Abstract
The rat basolateral nucleus of the amygdala continues to develop connectivity with the frontal cortex through the periadolescent period and even into young adulthood. Although neuronal loss in the prefrontal cortex has been found during the periadolescent period, prior literature has not examined whether neuron number in the basolateral amygdala is stable through this period. In addition, aging of the rat basolateral nucleus is accompanied by significant increases in the dendritic tree of its principal neurons, but whether this occurs in the context of neuronal death has not been previously explored. In the present study, a stereological examination of neuron and glia numbers in the rat basolateral amygdalar nucleus was undertaken in male and female hooded rats at one of four ages across the lifespan. Our findings indicate (1) a significant decrease in the number of neurons and glia in the basolateral nucleus between adolescence and adulthood, and (2) the number of glia, as well as the volume of the basolateral nucleus, increase between adulthood and old age, while neuron number remains stable. These findings provide an important cellular context for interpretation of the neurochemical and other alterations documented in developmental and age-related literature on the rat basolateral amygdala, and underline the substantial development of this brain area during adolescence, as well as its comparative preservation during aging.
Keywords: basolateral amygdala, stereology, development, adolescence, aging, sex differences
Introduction
Traditional views that neuroanatomical development is largely organized in the perinatal period have been updated in light of the overwhelming evidence that the periadolescent period is accompanied by major alterations in brain organization in humans (Swaab et al., 1995; Giedd et al., 1999; Sowell et al., 1999, 2002; De Bellis et al., 2001; Giedd, 2004) and other animals (Andersen et al., 1997, 2000; Spear, 2000; Nunez et al., 2002; Cunningham et al., 2002, 2007; Koshibu et al., 2004). This late wave of developmental events may follow sex-specific patterns (Giedd, 2004), including the first appearance of sex differences in neuron number, in many reproductive and ‘cognitive’ brain areas (Swaab et al., 1995; Chung et al., 2002; Markham et al., 2007). Some of these dimorphisms have been directly linked to the appearance of pubertal hormones (reviewed in Sisk and Zehr, 2005); in rat visual cortex, for example, the adult sex difference in neuron number is abolished by removal of the ovaries prior to puberty onset (Nunez et al., 2002).
The amygdala is one of the brain regions noted to undergo late developmental changes in both humans and other animals. In rats, the adolescent period stretches roughly from postnatal day 28 (P28) to P42 (Spear, 2000). Anatomical development has been reported during adolescence in different subnuclei of the rodent amygdala (Zehr et al., 2006; Cunningham et al., 2007), including the basolateral nucleus (Cunningham et al., 2002, 2007), which is noted for its cortical-like cytology and patterns of connectivity (Carlsen and Heimer, 1988). Late developmental changes observed in the basolateral nucleus (BLN) of the rat include increasing cholinergic innervation, indexed by acetylcholinesterase activity in BLN neuropil, which does not reach adult levels until P60 (Berdel et al., 1996), and progressive innervation by the posterior portion of the BLN to the medial prefrontal cortex (mPFC) through P65 (Cunningham et al., 2002). In mice, whole amygdalar volume increases by more than 50% between P30 (peripubertal) and P90, while overall brain volume only increases by 7% (Koshibu et al., 2004). A similar pattern is seen in humans: amygdalar volume increases between childhood and adulthood (Giedd et al., 1996,1997; Merke et al., 2003), while cerebral volume significantly decreases (Schumann et al., 2004; Lenroot and Giedd, 2006). Perhaps related to these developmental changes is the increased vulnerability to a number of psychopathologies during the adolescent period, including schizophrenia and depressive illness, which feature prominent affective problems (Spear, 2000; Rutter et al., 2003; Adriani and Laviola, 2004). These and other psychopathologies, including autism and post-traumatic stress disorder, have been linked to abnormalities in amygdalar-prefrontal function (Drevets, 1999; Grady and Keightley, 2002). Prior work from this laboratory reported significant decreases in neuron number in the ventral mPFC between adolescence and adulthood (Markham et al., 2007). One question addressed by the current study is whether the BLN, which does not establish adult levels of projections to the mPFC until early adulthood (Cunningham et al., 2002), is concomitantly undergoing changes in the number of neurons late in development.
Sex differences and effects of gonadal steroid hormones have been reported in anatomical measures of parts of the amygdala, including sexually dimorphic neuron number in several subnuclei associated with the vomeronasal system (Morris et al., 2008; Cooke et al., 2007; Guillamón and Segovia, 1997). The BLN, which has been the subject of far fewer studies that examined sex differences, is known to exhibit dimorphisms in spine density (Rubinow et al., 2007) and in the number of GABAergic neurons (Stefanova, 1998), but it is not known whether there are sex differences in neuron number overall. The BLN could develop dimorphisms following puberty as it contains steroid hormone receptors, although their expression is comparatively low in relation to other amygdalar subnuclei (Laflamme et al., 1998). In humans, functional imaging studies demonstrate striking sex differences in amygdalar hemispheric activation during tasks likely to involve the BLN (Cahill, 2003); however, to our knowledge sex differences in BLN anatomy have not previously been examined by hemisphere. Thus the present study examined volume, neuron and glia number in both hemispheres to determine whether there are sex by hemisphere interactions in these measures.
Most anatomical studies of the aging human amygdala are volumetric, and have provided mixed evidence including relative amygdalar preservation (Good et al., 2001; Jernigan et al., 2001; Grieve et al., 2005) and volumetric decreases (Coffey et al., 1992; Murphy et al., 1996; Mu et al., 1999; Walhovd et al., 2005). In rodents, we recently reported age-related increases in the dendritic tree of principal neurons in the rat BLN (Rubinow et al., 2007). Age-related changes in measures of catecholaminergic innervation and CRF-binding protein have also been reported in the basolateral amygdala of mice and rats respectively (Pisarska et al., 2000; von Bohlen und Halbach and Unsicker, 2002). However, it is not clear whether these alterations occur in the context of neuronal loss. Therefore, the present experiment was designed to provide a systematic study of age-related, as well as late-developmental, changes in volume and number of neurons in the BLN of rats of both sexes. Neuron and glia number were stereologically quantified in the basolateral nucleus in male and female rats sacrificed at one of four time points, corresponding to (1) pre-weaning/pre-puberty, (2) peri-puberty, (3) adulthood, and (4) old age.
Methods
Subjects
Subjects were Long-Evans rats, descended from stock originally obtained from Simonsen Laboratories (Gilroy, CA). There were 8 groups: males and females sacrificed at day 20 (preweaning/prepubertal; n = 8 males, 7 females), day 35 (peripubertal; 8 males, 7 females), day 90 (young adulthood; 8 males, 8 females), and old age (19-22 months; 6 males, 6 females). The rats were bred in the animal colony of the Psychology Department and weaned at day 25. They were housed with littermates in same-sex pairs, and maintained on a 12:12 light/dark cycle, with free access to rat chow and water. Aged rats were retired breeders. All the rats were handled weekly, to minimize stress effects of handling at sacrifice. All procedures were approved by the University of Illinois Institutional Animal Care and Use Committee. Cortical tissue from a subset of these subjects was utilized in two previous studies (Markham et al., 2007; Yates et al., 2008).
