Abstract
Objective
To evaluate the relative cost-effectiveness in different sub-Saharan African settings of presumptive treatment, field-standard microscopy and rapid diagnostic tests (RDTs) to diagnose malaria.
Methods
We used a decision tree model and probabilistic sensitivity analysis applied to outpatients presenting at rural health facilities with suspected malaria. Costs and effects encompassed those for both patients positive on RDT (assuming artemisinin-based combination therapy) and febrile patients negative on RDT (assuming antibiotic treatment). Interventions were defined as cost-effective if they were less costly and more effective or had an incremental cost per disability-adjusted life year averted of less than US$ 150. Data were drawn from published and unpublished sources, supplemented with expert opinion.
Findings
RDTs were cost-effective compared with presumptive treatment up to high prevalences of Plasmodium falciparum parasitaemia. Decision-makers can be at least 50% confident of this result below 81% malaria prevalence, and 95% confident below 62% prevalence, a level seldom exceeded in practice. RDTs were more than 50% likely to be cost-saving below 58% prevalence. Relative to microscopy, RDTs were more than 85% likely to be cost-effective across all prevalence levels, reflecting their expected better accuracy under real-life conditions. Results were robust to extensive sensitivity analysis. The cost-effectiveness of RDTs mainly reflected improved treatment and health outcomes for non-malarial febrile illness, plus savings in antimalarial drug costs. Results were dependent on the assumption that prescribers used test results to guide treatment decisions.
Conclusion
RDTs have the potential to be cost-effective in most parts of sub-Saharan Africa. Appropriate management of malaria and non-malarial febrile illnesses is required to reap the full benefits of these tests.
Résumé
Objectif
Evaluer le rapport coût-efficacité dans différents pays d’Afrique subsaharienne du traitement présomptif, de la microscopie classique sur le terrain et des tests diagnostiques rapides (TDR) dans le diagnostic du paludisme.
Méthodes
Nous avons fait appel à un modèle d’arbre de décisions et à une analyse probabiliste de sensibilité, qui ont été appliqués aux patients se présentant en ambulatoire dans des établissements de soins ruraux avec une présomption de paludisme. Nous avons évalué les coûts et les effets du traitement à la fois pour les patients positifs aux TDR (en les supposant sous traitement par une polythérapie à base d’artémisinine) et les patients fébriles négatifs à ces tests (en les supposant sous traitement antibiotique). Nous avons considéré que les interventions étaient efficientes sous l’angle économique si elles étaient moins coûteuses et plus efficaces ou si elles fournissaient un coût marginal par année de vie corrigée de l’incapacité évité inférieur à US $ 150. Les données ont été extraites de sources publiées et non publiées, complétées par des avis d’experts.
Résultats
Les TDR se sont révélés efficaces sur le plan économique par comparaison avec le traitement présomptif jusqu’à des valeurs élevées de la parasitémie due à Plasmodium falciparum. Les décideurs peuvent accorder à ce résultat un niveau de confiance de 50% lorsque la prévalence du paludisme est inférieure à 81% et de 95% lorsque cette prévalence est inférieure à 62%, niveau rarement dépassé dans la pratique. Au-dessous de 58% de prévalence, la probabilité que les TDR permettent des économies était supérieure à 50%. Par rapport à la microscopie, les tests avaient une probabilité de plus de 85% d’avoir un bon ratio coût-efficacité sur l’ensemble des niveaux de prévalence, en accord avec la meilleure précision qu’on s’attend à leur voir fournir en conditions réelles. Ces résultats ont résisté à une analyse de sensibilité approfondie. Le rapport coût-efficacité des TDR reflétait principalement l’amélioration des traitements et des issues des maladies fébriles non palustres, ainsi que les économies réalisées sur les coûts des antipaludiques. Ces résultats supposaient que les prescripteurs aient utilisé les réponses des tests pour guider les décisions thérapeutiques.
Conclusion
Les TDR sont en mesure d’avoir un bon rapport coût-efficacité dans la plupart des régions de l’Afrique subsaharienne. Une prise en charge appropriée du paludisme et des maladies fébriles non palustres est nécessaire pour tirer un bénéfice maximal de ces tests.
Resumen
Objetivo
Evaluar en diferentes entornos subsaharianos la costoeficacia relativa del tratamiento de sospecha, la microscopía estándar de campo y las pruebas de diagnóstico rápido (PDR) de la malaria.
Métodos
Se aplicó un modelo de árbol decisional y un análisis de sensibilidad probabilístico a pacientes ambulatorios que acudieron a establecimientos de salud rurales con presunta malaria. Se consideraron los costos y los efectos tanto para los pacientes positivos en la PDR (suponiendo que se administró tratamiento combinado con artemisinina) como para los pacientes febriles negativos en la PDR (suponiendo que se administraron antibióticos). Se definieron como costoeficaces las intervenciones que fueron menos costosas y más eficaces o que habían tenido un costo marginal por AVAD (año de vida ajustado en función de la discapacidad) ganado de menos de US$ 150. Los datos empleados se extrajeron de trabajos publicados o inéditos y se complementaron con la opinión de expertos.
Resultados
Las PDR fueron costoeficaces en comparación con el tratamiento de sospecha hasta valores altos de la prevalencia de parasitemia por Plasmodium falciparum. Las instancias decisorias pueden confiar con una probabilidad de al menos un 50% en ese resultado por debajo de una prevalencia de malaria del 81%, y del 95% por debajo de una prevalencia del 62%, nivel rara vez superado en la práctica. Las PDR tenían una probabilidad mayor del 50% de ser costoeficaces por debajo de una prevalencia del 58%. En lo que respecta a la microscopía, las PDR tenían más de un 85% de probabilidades de ser costoeficaces en todos los niveles de prevalencia, lo que refleja la mayor exactitud previsible en condiciones reales. Un extenso análisis de sensibilidad reveló que los resultados eran robustos. La costoeficacia de las PDR se debió principalmente al mejor tratamiento y resultados sanitarios conseguidos con las dolencias febriles no maláricas, más lo ahorrado en concepto de medicamentos antimaláricos. Los resultados dependen del supuesto de que los prescriptores tomaron sus decisiones terapéuticas en función de los resultados de las pruebas.
