Abstract
Objective
To standardize serological surveillance to compare rubella susceptibility in Australia and 16 European countries, and measure progress towards international disease-control targets.
Methods
Between 1996 and 2004, representative serum banks were established in 17 countries by collecting residual sera or community sampling. Serum banks were tested in each country and assay results were standardized. With a questionnaire, we collected information on current and past rubella vaccination programmes in each country. The percentage of seronegative (< 4 IU/ml) children (2–14 years of age) was used to evaluate rubella susceptibility, and countries were classified by seronegativity as group I (< 5%), group II (5–10%) or group III (> 10%). The proportion of women of childbearing age without rubella protection (≤ 10 IU/ml) was calculated and compared with WHO targets of < 5%.
Findings
Only Romania had no rubella immunization programme at the time of the survey; the remaining countries had a two-dose childhood schedule using the measles, mumps and rubella (MMR) vaccine. The percentage of susceptible children defined five countries as group I, seven as group II and four as group III. Women of childbearing age without rubella protection were < 5% in only five countries.
Conclusion
Despite the low reported incidence in many countries, strengthening the coverage of the routine two-dose of MMR vaccine among children is needed, especially in group III countries. Catch-up campaigns in older age groups and selective targeting of older females are needed in many countries to ensure necessary levels of protective immunity among women of childbearing age.
Résumé
Objectif
Standardiser la surveillance de la rubéole afin de pouvoir comparer la sensibilité à cette maladie entre l’Australie et 16 pays européens et mesurer les progrès vers les objectifs internationaux de lutte antirubéoleuse.
Méthodes
Entre 1996 et 2004, des banques de sérums représentatifs ont été mises en place dans 17 pays en collectant des résidus de sérum ou en réalisant des prélèvements dans les communautés. Ces banques de sérum ont fait l’objet d’analyses dans chaque pays et les résultats de ces analyses ont été standardisés. Au moyen d’un questionnaire, nous avons collecté des informations sur la situation antérieure et actuelle des programmes de vaccination antirubéoleuse de chaque pays. Le pourcentage d’enfants (2-14 ans) séronégatifs (< 4 UI/ml) a été utilisé pour évaluer la sensibilité à la rubéole et les pays ont été classés par niveau de séronégativité en 3 groupes : I (< 5%), II (5-10%) et III (> 10%). La proportion de femmes en âge de procréer sans protection contre la rubéole (≤ 10 UI/ml) a été déterminée et comparée à l’objectif fixé par l’OMS (< 5%).
Résultats
Seule la Roumanie n’avait pas de programme de vaccination lors de l’enquête, les autres pays appliquant un calendrier de vaccination durant l’enfance par deux doses de vaccin antirougeoleux-antiourlien-antirubéoleux (ROR). D’après le pourcentage d’enfants sensibles, cinq pays ont été classés dans le groupe I, sept dans le groupe II et quatre dans le groupe III. La proportion de femmes en âge de procréer non protégées contre la rubéole était inférieure à 5% dans cinq pays seulement.
Conclusion
Malgré la faible incidence signalée dans de nombreux pays, il faut renforcer la couverture par la vaccination systématique en 2 doses de ROR chez l’enfant, et notamment dans les pays du groupe III. Des campagnes de rattrapage visant les tranches d’âge supérieures et une vaccination sélective des femmes plus âgées s’imposent également dans nombre de pays pour garantir les niveaux d’immunité protectrice nécessaires chez les femmes en âge de procréer.
Resumen
Objetivo
Normalizar la vigilancia serológica para comparar la vulnerabilidad a la rubéola en Australia y en 16 países europeos, y medir los progresos hacia las metas internacionales de control de esa enfermedad.
Métodos
Entre 1996 y 2004 se establecieron serotecas representativas en 17 países reuniendo sueros residuales o mediante muestreo en las comunidades. Se analizaron las serotecas en cada país y se procedió a normalizar los resultados de los análisis. Mediante un cuestionario, se recopiló información sobre los programas de vacunación, antiguos y en vigor, en cada país. La vulnerabilidad a la rubéola se evaluó considerando el porcentaje de niños (2-14 años) seronegativos (< 4 UI/ml), y se clasificó a los países en función de la seronegatividad en tres grupos: grupo I (< 5%), grupo II (5%-10%) y grupo III (> 10%). Se calculó la proporción de mujeres en edad fecunda sin protección contra la rubéola (≤ 10 UI/ml) para compararla con la meta de la OMS (< 5%).
Resultados
Sólo Rumania carecía de un programa de inmunización contra la rubéola en el momento del estudio; los otros países habían adoptado una pauta de dos dosis en la niñez con la vacuna SPR (sarampión, parotiditis, rubéola). A partir del porcentaje de niños vulnerables se asignó a cinco países al grupo I, siete al grupo II, y cuatro al grupo III. La proporción de mujeres en edad fecunda sin protección contra la rubéola fue inferior al 5% en cinco países únicamente.
Conclusión
A pesar de la baja incidencia notificada en muchos países, es preciso reforzar la cobertura de la inmunización sistemática con dos dosis de vacuna SPR en la niñez, sobre todo en los países del grupo III. Se requieren también en muchos países campañas de puesta al día entre los grupos de más edad y una focalización selectiva en las mujeres mayores para asegurar el nivel necesario de inmunidad protectora en las mujeres en edad fecunda.
ملخص
الەدف
اضفاء المعايير القياسية على التـرصُّد السيرولوجي لمقارنة الاستعداد للإصابة في استـراليا و16 بلداً أوربياً، وقياس التقدُّم تجاە تحقيق الأەداف الدولية لمكافحة ەذا المرض.
الطريقة
أنشئت في الفتـرة ما بين 1996 و2004 بنوك أمصال تمثيلية في 17 بلداً، وذلك من خلال جمع ما يتبقى من الأمصال أو أخذ عينات من المجتمع. واختبرت ەذە البنوك في كل بلد، وأجري تقيـيس لنتائج ەذە الاختبارات. وجمع الباحثون معلومات حول البرامج الحالية والسابقة الخاصة بالتلقيح ضد الحصبة الألمانية في كل بلد من ەذە البلدان، مستخدمين استبياناً في ذلك. واستخدمت نسبة سلبية المصل (أقل من 4 وحدات دولية/مل) لدى الأطفال (2-14 سنة) لتقيـيم الاستعداد للإصابة بالحصبة الألمانية. وصنِّفت البلدان في مجموعات وفقاً للنسبة المئوية لسلبية المصل، المجموعة الأولى (أقل من 5%)، والمجموعة الثانية (5-10%)، والمجموعة الثالثة (أكثر من 10%). وحُسبت نسبة النساء في سن الإنجاب اللاتي لا تـتوفَّر لديەن حماية ضد الحصبة الألمانية (أقل من أو مساوياً لـ 10 وحدات دولية/مل)، وقورنت النسبة مع أەداف منظمة الصحة العالمية المتمثِّلة في خفض النسبة إلى أقل من 5%.
