Abstract
Reduction of indoor air pollution (IAP) exposure from solid fuel use is a potentially important intervention for childhood pneumonia prevention. This review updates a prior meta-analysis and investigates whether risk varies by etiological agent and pneumonia severity among children aged less than 5 years who are exposed to unprocessed solid fuels. Searches were made of electronic databases (including Africa, China and Latin America) without language restriction. Search terms covered all sources of IAP and wide-ranging descriptions of acute lower respiratory infections, including viral and bacterial agents.
From 5317 studies in the main electronic databases (plus 307 African and Latin American, and 588 Chinese studies, in separate databases), 25 were included in the review and 24 were suitable for meta-analysis. Due to substantial statistical heterogeneity, random effects models were used. The overall pooled odds ratio was 1.78 (95% confidence interval, CI: 1.45–2.18), almost unchanged at 1.79 (95% CI: 1.26–2.21) after exclusion of studies with low exposure prevalence (< 15%) and one high outlier. There was evidence of publication bias, and the implications for the results are explored. Sensitivity subanalyses assessed the impact of control selection, adjustment for confounding, exposure and outcome assessment, and age, but no strong effects were identified. Evidence on respiratory syncytial virus was conflicting, while risk for severe or fatal pneumonia was similar to or higher than that for all pneumonia.
Despite heterogeneity, this analysis demonstrated sufficient consistency to conclude that risk of pneumonia in young children is increased by exposure to unprocessed solid fuels by a factor of 1.8. Greater efforts are now required to implement effective interventions.
Résumé
Réduire l’exposition à la pollution de l’air intérieur due à l’emploi de combustible solide peut être une intervention importante pour prévenir la pneumonie chez l’enfant. Cette étude met à jour une méta-analyse antérieure et étudie la variation du risque de pneumonie en fonction de l’agent étiologique et de la gravité de la maladie chez les moins de 5 ans exposés à des combustibles solides non traités. Des recherches ont été effectuées dans des bases de données électroniques (notamment en Afrique, en Chine et en Amérique latine), sans limitations d’ordre linguistique. Les termes recherchés couvraient toutes les sources de pollution de l’air intérieur et des descriptions larges des infections aiguës des voies respiratoires inférieures, notamment par des virus et des agents bactériens.
Parmi les 5317 études entrées dans les bases de données principales (plus 307 études africaines et latino-américaines et 588 études chinoises dans des bases de données séparées), 25 ont été prises en compte dans l’étude et 24 se prêtaient à la méta-analyse. En raison de la forte hétérogénéité statistique de ces études, des modèles à effets aléatoires ont été utilisés. L’odds ratio groupé global était de 1,78 (intervalle de confiance à 95 %, IC = 1,45-2,18) et restait presque inchangé à 1,79 (intervalle de confiance à 95 %, IC = 1,26-2,21) après exclusion des études avec une faible prévalence de l’exposition (< 15 %) et d’une étude donnant des valeurs anormalement élevées. Il existait des preuves d’un biais de publication et les implications de ce biais sur les résultats sont examinées. Des sous-analyse de sensibilité ont évalué l’impact de la sélection des témoins, de l’ajustement pour les facteurs de confusion, des évaluations de l’exposition et des résultats sanitaires, ainsi que de l’âge, mais aucun effet important n’a été mis en évidence. Les données concernant le virus respiratoire syncytial étaient contradictoires, tandis que le risque de pneumonie grave ou mortelle était similaire ou plus élevé, pour toutes les pneumonies.
En dépit de l’hétérogénéité statistique, il a été prouvé que la cohérence de cette analyse était suffisante pour conclure à la majoration d’un facteur 1,8 du risque de pneumonie chez les jeunes enfants par l’exposition à des combustibles solides non traités. Il faut à présent consacrer des efforts plus importants à la mise en œuvre d’interventions efficaces.
Resumen
La reducción de la exposición a contaminación del aire en interiores (CAI) causada por el uso de combustibles sólidos es una intervención importante para la prevención de la neumonía en la niñez. En la presente revisión se actualiza un metanálisis anterior y se investiga si el riesgo depende del agente etiológico y de la gravedad de la neumonía entre los menores de cinco años expuestos a combustibles sólidos no procesados. Se hicieron búsquedas en bases de datos electrónicas (incluidas África, China y América Latina) sin restricción de idioma. Los términos de las búsquedas abarcaron todas las fuentes de CAI y descripciones amplias de las infecciones agudas de las vías respiratorias inferiores, incluidas causas virales y bacterianas.
De los 5317 estudios considerados en las principales bases de datos electrónicas (más 307 estudios africanos y latinoamericanos y 588 estudios chinos, en distintas bases de datos), se seleccionaron 25 para la revisión, y el metanálisis se realizó finalmente con 24 de ellos. Debido a la amplia heterogeneidad estadística, se emplearon modelos de efectos aleatorios. La razón de posibilidades global fue de 1,78 (intervalo de confianza (IC) del 95%: 1,45–2,18), casi idéntica a la cifra de 1,79 (IC95%: 1,26–2,21) obtenida al excluir los estudios con baja prevalencia de la exposición (< 15%) y un valor atípico alto. Había indicios de un sesgo de publicación, y se han analizado las implicaciones de ello para los resultados. En los subanálisis de sensibilidad se determinó el impacto de la selección de los controles, el ajuste respecto de las variables de confusión, la evaluación de la exposición y los resultados, y la edad, pero no se observó ningún efecto sustancial. Los datos probatorios sobre el virus sincitial respiratorio fueron contradictorios, mientras que el riesgo para la neumonía grave o mortal fue similar o superior al correspondiente a todos los casos de neumonía.
Pese a la heterogeneidad, el análisis realizado tiene la coherencia suficiente para que pueda concluirse que el riesgo de neumonía en los niños pequeños se ve multiplicado por 1,8 cuando hay exposición a combustibles sólidos no procesados. Hay que hacer un mayor esfuerzo para implementar intervenciones eficaces.