Histology
The rats were deeply anaesthetized with sodium pentobarbital and transcardially perfused with Ringer’s solution and 4% paraformaldehyde in phosphate-buffered saline for one week, at which time the brains were weighed and coded to avoid experimenter bias. The brains were then stored in 30% sucrose in fixative (4% paraformaldehyde) for 2-3 days.
The tissue was frozen and 60 μm thick coronal sections were taken on a sliding microtome. Small punches were made in the left hemisphere of all subjects so that hemispheric differences could be assessed. Every fourth section was mounted and stained with methylene blue/azure II for examination of BLN volume and neuron number. For a subset of animals, distributed across all groups, every fifth section was mounted and stained.
Quantification
The number of neurons and glia in the BLN were stereologically calculated in two steps as performed in previous studies from our laboratory (Reid and Juraska, 1992; Nunez et al., 2002; Markham et al., 2007). The BLN was parcellated and traced at 31.25X from each mounted section through the basolateral amygdala, using a camera lucida attachment to a light microscope. Parcellations were made throughout the rostrocaudal extent of the BLN according to cytoarchitectonic criteria differentiating the BLN from the dorsally situated lateral nucleus and from surrounding tissue (de Olmos et al., 2004). Rostral-to-caudal, the BLN shape and size changes markedly. The BLN first appears as a mass of large neurons medially adjacent to the external capsule and immediately caudal to the interstitial nucleus of the posterior limb of the anterior commissure. The rostral BLN is easily differentiated from the dorsally situated lateral nucleus of the amygdala, which is characterized by smaller, more densely packed neurons (Figure 1a). More caudally, the smaller neurons of the posterior or parvicellular BLN appear along the lateral boundary of the BLN, while the anterior or magnocellular BLN abuts the parvicellular BLN medially until it disappears entirely (Krettek and Price, 1978) (Figure 1b). To obtain volume, serial tracings of all mounted sections were scanned into a computer and the ImageJ software program was used to calculate the areas of all sections. Post-processing thickness was measured for each subject under high magnification during density measurements. Areas were multiplied by the distance between sections, determined from post-processing thickness and interval between mounted sections, to determine volume.
Figure 1.
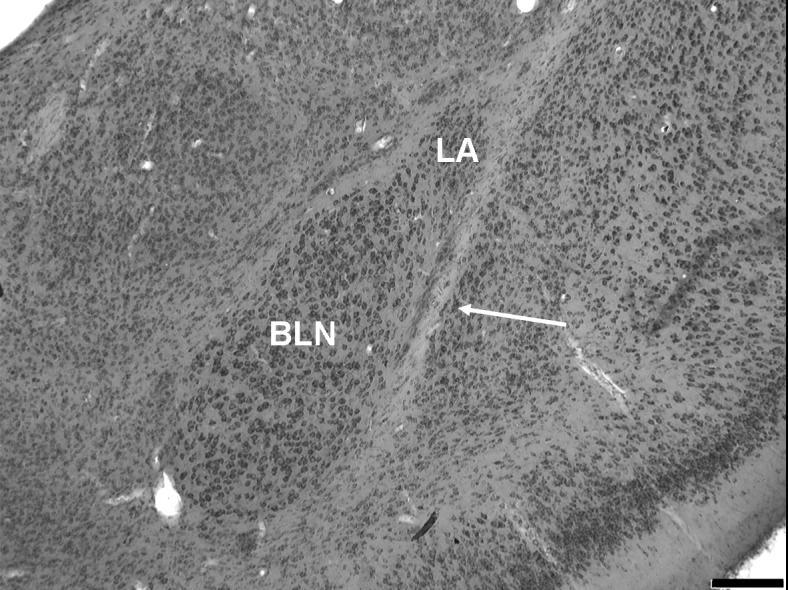
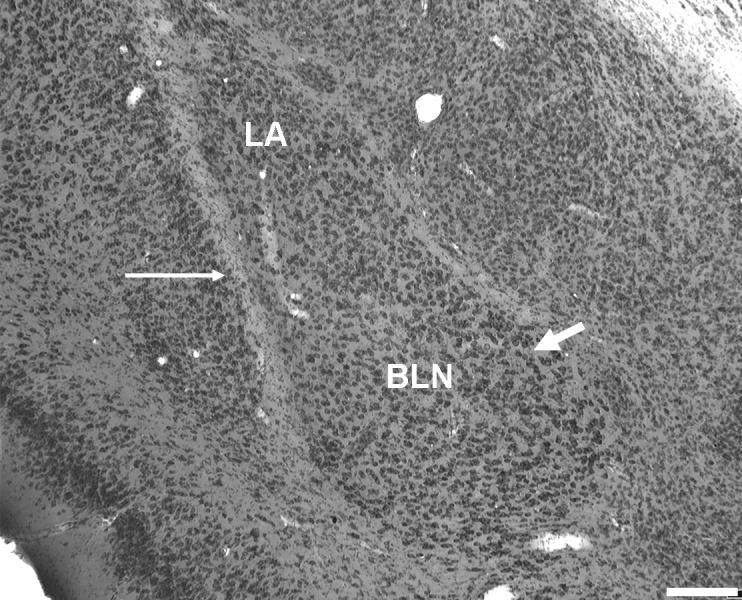
(a) Rostral-level photograph of the basolateral nucleus (BLN). The large neurons of the rostral BLN are readily distinguished from the small, densely packed neurons of the dorsally adjacent lateral nucleus in Nissl (methylene blue/azureII) sections. (b) The BLN at mid-level. The magnocellular division is still visible along the medial boundary (thick arrow). Thin arrows: external capsule. LA, lateral nucleus of the amygdala. Scale bars = 200 μm.
Neuronal and glia densities were determined using the optical disector, an unbiased stereological technique (Sterio, 1984). The StereoInvestigator (v.7.53.1, MicroBrightField Bioscience) program was used to systematically randomly sample the tissue to obtain neuron and glia counts. A counting frame of 35 × 35 μm, height 15 μm, containing the appropriate inclusion and exclusion lines, was used to sample cells. A step size of 185 μm (x,y) was used on all sections. Four to seven randomly determined coronal sections from both hemispheres were used for quantification, with 100-300 neurons (average 170) counted in the BLN of each subject.