Conclusión
Las PDR pueden ser costoeficaces en la mayoría del África subsahariana, pero para poder sacar el máximo provecho de esas pruebas hay que tratar adecuadamente la malaria y las enfermedades febriles no maláricas.
ملخص
الەدف
تقييم الفعَّالية النسبية لقاء التكاليف لاستخدام المعالجة الظنية، والفحص المجەري المعياري الميداني، والاختبارات التشخيصية السريعة في تشخيص الملاريا في مختلف المناطق الواقعة جنوب الصحراء الإفريقية.
الطريقة
لقد قمنا باستخدام نموذج شجرة القرارات، وتحليل الحساسية الاحتمالية، المطبق على المرضى الخارجيين القادمين إلى المرافق الصحية القروية للاشتباە في إصابتەم بالملاريا. وتم تحليل التكاليف والفعَّالية لكل من المرضى الإيجابيين وفقاً للاختبارات التشخيصية السريعة (بافتراض استخدام المعالجة التوليفية المرتكزة على الأرطمايسنين)، والمرضى المحمومين السلبيين وفقاً للاختبارات التشخيصية السريعة (بافتراض المعالجة بالمضاد الحيوي). وتُعَرَّف التدخلات بفعاليتەا لقاء التكاليف، إذا كانت تكلفتەا أقل، وفعَّاليتەا أكثر، أو أن تكون تكلفتەا التراكمية لكل سنة من سنوات العمر المصححة باحتساب سنوات العجز التي يمكن تفاديەا أقل من 150 دولاراً أمريكياً. وقد استُمِدَّت المعطيات من المصادر المنشورة وغير المنشورة، والتي تؤيدەا آراء الخبراء.
الموجودات
تتميز الاختبارات التشخيصية السريعة بفعالية عالية لقاء التكاليف بالمقارنة مع المعالجة الظنيَّة، التي تصل في حدەا الأعلى إلى درجات الانتشار المرتفعة من دوران المتصورات المنجلية في الدم. ومن ثـمَّ يمكن لصناع القرار أن يكونوا واثقين بنسبة 50% على الأقل من ەذە النتائج عندما يقل انتشار الملاريا عن 81%، وواثقين بنسبة 95% عندما يقل الانتشار عن 62%. وەي معدَّلات يندر تجاوزەا في الممارسات العملية على أرض الواقع. فاختبارات التشخيص السريع يمكنەا أن توفر التكاليف بنسبة تتجاوز 50% في حالات الانتشار الأقل من 58%. وبالمقارنة بالفحص المجەري، تتميز الاختبارات التشخيصية السريعة بفعالية أكبر لقاء التكاليف بنسبة تتجاوز 85% في كل مستويات الانتشار، مما يعكس درجة أعلى من الدقة المتوقعة في ظروف الحياة الحقيقية. وقد جاءت النتائج قوية بما يكفي وفقاً للتحاليل المكثفة للحساسية. وعكست فعالية الاختبارات التشخيصية السريعة لقاء التكاليف، تحَسُّن المعالجة والحصائل الصحية للأمراض الحموية الأخرى التي لا تنجم عن الملاريا، فضلاً عن توفير تكاليف الأدوية المضادة للملاريا. وارتكزت النتائج على افتراض مفادە، أن واصفي المعالجة اعتمدوا على نتائج الاختبار في توجيە قرارات المعالجة.
الاستنتاج
بمقدور الاختبارات التشخيصية السريعة تحقيق الفعَّالية لقاء التكاليف في معظم أجزاء المناطق الواقعة جنوب الصحراء الأفريقية. ومن ثـمَّ ينبغي على المعالجة الملائمة للأمراض الحموية سواء الناجمة عن الملاريا أو الأمراض الأخرى التي لا تنجم عن الملاريا، تحقيق أقصى استفادة من ەذە الاختبارات.
Introduction
The introduction of high-cost antimalarial drugs such as artemisinin-based combination therapy (ACT) is encouraging malaria-endemic countries in sub-Saharan Africa to reassess diagnostic practices. Traditional practice for outpatients has been to treat presumptively for malaria based on a history of fever,1,2 but a significant proportion of those treated may not have parasites (over 50% in many settings) and hence waste a considerable amount of drugs.3–6 Widespread prescription of chloroquine to patients not having malaria has been tolerated, partly because chloroquine is so cheap; however, ACT costs at least 10 times more per treatment.7 Moreover, overdiagnosis of malaria implies underdiagnosis and inappropriate treatment of non-malarial febrile illness: while a high proportion of such illnesses are self-limiting viral diseases, a significant minority, such as acute respiratory infections or bacterial meningitis, are bacterial diseases and potentially fatal.8,9
WHO currently makes the tentative recommendation that parasite-based diagnosis should be used in all cases of suspected malaria with the possible exception of children in high-prevalence areas and certain other situations.10,11 However, formal analyses have not estimated the epidemiological and economic thresholds at which different diagnostic strategies are preferable. As microscopy is generally limited to larger clinics, rapid diagnostic tests (RDTs) for malaria could be considered for most patients in endemic regions. However, there is very little evidence to guide decision-makers on the relative cost-effectiveness of presumptive treatment, RDTs and microscopy across epidemiological settings.
The objective of this study is to use a decision tree model and probabilistic sensitivity analysis to estimate the relative cost-effectiveness of RDTs, presumptive treatment and field standard microscopy in different epidemiological settings of sub-Saharan Africa where Plasmodium falciparum predominates.