ملخص
يُعَدُّ تقليص التعرُّض لتلوث الەواء الناجم عن استخدام الوقود الصلب في الأماكن المغلقة من التدخلات المەمة الممكنة لوقاية الأطفال من الالتەاب الرئوي. وتركز ەذە الدراسة على تحديث تحليل تلوي مسبق، كما تستقصي وجود تفاوت في احتمالات الخطر وفقاً للعامل السببي، ووفقاً لِحِدَّة الالتەاب الرئوي لدى الأطفال دون سن الخامسة ممن يتعرضون للوقود الصلب غير المعالَج. وأجريت عمليات البحث في قواعد البيانات الإلكترونية (بما فيەا أفريقيا، والصين، وأمريكا اللاتينية) دون التقيد بلغة. وقد غطت عبارات ومصطلحات البحث كل مصادر تلوث الەواء في الأماكن المغلقة، وتوصيفات واسعة النطاق للالتەابات التنفسية السفلية الحادة بما فيەا العوامل الفيروسية والجرثومية.
فمن بين 5317 دراسة كانت موجودة في قواعد البيانات الإلكترونية الأساسية (بالإضافة إلى 307 دراسة في أفريقيا وأمريكا اللاتينية و588 دراسة في الصين كانت موجودة في قواعد بيانات منفصلة) تم تضمين 25 دراسة في ەذە المراجعة، كما ثبت ملاءمة 24 دراسة للتحليل التلوي. ونظراً للتغايرية الإحصائية الكبيرة، فقد تم استخدام نماذج للتأثير العشوائي. ووجد أن الأرجحية الكلية المجمعة 1.78 (بفاصل ثقة 95%: إذ تراوحت الأرجحية الكلية المجمعة بين 1.45 و2.18) ولم يشەد تغيـيراً تقريباً عند 1.79 (بفاصل ثقة 95%: إذ تراوحت الأرجحية الكلية المجمعة بين 1.26 – 2.21) بعد استبعاد الدراسات ذات معدلات انتشار التعرض المنخفضة (التي يقل فيەا معدل انتشار التعرض عن 15%)، وقيمة خارجية واحدة مرتفعة. وقد وجدت بينات على التحيز في النشر، ومن ثم تم استكشاف مغزى ەذە النتائج. وقيِّمَتْ تحاليل الحساسية التفصيلية تأثير انتقاء المكافحة، والتصحيح لغرض التدقيق، والتعرُّض، وتقييم النتائج، والسن، دون التعرف على أي آثار قوية. وجاءت البينات بالنسبة للفيروس التنفسي المخلوي متضاربة، وإن كان خطر التعرُّض للالتەاب الرئوي الحاد أو المميت متشابەة أو أعلى في كل أنواع الالتەاب الرئوي.
فبالرغم من التغايرية، أثبت ەذا التحليل اتساقاً كافياً بما يخلص إلى أن خطر إصابة صغار الأطفال بالالتەاب الرئوي يزيد بسبب التعرض للوقود الصلب غير المعالج بعامل 1.8. ومن ثم فإننا بحاجة إلى بذل المزيد من الجەود لتنفيذ التدخلات الفعالة.
Introduction
With annual deaths from pneumonia in children under 5 years old exceeding 2 million and scant evidence of a decline in this number in the last 5–10 years, prevention remains a critical component of control strategy.1 In 1995, Kirkwood et al. identified indoor air pollution (IAP) from household use of solid fuels (wood, animal dung, crop wastes and coal) as one of several modifiable risk factors requiring evaluation.2 Solid fuels remain the principal household fuel for around 3 billion people, and since their use is closely linked to poverty, this is also a population with generally poor access to health care.
Several reviews have examined the available evidence linking IAP with childhood pneumonia, culminating in the meta-analyses carried out for the 2004 WHO comparative risk assessment.3,4 A total of 15 studies were reviewed in detail, and eight included in the meta-analyses. Exclusions were for specific methodological issues including low-exposure prevalence and inadequate assessment of exposure and outcome. The authors reported an overall pooled odds ratio (OR) of 2.3 (95% confidence interval, CI: 1.9–2.7). Subanalyses examining the effects of different exposure measures, degree of adjustment for confounding, and children’s age were quite consistent, although limited by small numbers of studies. Since very few of the studies measured exposure, the review could not relate risk of pneumonia to actual levels of pollutants.
Since completion of the meta-analysis by Smith et al.,3 several new studies have been published, and the first randomized control trial (RCT) testing the impact of a chimney wood stove (compared with a 3-stone open fire) in Guatemala has been completed.5–7 It was therefore important to update this systematic review and evaluate exposure-response data available from two recent studies.7,8 Additional objectives were to assess whether risk of acute lower respiratory infection (ALRI) differed by: (i) etiological agent (viral versus bacterial), and (ii) severity, as both issues have implications for the fraction of ALRI disease burden attributable to solid fuels.
Methods
Inclusion criteria relating to exposure, outcome and population were used to identify observational (cross-sectional, case–control, cohort) and intervention studies investigating risk of childhood (< 5 years) ALRI with household use of solid fuels (Box 1). Two reviewers (Mukesh Dherani and Maya Mascarenhas) independently interrogated the main published and unpublished literature databases (Table 1) to identify relevant studies using the search terms listed in Table 2, with an additional reviewer conducting searches of Chinese language databases (Table 1 and Table 2, available at: http://www.who.int/bulletin/volumes/86/5/07-044529/en/index.html).
Box 1. Inclusion criteria for the systematic review.
