Abstract
There is a significant emerging burden of chronic and end-stage kidney disease in low- and middle-income countries, driven by population ageing and the global epidemic of type 2 diabetes. Sufferers of end-stage kidney disease require ongoing dialysis or kidney transplantation to survive; however, in many low- and middle-income countries, treatment options are strictly limited or unaffordable. Low numbers of maintenance dialysis patients and transplant recipients reflect profound economic and service provision challenges for health-care systems in low- and middle-income countries in sustaining renal replacement therapy programmes. Underdeveloped organ donor and transplant programmes, health system and financing issues, ethical regulation of transplantation and the cost of pharmaceuticals commonly pose additional barriers to the delivery of efficient and cost-effective renal replacement therapy.
Development of locally appropriate transplant programmes, effective use of nongovernmental sources of funding, service planning and cost containment, use of generic drugs and local manufacture of dialysis consumables have the potential to make life-saving renal replacement therapy available to many more in need. Select low- and middle-income countries demonstrate more equitable provision of renal replacement therapy is possible outside high-income countries. For other low- and middle-income countries, education, the development of good public policy and a supportive international environment are critical. Prevention of end-stage kidney disease, ideally as part of an integrated approach to chronic vascular diseases, must also be a key objective.
Résumé
Les pays à revenu faible ou moyen assistent à l’apparition d’une charge importante de néphropathies chroniques ou en phase terminale, dues au vieillissement de la population et à l’épidémie mondiale de diabète de type 2. Les personnes atteintes d’une néphropathie en phase terminale doivent être dialysées en continu ou subir une transplantation rénale pour survivre. Néanmoins, dans un grand nombre de pays à revenu faible ou moyen, ces options thérapeutiques sont très peu accessibles ou inabordables. Le faible nombre des patients sous dialyse d’entretien ou transplantés reflète les profondes difficultés économiques et pratiques que rencontrent les systèmes de santé de ces pays pour faire fonctionner des programmes de transplantation rénale. Le développement insuffisant des programmes de dons d’organes et de transplantation, les problèmes de financement et les difficultés des systèmes de santé, la réglementation éthique en matière de transplantation et le coût des produits pharmaceutiques constituent souvent des obstacles supplémentaires à la délivrance de prestations efficaces et économiques dans le domaine de la transplantation rénale.
Le développement de programmes de transplantation locaux adaptés, le recours efficient à des sources de financement autres que publiques, la planification des services, la maitrise des coûts, l’utilisation de médicaments génériques et la production locale de consommables pour dialyse pourraient permettre à beaucoup plus de personnes qui en ont besoin d’accéder à une transplantation rénale salvatrice. Un certain nombre de pays à revenu faible ou moyen prouvent qu’un accès plus équitable à la transplantation rénale est possible en dehors des pays à haut revenu. Pour les autres pays appartenant à cette tranche de revenu, l’éducation, le développement de bonnes politiques publiques et un environnement international propice sont essentiels. La prévention des néphropathies en phase terminale, idéalement dans le cadre d’une approche intégrée des maladies vasculaires chroniques, doit également être un objectif majeur.
Resumen
Existe una importante carga emergente de nefropatía crónica y terminal en los países de ingresos bajos y medios, propiciada por el envejecimiento de la población y por la epidemia mundial de diabetes de tipo II. Los afectados necesitan diálisis continuas o un trasplante renal para sobrevivir; sin embargo, en muchos países de ingresos bajos y medios esas opciones terapéuticas están muy restringidas o son inasequibles. La baja cifra de pacientes sometidos a diálisis o trasplante refleja los grandes retos económicos y de prestación de servicios a que han de hacer frente los sistemas de salud de los países de ingresos bajos y medios para mantener sus programas de diálisis y trasplante renal. Las carencias de los programas de donantes de órganos y de trasplantes, los problemas relacionados con los sistemas de salud y su financiación, la regulación ética de los trasplantes y el costo de los productos farmacéuticos suponen por lo general barreras adicionales para un tratamiento eficiente y eficaz de la insuficiencia renal.
El desarrollo de programas de trasplantes adaptados a las condiciones locales, el uso eficaz de fuentes de financiación no gubernamentales, la planificación de los servicios y la contención de costos, el uso de genéricos y la fabricación local de productos fungibles para diálisis pueden salvar muchas vidas poniendo tanto ese método como los trasplantes al alcance de muchas más personas necesitadas. Algunos países de ingresos bajos y medios han demostrado que también es posible ofrecer de forma más equitativa esos tratamientos fuera de los países de ingresos altos. Para otros países de ingresos bajos y medios, la educación, la formulación de políticas públicas acertadas y un entorno internacional propicio son fundamentales. La prevención de la insuficiencia renal, a ser posible como parte de un manejo integrado de las vasculopatías crónicas, también ha de ser un objetivo clave.
