Abstract
Objective
To assess the field-test version of the new WHO Japanese encephalitis (JE) surveillance standards.
Methods
We applied the clinical case definition of acute encephalitis syndrome (AES), laboratory diagnostic criteria and case classifications to patients with suspected central nervous system (CNS) infections in southern Viet Nam.
Findings
Of the 380 patients (149 children) recruited with suspected CNS infections, 296 (96 children) met the AES case definition. 54 children were infected with JE virus (JEV), of whom 35 (65%) had AES, giving a sensitivity of 65% (95% CI: 56–73) and specificity of 39% (95% CI: 30–48). Nine adults with JEV presented with AES. 19 JEV-infected children missed by surveillance included 10 with acute flaccid paralysis, two with flaccid hemiparesis and six with meningism only. Altering the case definition to include limb paralysis and meningism improved sensitivity to 89% (95% CI: 83–95), while reducing specificity to 23% (95% CI: 15–30). Six children that did not have AES on admission had reduced consciousness after admission. Cerebrospinal fluid (CSF) analysis diagnosed seven patients negative on serum analysis. Five patients with neurological manifestations of dengue infection had JEV antibodies in serum and would have been misdiagnosed had we not tested for dengue antibodies in parallel.
Conclusion
Children infected with JEV that presented with acute limb paralysis or neck stiffness only were missed by the surveillance standards, although some of them subsequently became encephalopathic. A footnote in the surveillance standards drawing attention to these presentations would be helpful. An acute CSF sample is more sensitive and specific than an acute serum sample.
Résumé
Objectif
Evaluer la version d’essai sur le terrain de la nouvelle norme OMS de surveillance de l’encéphalite japonaise (EJ).
Méthodes
Nous avons appliqué la définition de cas clinique du syndrome encéphalitique aigu (SEA), les critères de diagnostic analytique et la classification des cas à des patients suspects d’infection du système nerveux central (SNC) dans le sud du Viet Nam.
Résultats
Parmi les 380 patients (dont 149 enfants) inclus dans l’étude avec une suspicion d’infection du SNC, 296 (96 enfants) répondaient à la définition de cas su SEA. Le virus de l’encéphalite japonaise (VEJ) infectait 54 des enfants, dont 35 (65%) présentaient un SEA, ce qui donne une sensibilité de 65% (IC à 95% : 56-73) et une spécificité de 39% (IC à 95% 30-48). Neuf adultes infectés par le virus présentaient un SEA. Parmi 19 enfants infectés par le VEJ et non détectés par la surveillance, on comptait 10 cas de paralysie flasque aiguë, dont deux avec une hémiparésie flasque et six avec un méningisme uniquement. Modifier la définition de cas pour y inclure la paralysie des membres et le méningisme permet d’amener la sensibilité à 89% (IC à 95% : 83-95), tout en réduisant la spécificité à 23% (IC à 95% : 15-30). Six enfants ne présentant pas de SEA à leur admission ont subi par la suite une dégradation de leur état de conscience. L’analyse du liquide céphalorachidien (LCR) a permis de diagnostiquer la maladie chez sept patients dont l’analyse du sérum était négative. Cinq patients avec des signes neurologiques de la dengue présentaient des anticorps contre le VEJ dans leur sérum et auraient fait l’objet d’une erreur de diagnostic si l’on n’avait pas recherché les anticorps de la dengue en parallèle.
Conclusion
Les enfants infectés par le VEJ présentant uniquement une paralysie aiguë des membres ou une raideur de la nuque n’ont pas été pris en compte par la norme de surveillance, bien que certains d’entre eux fussent atteints d’encéphalopathie. Il serait utile, dans cette norme, qu’une note de bas de page attire l’attention sur ces tableaux cliniques. L’analyse d’un échantillon de LCR provenant d’un cas aigu est plus sensible et spécifique que celle d’un échantillon de sérum pour le même type de cas.
Resumen
Objetivo
Evaluar la versión de prueba sobre el terreno de las nuevas normas de la OMS para la vigilancia de la encefalitis japonesa (EJ).
Métodos
Se analizó a pacientes con presunta infección del sistema nervioso central (SNC) en el sur de Viet Nam aplicando la definición de caso clínico de síndrome de encefalitis aguda (SEA), criterios de diagnóstico de laboratorio y clasificaciones de casos.
Resultados
De los 380 pacientes (149 niños) estudiados con presunta infección del SNC, 296 (96 niños) cumplían la definición de caso de SEA. Un total de 54 niños estaban infectados por el virus de la EJ (VEJ), y 35 (65%) de ellos presentaban SEA, con una sensibilidad del 65% (IC95%: 56 - 73) y una especificidad del 39% (IC95%: 30 - 48). Nueve adultos con VEJ presentaron inicialmente SEA. Entre los 19 niños infectados por el VEJ no detectados por el sistema de vigilancia había 10 con parálisis flácida aguda, dos con hemiparesia flácida y seis sólo con meningismo. El hecho de modificar la definición de caso para incluir la parálisis de miembros y el meningismo mejoró la sensibilidad hasta el 89% (IC95%: 83 - 95), pero redujo la especificidad al 23% (IC95%: 15 - 30). Seis niños sin SEA en el momento del ingreso presentaron una disminución de la conciencia una vez ingresados. El análisis del líquido cefalorraquídeo (LCR) permitió diagnosticar a siete pacientes con resultado negativo en el análisis del suero. Cinco pacientes con manifestaciones neurológicas de dengue presentaban anticuerpos contra el VEJ en el suero y habrían sido mal diagnosticados de no haberse analizado en paralelo los anticuerpos contra el virus del dengue.
Conclusión
Los niños infectados por el VEJ que presentaron al principio sólo parálisis de miembros o rigidez de cuello agudas escaparon a la detección con las normas de vigilancia empleadas, aunque algunos de ellos desarrollaron posteriormente encefalopatía. Convendría en este sentido insertar en las normas de vigilancia una nota a pie de página que advirtiera de la posibilidad de esa presentación inicial. En la fase aguda el análisis del LCR es más sensible y específico que el análisis del suero.