Criteria for outcome of child ALRI
1. Pneumonia assessed by recall (by caregiver) of key symptoms and signs within a specified time period (recall of up to two weeks)
2. Pneumonia assessed by report and/or recall (by caregiver) of key symptoms with direct observation of signs by staff trained under WHO guidelines
3. Assessment by a physician, leading to a diagnosis of pneumonia or other lower respiratory infection
4. In addition to any of the above, chest X-ray
5. In addition to any of the above, blood culture and/or culture of bronchioalveolar lavage
6. RSV disease (which may also be described as bronchiolitis)
Criteria for child exposure to household IAP
1. Fuel use: unprocessed solid fuels compared to clean(er) fuels such as liquefied petroleum gas, kerosene and electricity (fuels for comparison need to be specified)
2. Behavioural: time child spends near the (solid fuel) stove or other relevant behaviours
3. Behavioural: child carried while cooking
4. Structural: improved stove compared to traditional stove; cooking or heating inside compared to outside
5. Availability of actual measurements of IAP and/or exposure that demonstrate substantive exposure differential
Table 1. Electronic databases used for the systematic review.
| Databases of published literature | ||
|---|---|---|
|
Database |
Details |
|
| • MEDLINE | • 10 million references (52% United States of America). 1966 to present. | |
| • EMBASE | • 8 million references (33% USA). 1974 to present. | |
| • Cochrane Controlled Trials Register (CCTR) | • Peer reviewed published trials (approx. 270 000). | |
| • Cumulative Index to Nursing and Allied Health Literature (CINAHL) | • 6 million references. 1982 to present. | |
| • Database of Abstracts of Reviews of Effects (DARE) | • Structured abstracts of global systematic reviews. | |
| • Latin American and Caribbean Health Sciences Information System (LILACS) | • Health Sciences Latin American and Caribbean literature. 1982 to present. | |
| • Pascal Biomed | • World medicine and life sciences literature. 6 million references. 1987 to present. | |
| • China National Knowledge Infrastructures (CNKI) and Chinese Scientific Journal Database (VIP) | • CNKI: Over 7 200 core Chinese and English journals, 1980 to present. VIP: Largest full-text periodical database in China, 1989 to present. | |
| • Scientific Electronic Library Online (SciELO) – some of this resource accessed via LILACS | • Peer reviewed published articles from developing countries. | |
| • African Index Medicus (AIM) | • Index to African Health literature and resources. | |
| • Global Health | • 1.2 million references, 1973 to present. | |
|
Databases of “grey” literature | ||
|
Database |
Details |
|
| • System for Information on Grey Literature in Europe (SIGLE) | • European research reports and dissertations. 360 000 records. | |
| • Index to Conference Proceedings | • Details of conference proceedings including British Library Index to conference proceedings. | |
| • Pascal | • Conference proceedings, dissertations, patents and reports. | |
| • CAB abstracts | • Published abstracts in life sciences. 5 million records. 1973 to present. | |
Table 2. Search terms.
| Outcome | Exposure |
|---|---|
| 1. “ALRI” | 18. “IAP” |
| 2. “ARI” | 19. “indoor air” |
| 3. “pneumonia” | 20. “improved stoves” |
| 4. “respiratory illness” | 21. “wood smoke” |
| 5. “respiratory infection” | 22. “dung” |
| 6. “respiratory disease” | 23. “solid fuel” |
| 7. “fast breathing” | 24. “cooking fuel” |
| 8. “chest indrawing” “fast breath*” | 25. “cook* smoke” |
| 9. “rapid breath*” | 26. “stove” |
| 10. “raised respiratory rate” | 27. “chull*” |
| 11. “RSV” | 28. “heat*” |
| 12. “bronchiolitis” | 29. “coal” |
| 13. “streptococcus pneumoniae” | 30. “pollutant” |
| 14. “pneumococcus | 31. “pollution” |
| 15. “haemophilus influenza” | 32. “biomass” |
| 16. “H. influenza” | 33. “kerosene” |
| 17. 1 OR: 2 OR: 3 OR: 4 OR: 5 OR: 6 OR: 7….. OR: 16 | 34. “paraffin” |
| 35. 18 OR: 19 OR: 20 OR: 21 OR: 22..... OR: 34 | |
|
Combined terms | |
| 17. AND 35. | |
Pre-defined forms were used to extract information from selected studies, and methodological quality was assessed using design-specific bespoke instruments. Almost all studies were observational; as a consequence, particular care was required to identify bias and confounding so as to avoid arriving at erroneous but precise risk estimates from meta-analysis.9 All studies meeting criteria for review are summarized in Appendix A (available at: http://pcwww.liv.ac.uk/~ngb/) with a further explanation of quality assurance procedures.
The approach to meta-analysis was first to pool all eligible studies and then to carry out sensitivity analyses to assess the impact of methodological concerns. Eligible studies allowed distinction between upper and lower respiratory infection, and provided a risk estimate for ALRI with 95% CI (or data allowing calculation). The criterion for using random-effects meta-analysis was significant heterogeneity on Cochran’s Q (P < 0.1) and/or an I² statistic value > 50%. Sensitivity analyses were carried out for bias in control selection; exposure prevalence; exposure assessment; outcome assessment; control for confounding; age group. The information used to select studies for each sensitivity analysis is presented in Appendix A.
Publication bias was checked by funnel plot asymmetry and use of Begg’s and Egger’s tests.9 Statistical analyses were conducted using RevMan 4.2.10 (Cochrane Collaboration’s Information Management System, available at: http://www.cc-ims.net/RevMan) and Stata, version 9.1, software (Stata Corp., College Station, TX, United States of America). The impact of publication bias was assessed by (i) manual stepwise trimming removing studies with lowest precision and highest ORs until Egger’s test was non-significant, and (ii) using “metatrim” (Stata) which uses the Duval and Tweedie trim and fill procedure.10 Due to uncertainty about adjustment methods for publication bias in the presence of between-study heterogeneity (metatrim may over adjust), it is recommended that the resulting adjusted ORs be viewed as sensitivity analysis.11
Results
Systematic review
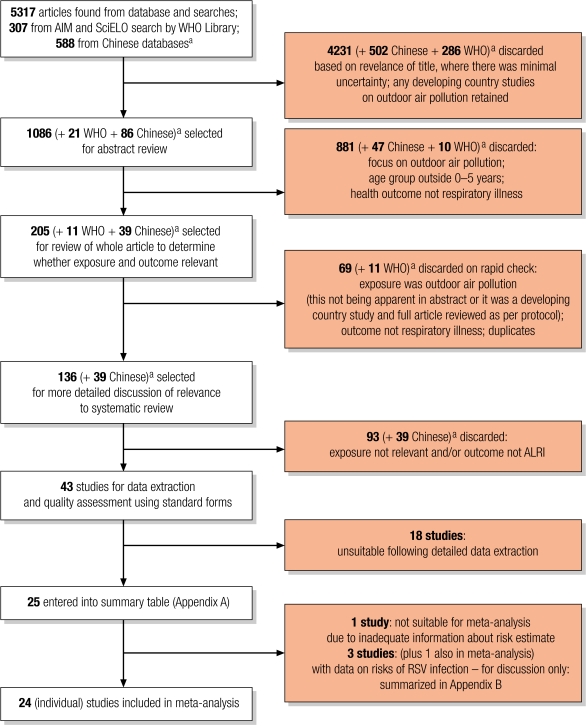
Fig. 1, (available at: http://www.who.int/bulletin/volumes/86/5/07-044529/en/index.html), summarizes the search and selection process. A total of 5317 studies from the main electronic databases were identified. In addition, 588 studies were found from Chinese language databases, China National Knowledge Infrastructures (CNKI) and Chinese Scientific Journal Database (VIP), and 307 African and Latin American studies were found from African Index Medicus (AIM) and Scientific Electronic Library Online (SciELO) respectively (Table 1). However, since they could not be electronically merged to identify duplicates, they are enumerated separately (Fig. 1). From all studies, 255 were selected for review, 43 for full data extraction and quality appraisal. Of these, 25 met criteria for the review.