ملخص
حدث عبء كبير بسبب الأمراض المزمنة والأمراض الكلوية في مراحلەا النەائية في البلدان ذات الدخل المنخفض والمتوسط، نتج عن الازدياد في معدل أعمار الناس وعن الوباء العالمي للنمط الثاني من السكري. ويحتاج ضحايا المرحلة النەائية من الأمراض الكلوية الى مواصلة الديال أو زرع الكلية لكي يبقوا أحياءً، إلا أن ەذين الخيارين في المعالجة يتعرضان لمحدودية خانقة أو عدم القدرة على دفع التكاليف في الكثير من البلدان المنخفضة أو المتوسطة الدخل، ويوضح نقص عدد المرضى الذين يعالجون بالديال المتواصل والذين يتلقون زراعة الكلية، مدى التحديات التي تعترض توفير تلك الخدمات ومدى وخامة ما تعانيە نظم تقديم الرعاية الصحية من تحديات في البلدان المنخفضة والمتوسطة الدخل، في محاولتەا لمواصلة فعالية برامج زراعة الكلية. ومن العوائق الإضافية التي تحول دون تقديم المعالجة بزرع الكلية بشكل فعال ويتصف بكفاءة عالية لقاء التكاليف، عدم تطور برامج زراعة الكلية والتبرع بالأعضاء، والمشكلات التي تثقل كاەل النُظُم الصحية وتمويلەا، والتشريعات الناظمة للزرع وتكاليف المستحضرات الدوائية اللازمة للزرع. إن تطوير برامج زرع ملائمة للظروف المحلية، والاستخدام الفعال للموارد غير الحكومية في التمويل، والتخطيط لتقديم الخدمة وتوفير التكاليف والاستخدام الرشيد للأدوية الجنيسة والتصنيع المحلي للمواد المستەلكة في الديال قد يساەم في توفيرالمعالجة بزرع الكلية المنقذة للحياة لعدد أكبر ممن يحتاجون إليە. إن اختيار البلدان المنخفضة والمتوسطة الدخل قد أظەر أن تقديم المعالجة بزرع الكلية على أسس أكثر عدالة أمر ممكن خارج البلدان المرتفعة الدخل. فيما تمس الحاجة في بعض البلدان الأخرى المنخفضة والمتوسطة الدخل إلى التثقيف وإعداد سياسات صحة عمومية جيدة والحصول على بيئة داعمة. ويعد اتقاء الوصول إلى المرحلة النەائية من أمراض الكلية جزءاً لا يتجزأ من الأسلوب المتكامل لمكافحة الأمراض الوعائية المرافقة، وينبغي مواصلة الاحتفاظ بە ضمن الأەداف الرئيسية.
Introduction
End-stage kidney disease (ESKD) can be defined by the requirement for life-saving dialysis or kidney transplantation. Worldwide, the number receiving renal replacement therapy (RRT) is estimated at more than 1.4 million,1 with incidence growing by approximately 8% annually.2 Driving this increase are population ageing, type 2 diabetes mellitus and hypertension,1 key risk-factors for chronic kidney disease. However, due to the expensive nature of RRT, treatment for ESKD is largely the domain of high-income countries (HIC).1 We consider the extent of the ESKD burden in low- and middle-income countries (LMIC) and impediments to the delivery of RRT, and propose a range of strategies for improving access to treatment for the world’s poorest ESKD sufferers.
The burden and causes of ESKD in LMIC
Because of a dearth of national registries and representative surveys, estimating the burden of ESKD in LMIC is difficult. This burden could, however, approach that of HIC. Diabetic nephropathy is now not only the leading cause of ESKD in much of Australasia, Europe and North America, but also in less affluent countries, including India, several Latin American countries, Malaysia and Turkey.3–7 Prevalence of diabetes is as high as 25% among Mexicans aged 25 to 40 years,8 and diabetic nephropathy causes 65% of ESKD in Puerto Rico.7 Diabetes is also a common cause of ESKD in Egypt, Kuwait, Lebanon and Saudi Arabia.9 It is predicted that, by 2030, 366 million adults worldwide will have diabetes, the majority of whom will be living in LMIC.10 Rising rates of diabetes in developing regions will inevitably be accompanied by increases in ESKD.
At the same time, many LMIC experience significant rates of infectious causes of kidney disease not typically seen in HIC, such as schistosomiasis, HIV, tuberculosis, amyloidosis, hepatitis B and C, and sickle cell anaemia.9,11 In India, north Africa and several Middle Eastern countries, environmental pollution, pesticides and other chemicals, analgesic abuse, herbal medicines and unregulated food additives have been attributed as causes of chronic kidney disease.9,11,12 Low socioeconomic status per se is also associated with higher rates of ESKD.13
Delivering RRT in LMIC
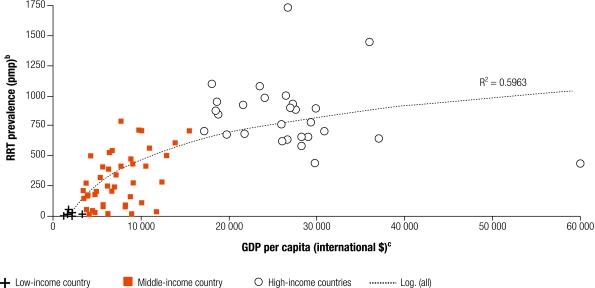
Despite a probable heavy burden of ESKD in LMIC, relatively few patients receive RRT (Fig. 1). Approximately 80% of the world’s RRT patients live in Europe, Japan or North America.2 By contrast, less than 10% of Indian ESKD patients receive RRT, while up to 70% of those starting dialysis die or stop treatment, due to cost, within the first 3 months.14 Approximately half of newly diagnosed ESKD patients living in major Chinese cities receive RRT.15 If the underlying prevalence of ESKD in mainland China approached that of the Province of Taiwan (1550 per million population), total patients requiring treatment would be double the current global number.16
Fig. 1.