ملخص
الەدف
تقيـيم إصدارة الاختبار الميداني للمعايـير الجديدة لمنظمة الصحة العالمية الخاصة بترصُّد التەاب الدماغ الياباني.
الطريقة
لقد قام الباحثون بتطبيق تعريف الحالة السريرية لمتلازمة الالتەاب الدماغي الحاد، ومعايـير التشخيص المختبري، وتصنيف الحالات على المرضى المشتبە في إصابتەم بعدوى الجەاز العصبي المركزي في جنوب فييت نام.
الموجودات
وجد الباحثون أن من بين 380 مريضاً (منەم 149 طفلاً) ممن تم اختيارەم للاشتباە في إصابتەم بالتەاب الجەاز العصبي المركزي، لم ينطبق تعريف الحالة الخاصة بمتلازمة الالتەاب الدماغي الحاد سوى على 296 مريضاً (منەـم 96 طفلاً)، حيث يعاني 54 طفلاً من العـدوى بفيـروس التەـاب الدمـاغ اليابانـي، منەـم 35 طفـلاً (65%) يعانون من متلازمة الالتەاب الدماغي الحاد، مما يعطي حساسية تقدر بنحو 65% (73 – 56: lc 95%)، ونسبة نوعيــة تقدر بنحو 39% (48 – 30: lc 95%). ووجد أن تسعة من البالغين المصابين بفيروس التەاب الدماغ الياباني يعانون من متلازمة الالتەاب الدماغي الحاد، كما وجد أن 19 طفلاً مصاباً بفيروس التەاب الدماغ الياباني ممن لم يكتشفوا خلال الترصُّد، منەم 10 أطفال مصابون بالشلل الرخو الحاد، وطفلان يعانون من الفالج الرخو، وستة أطفال يعانون من حالة سحائية فقط. وقد ساعد تغيـير تعريف الحالة ليشمل شلل أحد الأطراف والحالات السحائية، على تحسين الحساسية لتصل إلى 89% (95 – 83: lc 95%)، وإنە قلص نسبة التحديـد لتصـل إلـى 23% (30 – 15: lc 95%). وقد عانى ستة أطفال من انخفاض مستوى الوعي عقب الدخول إلى المستشفى، بالرغم من أنەم لم يكونوا يعانون من متلازمة الالتەاب الدماغي عند دخول المستشفى. وشخص تحليل السائل الدماغي النخاعي سبعة مرضى كحالات سلبية وفقاً لتحليل المصل. ووجد أن خمسة مرضى يعانون من تظاەرات عصبية ناشئة عن عدوى الضنك لديەم أضداد في المصل لفيروس الالتەاب الدماغي الياباني، وكان يحتمل تشخيص حالتەم على نحو خاطئ ما لم نقم بإجراء أضداد الضنك على التوازي.
الاستنتاج
إن معايـير الترصُّد لم تظەر أي قصور في اكتشاف الأطفال المصابين بفيروس التەاب الدماغ الياباني سوى ممن ظەر لديەم شلل أحد الأطراف أو تيبس بالرقبة فقط، بالرغم من إصابة بعضەم في ما بعد بالالتەاب الدماغي. ومن المفيد في ەذا المضمار إضافة حاشية في معايـير الترصُّد تلفت الانتباە إلى ەذە المظاەر السريرية. ومن ثم نخلص إلى أن فحص عينة من السائل الدماغي النخاعي تتسم بالمزيد من الحساسية، والنوعية بالمقارنة بفحص عينة من المصل في الفترة الحادة.
Introduction
Japanese encephalitis (JE) is one of the most common encephalitides worldwide, with an estimated 30 000–50 000 cases and 10 000–15 000 deaths annually. The disease is caused by a mosquito-borne flavivirus (family Flaviviridae, genus Flavivirus), which is related to dengue and West Nile viruses and found across most of south and east Asia.1 In parts of rural Asia, almost all individuals are infected during childhood. About one in 300 infections cause symptoms2 ranging from non-specific febrile illness to severe meningoencephalitis, characterized by fever, reduced consciousness, seizures and focal neurological signs. In addition, the virus can cause aseptic meningitis or a poliomyelitis-like acute flaccid paralysis.
JE can be controlled by vaccination.3 The implementation of control programmes requires accurate data on disease burden, which in turn are dependent on disease surveillance. WHO has produced surveillance standards for JE, which are available as a field-test version. The standards include clinical case definitions to identify patients with an acute encephalitis syndrome (AES), recommended laboratory criteria for confirmation of JE virus (JEV) infection and case classification based on the results of these tests (Box 1).4
Box 1. WHO recommended case definition for Japanese encephalitis (JE) – field-test version4.
Clinical case definition
Clinically, a case of acute encephalitis syndrome (AES) is defined as a person of any age, at any time of year, with the acute onset of fever and a change in mental status (including symptoms such as confusion, disorientation, coma, or inability to talk) AND/OR new onset of seizures (excluding simple febrile seizures).a Other early clinical findings can include an increase in irritability, somnolence or abnormal behaviour greater than that seen with usual febrile illness.
Case classification
Suspected case: A case that meets the clinical case definition for AES. Suspected cases should be classified in one of the following four ways.
Laboratory-confirmed JE: A suspected case that has been laboratory-confirmed as JE.
Probable JE: A suspected case that occurs in close geographic and temporal relationship to a laboratory-confirmed case of JE, in the context of an outbreak.
Acute encephalitis syndrome – other agent: A suspected case in which diagnostic testing is done and an etiological agent other than JE virus is identified.
Acute encephalitis syndrome – unknown: A suspected case in which no diagnostic testing is done, or in which testing identified no etiological agent, or in which the test results were indeterminate.