الموجودات
تبين أن رومانيا فقط لم تكن لديەا برنامج للتمنيع ضد الحصبة الألمانية وقت إجراء المسح. بقية البلدان كان لديەا جدول تلقيح للأطفال مؤلَّف من جرعتين من اللقاح ضد الحصبة والنكاف والحصبة الألمانية. وصنِّفت خمسة بلدان في المجموعة الأولى، وسبعة في الثانية، وأربعة في الثالثة، وفقاً لنسبة الأطفال الذين لديەم استعداد للإصابة. وكانت نسبة النساء في سن الإنجاب اللاتي لا تـتوفر لديەن حماية ضد الحصبة أقل من 5% في خمسة بلدان فقط.
الاستنتاج
على الرغم من انخفاض الوقوعات المبلغ عنەا في العديد من البلدان، إلا أن الحاجة قائمة لتعزيز التغطية بالتمنيع الروتيني للأطفال المؤلف من جرعتين من لقاح الحصبة والنكاف والحصبة الألمانية، لاسيَّما في بلدان المجموعة الثالثة، كما أن ەناك حاجة أيضاً إلى حملات تداركية للمجموعات الأكبر سناً، مع استەداف انتقائي للنساء الأكبر سناً، في العديد من البلدان، لضمان تحقيق المستويات المطلوبة من المناعة المحصِّنة بين النساء في سن الإنجاب.
Introduction
Rubella is a mild viral disease of little clinical significance in children and adult males. However, rubella infection in pregnancy is of major public health importance due to the teratogenic effects that can result from congenital rubella infection, which can lead to miscarriage, fetal death or birth of an infant with congenital rubella syndrome.1
Since the licensing of the first rubella vaccine in the late 1960s, the aim of rubella vaccination programmes has been the prevention of congenital rubella syndrome as a complication of rubella infection during pregnancy. Many countries first selectively vaccinated adolescent females, thus creating a cohort of immunized women of childbearing age.2 Since the introduction of a combined measles, mumps and rubella (MMR) vaccine, many countries have introduced childhood immunization programmes for rubella.3 Such programmes are established in most European countries,4 thus preventing the transmission of rubella and providing indirect protection to pregnant women.
If childhood rubella vaccination programmes do not attain the herd immunity threshold of about 80%, then a paradoxical increase in congenital rubella syndrome can occur due to a decreased circulation of the virus and an accumulation of susceptible adult females.5 This increase in the syndrome in the presence of a suboptimum vaccination programmes has been reported in Europe6,7 and underpinned the earlier WHO European recommendation of less than 5% susceptibility among women of childbearing age8 and the policy in many European countries of continuing to target these women with rubella vaccine.9
WHO has set targets, within the framework of a control strategy, for the elimination of both measles and rubella and the prevention of congenital rubella infection in the WHO European Region by 2010.10 Serological surveillance is vital to support such a strategy and to monitor progress towards international disease control targets. Serological surveillance avoids many of the limitations of passive disease reporting systems for rubella, in which many infections are either mild or asymptomatic,1 and congenital rubella syndrome, in which there are few cases reported due to poor surveillance systems.11 Furthermore, the effectiveness of a rubella vaccination programme can be evaluated by the identification and subsequent vaccination of susceptible cohorts among women of childbearing age.
International comparisons of the serology of rubella have been limited by the different methods and assays used.12 The European Sero-Epidemiology Network (ESEN) was established in 1996, with the aim of standardizing serological surveillance13 – in particular ensuring the comparability of laboratory results14 – of vaccine preventable diseases, including rubella.15 In 2001, ESEN2 extended this network to include 17 countries (Australia and 16 in the WHO European Region).16 The comparison of rubella seroepidemiology in the participant countries of ESEN2 will inform progress towards international disease-control targets and allow countries to develop evidence-based and cost-effective interventions to achieve these international goals.
Methods
National serum-bank collection
Seventeen countries (Australia and 16 in WHO Europe Region) tested for rubella IgG antibody in sera specimens collected between 1996 and 2004 (Table 1, available at: http://www.who.int/bulletin/volumes/86/2/07-042010/en/index.html). The sera were obtained either by residual sera collected during routine laboratory testing (11 of 17), by population-based random sampling (five of 17), or a combination of these two methods (one of 17). Ethical approval was sought from the appropriate national authorities for all collections.
Table 1. Year and number of samples collected in national serum banks of participating countries and enzyme immunoassays used to test for IgG antibodies to rubella.
| Country or area | Sera collection method | Year of collection | Age range collected (years) | No. samples for 1–19 year age band | No. samples for 20–39 year age band | Assay |
|---|---|---|---|---|---|---|
| Australia | Residual | 2002 | 1–34 | 2694 | 1278 | Enzygnost |
| Belgium | Residual | 2002/2003 | 1–60+ | 1960 | 811 | DiaSorin |
| Bulgaria | Residual | 2001–2004 | 1–60+ | 969 | 697 | Platelia |
| Cyprus | Residual/Population | 2003 | 1–50 | 1929 | 800 | RADM |
| Czech Republic | Population | 2001 | 1–60+ | 1692 | 803 | DiaSorin |
| England and Wales | Residual | 2000 | 1–60+ | 1704 | 892 | Microgen |
| Hungary | Residual | 2003 | 1–60+ | 1914 | 1156 | DiaSorin |
| Ireland | Residual | 2003 | 1–60+ | 1208 | 892 | BioTech |
| Israel | Residual | 1998 | 1–60+ | 1846 | 853 | Enzygnost |
| Latvia | Population | 2003 | 1–60+ | 1592 | 806 | BioRad |
| Lithuania | Residual | 2003 | 1–60+ | 1782 | 879 | Enzygnost |
| Luxembourg | Population | 2000/2001 | 4–60+ | 1381 | 736 | Enzygnost |
| Malta | Residual | 2003 | 1–60+ | 825 | 469 | Abbott |
| Romania | Residual | 2002 | 1–60+ | 2182 | 6844 | Enzygnost |
| Slovakia | Population | 2002 | 1–60+ | 2065 | 869 | DiaSorin |
| Slovenia | Residual | 1999/2000 | 1–60+ | 1918 | 800 | Enzygnost |
| Sweden | Population | 1996/1997 | a | 709 | 201 | Enzygnost |
a Sera collected from groups aged 2, 5, 8, 10, 14, 17, 20–34 and 65+ years.
Sera were collected from all age groups, were evenly distributed between males and females and were geographically representative of each country (Table 1). Project guidelines recommended that about 100 samples be tested in each 1-year age band of those less than 20 years of age and 200 for each 5-year age band from 20 to 40 years of age.13 Project targets were achieved in all countries except Ireland (50–75 samples per 1-year age band), Latvia (75–90), Bulgaria (50) and Malta (50). Fewer than 100 samples were tested in those aged 30–34 years in the Czech Republic and Malta.