Fig. 1.
Flowchart for study selection
AIM, African Index Medicus; ALRI, acute lower respiratory infection; RSV, respiratory syncytial virus; SciELO, Scientific Electronic Library Online.
a Results of searches by WHO Library (SciELO; EasternMed; LILACS; Western Pacific) and of Chinese databases could not be merged electronically, so the number of duplicates could not easily be identified. None of the located Chinese-language studies met the criteria for data extraction.
These 25 studies are summarized by study design (Appendix A) and comprise 3 cross-sectional, 16 case–control, 5 cohort, and 1 RCT. Apart from two studies among Native Americans,12,13 all were conducted in developing countries or urban areas of countries in transition, such as Brazil14,15 and Malaysia.16 Three other studies, plus the RCT, included respiratory syncytial virus (RSV) illness as an outcome, and allowed examination of the impact of solid fuel use on the incidence and/or severity of RSV disease (Appendix B; available at: http://pcwww.liv.ac.uk/~ngb/).17–19 An overriding feature of this review is the amount of variation among studies in terms of settings, design, exposure and outcome assessment, and factors affecting quality. This information is summarized in Appendix A and Appendix B, and key issues are discussed below.
Study setting and exposure prevalence
The selected studies include populations in all major continents, urban and rural communities, using most types of household fuel. Elevation varies from sea level to around 3000 m, and climate and seasonal patterns differ widely, with consequent potential for influence by seasonal epidemics (e.g. RSV illness), and by diseases such as malaria, which may be confused with pneumonia.20 The prevalence of exposure to smoke from household solid fuel use varies from less than 10% in urban areas14–16,21 to more than 90% in rural areas of the United Republic of Tanzania.22 Populations with low exposure prevalence (for this review defined as < 15%) may not be typical of poor biomass and coal-using communities in general, and furthermore, in urban areas, solid fuels may be processed (e.g. as charcoal, which is less polluting than wood) and often used along with modern fuels (e.g. kerosene). None of the studies examined the possible impact of HIV, but given the timing and location of each, this is unlikely to be a major factor for most – with the possible exception of a recent study in South Africa.23
Study design
Case–control studies were most numerous. We had concerns in a number of these studies about bias from control selection where these were mildly ill outpatients (e.g. acute upper respiratory infection) or children attending immunization clinics at the same hospital that was used to recruit pneumonia cases (details in Appendix A). Bias in the direction of the hypothesis (larger risk) could arise since, while children with pneumonia from poorer biomass-using homes may reach an urban referral centre, children from such homes are less likely to attend the same institution with mild illness or for well-child care. This will result in controls having a non-representative low prevalence of solid fuel use, and generate a falsely high OR. Some authors recognized this issue, for example Morris et al.,12 who quoted high immunization rates (90%) as possible evidence that controls were representative of the hospital-attending case population. By contrast, some of the other case–control studies selected controls that were more likely to be drawn from the same population from which cases arose, for example, Fonseca et al.15 and Weber et al.18
Exposure assessment
A wide range of methods were used for assessing exposure with few directly measuring IAP (Box 2). Because of complexity in (i) the way different fuels and devices are used for cooking, heating and lighting, and (ii) behaviours that determine child exposure, there is likely to be misclassification of exposure in most studies. This will tend to bias risk estimates towards no effect. Despite this, substantive differences between group average exposures should have been captured by most studies comparing solid and modern fuels, as exposure studies comparing homes that use mainly biomass for cooking with those that use clean fuels such as liquefied petroleum gas or electricity have demonstrated substantially lower levels with the latter.24 Several studies have also shown that improved solid fuel stoves can deliver important reductions in kitchen levels25,26 and child exposure27 but, since other studies have shown minimal or no reduction even in kitchen air pollution levels,28 it is important not to assume that a stove described as “improved” will actually reduce child exposure unless so demonstrated.
Box 2. Exposure assessment methods.
Questions on fuel type(s) mainly used for cooking and, in some studies, also for heating30
Behavioural measures, most commonly whether “child is carried by the mother” while she is cooking,18,31,37,41 also “time spent near fire”,32 and vaguer descriptions including “child stays in smoke”33
Questions on location of the child relative to cooking place, e.g. “cooking done in same room as where child sleeps”38
Type of solid fuel stove used, e.g. comparing traditional open fire stoves with improved chimney stoves,42 which in the case of the trial in Guatemala was the basis of the intervention
Measurement of house pollution and/or child exposure, in all subjects5,8,13 or a subgroup43
ALRI case ascertainment
There is similarly a wide range of methods used for ascertaining ALRI cases (Box 3). All of these definitions were included in the selection criteria as each should have some validity for ALRI, although maternal recall as used in the Demographic and Health Surveys (DHS) can be expected to have low specificity and possibly poor validity.23,29,30 Physician and radiological diagnosis should have higher specificity and microbiological investigations can indicate predominant viral or bacterial etiology. Pneumonia deaths indicate severe disease (as well as reflecting access to effective care) but validity depends on the method used to determine cause of death: the accuracy of verbal autopsy may (for example) be poorer in areas of endemic malaria.20,22
Box 3. ALRI definitions and case ascertainment.