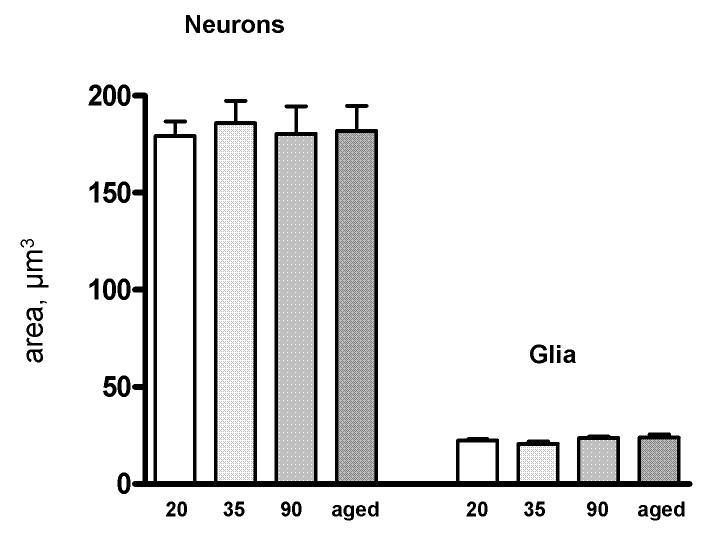
An upper guard zone of 2.5 μm was applied to prevent biased counts due to cellular irregularities at the cutting site (Mouton, 2002). Cells were counted only if their tops first came into focus within the 15 μm height of the counting frame. Glia and neurons were distinguished on the basis of several features: neurons can be distinguished by the clear presence of the nucleolus and by dendritic and axonal processes, both absent in glial cells; under Methylene Blue/Azure II staining, neurons and glia stain notably different shades of blue; and neurons tend to be much larger than glia, particularly in a magnocellular region such as the BLN. Figure 2 shows a high-magnification photo of BLN cells under the 63x oil objective used for cell counts. Figures 1 and 2 were taken in grayscale with an Optronincs MicroFire Progressive Scan CCD camera on a Zeiss AxioImager A1 light microscope and were not manipulated except for the addition of text markers. The validity of cell size as one of the distinguishing criteria was verified by areal measures of neurons and glia sampled in four subjects per age group using the Isotropic Nucleator (see Figure 3 and Results, below).
Figure 2.
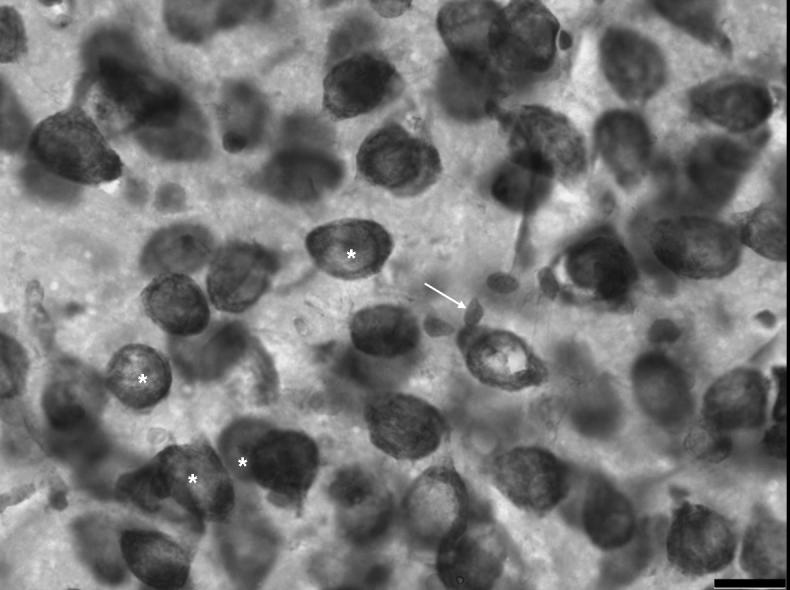
A high magnification photo of cells of the basolateral nucleus stained with methylene blue/azureII. Arrow indicates a glial cell. Asterisks are placed directly below examples of neuronal nucleoli. Scale bar = 16 μm.
Figure 3.
Average neuronal and glial cell areas across groups. The data are shown collapsed across sex. Error bars are +/- standard errors of the mean.
Density was calculated as the number of cells of each type counted over the volume of the total counting frames per subject. Tissue height used in calculating reference volume was measured at every fourth sampling site. Neuron and glia numbers were calculated by multiplying density by reference volume for each subject (Mouton, 2002).
Statistical Calculations
Coefficient of error (CE) was calculated for neurons and glia in all groups according to Howard and Reed (1998). CEs were less than 0.10 for all subjects, with average CE of .079 for neurons and .056 for glia (Table 1).
Table 1.
Average coefficient of error (CE) for neuron and glia counts by age group1
| Neuron CE (M,F) | Glia CE (M,F) | |
|---|---|---|
| day 20 | 0.071 (0.073, 0.070) | 0.052 (0.050,0.054) |
| day 35 | 0.084 (0.088, 0.078) | 0.054 (0.057,0.050) |
| day 90 | 0.081 (0.082, 0.081) | 0.060 (0.062,0.058) |
| 19-22 months | 0.080 (0.075, 0.085) | 0.059 (0.053,0.065) |
Breakdown by sex is given in parentheses.
Separate analyses were run for the development groups (days 20, 35 and 90) and the aged comparison (day 90 and 19-22 months) for two reasons. One is that litter of origin was not available for the aged group, and more importantly, potential statistical differences between the developmental and aged groups are not meaningful. Group differences in neuron number, volume, and number of glia were analyzed with mixed ANOVAs using hemisphere as a repeated measure and age, sex and litter (development groups) as independent factors. Post-hoc comparisons were made with 2-way ANOVAs in the developmental groups to compare any effects of age while keeping litter in the analysis.
Results
(1) Neuron and glial areal measurements
Areal measurements of neuronal and glial size, sampled in two males and two females per age group in order to verify the perceptible size differences used as one of several criteria to distinguish between the two cell types, were analyzed. Neuronal and glial areas were markedly different (P<.000001), and the differences were constant across sex and age group (Figure 3).
(2) Development
Volume
The volume of the BLN significantly changed with age (P=0.018). Post-hoc comparisons revealed a significant volumetric increase between day 20 and day 35 (P=0.029) but no change between days 35 and 90 (Figure 4a). Sex did not influence the volume of the BLN or interact with age. There were no hemispheric differences in volume and no interactions between hemisphere and sex or age.
Figure 4.