Methods
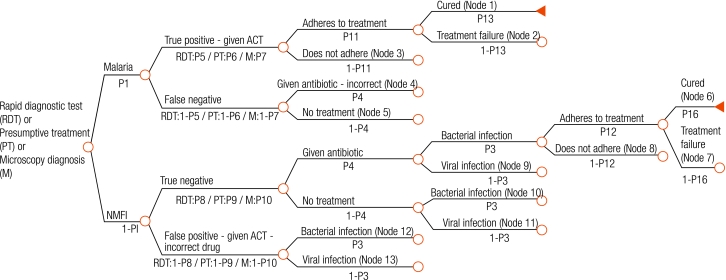
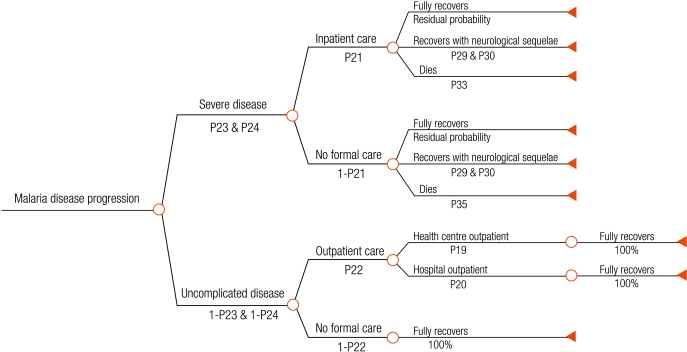
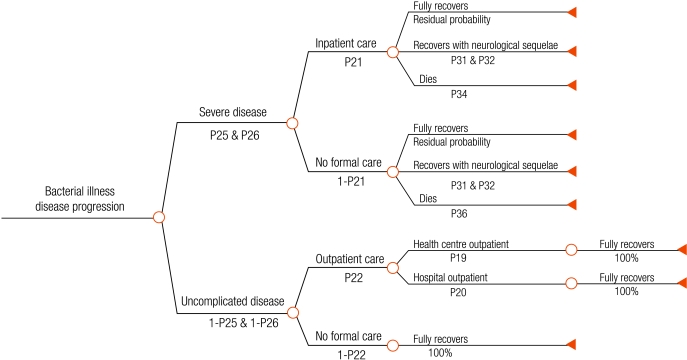
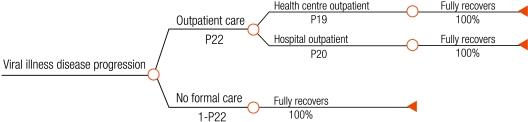
We developed a decision tree that begins with ambulatory patients presenting with fever to health facilities in rural sub-Saharan Africa (Fig. 1, Fig. 2, Fig. 3, Fig. 4), and proceeds through diagnosis and treatment to disease outcomes according to the sensitivity and specificity of each diagnostic strategy, the patient’s age and malaria prevalence among patients. Typical facilities would include health centres and dispensaries staffed by nurses and perhaps clinical officers, and outpatient departments of district hospitals. Once given first-line treatment, patients were assumed to face the same probabilities, health outcomes and costs regardless of diagnostic method. Parameter estimates for initial diagnosis and treatment were extracted from recently published data. Parameters describing treatment seeking patterns, costs for programme implementation and secondary treatment, and duration of disease were based mainly on those used in previous models.12,13 Expert opinion was relied on for probabilities of disease progression and mortality without appropriate treatment where reliable published data do not exist. Parameter values, sources, best estimates and probability distributions representing parameter uncertainty are available at: http://www.wpro.who.int/sites/rdt.
Fig. 1.
Root decision tree applying to all diagnostic strategies, mapping diagnosis and subsequent events according to malaria and non-malarial febrile illness (NMFI)
ACT, artemisinin-based combination therapy.
Fig. 2.
Malaria disease outcome tree after treatment failure, non-adherence, no first-line treatment or incorrect drug given to the patient after diagnosisa
a This tree arises from chance nodes 2, 3, 4 and 5 on the right hand side of the root tree (Fig. 1).
Fig. 3.
Bacterial disease outcome tree after treatment failure, non-adherence, no first-line treatment, or incorrect drug given to the patient after diagnosisa
a This tree arises from chance nodes 7, 8, 10 and 12 on the right hand side of the root tree (Fig. 1).
Fig. 4.
Disease outcome tree for all patients with viral illnessa
a This tree arises from chance nodes 9, 11 and 13 on the right hand side of the root tree (Fig. 1).
We assumed that health workers used the diagnostic test result in their clinical decision-making and that patients diagnosed positive for malaria received ACT and patients negative for malaria received an antibiotic such as amoxicillin. The proportion receiving antibiotics was varied in the sensitivity analysis. Best (most likely) estimates for drug efficacy were set at 85% for ACT in cases of malaria and 75% for antibiotics in bacterial disease. We assumed that antibiotics were not efficacious for malaria or viral illness, and that antimalarials did not cure bacterial disease. We assumed no coinfection between malaria and bacterial infections. Presumptive treatment on the basis of a history of fever was assumed to have perfect sensitivity and zero specificity. For RDTs we assumed a test detecting histidine-rich protein-2 (HRP-2) specific for P. falciparum, as 90% of malaria in sub-Saharan Africa is P. falciparum, with best estimates for RDT sensitivity and specificity of 96% and 95%, respectively.14–19 Microscopic diagnosis was based on best standard practice of district-hospital and health-centre general laboratories in sub-Saharan Africa, and assumed best estimates for sensitivity and specificity of 82% and 85%, respectively.20,21 We made comparisons according to all possible levels of endemicity of malaria expressed in terms of prevalence of parasitaemia in febrile outpatients presenting at facilities.
The chances of a febrile episode being fatal are far higher if associated with HIV infection.9,22,23 Very high HIV prevalence would affect the decision tree parameters. To avoid a very complex decision tree structure, parameter values were set assuming that HIV prevalence was relatively low (about 10% of people five years old or over), which is typical outside southern Africa.