Prevalence of patients receiving RRT, as at 31 December 2002, and GDP per capitaa
GDP, gross domestic product; pmp, per million population; RRT, renal replacement therapy.
a Classification of countries into low-, middle- and high-income are according to World Bank Analytical Classifications based on GNI per capita in US$ (2002).
b Data on RRT prevalence from: USRDS Annual Data Reports 2004 & 2005 (www.usrds.org); ERA-EDTA Registry Annual Reports 2002, 2003 & 2004 (www.era-edta-reg.org); Registro Latinoamericano de Diálisis y Trasplante Renal: Informe 2003 (www.slanh.org/registro/); United Kingdom Renal Registry Report, 2003 (www.renalreg.com); Barsoum R, Kidney Int Suppl 2003;63:S111; Sitprija V, Kidney Int Suppl 2003;63:S128; Naicker S, Kidney Int Suppl 2003;63:S119; D’Amico G, Kidney Int Suppl 2005;98:S46.
c Data on GDP per capita refers to purchasing power parity (PPP) international dollars 2002, obtained from World Bank’s World Development Indicators, 2005.
International variation in the number of people receiving RRT might reflect underlying variation in rates of kidney disease, due to a differing risk factor burden, genetic or environmental factors. However, it also reflects a limited economic capacity of LMIC to provide resource-intensive interventions. Accuracy of figures on RRT prevalence may vary, as few countries have comprehensive dialysis and transplant registries, hence underreporting may be an issue. It is clear, however, that large numbers of people in LMIC die from kidney failure without receiving any treatment.
Costs and rationing of dialysis
Examples of estimated annual haemodialysis costs per patient include US$ 7332 in Brazil,17 US$ 7500 in China,15 US$ 5000 in India14 and US$ 6240 in Indonesia.18 Given the vast gulf between per capita spending on health care and costs of haemodialysis in LMIC, it is not surprising that maintenance haemodialysis is rarely a government priority and often confined to the private sector. Budgetary constraints and the lack of trained personnel necessitate strict rationing of RRT and encourage user-pays systems. A South African observational study found more than half of new ESKD patients were not offered RRT.19 Reasons, in most cases, were poverty-related – contraindications including unsuitable living circumstances, unemployment and lack of education. Dialysis rationing is therefore fraught with inequity. In the South African study, white patients were almost four times more likely to receive treatment. In many countries, provision of RRT depends primarily on whether the patient has health insurance or can otherwise afford treatment via means such as taking on loans, selling property, support from employers or charity.14
Kidney transplantation
Successful kidney transplantation is associated with significant improvements in survival and quality of life, as well as substantial cost-savings, compared with dialysis. In the United States of America, recipients aged 20–39 years are projected to live almost 17 years longer than patients remaining on the waiting list.20 Transplantation imposes far fewer restrictions on day-to-day activities than does dialysis – most recipients report close to normal physical function and higher scores for mental functioning.21 In HIC, the ongoing annual cost of maintaining a functioning transplant is approximately one-third to one-quarter that of dialysis.22 With better outcomes for patients and the potential for reduced per patient costs, shifting more of the RRT population from dialysis to transplantation is on the health-care agenda of many HIC, where the main impediment remains low rates of organ donation.
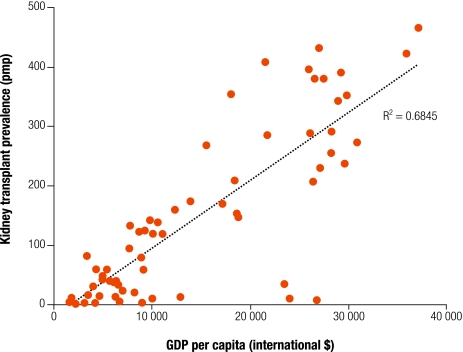
Rates of kidney transplantation vary widely internationally and serious global inequities in access to transplantation exist. For most LMIC, transplantation is rare due to lack of infrastructure (Fig. 2), and survival can be complicated by the affordability of immunosuppressive drugs, malnutrition and infectious disease, in particular tuberculosis.23 Antipathy towards organ donation may also be an issue. In Malaysia, although dialysis programmes have expanded rapidly over the past 10 years, kidney transplants have plateaued at an annual rate of 5–7 transplants per million population.24 This is despite access to the most recent immunosuppressive therapy and demonstrated ability to sustain long-term graft function at rates comparable to HIC.25 In some settings, gender inequality limits access to transplantation.26 For example, in India, transplants from living, related donors typically go from female donors to male recipients.14
Fig. 2.
Prevalence of kidney transplantation and GDP per capitaa
GDP, gross domestic product; pmp, per million population.
a Data obtained as for Fig. 1.