Recall by parent/carer of symptoms and signs (predominantly “respiratory illness with fast breathing”), usually over the previous 2 weeks
Fieldworker surveillance at weekly home visits to identify illness episodes with cough and/or difficulty breathing, and signs defined by WHO for recognition of ALRI44
Physician diagnosis, although very few studies reported standardized protocols and/or training6
Radiological pneumonia, varying from “positive findings” to detailed description of pneumonic infiltrate, lobar consolidation and pleural effusion. Few report standardized protocols or independent reading6
Investigations including oxygen saturation (pulse oximetry), respiratory viruses (mainly RSV)6,17–19 and pneumococcal disease37
Deaths among hospitalized cases38 and among population samples using verbal autopsy20,22
Dealing with confounding
In assessing how fully confounding was addressed, evidence was sought that the following ALRI risk factors had been matched and/or examined and adjusted for: socioeconomic status, parental education, breastfeeding, nutritional status, environmental tobacco smoke, crowding and vaccination status. The adequacy of control of and/or adjustment for confounding varied considerably, and is described in Appendix A and Appendix B. The sole RCT achieved effective balance of confounders through randomization.5
Meta-analysis
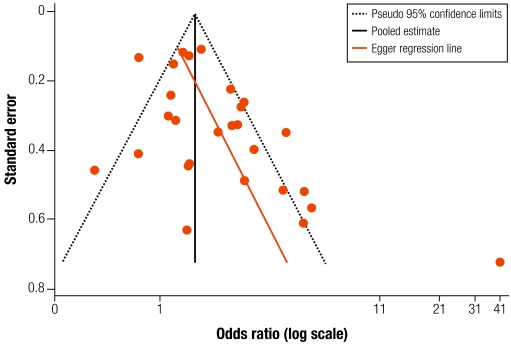
All studies in Appendix A were included in the meta-analysis, except Mtango et al. as insufficient data were provided for pneumonia deaths.22 Some 27 estimates from 24 studies are included, as that by Armstrong & Campbell has separate results for males and females,31 that by Pandey et al. has two groups,32 and the Guatemala trial provides distinct intention to treat and exposure-response analyses.5,7 The funnel plot shows asymmetry (Fig. 2), with significant Begg’s (P = 0.027) and Egger’s tests (P = 0.005). Exclusion (as an extreme outlier) of the high OR from Group II in Pandey et al.’s study,32 does not eliminate the asymmetry [Begg’s test (P = 0.098); Egger’s test (P = 0.016)]. With low exposure prevalence (< 15%) studies also excluded,14–16,21 Begg’s test is non-significant (P = 0.13) but Egger’s remains significant (P = 0.009).
Fig. 2.
Funnel plot for all studies included in meta-analysis
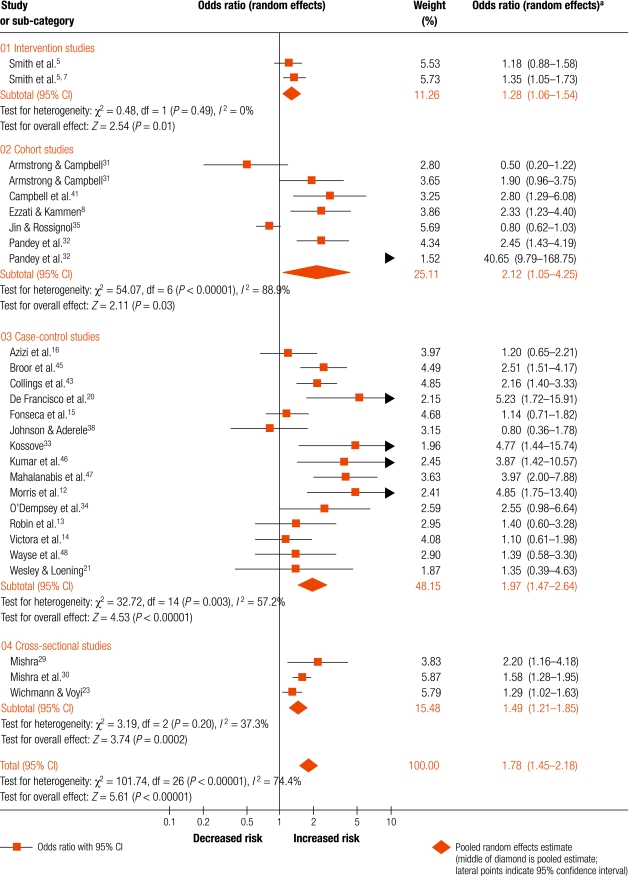
Fig. 3, (available at: http://www.who.int/bulletin/volumes/86/5/07-044529/en/index.html), shows the forest plot for all 27 estimates (24 studies) grouped by study design. The exposure comparisons made and outcome definitions used for each OR are presented in Table 3. There was substantial heterogeneity with I2 = 74.4% (P < 0.0001). The pooled OR was 1.78 (95% CI: 1.45–2.18). Following exclusion of the high outlier,32 the OR reduced to 1.67 (95% CI: 1.39–2.01; Table 3), and with additional exclusion of low exposure prevalence studies, the OR is 1.79 (95% CI: 1.46–2.21). The pooled ORs for individual study designs did not differ greatly (Fig. 3 and Table 3) , although the one RCT provided the lowest estimate. To assess the impact of publication bias, in addition to removal of the low exposure prevalence studies and the high outlier, we trimmed three studies with the lowest precision/highest ORs to obtain non-significant Begg’s (P = 0.81) and Egger’s tests (P = 0.068), and an OR of 1.64 (95% CI: 1.34–2.01).12,20,33 Adjustment with metatrim involved five studies and yielded an OR estimate of 1.54 (95% CI: 1.25–1.89).
Fig. 3.
Forest plot for all studies included in meta-analysis: comparison of higher versus lower exposure
a Values in parentheses are 95% confidence intervals.
