100(3):1328–1333. doi: 10.1073/pnas.0337480100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23(35):11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rowe WB, Kar S, Meaney MJ, Quirion R. Neurotensin receptor levels as a function of brain aging and cognitive performance in the Morris water maze task in the rat. Peptides. 2006;27(10):2415–2423. doi: 10.1016/j.peptides.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 84.Rubinow MJ, Arseneau LM, Beverly JL, Juraska JM. Effect of the estrous cycle on water maze acquisition depends on the temperature of the water. Behav Neurosci. 2004;118(4):863–868. doi: 10.1037/0735-7044.118.4.863. [DOI] [PubMed] [Google Scholar]
- 85.Rubinow MJ, Hagerbaumer DA, Juraska JM. 2006 Abstract Viewer and Itinerary Planner. Atlanta, Ga: Society for Neuroscience; 2006. Influence of sex, adolescence, and aging on performance of the conditioned place preference task. Program No. 811.16. [Google Scholar]
- 86.Schroeder JP, Packard MG. Posttraining intra-basolateral amygdala scopolamine impairs food- and amphetamine-induced conditioned place preferences. Behav Neurosci. 2002;116(5):922–927. doi: 10.1037//0735-7044.116.5.922. [DOI] [PubMed] [Google Scholar]
- 87.Schwartz NB. The role of FSH and LH and their antibodies on follicle growth and on ovulation. Biol Reprod. 1974;10(2):236–272. doi: 10.1095/biolreprod10.2.236. [DOI] [PubMed] [Google Scholar]
- 88.Sholl DA. The organization of the cerebral cortex. London: Methuen; 1956. [Google Scholar]
- 89.Sotres-Bayon F, Bush DEA, LeDoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem. 2004;11(5):525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- 90.Stemmelin J, Cassel JC, Kelche C. Morphological alterations in the occipital cortex of aged rats with impaired memory: a Golgi-Cox study. Exp Brain Res. 2003;151(3):380–386. doi: 10.1007/s00221-003-1510-9. [DOI] [PubMed] [Google Scholar]
- 91.Stoehr JD, Wenk GL. Effects of age and lesions of the nucleus basalis on contextual fear conditioning. Psychobiology. 1995;23(3):173–177. [Google Scholar]
- 92.Tessitore A, Hariri AR, Fera F, Smith WG, Das S, Weinberger DR, Mattay VS. Functional changes in the activity of brain regions underlying emotion processing in the elderly. Psychiatry Res. 2005;139(1):9–18. doi: 10.1016/j.pscychresns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 93.Van der Loos H. Une combination de deux vielles methods histologiques pour le systeme nerveux central. Monatsschr Psychiatr Neurol. 1956;132(5–6):330–334. [PubMed] [Google Scholar]
- 94.Vinader-Caerols C, Collado P, Segovia S, Guillamón A. Sex differences in the posteromedial cortical nucleus of the amygdala in the rat. Neuroreport. 1998;9(11):2653–2656. doi: 10.1097/00001756-199808030-00042. [DOI] [PubMed] [Google Scholar]
- 95.von Bohlen und Halbach O, Unsicker K. Age-related decline in the tyrosine hydroxylase immunoreactive innervation of the amygdala and dentate gyrus in mice. Cell Tissue Res. 2003;311(2):139–143. doi: 10.1007/s00441-002-0662-4. [DOI] [PubMed] [Google Scholar]
- 96.von Bohlen und Halbach O, Unsicker K. Morphological alterations in the amygdala and hippocampus of mice during ageing. Eur J Neurosci. 2002;16(12):2434–2440. doi: 10.1046/j.1460-9568.2002.02405.x. [DOI] [PubMed] [Google Scholar]
- 97.Vouimba RM, Munoz C, Diamond DM. Differential effects of predator stress and the antidepressant tianeptine on physiological plasticity in the hippocampus and basolateral amygdala. Stress. 2006;9(1):29–40. doi: 10.1080/10253890600610973. [DOI] [PubMed] [Google Scholar]
- 98.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26(9):1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 100.Warren SG, Humphreys AG, Juraska JM, Greenough WT. LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Res. 1995;703(1–2):26–30. doi: 10.1016/0006-8993(95)01059-9. [DOI] [PubMed] [Google Scholar]
- 101.Williams LM, Brown KJ, Palmer D, Liddell BJ, Kemp AH, Olivieri G, Peduto A, Gordon E. The mellow years?: neural basis of improving emotional stability over age. J Neurosci. 2006;26(24):6422–6430. doi: 10.1523/JNEUROSCI.0022-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wilson MA, Mascagni F, McDonald AJ. Sex differences in delta opioid receptor immunoreactivity in rat medial amygdala. Neurosci Lett. 2002;328(2):160–164. doi: 10.1016/s0304-3940(02)00481-0. [DOI] [PubMed] [Google Scholar]
- 103.Womble MD, Andrew JA, Crook JJ. 17β-Estradiol reduces excitatory postsynaptic potential (EPSP) amplitude in rat basolateral amygdala neurons. Neurosci Lett. 2002;331(2):83–86. doi: 10.1016/s0304-3940(02)00871-6. [DOI] [PubMed] [Google Scholar]
- 104.Wood GE, Beylin AV, Shors TJ. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behav Neurosci. 2001;115(1):175–187. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- 105.Works SJ, Wilson RE, Wellman CL. Age-dependent effect of cholinergic lesion on dendritic morphology in rat frontal cortex. Neurobiol Aging. 2004;25(7):963–974. doi: 10.1016/j.neurobiolaging.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 106.Xiao C, Sartin J, Mulchahey JJ, Segar T, Sheriff S, Herman JP, Kasckow JW. Aging associated changes in amygdalar corticotropin-releasing hormone (CRH) and CRH-binding protein in Fischer 344 rats. Brain Res. 2006;1073–1074:325–331. doi: 10.1016/j.brainres.2005.12.063. [DOI] [PubMed] [Google Scholar]
- 107.Yang Y, Cao J, Xiong W, Zhang J, Zhou Q, Wei H, Liang C, Deng J, Li T, Yang S, Xu L. Both stress experience and age determine the impairment or enhancement effect of stress on spatial memory retrieval. J Endocrinol. 2003;178(1):45–54. doi: 10.1677/joe.0.1780045. [DOI] [PubMed] [Google Scholar]
- 108.Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev. 2003;41(1):88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]