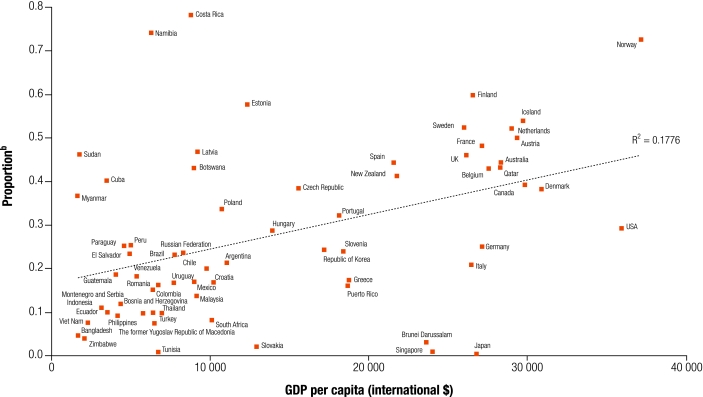
High rates of kidney transplantation can be achieved outside of HIC. Costa Rica is one example: in 2002, 78% of its RRT population had received transplants, among the highest proportion in the world (Fig. 3). This is attributed to a public health system which, covering 98% of the population, provides equitable access to RRT; and to high rates of living kidney donation (although deceased donation remains low).27
Fig. 3.
Proportion of overall RRT made up by kidney transplantation by GDP per capitaa
GDP, gross domestic product; RRT, renal replacement therapy.
a Data obtained as for Fig. 1.
b Data show the prevalence of functioning kidney transplants as a proportion of overall renal replacement therapy.
Addressing the burden of ESKD in LMIC
In the lowest-income countries where, realistically, most patients will not have access to RRT, prevention will be the key objective. Preventive interventions must be compatible with limited government revenues and health expenditure. Three-times a country’s gross national income (GNI) per capita is a recommended threshold for determining cost-effectiveness in developing settings.28 Multidrug regimens for the prevention of cardiovascular disease in at-risk populations of developing regions have been shown to be cost-effective according to this threshold.29 Data on the cost-effectiveness of intervention in chronic kidney disease is lacking, however it is recognized that chronic kidney disease prevention would be most cost-effective as part of an integrated strategy targeting chronic vascular diseases.30 An example of this type of integrated intervention has shown success in rural India, achieving blood pressure and diabetes targets, and lowering prevalence of chronic kidney disease at an annual cost of US$ 0.43 per capita of population.31 This programme minimized costs by using non-physician health workers and cheapest available diagnostic tests and drugs. Combination pharmacotherapy, a fixed dose of aspirin, a statin, an ACE-inhibitor and a diuretic/beta-blocker, may also have potential as an integrated approach to chronic vascular disease in LMIC.32
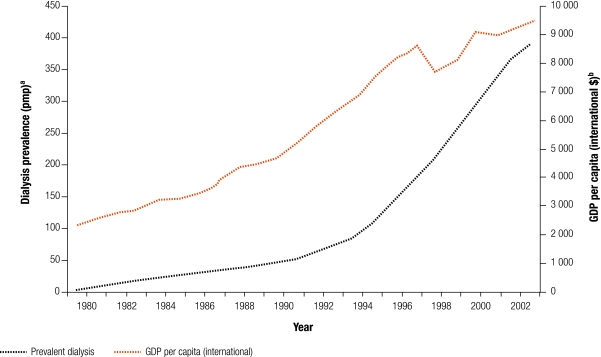
However, options for treatment should be available to those in LMIC who develop ESKD. Moreover, with economic growth, demand for RRT will increase (Fig. 4). This is due to:
Fig. 4.
Dialysis prevalence rate compared with GDP per capita in Malaysia from 1980 to 2003
GDP, gross domestic product; pmp, per million population.
a Data on dialysis prevalence from: Eleventh Report of the Malaysian Dialysis and Transplant Registry 2003 (www.msn.org.my/nrr/).
b Data on GDP per capita refers to purchasing power parity (PPP) international dollars 2002, obtained from World Bank’s World Development Indicators, 2005.
growth of the underlying burden of chronic kidney disease due to population ageing, urbanization and changing lifestyles;
development of the health sector and health insurance, making RRT affordable for more who need it;
increasing expectation of the population that, if they are sick, they will be treated;
increased willingness and ability of individuals to pay for treatment; and,
improvements in infrastructure, pharmaceutical availability and numbers of skilled health personnel.
A low level of economic development will almost certainly impose restrictions on the availability of RRT, not only due to the cost of RRT itself, but also because of the level of health and civil infrastructure required for dialysis and transplant programmes to deliver acceptable outcomes. However, underdeveloped organ donor and transplant programmes, health system and financing issues, issues of the ethical regulation of the transplantation process and the cost of pharmaceuticals commonly pose additional barriers to the delivery of efficient and cost-effective RRT.
Establishing organ donor and transplant programmes
Transplantation tends to be more cost-effective than dialysis, with successful transplantation incurring lower costs of treatment and producing better outcomes in terms of quality of life. Transplantation also results in a greater ability of patients to participate productively in the community. For persons living in LMIC, where there is little or no social safety net, transplantation might therefore constitute a particularly attractive treatment option. Establishing organ donor and transplant programmes requires that first the cultural and organization challenges to such programmes are recognized and addressed.