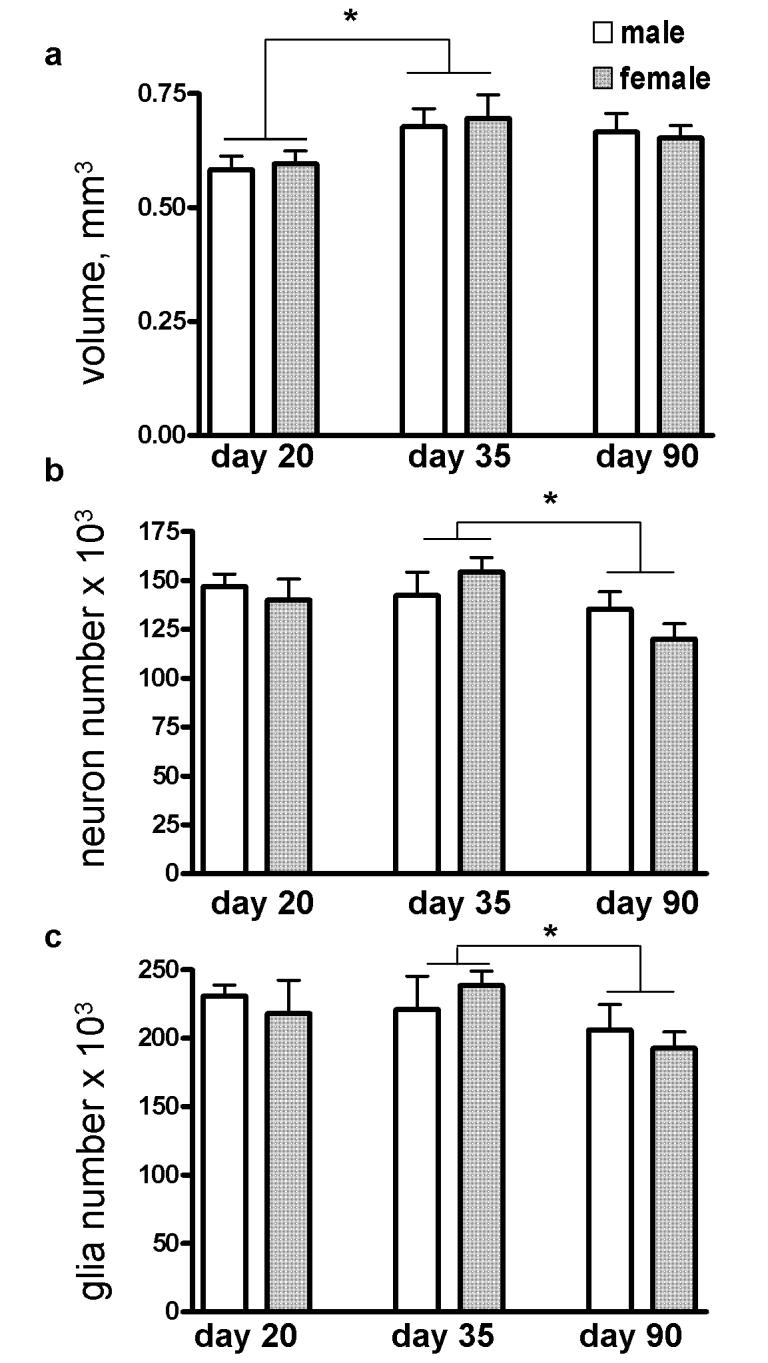
(a) The volume of the basolateral nucleus increased significantly between days 20 and 35. Both neuron number (b) and glia number (c) significantly decreased between days 35 and 90. Error bars are +/- standard errors of the mean. *P<0.03.
Neuron Number
Neuron number significantly changed with age ( P=0.010) (Figure 4b). Post-hoc comparison of neuron number between days 35 and 90 showed a decrease of 13% (P=0.007). Neuron number was unchanged between days 20 and 35. There were no effects of sex nor an interaction between sex and age on neuron number. There were no hemispheric differences in neuron number and no interactions between hemisphere and sex or age.
Number of Glia
The number of glia significantly changed with age (P=0.010). Glia number decreased by 13% (P=0.007) between days 35 and 90, but was unchanged between days 20 and 35 (Figure 4c). There was no effect of sex nor an interaction between sex and age on the number of glia. There were no hemispheric differences in glia number or interactions of hemisphere with sex or age.
(3) Aging
Volume
BLN volume significantly increased by 15% with age (P=0.002; Figure 5a). Sex did not influence volume or interact with age. There were no hemispheric differences or interactions of hemisphere with sex or age.
Figure 5.
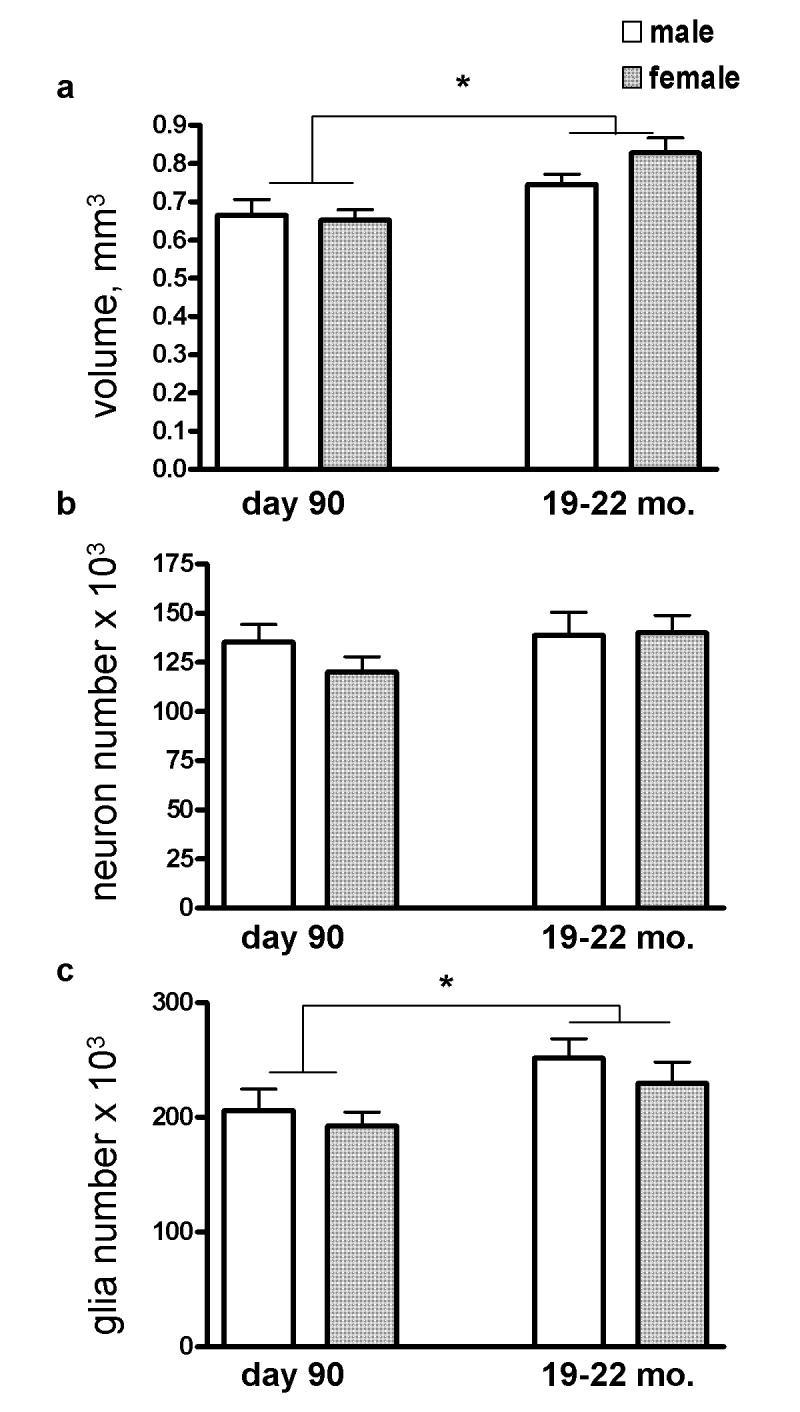
(a) The volume of the basolateral nucleus increased significantly between day 90 and 19-22 months. (b) Neuron number was unchanged. (c) Glia number increased significantly between day 90 and 19-22 months. Day 90 groups are the same as in Figure 4. Error bars are +/- standard errors of the mean. *P<0.02.
Neuron Number
Neither age nor sex significantly influenced neuron number, and there was no interaction (Figure 5b). There were no hemispheric differences or interactions of hemisphere with sex or age.
Number of Glia
The number of glia increased significantly by 18% with age (P=0.013; Figure 5c). There was no effect of sex on glia number and no interaction of sex with age. There were no hemispheric differences or interactions of hemisphere with sex or age.