Laboratory criteria for confirmation
Clinical signs of JE are indistinguishable from other causes of AES. Laboratory confirmation is therefore essential for accurate diagnosis of JE. Laboratory confirmation of a JE virus infection includes:
1. Presence of IgM antibodies specific to JE virus in a single sample of cerebrospinal fluid (CSF) or serum,b as detected by an IgM-capture ELISA specifically for JE virus;c
2. Detection of JE-virus antigens in tissue by immunohistochemistry;
3. Detection of JE-virus genome in serum, plasma, blood, CSFd or tissue by reverse transcriptase polymerase chain reaction (PCR) or an equally sensitive and specific nucleic acid amplification test;
4. Isolation of JE virus in serum, plasma, blood, CSFd or tissue;
5. Detection of a fourfold or greater rise in antibodies specific to JE virus as measured by haemagglutination inhibition (HI) or plaque reduction neutralization assay (PRNT) in serum collected during the acute and convalescent phase of illness. The two specimens for IgG should be collected at least 14 days apart. The IgG test should be done in parallel with other confirmatory tests to eliminate the possibility of cross-reactivity, as indicated in footnote c.
Most JE infections are asymptomatic. Therefore, in areas that are highly endemic for JE, it is possible to have AES due to a cause other than JE virus and have JE virus-specific IgM antibody present in serum. To avoid implicating asymptomatic JE as the cause of other AES illnesses, sterile collection and testing of a CSF sample from all persons with AES are recommended when feasible.
Only the first five to 10 cases of an outbreak need be confirmed through laboratory testing. During periods of epidemic transmission of JE virus, laboratory confirmation of every case may not be necessary.
aA simple febrile seizure is one in a child aged six months to less than six years old, the only finding is fever and a single generalized convulsion lasting less than 15 min, and who recovers consciousness within 60 min of the seizure.
bA serum sample should be obtained at admission. Because it may not yet be positive in a JE-infected person, a second serum sample should be collected at discharge or on the 10th day of illness onset or at the time of death and tested for presence of JE virus specific IgM.
cFurther confirmatory tests (e.g. looking for cross-reactivity with other flaviviruses circulating in the geographical area) should be done: when there is an ongoing dengue or other flavivirus outbreak; when vaccination coverage is very high; or in cases in areas where there are no epidemiological and entomological data supportive of JE transmission.
dDetection of virus genome or virus isolation in serum, plasma or blood is very specific for JE diagnosis; however, it is not sensitive as virus levels are usually undetectable in a clinically ill JE case. Therefore a negative result by these methods should not be used to rule out JE in a suspected case. Similarly detection of virus genome or virus isolation in CSF is usually only found in fatal cases and therefore not very sensitive and should not be used for ruling out a diagnosis of JE
Laboratory confirmation of JEV infection is not straightforward because attempts to isolate the virus are usually negative.5 Diagnosis is, therefore, usually based on detection of antibodies in serum or cerebrospinal fluid (CSF); however, serum antibodies can result from recent coincidental non-neurological, rather than causative, infection with the virus.6 There is also cross-reactivity of antibody with other flaviviruses, particularly dengue, which means that dengue infection can be misdiagnosed as JE unless antibodies for both are tested for in parallel.7
The JE surveillance standards were drawn up according to recommendations provided by an expert committee. The need to base guideline development on the best available evidence is being emphasized increasingly at WHO,8 which recommends that they are assessed by use in patients with detailed clinical and diagnostic information. In this report, we apply the standards to patients with suspected central nervous system (CNS) infections in southern Viet Nam, where JE and dengue are endemic, who had detailed clinical and virological work-up as part of previous prospective studies.9–11
Materials and methods
Patients were recruited in the paediatric and adult intensive care units at the Centre for Tropical Diseases, Ho Chi Minh City, Viet Nam, an infectious-diseases referral hospital for much of the south of the country. Study protocols were approved by the hospital’s scientific and ethical committee; consent was obtained from patients or accompanying relatives.
Clinical and virological methods
For one year, from January 1995, all children (< 15 years) and adults with a suspected CNS infection were studied. CNS infections were suspected in patients with a fever, or history of fever and at least one of the following: reduced consciousness (Glasgow coma score of 14 or lower for adults8 or Blantyre coma score less than 4 for children aged less than six years),9 severe headache, neck stiffness, focal neurological signs, tense fontanelle or convulsions. Patients with slide-positive cerebral malaria or clinical features of tetanus were admitted to specialized wards and not included in this series. Children age six months to less than six years with a single convulsion lasting less than 15 minutes who recovered consciousness within 60 minutes were considered to have had a simple febrile convulsion10 and were not studied. Clinical-history examination, methods of investigation and treatment have been described previously.9–11
Measurement of IgM and IgG antibodies against JEV and dengue in serum and CSF and criteria for distinguishing the two infections and distinguishing patients with a primary infection from those previously infected with a different flavivirus (a secondary infection) were as described previously.7,12–14 JEV was distinguished from dengue on the basis of greater antibody titres, and primary infections were distinguished from secondary infections by ratio of IgM to IgG. Virus isolation, reverse transcriptase polymerase chain reaction (PCR) and immunohistochemical detection of virus in autopsy material were described previously.9,10,15,16
Clinical diagnoses
Patients with reduced consciousness were diagnosed as having encephalitis if there was no metabolic or other explanation and they had focal neurological signs or CSF pleocytosis (corrected white-cell count > 5/µl) or convulsions other than simple febrile convulsions;10 patients with none of these features were considered to have an acute encephalopathy. If they had a positive culture for Salmonella typhi or strongly positive Widal test, they were diagnosed as typhoid encephalopathy; if other organisms were cultured from the blood, the diagnosis was encephalopathy associated with septicaemia.
Pyogenic meningitis was diagnosed in patients with CSF cell counts greater than 100/µl with a predominance of polymorphonuclear cells and low CSF-to-plasma glucose ratio (< 50%) or CSF cell count greater than 1000/µl; this was classified as culture positive or culture negative depending on the results of CSF culture. Aseptic meningitis was diagnosed in fully conscious patients, with lymphocytic CSF (< 1000/µl) and normal glucose ratio (≥ 50%). Tuberculous meningitis was diagnosed in patients with lymphocytic CSF and low glucose ratio (typically < 30%), or with any CSF pleocytosis (< 500 cells/µl) if the history was suggestive of chronic meningitis, or the patient had evidence of systemic tuberculosis, or there was a family member with tuberculosis. Patients with neck stiffness but no CSF pleocytosis were diagnosed as having meningism. Brain abscesses, tumour and strokes were diagnosed with computed tomography scans at a nearby hospital. Subarachnoid haemorrhage was diagnosed on the basis of history and a high CSF red-cell count that did not change between sequential CSF bottles. Complex febrile convulsions were diagnosed in children with fever and convulsions that did not meet the simple febrile convulsions case definition, for example, because they had focal convulsions or multiple generalized convulsions. Myelitis was diagnosed in those with acute flaccid paralysis associated with acute febrile illness, and Guillain-Barré syndrome was diagnosed as described previously.9 Clinical judgement was used to determine the most likely diagnosis for patients fitting more than one or no diagnostic category.