In many cultures, deceased donation/transplantation is not a widely acceptable practice.33 Japan has the highest prevalence of dialysis patients in the world, however less than 5% of the quarter of a million on maintenance dialysis have chosen to be registered with Japan’s Kidney Transplant Network.34 Belief about what happens after death, cultural resistance to mutilating the body and the idea of impurity associated with a dead body mean that brain death is not generally accepted.35 Brain death has also been a controversial issue for those of Islamic faith. Discussion and debate among Muslim religious clergy led to a 1996 declaration of the Islamic Organization of Medical Sciences allowing recovery of organs from brainstem-dead persons.33 Policy development regarding organ donation and transplantation must take into account people’s beliefs and values and the broader sociocultural context in which they live, and discussion and debates are important. Existing living-related donor transplantation should not be discouraged, although such programmes are unlikely to fully meet demand. There are other reasons to support deceased donor programmes – the risks and burdens to the living donor are avoided, and the deceased donor can potentially help multiple individuals through additional donations such as corneas. For deceased donor transplant programmes to develop in LMIC, education of health professionals and the public is needed to generate support for organ donation and increase the pool of potential donors. In the end, the balance of living and deceased donation needs to be tailored to the local situation and cultural context.
To maximize deceased donation, effective coordination is critical, alongside adequate infrastructure, trained personnel and conducive government policy. Centralized approaches to organ procurement tend to be most effective. In Brazil, increases in deceased donor kidney transplants have been achieved through a combination of improved organ procurement, education of transplant teams, better preservation techniques and the creation of a single waiting list.36 In Malaysia, an increase in deceased donor transplants followed the establishment of hospital-based Tissue Organ Procurement teams and centralization of the coordination of deceased donor transplantation.25 Global professional standards would assist optimization of provision and management of transplantation.
Health systems and financing
In low-income countries, government spending on health care is likely to be heavily supplemented by external aid and user fees. In middle-income countries, along with growth in formal employment, the economy and the security of institutions, comes growth of social-security systems or national health services, often with concurrent development of private insurance and private health systems.37 Financing of ESKD prevention and treatment should not rely exclusively on government funding, but should also make effective use of private and nongovernmental funding, as in the example of the living-related transplant sponsorship scheme conducted by the Sindh Institute of Urology and Transplantation in Karachi, Pakistan.38 Such schemes can have the advantage of containing costs as the providers are directly accountable for expenditure. Malaysia provides its dialysis services via an approximately equal mix of public hospitals, private centres and nongovernmental, not-for-profit centres run by groups such as Rotary, religious groups or the National Kidney Foundation. The Malaysian government provides fully-subsidized dialysis treatment to approximately 50% of patients, with additional funding from nongovernmental organizations (NGOs) and self-funding.24
Policy and regulation
National planning of RRT delivery is essential to contain costs and to promote equity in resource allocation.39 Different methods of provision of RRT will be appropriate for different settings. As rationing of dialysis is the norm for most LMIC, clear policies should state who is and who is not eligible for treatment.
Clear legal frameworks are lacking in several LMIC already undertaking transplantation, leading to ambiguity on complex ethical issues and creating environments which facilitate commercial organ trafficking and tourism. In the absence of deceased donor transplant programmes and limited living-related donors, many transplants come from paid living-unrelated donors. The largely unregulated environment in which commercial donation takes place, and the poverty in which donors live, mean that donors, and often recipients, generally lack protection from economic and physical exploitation from a trade driven largely by third parties. For example, donors often do not receive the full promised remuneration, and may face loss of income or employment due to persistent pain, decreased physical functioning, depression and stigmatization.40 Finally, transplant tourism leaves LMIC less able to meet the need for kidney transplants in their own communities. Moves towards national self-sufficiency with respect to kidney donation within HIC would counter this problem.
Legalization of payment for organs has been proposed in some HIC as a means of overcoming donor shortages.41 However in LMIC, sustainable increases in (and equitable access to) transplantation are more likely to be achieved through the development of regulated deceased and living-related donor programmes. Legislation, regardless of the scale of programmes, ultimately needs to cover criteria for brainstem death, consent, donor registration, ethical regulation of institutions and health professionals, and prevention of commercial transplantation, with adequate resources channelled into effective implementation of legislation.33
Cost of drugs and dialysis consumables
There is an urgent need to explore ways of providing high quality, lower cost RRT services. In LMIC, the high cost of imported consumables contributes to the expense of dialysis. Domestic manufacture might significantly reduce costs.12 Given the scale of local demand, domestic manufacture could be made feasible by serving export as well as local markets. Governments might provide incentives to foreign companies to facilitate licensing and registration for dialysis items, or implement programmes of cost containment in coordination with suppliers.42
Maximizing graft survival through exploration of barriers to optimal care such as poor affordability of immunosuppressive medications is also critical.23 The expiry in 1995 of the patent on Cyclosporine A opened the gateway to generic derivatives, making survival with a kidney transplant more of a possibility for the world’s poorest recipients.