Discussion
This is the first stereological study to examine whether neuron and glia numbers in the rat basolateral nucleus change across adolescence or between adulthood and old age. Evidence of neuron loss was found in the BLN of both sexes post-puberty, between days 35 and 90. Glia number also decreased at this time. In contrast, neuron number was stable into old age and glia number was higher in the aged.
Development
Neuron number decreased by 13% in the BLN between days 35 and 90. Previous findings from this laboratory indicate that neuron number decreases overall during the same period in the ventral (prelimbic and infralimbic; PL and IL) medial prefrontal cortex, although the changes were larger in females than in males (Markham et al., 2007). The PL and IL are specific projection targets of the posterior division of the BLN (Krettek and Price, 1977), and the projections from the posterior BLN to the IL increase through day 65 or later in rats (Cunningham et al., 2002). Taken together, these findings add the basic measure of neuron number to other evidence that the BLN and prefrontal cortex may be interrelated in their late development. GABAergic neurons of the mPFC represent a major target of new BLN efferents (Cunningham et al., 2007), as they are in adulthood (Gabbott et al., 2006). The current study did not address the phenotype of neurons lost between adolescence and adulthood. Future studies distinguishing principal neurons, which are glutamatergic and represent the projection neurons of the BLN (McDonald et al., 2004), from interneurons are needed to delineate whether either cell type is particularly vulnerable, and if the latter, interneuron phenotype may be distinguished.
Glial cell number also decreased by 13% between days 35 and 90. We did not distinguish among glial cell subtypes in this first analysis of BLN cell numbers across the lifespan of male and female rats. Thus astrocytes, oligodendrocytes, as well as microglia are expected to be represented in the glia numbers quantified, although the latter would be expected to make the smallest contribution (Grady et al., 03; Hamidi et al., 04). Astrocytes, and subsets of oligodendrocytes, contribute to synaptic functioning (He and Sun, 2007; van Landeghem et al., 2007), suggesting the co-occurring loss of neurons and glia might reflect concomitant synapse losses; a loss of oligodendrocytes might also reflect loss of myelination in local and/or projecting fibers, although further speculation on the functional significance of glial cell loss between adolescence and adulthood will require future work distinguishing among glial cell subtypes.
The late development of both the BLN and mPFC has significant implications for emotional aspects of cognitive development (Drevets, 1999; Likhtik et al., 2005). For example, human studies indicate a greater amygdalar response to emotional stimuli in adolescents versus adults, who show a greater frontal cortical response (Killgore et al., 2001; Monk et al., 2003), suggesting greater prefrontal control over emotional response in adulthood (Monk et al., 2003). Furthermore, the late establishment of BLN-mPFC circuitry is thought to contribute to the adolescent vulnerability to the onset of affective and psychotic disorders (Cunningham et al., 2007). The late development of the BLN, seen here in neuronal decreases following puberty, and in other studies of connectivity with the mPFC (Cunningham et al., 2002), altered expression of GABAergic neuron populations (Berdel and Morys, 2000), and increasing cholinergic innervation through day 60 (Berdel et al., 1996) may have implications for the development of cognitive abilities which rely on amygdala function (Rubinow et al., unpublished data).
One difference between the neuron losses late in development in the mPFC and the BLN is that in the former, neuron losses occur in context of volumetric decreases (Markham et al., 2007). However in the case of the BLN, volume does not decrease concomitantly, and number of glia cells is decreasing. A dissociation between volume and cell numbers has also been seen in the posterodorsal portion of the medial amygdala (Cooke et al., 2007; Morris et al., 2008). Thus, it appears that the neuropil and/or soma size may be increasing. One previous study examining neuronal soma of the rat BLN found no significant changes in soma size beyond postnatal day 14 (Berdel et al., 1997), and a second study reported decreases in size of BLN somata between days 20 and 40 (Escobar and Salas, 1993). While it is not known whether dendritic material in the rat increases between days 20 and 90, our previous finding that dendritic material increases in the BLN in old age (Rubinow et al., 2007) indicates that adulthood represents a period of plasticity for BLN neuropil. Future studies examining dendritic material in the BLN between puberty and adulthood will be important in addressing the question of the basis for volumetric stasis in the context of significant losses in number of neurons during this period.
The developmental trajectory of volumetric changes in both the BLN and the mPFC (Markham et al., 2007) follows the direction seen in humans: frontal cortical volume decreases (Giedd, 2004), and amygdalar volume increases (Giedd et al., 1996, 1997; Merke et al., 2003; Schumann et al., 2004) in adolescence. While it is not known whether these volumetric changes in the human brain are accompanied by decreases in neuron number as they are in the rat, it is noteworthy that in rats the same neuronal changes are associated with opposite volumetric changes in the BLN versus the mPFC. This dissociation underlines the importance of caution in interpreting volumetric data in the absence of information on underlying cellular alterations.
Sex differences
No sex differences were found in the volume or number of neurons in the BLN, and sex did not interact with hemisphere. This is in contrast with much of the amygdala which is known to exhibit sexual dimorphisms and anatomical changes in response to manipulations of gonadal steroids. The large majority of studies in this area have focused on the reproductively important nuclei closely associated with the vomeronasal system. Sex differences have been reported in the bed nucleus of the accessory olfactory tract, medial amygdala (MeA), posteromedial cortical nucleus, and parts of the bed nucleus of the stria terminalis (for reviews see Madeira and Lieberman, 1995; Guillamón and Segovia, 1997), and male rats have greater numbers of neurons in subdivisions of all of these nuclei (Guillamón and Segovia, 1997; Vinader-Caerols et al., 2000; Morris et al., 2008). Much research in this area has focused on the posterodorsal division of the MeA (MeApd), which contains the highest amygdalar concentrations of estrogen receptor-alpha and -beta (Laflamme et al., 1998) and high concentrations of androgen receptors (Gréco et al., 1996). The MeApd is significantly larger in male rats than in females (Hines et al., 1992), and exhibits sexual dimorphisms in neurochemistry (de Vries and Miller, 1998), opioid receptor immunoreactivity (Wilson et al., 2002), dendritic morphology (Rasia-Filho et al., 1999, 2004; Cooke et al., 2007), and numbers of excitatory synapses per neuron (Cooke and Woolley, 2005). In addition, males have more MeApd neurons and glia than females (Morris et al., 2008), and neuron number dimorphism is present before puberty (Cooke et al., 2007). However, these sex differences are altered in some respects between pre-puberty and adulthood, for example in aspects of MeApd laterality (Cooke et al., 2007; Morris et al., 2008).
The basolateral amygdala differs from these areas in several respects. The cytology and connectivity of the BLN are cortical-like (Carlsen and Heimer, 1988), and it is not part of the vomeronasal amygdala. Nevertheless, neuron loss in the BLN occurred sometime between puberty and adulthood, suggesting the possibility of a role for gonadal hormones in both sexes. Low levels of estrogen receptor-alpha and -beta have been detected in the BLN (Laflamme et al., 1998), and estrogen has been linked to developmental CNS apoptosis. For example, removal of the ovaries at day 20 eliminates the adult sex difference in neuron number in the visual cortex, suggesting that pubertal estrogen may have an important role in organization of the female visual cortex (Nunez et al., 2002). A direct examination of gonadectomy effects on neuron number in the rat BLN is needed to determine what role if any is played by pubertal hormones in the late wave of neuronal loss observed here.