We calculated the incremental cost in US dollars (2002 prices) of changing from one diagnostic approach to another from the joint perspective of providers and patients, using the ingredients approach to calculate diagnosis costs, first-line drug costs and variable costs of second-line treatment.24 The costs of microscopy diagnosis included materials, staff time, training and supervision. RDT diagnosis included the unit cost of the test; diagnosis according to presumptive treatment was assumed to cost nothing. We assumed drug cost per adult dose to be US$ 1–2.4 for ACT and US$ 0.61–0.93 for antibiotics. We set the cost of RDT kits at US$ 0.6–1 and that of microscopy at US$ 0.32–1.27. Microscopy costs are dependent on workload and were based on a range of 1000 to 6800 or more diagnoses per year. For simplicity we assumed that microscopy was used only for malaria diagnosis, not for other diseases. All other costs of first-line treatment were excluded as they were assumed to be the same across diagnostic strategies. We included variable costs to providers and patients of any second-line treatment (drugs, reagents, food), but excluded fixed costs (buildings, equipment, supervision and most staff costs) as these would not change with numbers of patients. We assumed that unresolved uncomplicated malaria was treated with a second-line drug of the same price and efficacy as the first-line antimalarial. We assumed that secondary treatment for severe bacterial infection was an alternative antibiotic costing twice as much as first-line treatment. Costs associated with the management of neurological sequelae were excluded.
We measured health outcomes in terms of disability-adjusted life years (DALYs) averted, calculated according to the methods of Lopez et al. without age weights.25 We based life expectancies on a west African life table with a life expectancy at birth of 50 years.
The causes of non-malarial febrile infection vary from region to region and encompass diseases such as acute respiratory infections and bacterial meningitis. For simplicity, disability weights and durations for uncomplicated and severe non-malarial febrile illnesses were assumed to be the same as those for malaria. We assumed that bacterial illness was more likely than malaria to become severe, but that only 5–15% of these infections had bacterial causes, with the rest being self-limiting viral infections.
We did probabilistic sensitivity analysis with Monte-Carlo simulations (Palisade @Risk add-in tool to Microsoft Excel), and cost and health outcomes were generated stochastically (10 000 simulations). Policy-makers will wish to identify interventions that are less costly than the comparator and have better health outcomes, called dominant, and rule out those that are more costly and less effective, termed dominated. More costly and more effective interventions may be selected if they are thought to be good value for money. An intervention was defined as cost-effective if it was dominant or had an incremental cost per DALY averted under US$ 150. The value of US$ 150 was chosen in the base case, to represent a decision-maker’s valuation of a healthy year of life. This was based on recommendations of the Ad Hoc Committee on Health Research Priorities, which stated that any intervention costing less than US$ 150 per DALY averted should be considered attractive in low-income countries.26
Additional sensitivity analyses were done by varying the parameter of interest and malaria prevalence according to the ranges in Table 1. A full report of all results is available at: http://www.wpro.who.int/sites/rdt, where customized results specific to local settings can be generated online using an interactive model.
Table 1. Sensitivity analysis: threshold level for parameters tested at which RDTs are preferable to presumptive treatment at 5% and 40% malaria prevalence among febrile outpatients.
| Parameters tested | Threshold level at which RDTs are preferable to presumptive treatment |
||||
|---|---|---|---|---|---|
| 50% certainty |
95% certainty |
||||
| 5% malaria prevalence | 40% malaria prevalence | 5% malaria prevalence | 40% malaria prevalence | ||
| Cost of RDT (US$ 0–4) | Any cost | Any cost | Any cost | < US$ 2.30 | |
| Cost of an adult dose of ACT (US$ 0–4) | Any cost | Any cost | Any cost | > US$ 0.20 | |
| Adherence to ACT (0–100%) | Any level | Any level | Any level | Any level | |
| Adherence to antibiotic (0–100%) | Any level | > 4% | > 25% | > 39% | |
| Probability that non-malarial febrile illness is bacterial (0–100%) | Any level | > 4% | > 20% | > 23% | |
| Probability that patient diagnosed negative for malaria receives antibiotica (0–100%) | Any level | Any level | Any level | > 23% | |
| Probability that a patient with bacterial infection became severe (≥ 5 years: 10–25%) (< 5 years: 20–40%) | Any level | Any level | Any level | Any level | |
| Proportion of presenting population 5 years old or over (0–100%) | Any level | Any level | Any level | Any level | |
| Valuation of a DALY averted (US$ 0–500) | Any value | Any value | > US$ 48 | > US$ 70 | |
ACT, artemisinin-based combination therapy; DALY, disability-adjusted life year; RDT, rapid diagnostic test. a When less than 100%, we assumed that antibiotics were randomly allocated to patients with non-malarial febrile illness.
Results
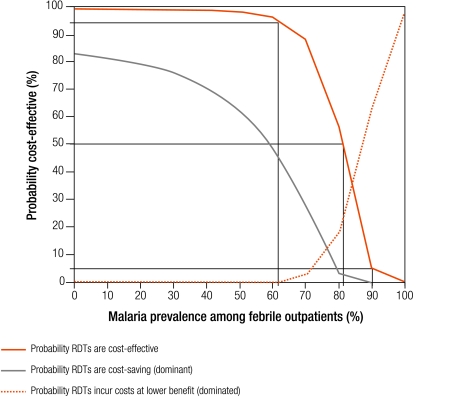
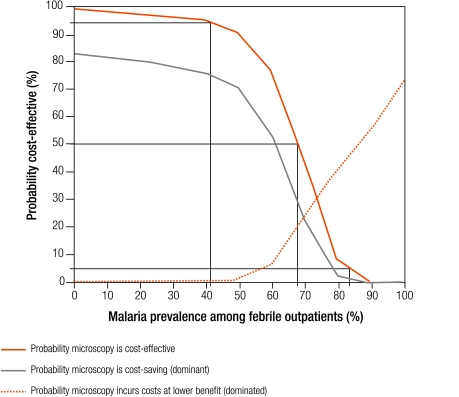
The incremental cost-effectiveness of RDTs and microscopy relative to presumptive treatment is shown in Fig. 5 and Fig. 6. Each graph shows the probability that the strategy is cost-effective, dominant or dominated. These probabilities can be interpreted as the level of risk associated with choosing one strategy over another. For example, at 40% malaria prevalence, a rough average for sub-Saharan Africa,3 we can be over 97% certain that RDTs are cost-effective compared with presumptive treatment, and over 70% certain that they are dominant. We provide results in terms of 50% risk and 95% risk as reference points, acknowledging that these thresholds tend to the extremes of risk acceptability and aversion.