Improving equity of access to RRT
Several international programmes have already been commissioned in response to the current and potential future burden of ESKD in LMIC and global inequities in access to treatment. Working groups include the Commission for the Global Advancement of Nephrology (COMGAN), Kidney Disease Improving Global Outcomes (KDIGO) and the Global Alliance for Transplantation (GAT). COMGAN, which has operated for over a decade under the auspices of the International Society of Nephrology, aids education, research, institutional partnerships and leadership in developing countries, principally through the Continuing Medical Education programme.43 KDIGO promotes worldwide coordination regarding clinical practice guidelines, to achieve broad dissemination of information and continuous quality improvement on a global scale.44 The Transplantation Society (an NGO in official relations with the WHO) launched GAT in 2004, an initiative which draws together international governmental and professional organizations (including the pharmaceutical industry as observers) with the mission of advancing safe, effective and ethical practice of transplantation for all patients in need through building and supporting relationships (cooperative planning, risk and resource sharing), improving the quality and flow of information, developing appropriate continuing education, and creating relevant professional guidelines such as those on living donor assessment and care of the transplant recipient.45–47
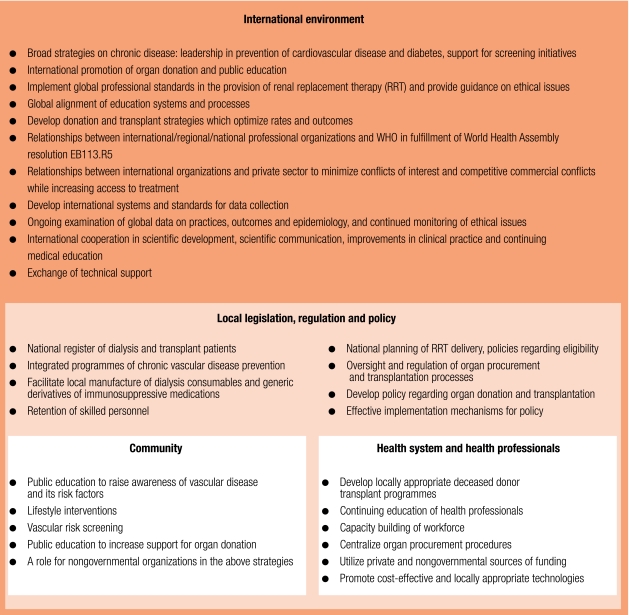
We outline a framework for improving global equity of access to treatment for ESKD in Fig. 5. Education and lifestyle intervention in the community, capacity building of health professionals, health system organization, and development of appropriate government policy have genuine potential to bring RRT to many more in need, and to safeguard the ethical delivery of dialysis and transplant services. Change within individual countries depends on a facultative international environment whereby professional organizations, governments, nongovernmental bodies and industry provide: i) leadership on prevention; ii) technical support, continuing medical education and financial aid; iii) evidence based guidelines and professional standards, and; iv) affordable drugs and dialysis consumables. However, success of such strategies may be precluded by local failure of governance to ensure implementation, especially attempts to regulate transplantation.48 Inadequate capacity of governments to regulate or capture the interests of relevant stakeholders will reduce the efficacy of policy initiatives, and therefore effective policy implementation mechanisms at the local level are a prerequisite.
Fig. 5.
A strategic framework for reducing the global burden of ESKD and improving equity of access to RRT
ESKD, end-stage kidney disease; RRT, renal replacement therapy.
Conclusions
The global burden of ESKD is concealed behind statistics which reflect only the number of people treated, not those who die of kidney failure or cardiovascular complications. This is particularly the case for LMIC, where resources to provide RRT are severely limited and where substantial underreporting of ESKD probably reflects a vast unmet need. Attention to both the prevention and management of ESKD is required. Successful RRT programmes have been established in select LMICs, and are testament to the viability of such programmes with the appropriate mix of local factors. Dialysis and transplant services need to be affordable, cost-effective and suited to local circumstances. The economic and quality-of-life advantages of transplantation make it an attractive modality over dialysis, and coordinated efforts to facilitate safe and ethical transplantation in LMIC are underway.46 Overall, global equity in provision of RRT will only be achieved through extensive public, patient and provider education, effective public policy, and ongoing support from international professional bodies, government and nongovernmental organizations. ■
Acknowledgements
We thank Dr Lai Seong Hooi and Associate Professor John Ayanian for their comments on this manuscript, and Dr Peter Arnold for his assistance in its preparation. Sarah White is supported by an Australian Postgraduate Award from the University of Sydney. Alan Cass is supported by a Senior Research Fellowship from the National Health and Medical Research Council of Australia. Stephen Jan is supported by a Career Development Award from the National Health and Medical Research Council of Australia.
Footnotes
Competing interests: None declared.
References
- 1.Moeller S, Gioberge S, Brown G. ESRD patients in 2001: global overview of patients, treatment modalities and development trends. Nephrol Dial Transplant. 2002;17:2071–6. doi: 10.1093/ndt/17.12.2071. [DOI] [PubMed] [Google Scholar]
- 2.Schieppati A, Remuzzi G. Chronic renal disease as a public health problem: epidemiology, social, and economic implications. Kidney Int Suppl. 2005;68(S98):7–10. doi: 10.1111/j.1523-1755.2005.09801.x. [DOI] [PubMed] [Google Scholar]
- 3.Modi GK, Jha V. The incidence of end-stage renal disease in India: a population-based study. Kidney Int. 2006;70:2131–3. doi: 10.1038/sj.ki.5001958. [Epub Oct 2006] [DOI] [PubMed] [Google Scholar]
- 4.Annual data report 2005 Bethesda, MD: United States Renal Data System, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2005. [Google Scholar]
- 5.ERA-EDTA registry: 2004 annual report Amsterdam: Academic Medical Centre; 2006.