Aging
Both volume and the number of glia cells were found to increase between adulthood and old age, while neuron number was unchanged. A contributing factor to volumetric increases is likely to be the increase in dendritic material documented in the BLN between adulthood and old age (Rubinow et al., 2007). Increases in the number of glia cells may reflect an increase in the number of synapses, as astrocytes form concurrently with synapses and are believed to function in support of synapse maintenance (He and Sun, 2007). Spine density is stable while dendritic material increases with age (Rubinow et al., 2007), so that spine number and perhaps excitatory synapses are increased in the aged BLN, potentially creating the need for a greater number of glia associated with synapses.
Two caveats apply. First, litter information was not available for the aged rats. However, significant effects on volume and glia numbers were observed in spite of variability due to litter differences. Second, the aged animals were retired breeders, while the younger groups were sexually inexperienced. This points to a difficulty in aging studies of laboratory rats, in that efforts to create parallel experiences between aged and younger rats can be at odds with natural models of aging. It could be argued that it would not be natural for rats to live so long without breeding experience (or in cages with little variety). Sexually experienced young adults, on the other hand, would undergo the behavioral and hormonal aspects of breeding much closer to the time of sacrifice than would the aged rats which could be reflected in temporary neural changes. While the aged rats differed from the younger groups in terms of breeding experience, this caveat is mitigated by the fact that increases in volume and number of glia in the BLN were seen in aged rats of both sexes, who undergo quite different hormonal and behavioral experiences during breeding.
The finding that neuron number is maintained and that there are increases both in the volume of the aged BLN and in the number of glia cells underlines the differences between aging processes in the BLN what is known about aging in other cognitive brain areas. In rats, the number of neurons of the primary visual cortex, as well as its volume, undergoes marked decreases with age, and there are also neuron and volume losses in the mPFC, although these alterations are comparatively minor (Yates et al., 2008). The rat BLN exhibits age-related dendritic growth (Rubinow et al., 2007), while decreases in dendritic material have been observed in the mPFC (Markham and Juraska, 2002) and in area CA1 of the hippocampus (Markham et al., 2005). In humans, aging is associated with notable shrinkage of the prefrontal cortex and the hippocampus (Convit et al., 2001; Raz et al., 2007; Xu et al., 2007). In contrast, findings of age-related human amygdala shrinkage are mixed at best, with a variety of reports using a range of methodologies, some describing shrinkage (Murphy et al., 1996; Mu et al., 1999; Walhovd et al., 2005) and others reporting preservation of amygdalar volume in old age (Good et al., 2001; Jernigan et al., 2001; Grieve et al., 2005).
A similar contrast exists between cognitive function in the aged basolateral amygdala (BLA) compared to the aged hippocampus and prefrontal cortex. In humans, prefrontal (Resnick et al., 2007) and hippocampal (Driscoll and Sutherland, 2005) function are widely recognized for vulnerability to age-related decline. However, the literature on amygdala function in human aging is much more equivocal, with evidence for preservation (Denburg et al., 2003; Mather et al., 2004) and, particularly when negative or fearful stimuli are used, impairment (Iidaka et al., 2002; Fischer et al., 2005). The same pattern is true of studies of cognitive aging in the rat, with performance of PFC- and hippocampus-mediated tasks considered most vulnerable to aging (reviewed in Della-Maggiore et al., 2002; Burke and Barnes, 2006), but several reports of preserved performance in BLA-dependent tasks (Stoehr and Wenk, 1995; Oler and Markus, 1998; Doyere et al., 2000). Nevertheless, both morphological and physiological alterations have been described in the aged rodent BLA (Almaguer et al., 2002; von Bohlen und Halbach and Unsicker, 2002, 2003; Rubinow et al., 2007), suggesting that the BLA certainly ages on a number of measures.
The present data underline the importance of the adolescent period in basolateral amygdala development. Future studies are needed to explore the significant neuronal loss observed between the peri-pubertal period and adulthood, particularly to address questions of neuronal phenotype and gonadal steroid sensitivity. BLA plasticity in cell numbers and neuronal morphology (Rubinow et al., 2007) appears to be salient in adolescence and between adulthood and old age.
Acknowledgements
We thank Dr. Julie Markham, Dr. Melissa Yates, Tiffany Stroup and Scott Robinson for their contributions to the study.
Grant sponsor: National Institute of Aging; AG022499
Grant sponsor: National Institute on Alcohol Abuse and Alcoholism; AA017354
Grant sponsor: National Institute of Child Health and Human Development; Training grant HD07333 (MJR)
Literature Cited
- Adriani W, Laviola G. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behav Pharmacol. 2004;15:341–352. doi: 10.1097/00008877-200409000-00005. [DOI] [PubMed] [Google Scholar]
- Almaguer W, Estupinan B, Uwe Frey J, Bergado JA. Aging impairs amygdala-hippocampus interactions involved in hippocampal LTP. Neurobiol Aging. 2002;23:319–324. doi: 10.1016/s0197-4580(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. NeuroReport. 1997;8:1495–1498. doi: 10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Berdel B, Moryś J. Expression of calbindin D28k and parvalbumin during development of rat’s basolateral amygdaloid complex. Int J Dev Neurosci. 2000;18:501–513. doi: 10.1016/s0736-5748(00)00024-1. [DOI] [PubMed] [Google Scholar]
- Berdel B, Moryś J, Maciejewska B. Neuronal changes in the basolateral complex during development of the amygdala of the rat. Int J Dev Neurosci. 1997;15:755–765. doi: 10.1016/s0736-5748(97)00022-1. [DOI] [PubMed] [Google Scholar]
- Berdel B, Moryś J, Maciejewska B, Narkiewicz O. Acetylcholinesterase activity as a marker of maturation of the basolateral complex of the amygdaloid body in the rat. Intl J Dev Neurosci. 1996;14:543–549. [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the aging brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Cahill L. Sex- and hemisphere-related influences on the neurobiology of emotionally influenced memory. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1235–41. doi: 10.1016/j.pnpbp.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Carlsen J, Heimer L. The basolateral amygdaloid complex as a cortical-like structure. Brain Res. 1988;441:377–380. doi: 10.1016/0006-8993(88)91418-7. [DOI] [PubMed] [Google Scholar]
- Chung WCJ, De Vries GJ, Swaab DF. Sexual differentiation of the bed nucleus of the stria terminalis in humans may extend into adulthood. J Neurosci. 2002;22:1027–1033. doi: 10.1523/JNEUROSCI.22-03-01027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey CE, Wilkinson WE, Parashos IA, Soady SA, Sullivan RJ, Patterson LJ, Figiel GS, Webb MC, Spritzer CE, Djang WT. Quantitative cerebral anatomy of the aging human brain: a cross-sectional study using magnetic resonance imaging. Neurology. 1992;42:527–536. doi: 10.1212/wnl.42.3.527. [DOI] [PubMed] [Google Scholar]
- Convit A, Wolf OT, de Leon MJ, Patalinjug M, Kandil E, Caraos C, Scherer A, Louis LA, Cancro R. Volumetric analysis of the pre-frontal regions: findings in aging and schizophrenia. Psychiatry Res. 2001;107:61–73. doi: 10.1016/s0925-4927(01)00097-x. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Stokas MR, Woolley CS. Morphological sex differences and laterality in the prepubertal medial amygdala. J Comp Neurol. 2007;501:904–915. doi: 10.1002/cne.21281. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Sexually dimorphic synaptic organization of the medial amygdala. J Neurosci. 2005;25:10759–10767. doi: 10.1523/JNEUROSCI.2919-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Increasing interaction of amygdalar afferents with GABAergic interneurons between birth and adulthood. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm183. doi:10.1093/cercor/bhm 183.