Surveillance case definitions
After the clinical recruitment had been completed and data entered onto a database, patients were classified according to the WHO clinical case definitions (Box 1).4 Patients were defined as having altered mental status if they had confusion, altered behaviour, disorientation, coma or inability to talk, either in the history or on initial examination. Patients were defined as having new-onset seizures if history was suggestive of a seizure or they were having seizures on examination. Patients with an acute febrile illness, and altered mental status or new-onset seizures, were defined as meeting WHO criteria for acute encephalitis syndrome (AES).4 To examine the possible role of other clinical features in case definitions for surveillance, we defined patients as having meningism if there was history or examination findings of neck stiffness. In patients whose consciousness level would allow assessment, acute paralysis was defined as rapid onset of weakness (reduced movement and power); if associated with reduced or absent deep-tendon reflexes and reduced tone, this was classified as acute flaccid paralysis.17 Patients with acute paralysis, cranial-nerve signs, other brainstem signs, lateralising signs or pyramidal or extrapyramidal signs were defined as having focal neurological signs.
Statistical methods
The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of the AES case definition and modifications to it for detecting JEV infection were assessed.
Results
Demographics
Of 386 patients (152 children) recruited, 380 (149 children) had sufficient information for inclusion (Fig. 1 and Fig. 2). The median age of children was 5 years (range: 0.167–15 years) and of adults was 32 years (range: 15–85 years). 71 patients died, 119 had neurological sequelae and 162 recovered with minor or no sequelae; for 28 the outcome was unknown because they were lost to follow-up at another hospital or they self-discharged. 54 children and nine adults were infected with JEV; 16 patients (seven children) were infected with dengue viruses; 217 patients had no serological evidence of infection with JEV or dengue, and in 84 patients serological diagnosis was not possible because only acute negative samples were available. These patients had the status JEV unknown.
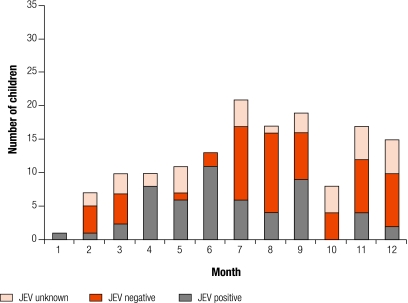
Fig. 1.
Admissions of children with suspected CNS infections who were JEV positive, negative or unknown
CNS, central nervous system; JEV, Japanese encephalitis virus.
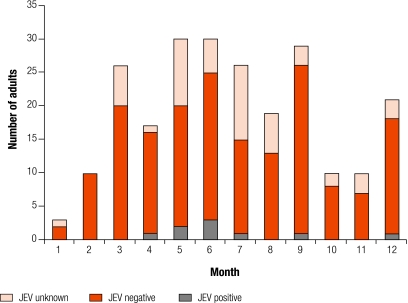
Fig. 2.
Admissions of adults with suspected CNS infections who were JEV positive, negative or unknown
CNS, central nervous system; JEV, Japanese encephalitis virus.
Most patients with JEV were admitted during the rainy season (April–September), but occasional cases were admitted throughout the year (Fig. 1 and Fig. 2). The median length of illness was 4 days (range: 0.5–28 days) for all children; 119 (80%) had been ill for 7 days or less, and 140 (94%) for 14 days or less. For the 54 children that were JEV positive, the median length of illness was 5 days (range: 2–15 days); 45 (83%) had been ill for 7 days or less, and 53 (98%) for 14 days or less. The median illness length for all adults was 6 days (range: 0.5–120 days); 137 (59%) had been ill for 7 days or less, and 195 (84%) for 14 days or less.
Clinical case definitions
The 380 patients were classified according to whether they met the WHO case definition of AES (i.e. symptoms of a change in mental status or new onset seizures) and the status of JEV infection (Table 1). Among the 278 patients with altered mental status, 244 had both a history of altered consciousness and reduced coma score on admission, 12 had a history only but were fully conscious on initial assessment in hospital, and 22 had altered consciousness on examination not in history. Among the 124 patients with new onset seizures, 72 had histories of seizures, 46 had seizures on examination and gave histories of seizures and six had seizures on examination (mostly focal seizures) but not in their histories. 16 children had new onset seizures without a change in mental status; typically these were children with focal seizures or with several brief generalized seizures with rapid recovery of consciousness between seizures (i.e. they had complex febrile convulsions).
Table 1. AES classification on admission for 380 patients, according to JEV status.
| AES classification | Children |
Adults |
Total (N = 380) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| JEV positive (N = 54) | JEV negative (N = 62) | JEV unknown (N = 33) | All (N = 149) | JEV positive (N = 9) | JEV negative (N = 171) | JEV unknown (N = 51) | All (N = 231) | ||||
| Change in mental status, new-onset seizures or botha | 35 | 38 | 23 | 96 | 9 | 145 | 46 | 200 | 296 | ||
| Change in mental status onlya | 14 | 15 | 9 | 38 | 7 | 101 | 26 | 134 | 172 | ||
| New onset seizures only | 1 | 10 | 5 | 16 | 0 | 1 | 1 | 2 | 18 | ||
| Both change in mental status and seizures | 20 | 13 | 9 | 42 | 2 | 43 | 19 | 64 | 106 | ||
| Neither change in mental status nor seizures | 19 | 24 | 10 | 53 | 0 | 26 | 5 | 31 | 84 | ||
AES, acute encephalitis syndrome; JEV, Japanese encephalitis virus. a Acute encephalitis syndrome is defined as an acute febrile illness with change in mental status, new-onset seizures or both.