Vaccine programme, coverage and rubella incidence
In March 2002, all ESEN national representatives were sent a standardized questionnaire that collected current and historical information regarding both the rubella vaccination programmes (age groups targeted, vaccines used and vaccine coverage) and the incidence of reported disease. This information was updated in 2006 with data from the WHO Health for All databases.
Standardization and reference assay
The methodology and results of the standardization of the rubella assays have been described elsewhere.17,18 A designated reference centre (Robert Koch Institute, Berlin, Germany) prepared a panel of 151 sera that were tested as negative, equivocal and positive using the enzyme immunoassay of Dade Behring Marburg GmbH (Enzygnost®).17 These panels were distributed to participant laboratories where they were tested with the quantitative enzyme immunoassay normally used by the participating laboratory. Seven of the 17 countries used the same assay as the reference centre to test their national serum banks (Table 1).
In two countries (Israel and Sweden), the national serum banks had been tested over a year before the distribution of the reference panel. A back-standardization, described elsewhere,18 was done in those countries to standardize their results to common project units.
Local titres were converted to standard titres by regressing the results of the panel testing of the national centre against those of the reference centre and thus obtaining standardization equations.17,18 Each national serum bank was tested with the same validated assay used for the reference panel. The country-specific standardization equations were used to convert the local quantitative results into standardized reference titres.
Data analysis
The following cut-offs were applied to standardized antibody titres: < 4 IU/ml were seronegative samples, 4–7 IU/ml were equivocal and > 7 IU/ml were seropositive. Equivocal samples were included with seropositives only if stated. Among women of childbearing age, protective immunity was defined as a rubella antibody titre > 10 IU/ml.19
Countries with childhood vaccination programmes were allocated into one of three groups according to the percentage seronegative children (2–14 years of age):
Group I (< 5% seronegativity)
Group II (5–10% seronegativity)
Group III (> 10% seronegativity).
Results
Rubella vaccination programmes
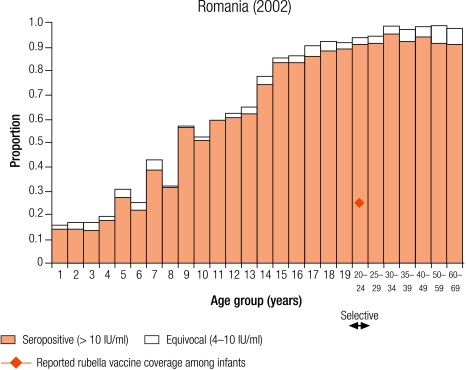
Of the 17 countries, only Romania had no rubella vaccination programme at the time of the serum bank collection in 2002 (Table 2, available at: http://www.who.int/bulletin/volumes/86/2/07-042010/en/index.html). A one-off rubella vaccination campaign targeting young females age 15–19 years had been undertaken in 1998, so by the time of the serum-survey done in Romania in 2002, these females were 20–24 years old (Annex 1, available at: http://www.who.int/bulletin/volumes/86/2/07-042010/en/index.html).
Table 2. Vaccination policies for rubella in the 17 participant countries at the time of serum-surveys, 2003.
| Country or area | Year childhood rubella vaccination introduced |
Age second dose recommended | Average vaccine coverage among infants (%)a | Adolescent female vaccination (years) | Current antenatal screening | Average incidence of rubella (per 100 000 population)a | |
|---|---|---|---|---|---|---|---|
| One-dose | Two-dose | ||||||
| Australia | 1989 | 1993 | 4 | 93 (1999–2003) | 1971–1993 | Yes | 1.3 (1999–2003) |
| Belgium | 1985 | 1994 | 11 | 82 (1999) | 1973–1994 | Yes | 0.2 (2001–03) |
| Bulgaria | 1992 | 2001 | 12 | 93 (1999–2003) | 1988–2001 | – | 86.8 (1999–2003) |
| Cyprus | – | 1989 | 4–6 | 86 (1999–2003) | 1974–1989 | Yes | 0.2 (1999–2003) |
| Czech Republic | – | 1986 | 2 | 97 (1999, 2001, 2003) | 1982–1997 | – | 11.3 (1999–2003) |
| England and Wales | 1988 | 1996 | 4–5 | 85 (1999–2003) | 1970–1988 | Yes | 0.1 (1999–2003) |
| Hungary | 1990 | 1999 | 11 | 100 (1999, 2002, 2003) | – | – | 0.7 (1999–2003) |
| Ireland | 1987 | 1992 | 4–5 | 76 (1999–2003) | 1971–1988 | Yes | 1.6 (1999–2003) |
| Israel | 1988 | 1995 | 6–7 | 95 (1999–2003) | 1973–1999 | – | 0.2 (1999–2003) |
| Latvia | 1993 | 2002 | 7 | 97 (1999–2003) | 1993–2002 | – | 29.0 (1999–2003) |
| Lithuania | 1992 | 1996 | 6–7 | 97 (1999–2003) | 1992–1996 | – | 20.2 (1999–2003) |
| Luxembourg | 1986 | 1994 | 5–6 | – (–) | – | Yes | 0.8 (2000–2001) |
| Malta | 1989 | 1992 | 12 | 74 (1999–2003) | 1976–1992 | – | 1.0 (1999–2003) |
| Romania | – | – | – | – (–) | – | – | 136.3 (1999–2003) |
| Slovakia | 1985 | 1992 | 11 | 99 (1999–2003) | 1985–1992 | – | 0.3 (1999–2003) |
| Slovenia | – | 1990 | 6–7 | 93 (1999–2003) | 1973–1990 | – | 0.5 (1999–2003) |
| Sweden | – | 1982 | 12 | 92 (1999–2002) | 1972–1982 | – | < 0.1 (1999–2003) |
a Period (years) presented in parentheses.
Annex 1.