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- de Olmos JS, Beltramino CA, Alheid GF. Amygdala and extended amygdala of the rat: a cytoarchitectonical, fibroarchitectonical, and chemoarchitectonical survey. In: Paxinos G, editor. “The Rat Nervous System, Third Edition”. Elsevier Academic Press; NewYork: 2004. pp. 509–603. [Google Scholar]
- de Vries GJ, Miller MA. Anatomy and function of extrahypothalamic vasopressin systems in the brain. Prog Brain Res. 1998;119:3–20. doi: 10.1016/s0079-6123(08)61558-7. [DOI] [PubMed] [Google Scholar]
- Della-Maggiore V, Grady CL, McIntosh AR. Dissecting the effect of aging on the neural substrates of memory: deterioration, preservation or functional reorganization? Rev Neurosci. 2002;13:167–181. doi: 10.1515/revneuro.2002.13.2.167. [DOI] [PubMed] [Google Scholar]
- Denburg NL, Buchanan TW, Tranel D, Adolphs R. Evidence for preserved emotional memory in normal older persons. Emotion. 2003;3:239–253. doi: 10.1037/1528-3542.3.3.239. [DOI] [PubMed] [Google Scholar]
- Doyere V, Gisquet-Verrier P, de Marsanich B, Ammassari-Teule M. Age-related modifications of contextual information processing in rats: role of emotional reactivity, arousal and testing procedure. Behav Brain Res. 2000;114:153–165. doi: 10.1016/s0166-4328(00)00223-0. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Sutherland RJ. The aging hippocampus: navigating between rat and human experiments. Rev Neurosci. 2005;16:87–121. doi: 10.1515/revneuro.2005.16.2.87. [DOI] [PubMed] [Google Scholar]
- Escobar C, Salas M. Neonatal undernutrition and amygdaloid nuclear complex development: an experimental study in the rat. Exp Neurol. 1993;122:311–318. doi: 10.1006/exnr.1993.1130. [DOI] [PubMed] [Google Scholar]
- Fischer H, Sandblom J, Gavazzeni J, Fransson P, Wright CI, Bäckman L. Age-differential patterns of brain activation during perception of angry faces. Neurosci Lett. 2005;386:99–104. doi: 10.1016/j.neulet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Busby SJ. Amygdala input monosynaptically innervates parvalbumin immunoreactive local circuit neurons in rat medial prefrontal cortex. Neuroscience. 2006;139:1039–1048. doi: 10.1016/j.neuroscience.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4-18 years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grady CL, Keightley ML. Studies of altered social cognition in neuropsychiatric disorders using functional neuroimaging. Can J Psychiatry. 2002;48:327–336. doi: 10.1177/070674370204700403. [DOI] [PubMed] [Google Scholar]
- Grady MS, Charleston JS, Maris D, Witgen BM, Lifshitz J. Neuronal and glial cell number in the hippocampus after experimental traumatic brain injury: analysis by stereological estimation. J Neurotrauma. 2003;20:929–41. doi: 10.1089/089771503770195786. [DOI] [PubMed] [Google Scholar]
- Gréco B, Edwards DA, Michael RP, Clancy AN. Androgen receptor immunoreactivity and mating-induced Fos expression in forebrain and midbrain structures in the male rat. Neuroscience. 1996;75:161–71. doi: 10.1016/0306-4522(96)00183-2. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E. Preservation of limbic and paralimbic structures in aging. Hum Brain Mapp. 2005;25:391–401. doi: 10.1002/hbm.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillamón A, Segovia S. Sex differences in the vomeronasal system. Brain Res Bull. 1997;44:377–382. doi: 10.1016/s0361-9230(97)00217-7. [DOI] [PubMed] [Google Scholar]
- Hamidi M, Drevets WC, Price JL. Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol Psychiatry. 2004;55:563–9. doi: 10.1016/j.biopsych.2003.11.006. [DOI] [PubMed] [Google Scholar]
- He F, Sun YE. Glial cells more than support cells? Int J Biochem Cell Biol. 2007;39:661–665. doi: 10.1016/j.biocel.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Hines M, Allen LS, Gorski RA. Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Res. 1992;579:321–326. doi: 10.1016/0006-8993(92)90068-k. [DOI] [PubMed] [Google Scholar]
- Howard CV, Reed MG. Springer; New York: 1998. Unbiased stereology: three-dimensional measurement in microscopy. [Google Scholar]
- Iidaka T, Okada T, Murata T, Omori M, Kosaka H, Sadato N, Yonekura Y. Age-related differences in the medial temporal lobe responses to emotional faces as revealed by fMRI. Hippocampus. 2002;12:352–362. doi: 10.1002/hipo.1113. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Oki M, Yurgelun-Todd DA. Sex-specific developmental changes in amygdala responses to affective faces. Neuroreport. 2001;12:427–433. doi: 10.1097/00001756-200102120-00047. [DOI] [PubMed] [Google Scholar]
- Koshibu K, Levitt P, Ahrens ET. Sex-specific, postpuberty changes in mouse brain structures revealed by three-dimensional magnetic resonance microscopy. Neuroimage. 2004;22:1636–1645. doi: 10.1016/j.neuroimage.2004.03.051. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. J Comp Neurol. 1977;172:687–722. doi: 10.1002/cne.901720408. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J Comp Neurol. 1978;178:255–280. doi: 10.1002/cne.901780205. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ERα and ERβ) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol. 1998;36:357–78. doi: 10.1002/(sici)1097-4695(19980905)36:3<357::aid-neu5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Paz R, Paré D. Prefrontal control of the amygdala. J Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira MD, Lieberman AR. Sexual dimorphism in the mammalian limbic system. Prog Neurobiol. 1995;45:275–333. doi: 10.1016/0301-0082(94)00052-j. [DOI] [PubMed] [Google Scholar]
- Markham JA, Juraska JM. Aging and sex influence the anatomy of the rat anterior cingulate cortex. Neurobiol Aging. 2002;23:579–588. doi: 10.1016/s0197-4580(02)00004-0. [DOI] [PubMed] [Google Scholar]
- Markham JA, McKian KP, Stroup TS, Juraska JM. Sexually dimorphic aging of dendritic morphology in CA1 of hippocampus. Hippocampus. 2005;15:97–103. doi: 10.1002/hipo.20034. [DOI] [PubMed] [Google Scholar]
- Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144:961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Mather M, Canli T, English T, Whitfield S, Wais P, Ochsner K, Gabrieli JD, Carstensen LL. Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychol Sci. 2004;15:259–263. doi: 10.1111/j.0956-7976.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Muller JF. Immunocytological localization of GABABR1 receptor subunits in the basolateral amygdala. Brain Res. 2004;1018:147–158. doi: 10.1016/j.brainres.2004.05.053. [DOI] [PubMed] [Google Scholar]