Fig. 5.
Probability that RDTs are cost-effective, dominant or dominated relative to presumptive treatment
RDT, rapid diagnostic test.
Fig. 6.
Probability that field-standard microscopy is cost-effective, dominant or dominated relative to presumptive treatment
A decision-maker can be 95% certain that RDTs are cost-effective relative to presumptive treatment at any prevalence below 62% (Fig. 5). There is 95% certainty that RDTs are not cost-effective above 90% prevalence. RDTs are cost-effective relative to presumptive treatment with 50% certainty even when prevalence is less than 81% (where the curve showing the probability that RDTs are cost-effective crosses 50% on the y-axis). RDTs are likely to be dominant with 50% certainty below 58% malaria prevalence, and dominated above 87% malaria prevalence. At low levels of malaria prevalence there is massive overdiagnosis of malaria with presumptive treatment, and use of parasitic diagnosis results in large cost savings through avoided ACT prescriptions. At very high malaria prevalences, presumptive treatment is cost-saving because the costs of diagnosis are avoided, and antimalarials are appropriate for nearly all patients.
Microscopy is likely to be cost-effective relative to presumptive treatment with 50% certainty when malaria prevalence is less than 67% (Fig. 6). This threshold drops to less than 41% prevalence when 95% certainty is required. Microscopy is not cost-effective (95% certainty) above 83% malaria prevalence. Microscopy is dominant with 50% certainty below 62% prevalence, and dominated above 85%.
RDTs are more than 85% likely to be cost-effective relative to microscopy across all levels of prevalence, and are more than 15% likely to be dominant (data not shown). Although RDTs have a higher initial unit cost than microscopy, fewer DALYs are incurred with RDTs as they have better sensitivity and specificity in an operational setting.
Sensitivity analysis
We tested the robustness of the cost-effectiveness results extensively. Results are discussed here for the six parameters with the greatest impact (presented in Table 1 for prevalence rates of 5% and 40%; further details can be found in figures available at: http://www.wpro.who.int/sites/rdt). RDTs are consistently more attractive than microscopy in the base case analysis, and sensitivity analyses has little effect on this comparison, so we restrict our discussion to RDTs relative to presumptive treatment.
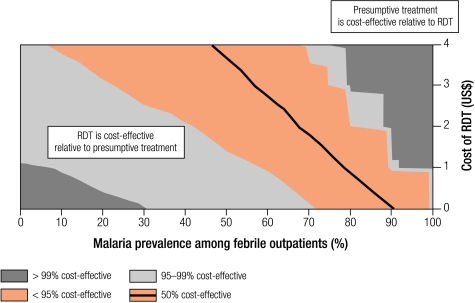
Cost-effectiveness is sensitive to the cost of the initial diagnostic test, with RDTs becoming more cost-effective relative to presumptive treatment as their cost decreased. Results are presented in Fig. 7 with a cost-effectiveness probability plane, where RDT cost is varied along the y-axis, and malaria prevalence along the x-axis. For example, at 40% malaria prevalence, RDTs would be preferable to presumptive treatment with 50% certainty if an RDT cost less than US$ 4, and with 95% certainty with cost less than US$ 2.30 per test, well above the base case cost of US$ 0.80.
Fig. 7.
Probability planes showing sensitivity analysis of the cost-effectiveness of RDTs compared with presumptive treatment at different RDT costs
RDT, rapid diagnostic test.
The cost of an adult dose of ACT has a strong impact on the comparison of RDTs with presumptive treatment. RDTs become more cost-effective as ACT cost increases, although this trend is not uniform. At low malaria prevalence, everyone receives ACT under presumptive treatment, but the specificity of RDTs screens out 95% of patients with non-malarial febrile illnesses, who receive antibiotics. At high prevalence, almost every patient receives ACT with both presumptive treatment and RDT diagnosis, so raising the cost of ACT has little impact on their relative cost-effectiveness. At 40% prevalence, RDTs are cost-effective with a high degree of certainty at any ACT cost above US$ 0.20, well below current ACT costs (US$ 1–2.40).7
RDTs become more cost-effective as adherence to antibiotics increases. Better adherence means that first-line therapy for patients diagnosed with non-malarial febrile infection is less likely to fail, but this has very little impact on costs because second-line treatment of bacterial infection is much less costly than that for malaria.
Whether the illness is bacterial has a strong impact on the cost-effectiveness of RDTs compared with presumptive treatment. As non-malarial febrile illness becomes more severe, it is more cost-effective to distinguish between these diseases and malaria. At very high malaria prevalence, the probability that non-malarial febrile infection is bacterial is irrelevant. At all levels of malaria prevalence commonly found in sub-Saharan Africa, RDTs are cost-effective when more than 20% of non-malarial febrile patients have bacterial illnesses.
Whether a patient diagnosed as not having malaria receives an antibiotic has a moderate impact on the decision to change policy to RDTs from presumptive treatment. RDT cost-effectiveness improves as more patients with negative tests receive antibiotics because outcomes of non-malarial febrile illnesses are less severe despite higher antibiotic costs. RDTs remain cost-effective below 50% prevalence even when no patients testing negative for malaria receive an antibiotic, as RDTs are slightly less effective but much cheaper than presumptive treatment under these conditions.