Sensitivity, specificity, PPV and NPV were calculated after removing patients in whom the JEV status was not known (Table 2). For children, the AES definition was 65% (95% CI: 56–73%), the specificity was 39% (95% CI: 30–48%), PPV 48%, and NPV 56%. For adults the AES definition was 100% sensitive, 16% (95% CI: 10–21%) specific, PPV 6% and NPV 100%. 19 (35%) JEV-infected children did not have AES on admission to hospital: 10 had acute flaccid paralysis in one or more limbs (six of whom also had meningism), two had flaccid hemiparesis (one of whom had meningism), six had meningism only, and one had high fever and severe headache.
Table 2. Effect of different case definitions on the sensitivity, specificity and positive and negative predictive values for JEV infection in children.
| Case definition | JE positive (N = 54) | JE negative (N = 62) | JE unknown (N = 33) | All (N = 149) | Sensitivity (%)a | Specificity (%)a | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|
| AES | 35 | 38 | 23 | 96 | 65 (56–73) | 39 (30–48) | 48 | 56 |
| AES, acute paralysis or both | 47 | 46 | 26 | 119 | 87 (81–93) | 26 (18–34) | 51 | 70 |
| AES, meningism or both | 48 | 48 | 29 | 125 | 89 (83–95) | 23 (15–30) | 50 | 70 |
| AES at any time during hospitalization | 41 | 44 | 25 | 110 | 76 (68–84) | 29 (21–37) | 48 | 58 |
AES, acute encephalitis syndrome (defined as acute febrile illness and change in mental status or new onset seizures); JEV, Japanese encephalitis virus; NPV, negative predictive value; PPV, positive predictive value. a 95% confidence intervals are presented in parentheses.
Because the AES case definition for children on hospital admission had only 65% sensitivity for JEV-infected cases, we also examined modified case definitions. Changing the case definition to include fully conscious children with acute paralysis would include a further 12 JEV-infected children and eight JEV-negative children, thus improving the sensitivity to 87% (95% CI: 81–93%), but reducing the specificity to 26% (95% CI: 18–34%; Table 2). Alternatively including meningism would improve the sensitivity to 89%, with a change in specificity to 23%. Including both limb paralysis and meningism in the case definition would identify 53 of the 54 JEV-infected children and miss just one child with fever and severe headache. Six of 19 JEV-infected children who did not meet the AES case definition on entry did develop reduced consciousness (associated with seizures in three cases) after admission (Table 2). Six JEV-negative children also had altered mental status after admission. Thus, changing the case definition to include altered mental status or seizures at any time during hospitalization increased sensitivity for JEV-infection in children to 76% (95% CI: 68–84%), but reduced specificity to 29% (95% CI: 21–37%).
Laboratory criteria for diagnosis
The 63 patients with JEV infection comprised 62 with IgM antibodies to JEV and one with virus detected in post-mortem brain material by immunohistochemistry,16 for whom serum or CSF samples were unavailable. For the 62 diagnosed by IgM detection, 41 were positive on their initial serum sample; 25 of these patients had a CSF sample taken on the same day, which was IgM positive in 24, and negative in one. This patient had serial serum samples with decreasing titres of IgM and IgG, suggesting recent coincidental JEV infection, rather than a causal infection. Seven (11%) patients had negative admission serum samples, but the CSF on the same day was positive; for two of these a second serum sample was taken a few days later, which were both positive, confirming seroconversion. Two additional patients for whom no serum sample was available had positive initial CSF. A further 12 (19%) patients had negative serum and CSF samples on admission, but subsequent serum samples were positive. Two of these patients also had subsequent positive CSF samples. The median (range) interval between the first and second serum samples was 7 days (range: 5–26 days). Thus a single admission serum sample diagnosed 41 (68%) of the 60 JEV patients with this sample available, whereas an admission CSF sample diagnosed 33 (72%) of 46 patients. Serum IgM was positive for 24 of 31 patients with a positive CSF, and negative for 201 of 202 patients with a negative CSF [sensitivity 77% (95% CI: 72–82), specificity 99% (95% CI: 98–100), PPV 96% and NPV 97%].
For the 60 patients with a diagnosis based on serum antibodies, 39 had a primary flavivirus infection. By use of alternative criteria,13 29 were classed as primary, 21 as secondary and 10 could not be classified. Those with secondary infection were less likely to have high titres of IgM antibody in their first sample than those with primary infection [eight (62%) of 21 versus 33 (85%) of 39, P < 0.001]; this remained true if the alternative criteria were used to distinguish primary from secondary infection, 10 (48%) of 21 patients versus 25 (86%) of 29 patients (P = 0.009).
In addition to the 62 patients described above, five had JEV IgM (> 40 units) in their serum but were actually infected with dengue virus. For two of these patients, antibody against JEV was detected in the admission sample; for four of these patients, CSF was tested at the same time but was negative for JEV in all cases. All five patients had high titres of dengue antibody at least 1.8 times the JEV titres, and four also had dengue virus detected in their serum by culture or PCR.10 In addition to these five patients with dengue, 11 patients with suspected CNS infections were positive for dengue but did not have antibody against JEV.
Virus isolation was attempted on 67 samples (15 serum, 33 CSF and 19 post-mortem needle biopsy or autopsy samples) from 48 patients. Dengue virus was isolated from three patients as described previously,10 but there were no other virus isolates. Reverse transcriptase PCR for JEV was done on the CSF of 75 patients (39 with JEV infection diagnosed by IgM detection) and the serum of two patients who seroconverted for JEV, but results were negative in all cases.
Case classification
44 patients with AES had “laboratory-confirmed JE” (Box 1). 92 patients would have been classified as having “probable JE” – i.e. coming from rural provinces of southern Viet Nam where confirmed cases occurred during the April to September seasonal outbreak (Box 1); however, serological investigations revealed that 73 of these patients were negative for JEV. For the remaining 19, the JEV status was unknown. In Table 3, the final clinical and microbiological diagnosis for all 296 AES and 84 non-AES patients is given.