- 6.Excell L, McDonald S, editors. Registry report ANZDATA Adelaide: Australia and New Zealand Dialysis and Transplant Registry; 2005. [Google Scholar]
- 7.Cusumano A, Garcia GG, Di Gioia C, Hermida O, Lavorato C. The Latin American Dialysis and Transplantation Registry (RLDT) annual report 2004. Ethn Dis 2006;16:S2-10-13. [PubMed]
- 8.Correa-Rotter R, Gonzalez-Michaca L. Early detection and prevention of diabetic nephropathy: a challenge calling for mandatory action for Mexico and the developing world. Kidney Int Suppl. 2005;68(S98):69–75. doi: 10.1111/j.1523-1755.2005.09813.x. [DOI] [PubMed] [Google Scholar]
- 9.Shaheen F, Al-Khader AA. Preventive strategies of renal failure in the Arab world. Kidney Int Suppl. 2005;68(S98):37–40. doi: 10.1111/j.1523-1755.2005.09807.x. [DOI] [PubMed] [Google Scholar]
- 10.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 11.Barsoum R. End-stage renal disease in North Africa. Kidney Int Suppl. 2003;63(S83):111–4. doi: 10.1046/j.1523-1755.63.s83.23.x. [DOI] [PubMed] [Google Scholar]
- 12.Kher V. End-stage renal disease in developing countries. Kidney Int. 2002;62:350–62. doi: 10.1046/j.1523-1755.2002.00426.x. [DOI] [PubMed] [Google Scholar]
- 13.Young EW, Mauger EA, Jiang KH, Port FK, Wolfe RA. Socioeconomic status and end-stage renal disease in the United States. Kidney Int. 1994;45:907–11. doi: 10.1038/ki.1994.120. [DOI] [PubMed] [Google Scholar]
- 14.Sakhuja V, Sud K. End-stage renal disease in India and Pakistan: Burden of disease and management issues. Kidney Int Suppl. 2003;63(S83):115–8. doi: 10.1046/j.1523-1755.63.s83.24.x. [DOI] [PubMed] [Google Scholar]
- 15.Lin S. Nephrology in China: A great mission and momentous challenge. Kidney Int Suppl. 2003;63(S83):108–10. doi: 10.1046/j.1523-1755.63.s83.22.x. [DOI] [PubMed] [Google Scholar]
- 16.D’Amico G. Opportunities for a chronic disease outreach program in China. Kidney Int Suppl. 2005;68(S98):46–8. doi: 10.1111/j.1523-1755.2005.09809.x. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Iturbe B, Bellorin-Font E. End-stage renal disease prevention strategies in Latin America. Kidney Int Suppl. 2005;68(S98):30–6. doi: 10.1111/j.1523-1755.2005.09806.x. [DOI] [PubMed] [Google Scholar]
- 18.Sitprija V. Nephrology in South East Asia: Fact and concept. Kidney Int Suppl. 2003;63(S83):128–30. doi: 10.1046/j.1523-1755.63.s83.27.x. [DOI] [PubMed] [Google Scholar]
- 19.Moosa MR, Kidd M. The dangers of rationing dialysis treatment: The dilemma facing a developing country. Kidney Int. 2006;70:1107–14. doi: 10.1038/sj.ki.5001750. [DOI] [PubMed] [Google Scholar]
- 20.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–30. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 21.Evans RW, Manninen DL, Garrison LP, Jr, Hart LG, Blagg CR, Gutman RA, et al. The quality of life of patients with end-stage renal disease. N Engl J Med. 1985;312:553–9. doi: 10.1056/NEJM198502283120905. [DOI] [PubMed] [Google Scholar]
- 22.Annual data report 2005, Bethesda, MD: United States Renal Data System, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2005. [Google Scholar]
- 23.Verma BS, Bhandari M, Kumar A. Transplantation in developing countries. Economics, reality, and solutions. Transplant Proc. 2000;32:1482. doi: 10.1016/S0041-1345(00)01298-7. [DOI] [PubMed] [Google Scholar]
- 24.Lim YN, Lim TO, editors. Eleventh report of the Malaysian dialysis and transplant registry 2003 Kuala Lumpur: National Renal Registry; 2004. [Google Scholar]
- 25.Hooi LS, Lela YM, editors. First report of the national transplant registry, Kuala Lumpur: National Transplant Registry; 2004. Available from: http://www.mst.org.my/
- 26.Ghods AJ, Nasrollahzadeh D. Gender disparity in a live donor renal transplantation program: assessing from cultural perspectives. Transplant Proc. 2003;35:2559–60. doi: 10.1016/j.transproceed.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Cerdas M. Chronic kidney disease in Costa Rica. Kidney Int Suppl. 2005;68(S97):31–3. doi: 10.1111/j.1523-1755.2005.09705.x. [DOI] [PubMed] [Google Scholar]
- 28.Macroeconomics and Health. Investing in health for economic development - Report of the Commission on Macroeconomics and Health. Geneva: WHO;2001. [Google Scholar]