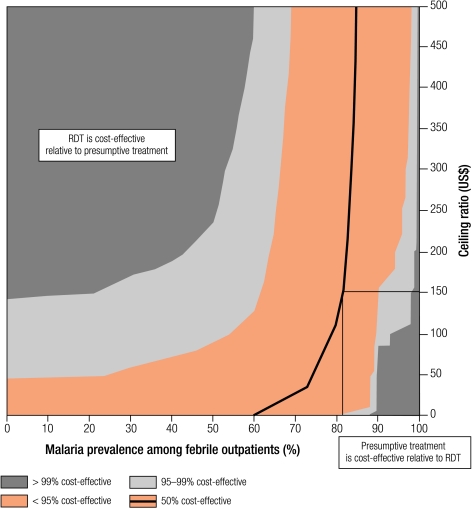
Cost-effectiveness is sensitive to changes in the decision-maker’s valuation of a healthy year of life (Fig. 8). Where malaria prevalence is low, RDTs are cost-effective at any value at 50% certainty, and above US$ 50 per DALY averted at 95% certainty. At high malaria prevalence such as 65%, our confidence that RDTs are cost-effective does not rise above 95% at any level of health gain valuation. This reflects the increased probability that presumptive treatment is dominant at high prevalence.
Fig. 8.
Probability planes showing sensitivity analysis of the cost-effectiveness of RDTs compared with presumptive treatment at different valuations of DALYs averted
DALY, disability-adjusted life year; RDT, rapid diagnostic test.
Discussion
This study demonstrates that taking both antimalarial and antibiotic treatments into account, RDTs are cost-effective compared with presumptive treatment up to high levels of P. falciparum malaria prevalence among patients with febrile illness presenting to rural health facilities.
Decision-makers can be at least 50% confident of this result at less than 80% malaria prevalence in febrile outpatients, and 95% confident below 62% prevalence. In practice, the proportion of febrile outpatients who are parasitaemic seldom exceeds 60%, with most proportions being much lower, so this analysis suggests that RDTs are cost-effective for most malaria-affected areas in sub-Saharan Africa. This finding supports those of previous studies27–29 showing that parasitic diagnosis had the potential to be cost-saving in sub-Saharan Africa and a simple model that indicated the potential for parasitic diagnosis to improve health outcomes for non-malarial febrile infection in epidemic settings.29 Major potential health benefits were also identified in a population-based model of sub-Saharan Africa that found that the introduction of a new diagnostic could lead to significant reductions in malaria-related deaths (assuming that clinical diagnosis was less than 100% sensitive) and unnecessary antimalarial treatments.30
The better health outcomes with RDTs relative to presumptive treatment do not reflect improved treatment of true malaria cases, as the sensitivity of RDTs is lower than that of presumptive treatment, but rather improved treatment of bacterial non-malarial febrile infections, which are assumed to be inappropriately treated with ACT under presumptive treatment, based on current practice. From a cost perspective, many unnecessary and costly ACT treatments could be avoided by the use of RDTs, and a substantial number of patients with non-malarial febrile illnesses would receive appropriate treatment and be less likely to incur second-line treatment costs. At a combined best estimate of US$ 1.41 for an RDT and antibiotic for an adult, the initial costs of negative malaria diagnoses are less than the current average cost of ACT (US$ 1.70).
The analysis suggests that, under the assumptions, RDTs would be preferable to microscopy. The RDT strategy is always more costly than microscopy at the case volumes assumed, but the better accuracy of RDTs under current routine conditions means that fewer DALYs are incurred for patients with both malaria and non-malarial illnesses, provided prescribers use test results to guide their treatment decisions. In large facilities, microscopy may offer benefits, ignored here, of providing parasite counts and diagnosing other diseases. However, the costs of improving microscopy standards to levels similar to RDTs in lower-level facilities are likely to be significant.
If policy-makers are comfortable with 50% certainty, RDTs in rural facilities are robustly cost-effective compared with presumptive treatment and microscopy under most common prevalence scenarios, except when malaria prevalence among patients with febrile illnesses is relatively high, the cost of RDTs is high, adherence to antibiotics is low, patients are unlikely to receive an antibiotic, a very low proportion of non-malarial febrile illnesses are bacterial, or the valuation of health gain is very low. If 95% certainty is required, the results are more dependent on prevalence. At 40% prevalence, we can be 95% confident that RDTs are cost-effective compared with presumptive treatment as long as valuation of health gain is at least US$ 75 per DALY averted, RDTs cost less than US$ 2.30 per test, antibiotic adherence is greater than 40%, the percentage of non-malarial febrile illnesses that are bacterial is greater than 20%, and the proportion of RDT-negative cases receiving antibiotics is at least 23%.
Models inevitably serve as simplified approximations of the true nature and complexity of behaviours of health-care providers and patients. In practice, treatment-seeking patterns are more complex,31,32 and providers often prescribe antimalarials even when tests are negative.20,21,33 Cost-effectiveness can be substantially reduced where clinicians do not adhere to treatment guidelines or prescribe according to diagnostic results, where inefficient procurement leads health facilities to run out of drugs or diagnostics frequently, or where quality assurance for drugs or diagnostics is poor.34 Little is known about the impact of introducing new diagnostics on patients’ behaviour, especially on facility use and adherence to therapy.
Better data could improve the model’s accuracy: the sensitivity and specificity of RDTs and microscopy in different operational settings, adherence to treatment (which affects progression to severe disease) and treatment-seeking behaviour after treatment failure are all of particular importance.
The composition of non-malarial febrile illnesses is likely to have a strong influence on cost-effectiveness and will differ between regions. However, data on alternative treatable diagnoses are surprisingly scarce and model parameter values relied largely on expert opinion. In addition, HIV complicates the picture. We assumed relatively low HIV prevalence, but where HIV is more common, case fatality rates in individuals with acute febrile illness could be higher, especially for adults with non-malarial illness; because this would increase the cost-effectiveness of diagnostic tools, it does not undermine the central conclusion.