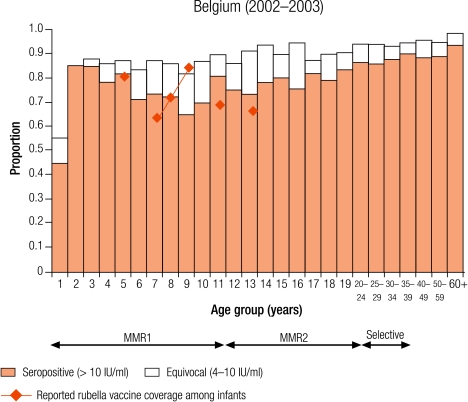
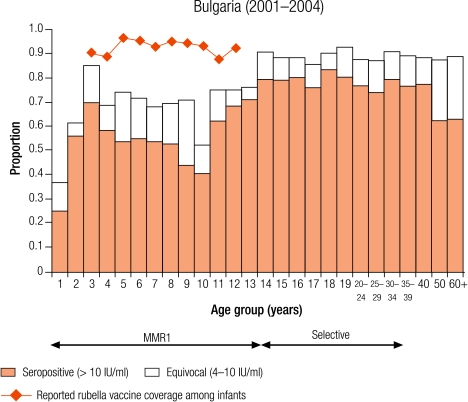
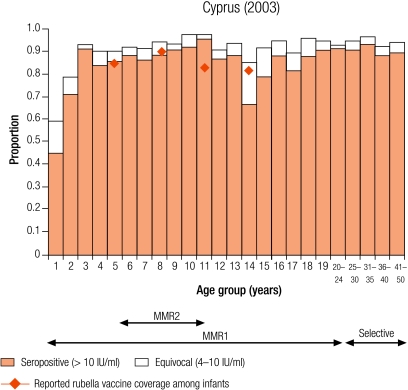
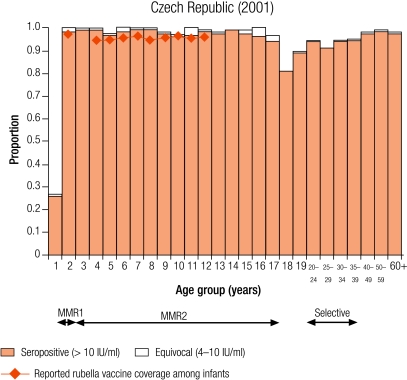
Age-specific rubella seroprevalence by country
MMR1, one-dose measles, mumps and rubella vaccine; MMR2, two-dose measles, mumps and rubella vaccine.
The remaining 16 countries all routinely immunized all children with a two-dose MMR vaccine schedule (Table 2). The first dose of the MMR vaccine was given between ages 13 and 20 months, but the recommended age for the second dose of the MMR vaccine varied from 21 months in the Czech Republic to 12 years in Bulgaria, Malta and Sweden (Table 2).
Twelve countries initially introduced a programme of selective vaccination of adolescent females (Table 2). Four countries (Cyprus, the Czech Republic, Slovenia and Sweden) implemented rubella vaccination of children as a two-dose regime. The remaining 11 countries first implemented rubella vaccination as a single infant dose and a two-dose regimen was introduced between 3 years (Malta) and 9 years (Belgium, Bulgaria and Latvia) later (Table 2). Antenatal screening for rubella susceptibility was done in six of the 17 countries (35%).
Prevaccination serology
In Romania, before the introduction of rubella vaccination, the seroprevalence of rubella increased with age (Table 3; Annex 1). Overall, 10.6% of women of childbearing age did not have protective immunity to rubella and the percentage unprotected was highest in 15–19 year olds (13.3%; Table 4). Among females targeted by the rubella catch-up campaign in 1998 and who in 2002 were 20–24 years old, the percentage without protective immunity was not significantly lower (12/116, 8.3%) than in those that were either too young (15–19 years old, 43/323, 13.3%) or too old (25–29 years old, 10/120, 10.3%) to have been vaccinated (χ² = 2.35, P = 0.31).
Table 3. Percentage seronegative samples (< 4 IU/ml) in children (2–14 years old) and young adults (15–39 years old) by gender in ESEN2 countries, 1996–2003.
| Country or area | Age group 2–14 years (%) |
Age group 15–39 years (%) |
||||||
|---|---|---|---|---|---|---|---|---|
| Overalla | 2–4 years | 5–9 years | 10–14 years | Overalla | Males | Females | ||
| Romania | 57.1 (1485) | 82.2 | 62.6 | 36.7 | 7.1 (1418) | 7.7 | 6.7 | |
| Group I | ||||||||
| Australiab | 4.6 (1594) | 5.0 | 4.5 | 4.5 | 9.3 (2011) | 16.0 | 2.7 | |
| Czech Republic | 0.9 (1178) | 0.0 | 0.9 | 1.4 | 6.4 (1302) | 11.6 | 1.1 | |
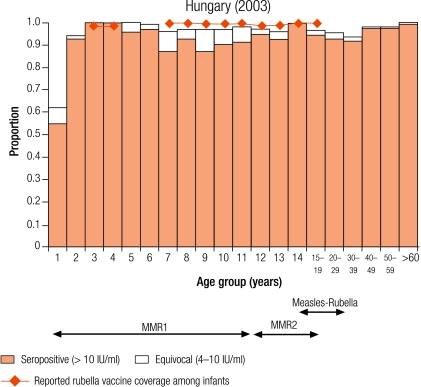
| Hungary | 2.2 (1313) | 2.0 | 2.2 | 2.4 | 4.8 (1300) | 4.4 | 5.3 | |
| Slovakia | 2.9 (1421) | 3.2 | 3.3 | 2.3 | 3.3 (1412) | 5.4 | 1.1 | |
| Slovenia | 3.7 (1312) | 7.1 | 1.6 | 3.8 | 2.4 (1300) | 3.9 | 1.3 | |
| Group II | ||||||||
| Cyprus | 8.7 (1307) | 13.3 | 7.7 | 6.5 | 6.1 (1286) | 5.8 | 6.3 | |
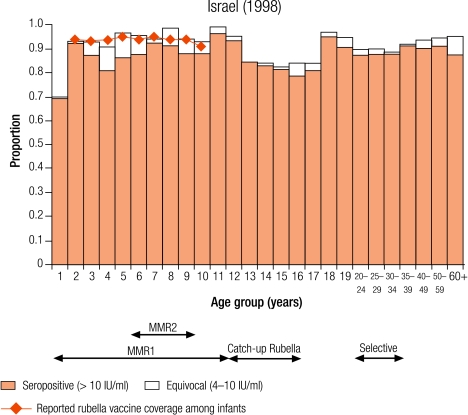
| Israel | 6.8 (1217) | 7.6 | 4.5 | 8.6 | 10.8 (1393) | 17.8 | 1.5 | |
| Latvia | 8.7 (1091) | 8.3 | 5.3 | 12.3 | 9.7 (1232) | 13.1 | 6.5 | |
| Lithuania | 5.6 (1213) | 3.6 | 3.9 | 8.1 | 5.1 (1373) | 5.3 | 5.0 | |
| Luxembourgc | 5.2 (994) | – | 5.2 | 5.5 | 6.9 (1123) | 7.7 | 6.2 | |
| Malta | 6.8 (570) | 9.6 | 6.3 | 6.2 | 4.4 (665) | 5.2 | 3.8 | |
| Swedend | 8.8 (604) | – | – | – | 2.6 (306) | 6.3 | 0.0 | |
| Group III | ||||||||
| Belgium | 12.8 (1219) | 11.0 | 13.2 | 8.5 | 5.8 (1457) | 7.7 | 3.8 | |
| Bulgaria | 28.4 (619) | 28.9 | 28.8 | 27.8 | 11.3 (799) | 10.5 | 11.8 | |
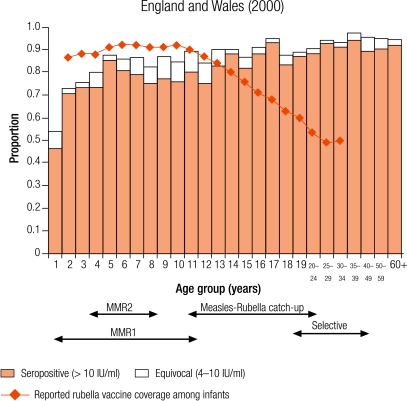
| England and Wales | 15.7 (1184) | 24.0 | 14.4 | 12.7 | 8.4 (1650) | 10.9 | 6.0 | |
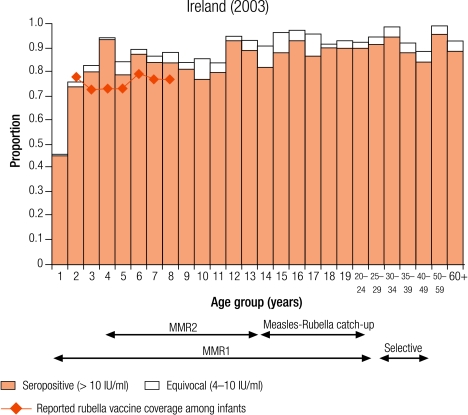
| Ireland | 12.9 (805) | 15.5 | 13.8 | 10.2 | 5.6 (1212) | 9.2 | 3.6 | |
ESEN2, European Sero-Epidemiology Network 2. a n presented in parentheses. b Sera collected up to the age of 34 years old. c Sample size in 2–4 years age group was too small. d Sera collected from select ages.