Table 3. Clinical diagnosis, initial AES classification and final AES classification for 149 children and 231 adults with suspected infections of the central nervous system.
| Clinical diagnostic groupa | Etiologyb | AES on admission? |
Final case classification for patients with AESc | |||||
|---|---|---|---|---|---|---|---|---|
| No |
Yes |
|||||||
| Children | Adults | Children | Adults | |||||
| Encephalitis | JEV | 6 | 0 | 35 | 9 | Laboratory-confirmed JE | ||
| Dengue | 0 | 0 | 2 | 1 | AES – other agent | |||
| Rabies | 0 | 0 | 1 | 1 | AES – other agent | |||
| Unknownd | 1 | 0 | 7 | 30 | AES – unknown | |||
| ADEM (post rabies vaccination) | 0 | 0 | 0 | 2 | AES – unknown | |||
| Encephalopathy | Dengue (DF or DHF) | 0 | 0 | 2 | 5 | AES – other agent | ||
| Typhoid | 0 | 0 | 3 | 4 | AES – other agent | |||
| Malaria | 0 | 0 | 2 | 3 | AES – other agent | |||
| Leptospirosis | 0 | 0 | 0 | 1 | AES – other agent | |||
| Unknown | 0 | 0 | 7 | 38 | AES – unknown | |||
| Sepsis – other | 0 | 0 | 1 | 11 | AES – other agent | |||
| Pyogenic meningitis | Culture positive | 5 | 2 | 1 | 14 | AES – other agent | ||
| Culture negative | 5 | 5 | 7 | 17 | AES – unknown | |||
| JEVe | 1 | 0 | 0 | 0 | N/A | |||
| Tuberculous meningitis | 1 | 10 | 7 | 28 | AES – unknown | |||
| Aseptic meningitis | JEV | 4 | 0 | 0 | 0 | N/A | ||
| Mumps | 0 | 1 | 0 | 0 | N/A | |||
| Unknown | 1 | 5 | 0 | 0 | N/A | |||
| Meningitis – fungal | 0 | 2 | 0 | 1 | AES – other agent | |||
| Meningism | JEV | 2 | 0 | 0 | 0 | N/A | ||
| Assoc with pneumonia | 2 | 0 | 1 | 0 | AES – unknown | |||
| Assoc with other viral illness | 2 | 2 | 0 | 0 | N/A | |||
| Myelitis | JEV | 6 | 0 | 0 | 0 | N/A | ||
| Dengue | 1 | 1 | 0 | 0 | N/A | |||
| Unknown | 2 | 0 | 2 | 1 | AES – unknown | |||
| Abscess | 2 | 2 | 0 | 5 | AES – other agent | |||
| Guillain-Barré syndrome | 6 | 0 | 1 | 0 | AES – unknown | |||
| Complex febrile convulsion | 0 | 0 | 12 | 0 | AES – unknown | |||
| Tense fontanelle | 6 | 0 | 1 | 0 | AES – unknown | |||
| Non-infectious cause | 0 | 1 | 4 | 29 | AES – other agent | |||
| Total | 52 | 32 | 96 | 200 | ||||
ADEM, acute disseminated encephalomyelitis; AES, acute encephalitis syndrome; DF, dengue fever; DHF, dengue haemorrhagic fever; JE, Japanese encephalitis; JEV, Japanese encephalitis virus; N/A, not applicable. a Clinical diagnostic group was based on the initial clinical and (cerebrospinal fluid) CSF findings. b Etiology is that determined after investigations were completed. c Laboratory-confirmed JE patients meet AES case definition on admission; AES – other agent, if an alternative agent or non-infectious cause was identified; AES – unknown, if no definite cause identified. d One JEV-negative child presented with fever, focal neurological signs, but no loss of consciousness and had encephalitis diagnosed on computed tomography. e One child with a neck stiffness, and extensor plantars, had a CSF white-cell count of 200/ml, 55% neutrophils, a glucose ratio of 53% and protein of 63.5 mg/dl was diagnosed clinically as having pyogenic meningitis, but CSF bacterial cultures were negative, and the CSF was positive for JEV IgM antibody.
Another cause was found for 90 patients. 15 had culture positive acute bacterial meningitis (seven with pneumococcus, six with other Streptococcus, four with meningococcus, two with Staphylococcus, two with Haemophilus influenzae, and one with Klebsiella); 19 had encephalopathy associated with typhoid (seven patients) or other systemic sepsis (including two with Pseudomonas, two with meningococcus, one with Escherichia coli, and one with non-cholera Vibrio); 34 had non-infectious causes, including 10 with strokes, eight with subarachnoid haemorrhage, seven with epilepsy and five with tumours. In five patients encephalopathy was attributed to malaria, which had been partially pretreated in four patients and was associated with Plasmodium vivax infection in one patient. 161 patients fell into the “AES – unknown” category; these included 45 patients with encephalopathy and 37 with a clinical diagnosis of encephalitis for whom the cause was unknown, 35 in whom tuberculous meningitis was suspected clinically but not confirmed, and 25 that had pyogenic meningitis clinically but in whom no agent was identified. 12 children with complex febrile seizures fell into the AES – unknown classification.
Discussion
Our study has shown that in this area of southern Viet Nam, where JE is endemic, the field-test version of the WHO JE surveillance standards would have detected about two-thirds of the 54 children and all of the nine adults with neurological disease caused by JEV. This area is typical of many parts of tropical Asia where JE occurs with most cases occurring in children, year-round disease with a summer peak and coexistent dengue disease. One-third of the JEV-infected children presented with acute limb paralysis, meningitis or both, and so would not have been detected by the current AES case definition. Although it was recognized when the surveillance standards were drawn up that patients with acute paralysis and meningitis would be missed, whether this would be a significant number of patients was then unclear. Given that these patients represent one-third of cases in this series, this group is important. Although pure aseptic (viral) meningitis is a self-limiting illness of no major consequence, the same cannot be said of paralytic illness, as most patients are left with substantial disability.9 Of the 12 patients with acute paralysis, 10 met the case definition of acute flaccid paralysis (implying viral myelitis or inflammation in the spinal cord), whereas the other two had hemiparesis. For JEV surveillance, the distinction is probably unimportant; the ability to recognize a child with an acute febrile illness and asymmetrical limb weakness would probably suffice.