Table 3. Pooled odds ratios from meta-analysis of all studies and sensitivity analyses.
| Group | Detail | Estimates including low exposure prevalence studies |
Estimates excluding low exposure prevalence studies |
|||||
|---|---|---|---|---|---|---|---|---|
| N | OR | 95% CI | N | OR | 95% CI | |||
| All studies | 27 | 1.78 | 1.45–2.18 | 23 | 1.93 | 1.54–2.42 | ||
| 26 | 1.67a | 1.39–2.01 | 22 | 1.79a | 1.46–2.21 | |||
| Study design | Randomized control trial (one study) | 2 | 1.28 | 1.06–1.54 | No studies with low prevalence | |||
| Cohort | 7 | 2.12 | 1.05–4.25 | No studies with low prevalence | ||||
| All case–control | 15 | 1.97 | 1.47–2.64 | 11 | 2.38 | 1.90–2.97 | ||
| Case–control with good control selection | (9) | 1.50 | 1.05–2.14 | (5) | 2.17 | 1.07–4.41 | ||
| Cross sectional | 3 | 1.49 | 1.21–1.85 | No studies with low prevalence | ||||
| Confounding | Adequate or good adjustmenta | 16 | 1.77 | 1.43–2.18 | 14 | 1.80 | 1.43–2.25 | |
| Exposure | Good categorizationa | 16 | 1.67 | 1.33–2.09 | 14 | 1.73 | 1.35–2.20 | |
| Solid versus clean fuela | 14 | 1.69 | 1.29–2.20 | 12 | 1.76 | 1.32–2.36 | ||
| Outcome | Excluding DHSa | 23 | 1.72 | 1.37–2.17 | 19 | 1.89 | 1.44–2.48 | |
| measure | Used physician diagnosis or more specific | 20 | 1.65 | 1.26–2.15 | 16 | 1.83 | 1.31–2.55 | |
| – | – | – | 15 | 1.97b | 1.44–2.70 | |||
| Age group | < 60 months | 11 | 1.62 | 1.21–2.15 | 10 | 1.67 | 1.22–2.30 | |
| < 36 months | 4 | 2.05 | 1.38–3.07 | 3 | 2.17 | 1.37–3.43 | ||
| < 24 monthsa | 12 | 1.96 | 1.36–2.82 | 9 | 1.85 | 1.27–2.69 | ||
Sensitivity analysis
Classification of key study characteristics used to determine exclusions in the following sensitivity analyses are summarized (in bold) in Appendix A. The resulting meta-analyses are presented in Table 3.
Control selection
We previously identified the possibility of bias from control selection in some case–control studies. The pooled OR for nine studies with more appropriate control selection was 1.50 (95% CI: 1.05–2.14), this lower estimate implying that bias may have occurred. There are, however, other substantive methodological limitations across this group of studies: thus, additional exclusion of low exposure prevalence studies14–16,21 left five estimates with an OR of 2.17 (95% CI: 1.07–4.41).
Control of confounding
Fifteen study estimates, of all designs, were judged to have adequate/good control of confounding. These had a pooled OR of 1.80 (95% CI: 1.43–2.25) after exclusion of low exposure prevalence studies (Table 3). The fact that the exclusion of studies with limited or no adjustment makes so little difference may be considered surprising. One possible reason is offered by some of the authors, namely that, in some settings, exposure contrasts may be observed with little heterogeneity of socioeconomic and related factors,8,32 but this may not be the full explanation.
Exposure prevalence and assessment
This analysis retained studies comparing clean versus solid fuel, or solid fuel stove types with evidence of substantial measured exposure differences. Exposure defined by “carriage on mother’s back while cooking” or by “more time spent by fire” was excluded as we are not aware of any studies demonstrating higher exposure among these children. The pooled OR with exclusion of low exposure prevalence studies was 1.73 (95% CI: 1.35–2.20; Table 3). When restricted to studies comparing clean versus solid fuel, the OR was 1.76 (95% CI: 1.32–2.36).
Outcome assessment
To determine the impact of variation in outcome assessment, we initially excluded studies based on the DHS surveys.23,29,30 The resulting OR, with additional exclusion of low exposure prevalence studies and the outlier, was 1.89 (95% CI: 1.44–2.48). For studies using the most specific outcomes, that is physician diagnosis, chest X-ray, laboratory confirmation of pneumococcal disease,34 and death (with cause determined by verbal autopsy),20 the pooled OR was 1.83 (95% CI: 1.31–2.55) with exclusion of low exposure prevalence studies. With exclusion of one further study35 that used physician diagnosis obtained from record cards over an 18 month period (the authors claim these records should be complete, but no validation is provided), the OR is 1.97 (95% CI: 1.44–2.70).
Age group
Pooled ORs were slightly higher for the younger two age groups, even when low exposure prevalence studies and the high outlier were excluded.
Risk of RSV disease/bronchiolitis
Appendix B summarizes four studies providing information on risk of RSV illness, one of which also studied human metapneumovirus,19 and results are conflicting. Weber et al. found that more frequent cooking (higher exposure) was protective for severe RSV with an adjusted OR of 0.31 (95% CI: 0.14–0.70).18 This was somewhat consistent with the Guatemala trial,5 which found no increase in risk of severe (hypoxaemic) RSV positive cases, OR (open fire versus intervention stove or “plancha”) = 0.95 (95% CI: 0.54–1.67), but an increased risk in the open-fire group for hypoxaemic RSV negative cases, OR (open fire versus plancha) = 1.64 (95% CI: 0.96–2.78). In contrast, Al-Sonboli et al. found an adjusted OR of 10.3 (95% CI: 2.2–48.0) for risk of severe RSV illness with exposure.19 Similarly, Jeena et al.’s data yields an unadjusted OR of 2.42 (95% CI: 0.84–6.83) for exposure to pollution (adjusted estimate not given but stated as non-significant).17 Although bias from control group selection is likely in both of the latter studies,19 this conflicting evidence requires further investigation.