Table 4. Women of a childbearing age without protective immunity (defined as a titre < 10 IU/ml) for rubella by age group.
| Country or area | Overall (%)a |
By age group (%)a |
|||
|---|---|---|---|---|---|
| 15–39 years | 15–19 years | 20–29 years | 30–39 years | ||
| Australiab | 2.7 (1020) | 4.0 (373) | 2.5 (489) | 0.6 (158) | |
| Belgium | 13.4 (739) | 17.5 (331) | 11.8 (204) | 8.3 (204) | |
| Bulgaria | 11.8 (466) | 10.8 (138) | 13.0 (216) | 10.7 (112) | |
| Cyprus | 9.7 (648) | 10.8 (249) | 6.0 (199) | 12.0 (200) | |
| Czech Republic | 1.4 (649) | 0.4 (249) | 0.3 (300) | 7.0 (100) | |
| England and Wales | 6.2 (837) | 12.9 (248) | 4.1 (389) | 2.0 (200) | |
| Hungary | 8.2 (646) | 5.1 (253) | 9.8 (194) | 10.6 (199) | |
| Ireland | 7.6 (778) | 10.7 (159) | 6.2 (307) | 7.4 (312) | |
| Israel | 5.0 (595) | 4.8 (334) | 3.7 (189) | 9.7 (72) | |
| Latvia | 8.7 (635) | 8.6 (220) | 7.9 (203) | 9.4 (212) | |
| Lithuania | 6.1 (864) | 2.2 (273) | 7.6 (288) | 8.3 (303) | |
| Luxembourg | 8.1 (615) | 11.0 (228) | 5.1 (215) | 8.1 (172) | |
| Malta | 5.1 (396) | 6.2 (97) | 5.5 (181) | 3.4 (118) | |
| Romania | 10.6 (780) | 13.3 (323) | 9.3 (236) | 8.1 (221) | |
| Slovakia | 2.2 (712) | 1.1 (272) | 2.5 (236) | 3.4 (204) | |
| Slovenia | 2.9 (758) | 2.5 (284) | 3.6 (251) | 2.7 (223) | |
| Swedenc | 2.2 (179) | – (–) | – (–) | – (–) | |
a n presented in parentheses. b Sera collected only from those aged < 35 years old. c Sera collected from selected ages only.
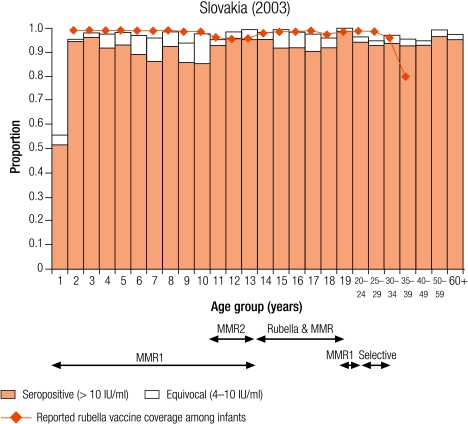
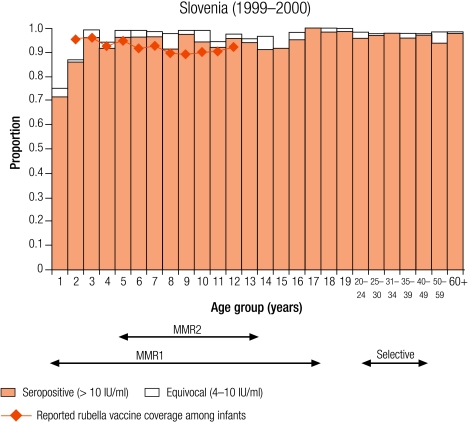
Group I countries
Five countries were defined as group I (less than 5% of children were seronegative for rubella): Australia (4.6%), the Czech Republic (0.9%), Hungary (2.2%), Slovakia (2.9%) and Slovenia (3.7%; Table 3). In all countries, the average reported infant vaccine coverage was at least 93%, while the average incidence of rubella disease was less than one per 100 000 population except in Australia (1.3 per 100 000) and the Czech Republic (11.3 per 100 000, Table 2).
In Hungary, Slovakia and Slovenia this level of control (< 5% seronegativity) was maintained in young adults (Table 3), but not in the Czech Republic and Australia, due to high seronegativity among adult males (11.6% and 16% respectively, Table 3) who had not been targeted by any rubella vaccination campaign. In Australia, The Czech Republic, Slovakia and Slovenia less than 5% of women of childbearing age did not have protective immunity (Table 4). In the Czech Republic and Hungary, the proportion of women of childbearing age without protective immunity reached a maximum in 30–39-year-old women (7.0% and 10.6% respectively) who would not have been targeted by any rubella vaccination programme.