Although acute paralysis due to JEV has been reported in other countries, including India and Japan,18–20 that it is sometimes labelled as Guillain-Barré syndrome18 emphasizes the under-recognition and highlights the importance of drawing attention to this presentation. JEV is increasingly recognized as a cause of aseptic meningitis,11,21 The surveillance standards currently make no mention of patients presenting with meningism or limb paralysis. The guidance should suggest that such patients are observed closely and classified as AES if they develop altered mental status (six of the patients that presented with paralysis in our study did develop AES after admission). Alternatively, the case definition could be modified to include patients with an acute febrile illness and limb paralysis or neck stiffness, for whom the term “acute CNS syndrome” could be used. However, broadening the case definition would greatly increase the number of samples from suspected JE patients, especially if meningism were included as well as paralysis with implications in terms of laboratory capacity and costs.
Our study makes important observations about the role of viral diagnostics in surveillance for JE, especially the utility of the different samples. For patients with both CSF and serum taken on the same day, serum IgM was positive in just over three quarters of patients with positive CSF. Unlike serum, CSF did not give a false JEV positive for patients with neurological disease due to dengue infection (in the few cases we studied). Had we relied solely on serum JEV-IgM testing, five patients with neurological dengue would have been misdiagnosed as JE. Vaccination against JE has increased in southern Viet Nam since the time of our study. With increasing vaccination against JE across Asia, the issue of testing CSF rather than serum samples becomes even more important. After vaccination, individuals develop IgM antibody against JEV in serum but probably not in CSF. When such individuals present to hospital with another cause of CNS disease, they can be misdiagnosed as having JE if their diagnosis is based solely on serological testing. This occurred during the first Nipah virus outbreak in Malaysia as some patients had recently been vaccinated against JE.22 We are likely to see much more misdiagnosis if JE diagnosis is based solely on serum IgM, which could lower confidence in JE-vaccination campaigns.
In our study, a single acute sample diagnosed approximately 70% of patients with JE. If the first sample is negative or the result is not yet available a second sample should be taken a few days later, ideally on the 10th day of illness (or after), by which time almost all patients have seroconverted.12,23 If the second sample is negative, this virtually excludes JE as the diagnosis. Most of our patients that seroconverted in hospital had an IgM and IgG profile characteristic of a secondary flavivirus infection (i.e. a slow IgM rise and more rapid IgG rise) and are presumed to have had a primary infection with dengue virus. In areas where JEV is the only flavivirus, more patients are likely to be diagnosed on their initial sample. If a patient dies or is to be discharged before the 10th day of illness, the second sample can be taken at this time. Approximately 80% of patients with JE have seroconverted by the fifth or sixth days of illness,12,23 and in our study one patient was diagnosed with a second sample taken just 5 days after the first. Some patients who die from JE without producing antibodies can only be diagnosed by detecting the virus.24 Our attempts at virus isolation and nucleic acid amplification were not successful – possibly because of issues relating to freezing and transporting samples, plus the limitations of PCR techniques in the 1990s. More modern methods of viral RNA detection would likely be more successful.21 However, for the purposes of disease surveillance in resource-poor parts of tropical Asia, efforts should probably still focus on getting appropriate samples for IgM ELISA diagnostics.
In summary, in an area endemic for JE and dengue, the field-test version of the JE surveillance standards detected about two-thirds of children hospitalized with JEV infection. Children that presented with acute limb paralysis or neck stiffness only were missed, although some of them subsequently became encephalopathic. A footnote in the surveillance standards that draws attention to these presentations would be helpful [such a footnote will be included in the next version of the WHO standards]. CSF was the most useful sample in terms of sensitivity and specificity; however, this test alone missed 20% of patients, and acute and convalescent serum samples are also recommended. The surveillance standards should also be assessed in other JE endemic and epidemic areas. There are many publications on confirmed JE, but the standards can only be assessed by study of all patients with suspected CNS disease. The formal evaluation of WHO Surveillance Standards and other guidelines during their development provides a useful evidence base to support their recommendation and should limit the controversy that sometimes is associated with their introduction.25,26 ■
Acknowledgements
We thank the Director and staff of the Centre for Tropical Diseases Ho Chi Minh City for their support; and David Vaughn and staff at AFRIMS, Bangkok, for virological support.
Footnotes
Funding: We thank the Wellcome Trust of Great Britain for funding the initial clinical studies, and the Bill and Melinda Gates Foundation for funding the JE Program at PATH, Seattle and the Medical Research Council of the United Kingdom for additional funding.
Competing interests: TS was on the WHO expert panel that produced the Japanese Encephalitis Surveillance Standards.