Impact on severe outcomes
Severe pneumonia, best predicted by hypoxaemia, has higher case fatality than less severe.36 Bacterial pneumonia also has higher case fatality than viral, although hypoxaemia is common in severe RSV illness. Risk estimates for severe and non-viral pneumonia are therefore important in assessing the fraction of ALRI disease burden preventable through exposure reduction. Five studies (all included in Appendix A) provide data to examine risk by severity with outcomes defined by one or more of (i) hypoxaemia, (ii) pneumococcal infection, (iii) RSV negative with hypoxaemia, and (iv) deaths from pneumonia.
Hypoxaemic and bacterial pneumonia
O’Dempsey et al. reported an adjusted OR of 2.55 (95% CI: 0.98–6.65) for pneumococcal disease (pneumonia, meningitis and septicaemia; 79% pneumonia) in under 5 year olds.37 Preliminary intention to treat analysis of the Guatemala trial found an OR for open fire versus plancha of 1.85 (95% CI: 1.04–3.23) for severe pneumonia as assessed by fieldworkers under WHO guidelines while for all physician-diagnosed hypoxaemic cases the OR for open fire versus plancha was 1.35 (95% CI: 0.92–2.00) and 1.64 (95% CI: 0.96–2.78) for RSV negative and hypoxaemic cases. Exposure-response analysis of the trial found similar, statistically significant reductions (adjusted) in risk for fieldworker-assessed severe pneumonia and physician-diagnosed hypoxaemic pneumonia.7
Deaths from pneumonia
Using verbal autopsies, de Francisco et al. reported adjusted ORs of 1.47 (95% CI: 0.54–4.02) for pneumonia deaths with “sometimes carrying the child while cooking” and 5.23 (95% CI: 1.72–15.92) for “always carrying the child while cooking”.20 Also using verbal autopsies, Mtango et al. reported an adjusted OR for all deaths when children slept in the room used for cooking of 2.78 (95% CI: 1.79–4.33).22 For pneumonia deaths (25% of deaths from all causes), the adjusted risk was 4.29 (95% CI not provided). Among 103 pneumonia cases, Johnson et al. reported mortality among cases of 31% for firewood users, compared with 3.6% for petroleum product users, an unadjusted OR of 12.3 (95% CI: 2.57–58.60), but an adjusted estimate was not reported.38
Discussion and conclusion
This review found considerable variation in design and quality, and substantial statistical heterogeneity. This has been addressed by taking an initially inclusive approach then using sensitivity analysis to identify factors that might have contributed to bias in the overall estimate. There was also evidence of publication bias among the 24 studies selected for meta-analysis, not eliminated by exclusion of one outlying high estimate.32 The overall pooled OR for all studies was 1.78 (95% CI: 1.45–2.18) which increased slightly on exclusion of the outlier and four studies (all case–control) with very low exposure prevalence. This estimate, and those from the sensitivity analyses, are lower than the overall pooled result from the previous meta-analysis (OR 2.3; 95% CI: 1.9–2.7),3 reflecting some differences in inclusion criteria, the larger number of studies included in this new review and findings from the additional studies now available.
Sensitivity analyses did not identify any substantial effects resulting from differences in exposure and outcome assessment, or other aspects of study design. Exclusion of studies with exposure prevalence less than 15% increased risk estimates. However, the thoroughness with which confounding was controlled appeared to make little difference, possibly due to relatively low levels of heterogeneity in other pneumonia risk factors in some of the studies with less complete adjustment. Quality of exposure assessment, and restriction to studies comparing solid versus clean fuels also made little difference. Exclusion of the DHS-based studies, and restriction to physician or more specific outcome definition, did result in higher risk estimates of around 1.9 to 2.0 but this may in part reflect the consequently greater influence of the case–control studies. Risk was higher in younger children and, although the differences were small, we would expect this due to their vulnerability and proximity to pollution sources.
Due to wide variation in study designs, methods and quality, it was not possible to obtain a pooled estimate for studies which satisfied all desirable quality criteria, as few would be retained. Consequently, and taking into account the lack of any strong effects from sensitivity analyses, we conclude that the most appropriate single estimate is that for all studies, excluding those with low exposure prevalence, and the high outlier from Pandey et al., that is 1.79 (95% CI: 1.46–2.21). Publication bias is potentially important: the two adjustment methods yielded ORs of 1.54 and 1.65, and it is recommended that these be considered for sensitivity analysis in assessment of disease impact and economic analysis.
The few studies with data on RSV risk are not in agreement and further studies are required to elucidate this relationship. The findings from all five studies with information on severe and fatal pneumonia are consistently in the direction of increased risk, the odds ratios are substantial and, where available, within-study comparisons show a larger effect on the more severe outcomes. One study found an exposure (by category)-response association.20 It is concluded that risk for severe pneumonia is similar to that for all pneumonia at least, and quite possibly greater.
Since only one trial is available, evaluation of the impact on ALRI of various types of intervention in different settings will need to draw on other sources as well, including risk of exposure (this review), data on exposure differentials observed between various fuel and stove combinations,24,39,40 and evidence on IAP and exposure reductions achieved with stoves and other interventions.25 Importantly, the two studies providing evidence on the exposure–response relationship report that risk falls progressively from higher to lower exposure.5,7,8
We conclude that reduction of household IAP from solid fuel use through switching to other fuels, improving combustion and ventilation, and possibly other measures, would make an important contribution to prevention of pneumonia morbidity and mortality. Additional intervention studies are desirable, where possible these should include randomized trials, but other designs should also be considered in the context of intervention programmes. Future studies should ensure careful description of exposure (and measure exposure directly in a subgroup at least) and adopt pneumonia case-ascertainment protocols that offer good specificity. However, despite the variations in methods and quality among the studies reviewed, there is sufficient evidence now available to justify much greater exposure-reduction efforts in the hundreds of millions of households using solid fuels worldwide. ■
Acknowledgements
We thank Alisa Jenny and Ray Liu (University of California, Berkeley) for assistance with the review and for searching the Chinese databases; and Shamin Qazi (Department of Child and Adolescent Health and Development, WHO). Funding support was provided by the United Nations Children’s Fund (UNICEF) and Ray Liu was supported by the CC Chen Fellowship Fund.