The appropriate protocol for the treatment of patients testing negative for malaria is unclear, and little evidence is available on antibiotic prescribing.35 In our base case we assumed that 100% of patients with non-malarial febrile illnesses, and 0% of those with malaria, received antibiotics. In reality clinicians may target antibiotics to non-malarial infections thought more likely to be bacterial; this would make RDTs more cost-effective. In addition, clinicians may prescribe antibiotics to some patients testing positive for malaria, as in the protocol for the Integrated Management of Childhood Illness. As many patients in sub-Saharan Africa will have malaria parasitaemia and bacterial illness, this approach is appropriate where clinical suspicion is high.36 Such prescribing patterns would reduce the incremental effectiveness and costs of RDTs relative to presumptive treatment, leaving the impact on cost-effectiveness uncertain.
The model considers formal outpatient facilities only, although in many settings a high proportion of careseekers obtain drugs from shops.31,32 Further analysis of the cost-effectiveness of RDTs at these informal providers is warranted. Finally, we have not considered future benefits from the reduction in antimalarial drug pressure, slowing the development of antimalarial resistance30 or of increasing antibiotic drug pressure through improved specificity of malaria diagnosis.
Conclusions
This study demonstrates that in an era of ACT, RDTs for malaria have the potential to be highly cost-effective compared with presumptive treatment across most of Africa. This is due as much to improved targeting of antibiotics to those who do not have parasites as to better targeting of antimalarials. This conclusion does, however, depend on ensuring accuracy of RDTs in the field and use of the tests to guide treatment decisions. Efforts to evaluate operational RDT use and test the cost-effectiveness of interventions to improve clinician adherence to treatment protocols should be a priority. ■
Acknowledgements
We thank Brian Greenwood and Hugh Reyburn (London School of Hygiene and Tropical Medicine) and Jay Berkley (Kenya Medical Research Institute/Wellcome Trust Research Programme) for advice on parameter values. The study was funded by the UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) and the United Kingdom Department for International Development through its grant to the Health Economics and Financing Programme. No organization with any financial or other material interest in diagnostic tests had any role in funding, study design, data collection, data analysis, data interpretation or writing of the paper.
Footnotes
Competing interests: None declared.
References
- 1.O’Dempsey TJ, McArdle TF, Laurence BE, Lamont AC, Todd JE, Greenwood BM. Overlap in the clinical features of pneumonia and malaria in African children. Trans R Soc Trop Med Hyg. 1993;87:662–5. doi: 10.1016/0035-9203(93)90279-Y. [DOI] [PubMed] [Google Scholar]
- 2.Chandramohan D, Jaffar S, Greenwood B. Use of clinical algorithms for diagnosing malaria. Trop Med Int Health. 2002;7:45–52. doi: 10.1046/j.1365-3156.2002.00827.x. [DOI] [PubMed] [Google Scholar]
- 3.Brinkmann U, Brinkmann A. Malaria and health in Africa: the present situation and epidemiological trends. Trop Med Parasitol. 1991;42:204–13. [PubMed] [Google Scholar]
- 4.Amexo M, Tolhurst R, Barnish G, Bates I. Malaria misdiagnosis: effects on the poor and vulnerable. Lancet. 2004;364:1896–8. doi: 10.1016/S0140-6736(04)17446-1. [DOI] [PubMed] [Google Scholar]
- 5.Reyburn H, Mbatia R, Drakeley C, Carneiro I, Mwakasungula E, Mwerinde O, et al. Over-diagnosis of malaria among patients with severe febrile illness in Tanzania: a prospective study. BMJ. 2004;329:1212. doi: 10.1136/bmj.38251.658229.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnish G, Bates I, Iboro J. Newer drug combinations for malaria. BMJ. 2004;328:1511–2. doi: 10.1136/bmj.328.7455.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arrow K, Panosian C, Gelband H. Saving lives, buying time: economics of malaria drugs in an age of resistance. Washington: National Academies Press; 2004. [PubMed] [Google Scholar]
- 8.Brent AJ, Ahmed I, Ndiritu M, Lewa P, Ngetsa C, Lowe B, et al. Incidence of clinically significant bacteraemia in children who present to hospital in Kenya: community-based observational study. Lancet. 2006;367:482–8. doi: 10.1016/S0140-6736(06)68180-4. [DOI] [PubMed] [Google Scholar]
- 9.Berkley JA, Lowe BS, Mwangi I, Williams T, Bauni E, Mwarumba S, et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 10.The role of laboratory diagnosis to support malaria disease management: focus on the use of rapid diagnostic tests in areas of high transmission. Geneva: WHO; 2006.
- 11.WHO technical consultation to review the role of the parasitological diagnosis to support malaria disease management: focus on the use of RDTs in areas of high transmission deploying ACT treatments. Geneva: WHO; 2005.