Group II countries
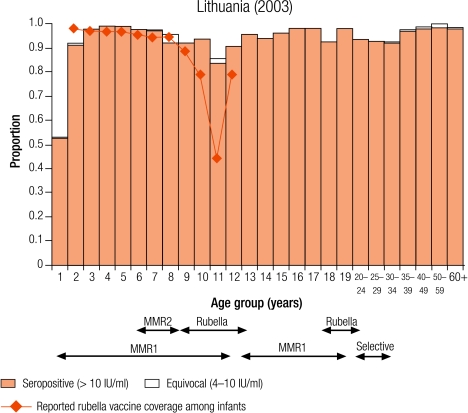
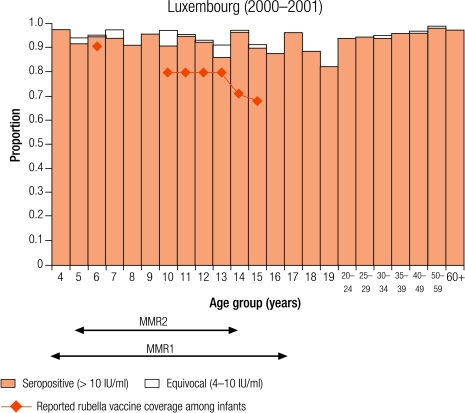
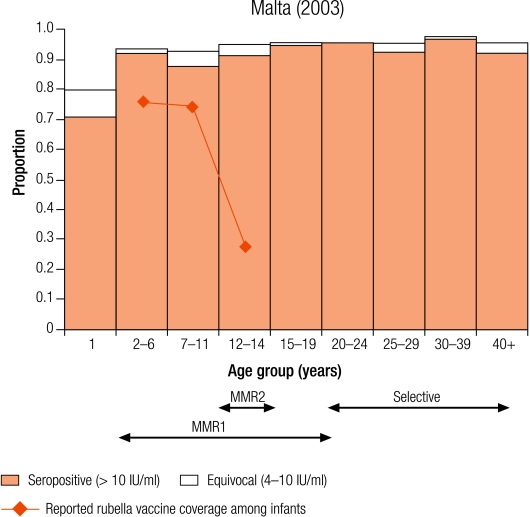
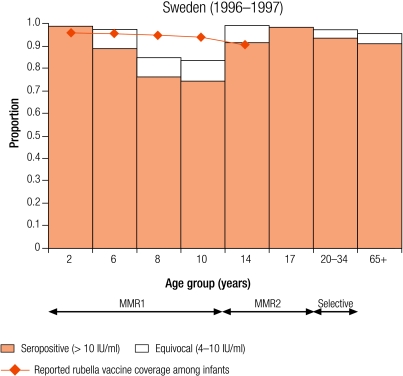
Seven countries were defined as group II (5–10% seronegativity in children): Cyprus (8.7%), Israel (6.8%), Latvia (8.7%), Lithuania (5.6%), Luxembourg (5.2%), Malta (6.8%) and Sweden (8.8%; Table 3). In Sweden, protective immunity among women of childbearing age was less than 5% (Table 4), but samples were only collected from selected age groups (Table 1). Two countries (Luxembourg and Sweden) have been included in this group although direct comparisons with other countries are difficult as samples were collected from restricted age groups (Table 1).
Four countries (Israel, Latvia, Lithuania and Sweden) reported an average infant vaccine coverage in the 5 years from 1999–2003 of more than 90% (no data for 2003 in Sweden), two of less than 90% (Cyprus and Malta), of which the lowest was Malta (74%), and Luxembourg did not report infant vaccine coverage (Table 2). Five countries reported an average incidence of notified rubella disease of, at most, one case per 100 000 population (Cyprus, Israel, Malta and Sweden for 1999–2003, and Luxembourg for 2000–01), whereas Latvia and Lithuania reported respectively averages of 29.0 and 20.2 cases per 100 000 population (Table 2).
In two countries (Cyprus and Malta), the percentage of seronegative people declined with increasing age, in Cyprus from 13.3% in 2–4 year olds to 6.5% in 10–14 year olds and in Malta from 9.6% to 6.2% (Table 3). This is probably due to the administration of a second does of the MMR vaccine for which no coverage data were available.
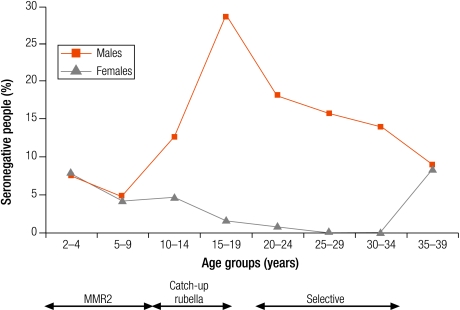
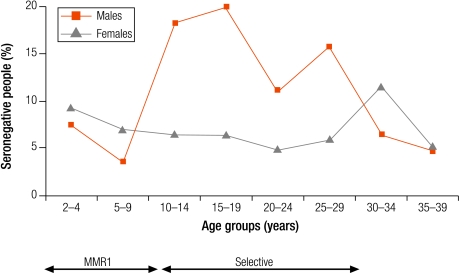
In Israel, Latvia and Lithuania, the highest levels of seronegativity were among children in the oldest age group (10–14 years; Table 3). In Lithuania, although older age groups had been targeted with one dose of rubella vaccine introduced for 12 year olds between 1992 and 2001, coverage was often low (Annex 1) and seronegativity was higher in older children (8.1% in 10–14 year olds) than in younger children (< 5%; Table 3). In Israel and Latvia, as boys older than 11 years old would not have been targeted by a rubella vaccination programme, the higher levels of seronegativity among older children was due to gender differences with higher seronegativity among older males than females (Fig. 1 and Fig. 2).
Fig. 1.
Percentage seronegative (< 4 IU/ml) for rubella antibodies by age group and sex in Israel (1998)
MMR2, two-dose measles, mumps and rubella vaccine.
Fig. 2.
Percentage seronegative (< 4 IU/ml) for rubella antibodies by age group and sex in Latvia (2003)
MMR2, two-dose measles, mumps and rubella vaccine.
In Luxembourg, seronegativity was just over 5% in children (5.2%). A higher percentage of seronegativity was observed in 15–19 year olds (11%; data not shown) as those older than 15 years had not been targeted by any rubella vaccination programme,20 and 11% of females in this age group were without protective immunity against rubella (Table 4). In Sweden, seronegativity increased from 1% in 2 year olds to a maximum of 16.2% in 10 year olds and declined to 1% in 14 year olds after the second dose of the MMR vaccine at 12 years of age (Annex 1).
Group III countries
Four countries were defined as group III with greater than 10% seronegativity in children: Belgium (12.8%), Bulgaria (28.4%), England and Wales (15.7%), and Ireland (12.9%; Table 3). The reported average infant vaccine coverage for the 5 years from 1999–2003 was greater than 90% in Bulgaria (93%) and 85% or less in the remaining three countries (1999 data for Belgium; Table 2). In Bulgaria, the average incidence of notified rubella disease for the same period was 86.8 per 100 000, while it was less than two per 100 000 in the other three countries (data 2001–2003 for Belgium; Table 2).