References
- 1.Tsai TR, Solomon T, Vaughn DW. Flaviviruses (yellow fever, dengue, dengue hemorrhagic fever, Japanese encephalitis, West Nile, St Louis encephalitis, tick-borne encephalitis). In: Mandell GL, Bennett JE, Dolin R, eds. Principles and practice of infectious diseases, 6th ed. Philadelphia: Elsevier; 2004. pp.1926-50. [Google Scholar]
- 2.Vaughn DW, Hoke CH. The epidemiology of Japanese encephalitis: prospects for prevention. Epidemiol Rev. 1992;14:197–221. doi: 10.1093/oxfordjournals.epirev.a036087. [DOI] [PubMed] [Google Scholar]
- 3.Solomon T. Control of Japanese encephalitis – within our grasp? N Engl J Med. 2006;355:869–71. doi: 10.1056/NEJMp058263. [DOI] [PubMed] [Google Scholar]
- 4.WHO – recommended standards for surveillance of selected vaccine-preventable diseases Geneva: WHO; 2006. Available from: http://www.who.int/vaccines-documents/DocsPDF06/843.pdf
- 5.Leake CJ, Burke DS, Nisalak A, Hoke CH. Isolation of Japanese encephalitis virus from clinical specimens using a continuous mosquito cell line. Am J Trop Med Hyg. 1986;35:1045–50. doi: 10.4269/ajtmh.1986.35.1045. [DOI] [PubMed] [Google Scholar]
- 6.Burke DS, Nisalak A. Detection of Japanese encephalitis virus immunoglobulin M antibodies in serum by antibody capture radioimmunoassay. J Clin Microbiol. 1982;15:353–61. doi: 10.1128/jcm.15.3.353-361.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Innis BL, Nisalak A, Nimmannitya S, Kusalerdchariya S, Chongswasdi V, Suntayakorn S, et al. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am J Trop Med Hyg. 1989;40:418–27. doi: 10.4269/ajtmh.1989.40.418. [DOI] [PubMed] [Google Scholar]
- 8.Hill S, Pang T. Leading by example: a culture change at WHO. Lancet. 2007;369:1842–4. doi: 10.1016/S0140-6736(07)60676-X. [DOI] [PubMed] [Google Scholar]
- 9.Solomon T, Kneen R, Dung NM, Khanh VC, Thuy TTN, Ha DQ, et al. Poliomyelitis-like illness due to Japanese encephalitis virus. Lancet. 1998;351:1094–7. doi: 10.1016/S0140-6736(97)07509-0. [DOI] [PubMed] [Google Scholar]
- 10.Solomon T, Dung NM, Vaughn DW, Kneen R, Thao LTT, Raengsakulrach B, et al. Neurological manifestations of dengue infection. Lancet. 2000;355:1053–9. doi: 10.1016/S0140-6736(00)02036-5. [DOI] [PubMed] [Google Scholar]
- 11.Solomon T, Dung NM, Kneen R, Thao LT, Gainsborough M, Nisalak A, et al. Seizures and raised intracranial pressure in Vietnamese patients with Japanese encephalitis. Brain. 2002;125:1084–93. doi: 10.1093/brain/awf116. [DOI] [PubMed] [Google Scholar]
- 12.Solomon T, Thao LTT, Dung NM, Kneen R, Hung NT, Nisalak A, et al. Rapid diagnosis of Japanese encephalitis by using an IgM dot enzyme immunoassay. J Clin Microbiol. 1998;36:2030–4. doi: 10.1128/jcm.36.7.2030-2034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Libraty DH, Nisalak A, Endy TP, Suntayakorn S, Vaughn DW, Innis BL. Clinical and immunological risk factors for severe disease in Japanese encephalitis. Trans R Soc Trop Med Hyg. 2002;96:173–8. doi: 10.1016/S0035-9203(02)90294-4. [DOI] [PubMed] [Google Scholar]
- 14.Winter PM, Dung NM, Loan HT, Kneen R, Wills B.Thu le T, et alProinflammatory cytokines and chemokines in humans with Japanese encephalitis. J Infect Dis 20041901618–26. 10.1086/423328 [DOI] [PubMed] [Google Scholar]
- 15.Raengsakulrach B, Nisalak A, Gettayacamin M, et al. An intranasal challenge model for testing Japanese encephalitis vaccines in rhesus monkeys. Am J Trop Med Hyg. 1999;60:329–37. doi: 10.4269/ajtmh.1999.60.329. [DOI] [PubMed] [Google Scholar]
- 16.German AC, Myint KS, Mai NT, Pomeroy I, Phu NH, Tzartos J, et al. A preliminary neuropathological study of Japanese encephalitis in humans and a mouse model. Trans R Soc Trop Med Hyg. 2006;100:1135–45. doi: 10.1016/j.trstmh.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Acute onset flaccid paralysis Geneva, WHO, 1993.
- 18.Ravi V, Taly AB, Shankar SK, Shenoy PK, Desai A, Nagaraja D, et al. Association of Japanese encephalitis virus infection with Guillain-Barre-syndrome in endemic areas of south India. Acta Neurol Scand. 1994;90:67–72. doi: 10.1111/j.1600-0404.1994.tb02681.x. [DOI] [PubMed] [Google Scholar]
- 19.Nakashima A, Horichi Y, Takagi Y, Miyamoto S, Takashima S, Inoue H. A case of Japanese encephalitis: CT and MRI findings in acute and convalescent stages. Radiat Med. 1999;17:369–71. [PubMed] [Google Scholar]
- 20.Misra UK, Kalita J. Anterior horn cells are also involved in Japanese encephalitis. Acta Neurol Scand. 1997;96:114–7. doi: 10.1111/j.1600-0404.1997.tb00250.x. [DOI] [PubMed] [Google Scholar]
- 21.Kuwayama M, Ito M, Takao S, Shimazu Y, Fukuda S, Miyazaki K, et al. Japanese encephalitis virus in meningitis patients, Japan. Emerg Infect Dis. 2005;11:471–3. doi: 10.3201/eid1103.040285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farrar JJ. Nipah-virus encephalitis – investigation of a new infection. Lancet. 1999;354:1222–3. doi: 10.1016/S0140-6736(99)90124-1. [DOI] [PubMed] [Google Scholar]
- 23.Burke DS, Nisalak A, Ussery MA, Laorakpongse T, Chantavibul S. Kinetics of IgM and IgG responses to Japanese encephalitis virus in human serum and cerebrospinal fluid. J Infect Dis. 1985;151:1093–9. doi: 10.1093/infdis/151.6.1093. [DOI] [PubMed] [Google Scholar]
- 24.Burke DS, Lorsomrudee W, Leake CJ, Hoke CH, Nisalak A, Chongswasdi V, et al. Fatal outcome in Japanese encephalitis. Am J Trop Med Hyg. 1985;34:1203–10. doi: 10.4269/ajtmh.1985.34.1203. [DOI] [PubMed] [Google Scholar]
- 25.Rigau-Perez JG. Severe dengue: the need for new case definitions. Lancet Infect Dis. 2006;6:297–302. doi: 10.1016/S1473-3099(06)70465-0. [DOI] [PubMed] [Google Scholar]
- 26.Deen JL, Harris E, Wills B, Balmaseda A, Hammond SN, Rocha C, et al. The WHO dengue classification and case definitions: time for a reassessment. Lancet. 2006;368:170–3. doi: 10.1016/S0140-6736(06)69006-5. [DOI] [PubMed] [Google Scholar]