- 12.Goodman C, Coleman PG, Mills AJ. Economic analysis of malaria control in sub-Saharan Africa. Geneva: Global Forum for Health Research; 2000:185. [Google Scholar]
- 13.Coleman PG, Morel CM, Shillcutt SD, Goodman CA, Mills AJ. A threshold analysis of the cost-effectiveness of artemisinin-based combination therapies in sub-Saharan Africa. Am J Trop Med Hyg. 2004;71:196–204. [PubMed] [Google Scholar]
- 14.Bell DR, Wilson DW, Martin LB. False-positive results of a Plasmodium falciparum histidine-rich protein 2-detecting malaria rapid diagnostic test due to high sensitivity in a community with fluctuating low parasite density. Am J Trop Med Hyg. 2005;73:199–203. [PubMed] [Google Scholar]
- 15.Beadle C, Long GW, Weiss WR, McElroy PD, Maret SM, Oloo AJ, et al. Diagnosis of malaria by detection of Plasmodium falciparum HRP-2 antigen with a rapid dipstick antigen-capture assay. Lancet. 1994;343:564–9. doi: 10.1016/S0140-6736(94)91520-2. [DOI] [PubMed] [Google Scholar]
- 16.Craig MH, Bredenkamp BL, Williams CH, Rossouw EJ, Kelly VJ, Kleinschmidt I, et al. Field and laboratory comparative evaluation of ten rapid malaria diagnostic tests. Trans R Soc Trop Med Hyg. 2002;96:258–65. doi: 10.1016/S0035-9203(02)90092-1. [DOI] [PubMed] [Google Scholar]
- 17.Bojang KA. The diagnosis of Plasmodium falciparum infection in Gambian children, by field staff using the rapid manual ParaSight-F test. Ann Trop Med Parasitol. 1999;93:685–7. doi: 10.1080/00034989957925. [DOI] [PubMed] [Google Scholar]
- 18.Premji Z, Minjas JN, Shiff CJ. Laboratory diagnosis of malaria by village health workers using the rapid manual Para-Sight-F test. Trans R Soc Trop Med Hyg. 1994;88:418. doi: 10.1016/0035-9203(94)90409-X. [DOI] [PubMed] [Google Scholar]
- 19.New perspectives: malaria diagnosis. Geneva: WHO; 2000. p.57. [Google Scholar]
- 20.Reyburn H, Ruanda J, Mwerinde O, Drakeley C. The contribution of microscopy to targeting antimalarial treatment in a low transmission area of Tanzania. Malar J. 2006;5:4. doi: 10.1186/1475-2875-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barat L, Chipipa J, Kolczak M, Sukwa T. Does the availability of blood slide microscopy for malaria at health centers improve the management of persons with fever in Zambia? Am J Trop Med Hyg. 1999;60:1024–30. doi: 10.4269/ajtmh.1999.60.1024. [DOI] [PubMed] [Google Scholar]
- 22.Gordon MA, Hastings TB, Macpherson G, Gordon SB, Boeree MJ, Walsh AL, et al. Non-typhoidal salmonella bacteremia among HIV-infected Malawian adults: high mortality and frequent recrudescence. AIDS. 2002;16:1633–41. doi: 10.1097/00002030-200208160-00009. [DOI] [PubMed] [Google Scholar]
- 23.Gordon SB, Chaponda ME, Walsh AL, Whitty CJM, Gordon MA, Machili CE, et al. Pneumococcal disease in HIV-infected Malawian adults: acute mortality and long-term survival. AIDS. 2002;16:1409–17. doi: 10.1097/00002030-200207050-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips M, Mills A, Dye C. Guidelines for cost-effectiveness analysis of vector control, PEEM guidelines series 3. Geneva: WHO; 1993. [Google Scholar]
- 25.Lopez A, Mathers C, Ezzati M, Jamison D, Murray C, editors. The global burden of disease and risk factors. Oxford: Oxford University Press/World Bank; 2006. [PubMed] [Google Scholar]
- 26.Investing in health research and development: report of the ad hoc committee on health research relating to future investment options. Geneva: WHO; 1996 (TDR/Gen/96.1).
- 27.Jonkman A, Chibwe RA, Khoromana CO, Liabunya UL, Chaponda ME, Kandiero GE, et al. Cost-saving through microscopy-based versus presumptive diagnosis of malaria in adult outpatients in Malawi. Bull World Health Organ. 1995;73:223–7. [PMC free article] [PubMed] [Google Scholar]
- 28.Goodman CA. The economic evaluation of malaria diagnosis.: Working paper prepared for informal consultation on malaria diagnostics at the turn of the century. Geneva: WHO/USAID; 1999. p. 18.
- 29.Rolland E, Checchi F, Pinoges L, Balkan S, Guthmann JP, Guerin PJ. Operational response to malaria epidemics: are rapid diagnostic tests cost-effective? Trop Med Int Health. 2006;11:398–408. doi: 10.1111/j.1365-3156.2006.01580.x. [DOI] [PubMed] [Google Scholar]
- 30.Rafael ME, Taylor T, Magill A, Lim YW, Girosi F, Allan R. Reducing the burden of childhood malaria in Africa: the role of improved diagnostics. Nature. 2006;444:39–48. doi: 10.1038/nature05445. [DOI] [PubMed] [Google Scholar]
- 31.McCombie SC. Self-treatment for malaria: the evidence and methodological issues. Health Policy Plan. 2002;17:333–44. doi: 10.1093/heapol/17.4.333. [DOI] [PubMed] [Google Scholar]
- 32.de Savigny D, Mayombana C, Mwageni E, Masanja H, Minhaj A, Mkilindi Y, et al. Care-seeking patterns for fatal malaria in Tanzania. Malar J. 2004;3:27. doi: 10.1186/1475-2875-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zurovac D, Midia B, Ochola SA, English M, Snow RW. Microscopy and outpatient malaria case management among older children and adults in Kenya. Trop Med Int Health. 2006;11:432–40. doi: 10.1111/j.1365-3156.2006.01587.x. [DOI] [PubMed] [Google Scholar]
- 34.Zurovac D, Larson BA, Akhwale W, Snow RW. The financial and clinical implications of adult malaria diagnosis using microscopy in Kenya. Trop Med Int Health. 2006;11:1185–94. doi: 10.1111/j.1365-3156.2006.01674.x. [DOI] [PubMed] [Google Scholar]
- 35.Radyowijati A, Haak H. Improving antibiotic use in low-income countries: an overview of evidence on determinants. Soc Sci Med. 2003;57:733–44. doi: 10.1016/S0277-9536(02)00422-7. [DOI] [PubMed] [Google Scholar]
- 36.Berkley JA, Maitland K, Mwangi I, Ngetsa C, Mwarumba S, Lowe BS, et al. Use of clinical syndromes to target antibiotic prescribing in seriously ill children in malaria endemic area: an observational study. BMJ. 2005;330:995. doi: 10.1136/bmj.38408.471991.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]