- Merke DP, Fields JD, Keil MF, Vaituzis AC, Chrousos GP, Giedd JN. Children with classic congenital adrenal hyperplasia have decreased amygdala volume: potential prenatal and postnatal hormonal effects. J Clin Endocrinol Metab. 2003;88:1760–1765. doi: 10.1210/jc.2002-021730. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Charney DS, Ernst M, Pine DS. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual dimorphism in neuronal number of the posterodorsal medial amygdala is independent of circulating androgens and regional volume in adult rats. J Comp Neurol. 2008;506:851–859. doi: 10.1002/cne.21536. [DOI] [PubMed] [Google Scholar]
- Mouton P. Johns Hopkins University; Baltimore: 2002. Principles and practices of unbiased stereology. [Google Scholar]
- Mu Q, Xie J, Wen Z, Weng Y, Shuyun Z. A quantitative MR study of the hippocampal formation, the amygdala, and the temporal horn of the lateral ventricle in healthy subjects 40 to 90 years of age. Am J Neuroradiol. 1999;20:207–211. [PMC free article] [PubMed] [Google Scholar]
- Murphy DGM, DeCarli C, McIntosh AR, Daly E, Mentis MJ, Pietrini P, Szczepanik J, Schapiro MB, Grady CL, Horwitz B, Rapoport SI. Sex differences in human brain morphometry and metabolism: an in vivo Magnetic Resonance Imaging and Positron Emission Tomography study on the effect of aging. Arch Gen Psychiatry. 1996;53:585–594. doi: 10.1001/archpsyc.1996.01830070031007. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Sodhi J, Juraska JM. Ovarian hormones after postnatal day 20 reduce neuron number in the rat primary visual cortex. J Neurobiol. 2002;52:312–321. doi: 10.1002/neu.10092. [DOI] [PubMed] [Google Scholar]
- Oler JA, Markus EJ. Age-related deficits on the radial maze and in fear conditioning: hippocampal processing and consolidation. Hippocampus. 1998;8:402–415. doi: 10.1002/(SICI)1098-1063(1998)8:4<402::AID-HIPO8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Pisarska M, Mulchahey JJ, Welge JA, Geracioti TD, Kasckow JW. Age-related alterations in emotional behaviors and amygdalar corticotrophin-releasing factor (CRF) and CRF-binding protein expression in aged Fischer 344 rats. Brain Res. 2000;877:184–190. doi: 10.1016/s0006-8993(00)02606-8. [DOI] [PubMed] [Google Scholar]
- Rasia-Filho AA, Fabian C, Rigoti KM, Achaval M. Influence of sex, estrous cycle and motherhood on dendritic spine density in the rat medial amygdala revealed by the Golgi method. Neuroscience. 2004;126:839–847. doi: 10.1016/j.neuroscience.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Rasia-Filho AA, Londero RG, Achaval M. Effects of gonadal hormones on the morphology of neurons from the medial amygdaloid nucleus of rats. Brain Res Bull. 1999;48:173–183. doi: 10.1016/s0361-9230(98)00160-9. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Haacke EM. Brain aging and its modifiers: insights from in vivo neuromorphometry and susceptibility weighted aging. Ann N Y Acad Sci. 2007;1097:84–93. doi: 10.1196/annals.1379.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid SN, Juraska JM. Sex differences in neuron number in the binocular area of the rat visual cortex. J Comp Neurol. 1992;321:448–455. doi: 10.1002/cne.903210311. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Lamar M, Driscoll I. Vulnerability of the orbitofrontal cortex to age-associated structural and functional brain changes. Ann N Y Acad Sci. 2007;1121:562–575. doi: 10.1196/annals.1401.027. [DOI] [PubMed] [Google Scholar]
- Rubinow MJ, Drogos LL, Juraska JM. Age-related dendritic hypertrophy and sexual dimorphism in rat basolateral amygdala. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.05.006. doi 10.1016/j.neurobiolaging.2007.05.006.
- Rutter M, Caspi A, Moffitt TE. Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies. J Child Psychol Psychiatry. 2003;44:1092–1115. doi: 10.1111/1469-7610.00194. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, Lammers CR, Reiss AL, Amaral DG. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24:6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2:859–61. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stefanova N. γ-Aminobutyric acid-immunoreactive neurons in the amygdala of the rat — sex differences and effect of early postnatal castration. Neurosci Lett. 1998;255:175–7. doi: 10.1016/s0304-3940(98)00735-6. [DOI] [PubMed] [Google Scholar]
- Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134:127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Stoehr JD, Wenk GL. Effects of age and lesions of the nucleus basalis on contextual fear conditioning. Psychobiology. 1995;23:173–177. [Google Scholar]
- Swaab DF, Gooren LJG, Hofman MA. Brain research, gender, and sexual orientation. J Homosex. 1995;28:283–301. doi: 10.1300/J082v28n03_07. [DOI] [PubMed] [Google Scholar]
- van Landeghem FK, Weiss T, von Deimling A. Expression of PACAP and glutamate transporter proteins in satellite oligodendrocytes of the human CNS. Regul Pept. 2007;142:52–9. doi: 10.1016/j.regpep.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Vinader-Caerols C, Collado P, Segovia S, Guillamón A. Estradiol masculinizes the posteromedial cortical nucleus of the amygdala in the rat. Brain Res Bull. 2000;53:269–273. doi: 10.1016/s0361-9230(00)00332-4. [DOI] [PubMed] [Google Scholar]
- von Bohlen und Halbach O, Unsicker K. Morphological alterations in the amygdala and hippocampus of mice during ageing. Eur J Neurosci. 2002;16:2434–2440. doi: 10.1046/j.1460-9568.2002.02405.x. [DOI] [PubMed] [Google Scholar]
- von Bohlen und Halbach O, Unsicker K. Age-related decline in the tyrosine hydroxylase immunoreactive innervation of the amygdala and dentate gyrus in mice. Cell Tiss Res. 2003;311:139–143. doi: 10.1007/s00441-002-0662-4. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Mascagni F, McDonald AJ. Sex differences in delta opioid receptor immunoreactivity in rat medial amygdala. Neurosci Lett. 2002;328:160–164. doi: 10.1016/s0304-3940(02)00481-0. [DOI] [PubMed] [Google Scholar]
- Xu Y, Valentino DJ, Scher AI, Dinov I, White LR, Thompson PM, Launer LJ, Toga AW. Age effects on hippocampal structural changes in old men: the HAAS. Neuroimage. 2008 doi: 10.1016/j.neuroimage.2007.12.034. doi:10.1016j.neuroimage.2007.12.034.
- Yates MA, Markham JA, Anderson SE, Morris JR, Juraska JM. Regional variability in age-related loss of neurons from the primary visual cortex and medial prefrontal cortex of male and female rats. Brain Res. 2008;1218:1–12. doi: 10.1016/j.brainres.2008.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr JL, Todd BJ, Schulz KM, McCarthy MM, Sisk CL. Dendritic pruning of the medial amygdala during pubertal development of the male Syrian hamster. J Neurobiol. 2006;66:578–90. doi: 10.1002/neu.20251. [DOI] [PubMed] [Google Scholar]