The percentage of seronegative people within the three childhood age groups ranged from 8.5% of 10–14 year olds in Belgium to 28.9% of 2–4 year olds in Bulgaria. Over 5% of women of childbearing age were without antibody levels needed for protective immunity (i.e. > 10 IU/ml), ranging from 6.2% in England and Wales to 13.4% in Belgium, with the highest percentages among younger women of childbearing age, reaching nearly 20% (17.5%) of 15–19-year-old Belgian females (Table 4).
Discussion
We report on an international study comparing the seroepidemiology of rubella in Australia and 16 countries in the WHO European Region. This present study updates the first ESEN study in six countries with national serum banks collected between 1994 and 1998.15 As with the first ESEN rubella study,15 the rubella antibody titres have been standardized to common units, thereby controlling for possible interassay and interlaboratory variations.14,17,18
The target set by the WHO European strategic plan for the prevention of congenital rubella infection (CRI) is to reduce the incidence of congenital rubella syndrome to less than one case per 100 000 live births annually and of rubella to less than one per 100 000 population by 2010.10 Nine of the 17 countries had achieved the target of rubella incidence. Many countries reported low rubella vaccine coverage of infants, although such estimates did not account for those vaccinated in either a second dose or catch-up campaigns in older age groups. Nonetheless, some countries have reported improved MMR vaccine coverage since the time of the serosurveys.21
In the absence of age-specific susceptibility targets for rubella elimination, as there are for measles, we have used the percentage of seronegative children to categorize a country’s susceptibility to rubella outbreaks. We have based these groups on mathematical models which demonstrated that in low-transmission and intermediate-transmission countries the proportion of infants needed to be immunized to eliminate the risk of infection in women of childbearing age should be greater than 80%, but in high transmission countries this needed to be greater than 90%.22 Wide variations in the herd immunity thresholds for rubella have been estimated in different European countries, although these thresholds are lower than for measles.23
In Romania, the absence of any control programme for rubella resulted in a rubella epidemic with subsequent increase in congenital rubella syndrome.24 In the remaining 15 countries, childhood immunity was above putative thresholds to block endemic transmission. Seronegativity among children was greater than 10% in the four group III countries (Belgium, Bulgaria, England and Wales, and Ireland), at which level modelling studies have estimated that smaller epidemics could occur.24 Efforts to improve rubella immunity among children are needed to prevent the occurrence of such outbreaks in these countries and ensure that the indirect protection offered to pregnant women is maintained. However, one assumes that vaccine coverage is uniform across geographical and social groups, and this is not always the case has observed by the recent outbreak of rubella in the Netherlands leading to cases of congenital rubella syndrome.25
In previous WHO strategies, targets of less than 5% rubella susceptibility among women of childbearing age have been set.8 Only Australia and four European countries (the Czech Republic, Slovakia, Slovenia and Sweden) had achieved a protective immunity of less than 5% among women of childbearing age. However, of the four countries most likely to experience smaller epidemics (Belgium, Bulgaria, England and Wales, and Ireland), the percentage of women of childbearing age without protective immunity was greater than 5%, with a maximum of 13.4% reported in Belgium. This highlights the importance of ensuring there is an appropriate vaccination strategy for these women, either by continued selective vaccination of adolescent females or by antenatal screening and subsequent postpartum vaccination of susceptible women.6
The percentage of susceptible people in Bulgaria would presage an outbreak of rubella despite reported high vaccine coverage with a reliable vaccine (RA27/3). The discrepancy between vaccine coverage and seroprevalence was also noted for the measles seroprofile26 and could represent a possible problem with sample collection and storage or with the assay even though the standardization panel results were good.18 Nonetheless, urgent measures, including vaccination of older age groups, may be needed to avoid another rubella epidemic in Bulgaria, the last was reported in 2000.
In situations where there is no circulation of wild virus due to effective rubella vaccination policies, antibody titres wane 15 years after vaccination;27 although, of the three components of the MMR vaccine, the rubella vaccine-induced antibody response has been reported as the strongest.28 This emphasizes the importance of ensuring that a two-dose rubella immunization is routinely given.29 The seroprofiles of several countries illustrate the possible boosting of the rubella antibody response upon administration of subsequent rubella doses (e.g. Sweden). Nonetheless, the timing of the second rubella dose is critical to ensure that rubella immunity is maintained in women of childbearing age.
In some countries, we have observed a failure to ensure adequate protection at the time of changes in rubella vaccination programmes, although these often tend to be short-lived and of limited public-health importance. However, in several countries there are large cohorts of susceptible males who were not targeted by selective vaccination and too old for the childhood vaccination programme and these can act as foci for rubella epidemics.30,31 This underlines the importance of ensuring proper levels of protection in males and, despite evident difficulties, that they are included in any catch-up vaccination campaigns.32
Two-dose childhood vaccination programmes for rubella have now been implemented in all countries that participated. However, international disease-control targets for rubella could be missed in many countries unless these programmes are strengthened by improved routine coverage of children and, where appropriate, catch-up campaigns in older age groups. Furthermore, rubella immunization programmes should be strengthened in conjunction with the corresponding measles programmes where appropriate. The low level of protective immunity among women of childbearing age underlines the importance of appropriate screening programmes for rubella susceptibility. Serological surveillance, when undertaken in a coordinated and standardized manner, has provided valuable information with which to evaluate vaccine programmes internationally. Such initiatives should play an important part in improving public health in Europe. ■
Acknowledgements
This work was done with funding from the European Commission (contract number QLK2-CT-2000–00542) as well as from national governments and universities.
Footnotes
Competing interests: None declared.
References
- 1.Plotkin SA, Reef S. Rubella vaccine. In: Vaccines 4th ed. Plotkin SA, Orenstein WA eds. London: WB Saunders Co.; 2004. pp. 707-743. [Google Scholar]
- 2.Dudgeon JA. Selective immunisation: protection of the individual. Rev Infect Dis. 1985;7:S185–90. doi: 10.1093/clinids/7.supplement_1.s185. [DOI] [PubMed] [Google Scholar]
- 3.Plotkin SA. Rubella eradication. Vaccine. 2001;19:3311–9. doi: 10.1016/S0264-410X(01)00073-1. [DOI] [PubMed] [Google Scholar]
- 4.Spika JS, Wassilak S, Pebody R, Lipskaya G, Deshevoi S, Guris D, et al. Measles and rubella in the World Health Organization European region: diversity creates challenges. J Infect Dis. 2003;187:S191–7. doi: 10.1086/368336. [DOI] [PubMed] [Google Scholar]
- 5.Anderson RM, May RM. Vaccination against rubella and measles: quantitative investigations of different policies. J Hyg (Lond) 1983;90:259–325. doi: 10.1017/s002217240002893x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panagiotopoulos T, Antoniadou I, Valassi-Adam E. Increase in congenital rubella occurrence after immunisation in Greece: retrospective survey and systematic review. BMJ. 1999;319:1462–7. doi: 10.1136/bmj.319.7223.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henquell C, Bournazeau JA, Vanlieferinghen P, Grangeot-Keros L, Chambon M, Lebel A, et al. Presse Med. 1999;28:777–80. [The re-emergence in 1997 of rubella infections during pregnancy. 11 cases in Clermont-Ferrand] [PubMed] [Google Scholar]
- 8.Strategic Plan for measles and congenital rubella infection in the European region of WHO Copenhagen: WHO Regional Office for Europe; 2003.