- 29.Gaziano TA, Opie LH, Weinstein MC. Cardiovascular disease prevention with a multidrug regimen in the developing world: a cost-effectiveness analysis. Lancet. 2006;368:679–86. doi: 10.1016/S0140-6736(06)69252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dirks JH, de Zeeuw D, Agarwal SK, Atkins RC, Correa-Rotter R, D’Amico G, et al. Prevention of chronic kidney and vascular disease: toward global health equity — the Bellagio 2004 Declaration. Kidney Int Suppl. 2005;68(S98):1–6. doi: 10.1111/j.1523-1755.2005.09800.x. [DOI] [PubMed] [Google Scholar]
- 31.Mani MK. Nephrologists sans frontieres: Preventing chronic kidney disease on a shoestring. Kidney Int. 2006;70:821–3. doi: 10.1038/sj.ki.5001793. [DOI] [PubMed] [Google Scholar]
- 32.Wise J. Polypill holds promise for people with chronic disease. Bull World Health Organ. 2005;83:885–7. [PMC free article] [PubMed] [Google Scholar]
- 33.Gabr M. Organ transplantation in developing countries. World Health Forum. 1998;19:120–3. [PubMed] [Google Scholar]
- 34.The Japan Society for Transplantation. 2006Factbook Available from: http://www.asas.or.jp/jst/factbook/2006/fact06_03.html
- 35.Daar AS, Marshall P. Culture and psychology in organ transplantation. World Health Forum. 1998;19:124–32. [PubMed] [Google Scholar]
- 36.Zatz R, Romao JE, Jr, Noronha IL. Nephrology in Latin America, with special emphasis on Brazil. Kidney Int Suppl. 2003;63(S83):131–4. doi: 10.1046/j.1523-1755.63.s83.28.x. [DOI] [PubMed] [Google Scholar]
- 37.Schieber G, Maeda A. Health care financing and delivery in developing countries. Health Aff (Millwood) 1999;18:193–205. doi: 10.1377/hlthaff.18.3.193. [DOI] [PubMed] [Google Scholar]
- 38.Rizvi SA, Naqvi SA. Need for increasing transplant activity: a sustainable model for developing countries. Transplant Proc. 1997;29:1560–2. doi: 10.1016/S0041-1345(96)00674-4. [DOI] [PubMed] [Google Scholar]
- 39.Barsoum R. Haemodialysis: cost-conscious end-stage renal failure management. Nephrology. 1998;4(Suppl 2):96–100. doi: 10.1111/j.1440-1797.1998.tb00482.x. [DOI] [Google Scholar]
- 40.Goyal M, Mehta RL, Schneiderman LJ, Sehgal AR. Economic and health consequences of selling a kidney in India. JAMA. 2002;288:1589–93. doi: 10.1001/jama.288.13.1589. [DOI] [PubMed] [Google Scholar]
- 41.Friedman AL. Payment for living organ donation should be legalised. BMJ. 2006;333:746–8. doi: 10.1136/bmj.38961.475718.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aviles-Gomez R, Luquin-Arellano VH, Garcia-Garcia G, Ibarra-Hernandez M, Briseno-Renteria G. Is renal replacement therapy for all possible in developing countries? Ethn Dis 2006;16:S2-70-72. [PubMed]
- 43.Dirks JH, Robinson SW. The global perspective of the International Society of Nephrology: a decade of experience with COMGAN. Kidney Int. 2005;68:1395–410. doi: 10.1111/j.1523-1755.2005.00549.x. [DOI] [PubMed] [Google Scholar]
- 44.Port FK, Eknoyan G. The Dialysis Outcomes and Practice Patterns Study (DOPPS) and the Kidney Disease Outcomes Quality Initiative (KDOQI): a cooperative initiative to improve outcomes for hemodialysis patients worldwide. Am J Kidney Dis. 2004;44:1–6. doi: 10.1053/j.ajkd.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 45.Delmonico F. A Report of the Amsterdam Forum on the care of the live kidney donor: Data and medical guidelines. Transplantation. 2005;79(Suppl 2):53–66. doi: 10.1097/01.TP.0000157524.61834.EC. [DOI] [PubMed] [Google Scholar]
- 46.Global Alliance for Transplantation. Strategic direction setting and operational guidelines Montreal: The Transplantation Society; 2006. Available from: http://www.transplantation-soc.org/globalalliance.php
- 47.Abbud-Filho M, Adams PL, Alberu J, Cardella C, Chapman J, Cochat P, et al. A report of the Lisbon Conference on the care of the kidney transplant recipient. Transplantation. 2007;83:S1–22. doi: 10.1097/01.tp.0000260765.41275.e2. [DOI] [PubMed] [Google Scholar]
- 48.Muraleedharan VR, Jan S, Ram Prasad S. The trade in human organs in Tamil Nadu: the anatomy of regulatory failure. Health Economics. Policy and Law. 2006;1:41–57. doi: 10.1017/S1744133105001052. [DOI] [PubMed] [Google Scholar]