- 9.Ukkonen P. Rubella immunity and morbidity: impact of different vaccination programs in Finland 1979-1992. Scand J Infect Dis. 1996;28:31–5. doi: 10.3109/00365549609027146. [DOI] [PubMed] [Google Scholar]
- 10.Eliminating measles and rubella and preventing congenital rubella infection. WHO European Region strategic plan 2005-2010 Copenhagen: WHO Regional Office for Europe; 2005.
- 11.Spika JS, Hanon FX, Wassilak S, Pebody R, Emiroglu N. Preventing congenital rubella infection in the European Region of WHO: 2010 target. Euro Surveill. 2004;9:4–5. [PubMed] [Google Scholar]
- 12.Galazaka A. Rubella in Europe. Epidemiol Infect. 1991;107:43–54. doi: 10.1017/s0950268800048664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osborne K, Weinberg J, Miller E. The European Sero-Epidemiology Network (ESEN). Euro Surveill. 1997;2:29–31. doi: 10.2807/esm.02.04.00167-en. [DOI] [PubMed] [Google Scholar]
- 14.Andrews N, Pebody RG, Berbers G, Blondeau C, Crovari P, Davidkin I, et al. The European Sero-Epidemiology Network: standardizing the enzyme immunoassay results for measles, mumps and rubella. Epidemiol Infect. 2000;125:127–41. doi: 10.1017/S0950268899004173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pebody RG, Edmunds WJ, Conyn-van Spaendonck M, Olin P, Berbers G, Ribeiere I, et al. The seroepidemiology of rubella in western Europe. Epidemiol Infect. 2000;125:347–57. doi: 10.1017/S0950268899004574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nardone A, Miller E. Serological surveillance of rubella in Europe: European Sero-Epidemiology Network (ESEN2). Euro Surveill. 2004;9:5–7. doi: 10.2807/esm.09.04.00456-en. [DOI] [PubMed] [Google Scholar]
- 17.Tischer A, Andrews N, Kafatos G, Nardone A, Berbers G, Davidkin I et al. Standardisation of measles, mumps and rubella assays to enable comparisons of seroprevalence data across 21 European countries and Australia. Epidemiol Infect Mar 30:1-11 [epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 18.Kafatos G, Andrews N, Nardone A. Model selection methodology for inter-laboratory standardisation of antibody titres. Vaccine. 2005;23:5022–7. doi: 10.1016/j.vaccine.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 19.Skendzel LP. Rubella immunity. Defining the level of protective antibody. Am J Clin Pathol. 1996;106:170–4. doi: 10.1093/ajcp/106.2.170. [DOI] [PubMed] [Google Scholar]
- 20.Mossong J, Putz L, Schneider F. Seroprevalence of measles, mumps and rubella antibodies in Luxembourg: results from a national cross-sectional study. Epidemiol Infect. 2004;132:11–8. doi: 10.1017/S0950268803001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vlaams infectieziektenbulletin 57/2006/3. Available from: www.wvc.vlaanderen.be
- 22.Edmunds WJ, van de Heijden OG, Eerola M, Gay NJ. Modelling rubella in Europe. Epidemiol Infect. 2000;125:617–34. doi: 10.1017/S0950268800004660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edmunds WJ, Gay NJ, Kretzschmar M, Pebody RG, Wachmann H. European Sero-epidemiology Network. The pre-vaccination epidemiology of measles, mumps and rubella in Europe: implications for modelling studies. Epidemiol Infect. 2000;125:635–50. doi: 10.1017/S0950268800004672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rafila A, Marin M, Pistol A, Nicolaiciuc D, Lupulescu E, Uzicanin A, et al. A large rubella outbreak, Romania - 2003. Euro Surveill. 2004;9:7–8. doi: 10.2807/esm.09.04.00457-en. [DOI] [PubMed] [Google Scholar]
- 25.van der Veen Y, Hahne S, Ruijs H, van Binnendijk R, Timen A, van Loon AM et al. Rubella outbreak in an unvaccinated religious community in the Netherlands leads to cases of congenital rubella syndrome. Euro Surveill 2005 10:E051124.3. [DOI] [PubMed]
- 26.Andrews N, Tischer A, Siedler A, Pebody RG, Barbara C, Cotter S, et al. Towards measles elimination: measles susceptibility in seventeen European countries and Australia. Bull World Health Organ. doi: 10.2471/BLT.07.041129. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davidkin I, Peltola H, Leinikki P, Valle M. Duration of rubella immunity induced by two-dose measles, mumps and rubella (MMR) vaccination. A 15-year follow-up in Finland. Vaccine. 2000;18:3106–12. doi: 10.1016/S0264-410X(00)00139-0. [DOI] [PubMed] [Google Scholar]
- 28.Vyse AJ, Gay NJ, Hesketh LM, Pebody R, Morgan-Capner P, Miller E. Interpreting serological surveys using mixture models: the seroepidemiology of measles, mumps and rubella in England and Wales at the beginning of the 21st century. Epidemiol Infect. 2006;134:1303–12. doi: 10.1017/S0950268806006340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pebody RG, Gay NJ, Hesketh LM, Vyse A, Morgan-Capner P, Brown DW, et al. Immunogenicity of second dose measles-mumps-rubella (MMR) vaccine and implications for serosurveillance. Vaccine. 2002;20:1134–40. doi: 10.1016/S0264-410X(01)00435-2. [DOI] [PubMed] [Google Scholar]
- 30.Castillo-Solorzano C, Carrasco P, Tambini G, Reef S, Brana M, de Quadros CA. New horizons in the control of rubella and prevention of congenital rubella syndrome in the Americas. J Infect Dis. 2003;187:S146–52. doi: 10.1086/368034. [DOI] [PubMed] [Google Scholar]
- 31.Cohen D, Muhsen KH, Aboudy Y, Harari H, Mendelson E, Green MS. Use of rubella seroepidemiological data for assessment of previous vaccination policy and for decision making in response to epidemics in Israel. Vaccine. 2006;24:5604–8. doi: 10.1016/j.vaccine.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 32.Kelly H, Worth L, Karapanagiotidis T, Riddell M. Interruption of rubella virus transmission in Australia may require vaccination of adult males: evidence from a Victorian sero-survey. Commun Dis Intell. 2004;28:69–73. doi: 10.33321/cdi.2004.28.3. [DOI] [PubMed] [Google Scholar]