Competing interests: None declared.
ALRI, acute lower respiratory infection; IAP, indoor air pollution; RSV, respiratory syncytial virus.
ALRI, acute lower respiratory infection; RSV, respiratory syncytial virus.
References
- 1.Rudan I, Tomaskovic L, Boschi-Pinto C, Campbell H. Global estimate of the incidence of clinical pneumonia among children under five years of age. Bull World Health Organ. 2004;82:895–903. [PMC free article] [PubMed] [Google Scholar]
- 2.Kirkwood BR, Gove S, Rogers S, Lob-Levyt J, Arthur P, Campbell H. Potential interventions for the prevention of childhood pneumonia in developing countries: a systematic review. Bull World Health Organ. 1995;73:793–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Smith KR, Mehta S, Feuz M. Indoor air pollution from household use of solid fuels. In: Ezzati M, ed. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors Geneva: WHO; 2004. [Google Scholar]
- 4.Reducing risks, promoting healthy life: the world health report Geneva: WHO; 2002. [DOI] [PubMed]
- 5.Smith KR, Bruce NG, Weber M, Hubbard A, Jenny A, Dherani M, et al. Impact of a chimney wood stove on risk of pneumonia in children aged less than 18 months in rural Guatemala: results from a randomized, controlled trial. Epidemiology. 2007;17:S45. doi: 10.2188/jea.17.45. [DOI] [Google Scholar]
- 6.Bruce N, Diaz A, Arana B, Jenny A, Thompson L, Weber M, et al. Pneumonia case-finding in the Guatemala indoor air pollution trial (RESPIRE): standardizing methods for resource poor settings. Bull World Health Organ. 2007;85:535–44. doi: 10.2471/BLT.06.035832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCracken JP, Schwartz J, Mittleman M, Ryan L, Diaz A, Smith KR, et al. Biomass smoke exposure and acute lower respiratory infections among Guatemalan children. Epidemiology. 2007;18:S185. doi: 10.1097/01.ede.0000254653.69858.88. [DOI] [Google Scholar]
- 8.Ezzati M, Kammen DM. Quantifying the effects of exposure to indoor air pollution from biomass combustion on acute respiratory infections in developing countries. Environ Health Perspect. 2001;109:481. doi: 10.2307/3454706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egger M, Davey Smith G, Schneider M. Systematic reviews of observational studies. In: Egger M, Davey Smith G, Altman DG, eds. Systematic reviews in health care: meta-analysis in context London: BMJ Publishing Group; 2001. p. 211. [Google Scholar]
- 10.Duval S, Tweedie R. A non parametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98. doi: 10.2307/2669529. [DOI] [Google Scholar]
- 11.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med. 2007;26:4544–62. doi: 10.1002/sim.2889. [DOI] [PubMed] [Google Scholar]
- 12.Morris K, Morgenlander M, Coulehan JL, Gahagen S, Arena VC. Wood-burning stoves and lower respiratory tract infection in American Indian children. Am J Dis Child. 1990;144:105–8. doi: 10.1001/archpedi.1990.02150250117047. [DOI] [PubMed] [Google Scholar]
- 13.Robin LF, Less PS, Winget M, Steinhoff M, Moulton LH, Santosham M, et al. Wood-burning stoves and lower respiratory illnesses in Navajo children. Pediatr Infect Dis J. 1996;15:859–65. doi: 10.1097/00006454-199610000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Victora CG, Fuchs SC, Flores JA, Fonseca W, Kirkwood B. Risk factors for pneumonia among children in a Brazilian metropolitan area. Pediatrics. 1994;93:977–85. [PubMed] [Google Scholar]
- 15.Fonseca W, Kirkwood BR, Victora CG, Fuchs SR, Flores JA, Misago C. Risk factors for childhood pneumonia among the urban poor in Fortaleza, Brazil: a case-control study. Bull World Health Organ. 1996;74:199–208. [PMC free article] [PubMed] [Google Scholar]
- 16.Azizi BH, Zulkifli HI, Kasim MS. Protective and risk factors for acute respiratory infections in hospitalized urban Malaysian children: a case-control study. Southeast Asian J Trop Med Public Health. 1995;26:280. [PubMed] [Google Scholar]
- 17.Jeena PM, Ayannusi OE, Annamalai K, Naidoo P, Coovadia HM, Guldner P. Risk factors for admission and the role of respiratory syncytial virus-specific cytotoxic T-lymphocyte responses in children with acute bronchiolitis. S Afr Med J. 2003;93:291–4. [PubMed] [Google Scholar]
- 18.Weber MW, Milligan P, Hilton S, Lahai G, Whittle H, Mulholland EK, et al. Risk factors for severe respiratory syncytial virus infection leading to hospital admission in children in the western region of the Gambia. Int J Epidemiol. 1999;28:157–62. doi: 10.1093/ije/28.1.157. [DOI] [PubMed] [Google Scholar]
- 19.Al-Sonboli N, Hart CA, Al-Aghbari N, Al-Ansi A, Ashoor O, Cuevas LE. Human metapneumovirus and respiratory syncytial virus disease in children, Yemen. Emerg Infect Dis. 2006;12:1437–9. doi: 10.3201/eid1209.060207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Francisco A, Morris J, Hall AJ, Armstrong Schellenberg JRM, Greenwood BM. Risk factors for mortality from acute lower respiratory tract infections in young Gambian children. Int J Epidemiol. 1993;22:1174. doi: 10.1093/ije/22.6.1174. [DOI] [PubMed] [Google Scholar]
- 21.Wesley AG, Loening WE. Assessment and 2-year follow-up of some factors associated with severity of respiratory infections in early childhood. S Afr Med J. 1996;86:365–8. [PubMed] [Google Scholar]
- 22.Mtango FD, Neuvians D, Broome CV, Hightower AW, Pio A. Risk factors for deaths in children under 5 years old in Bagamoyo district, Tanzania. Trop Med Parasitol. 1992;43:229–33. [PubMed] [Google Scholar]
- 23.Wichmann J, Voyi KVV. Impact of cooking and heating fuel use on acute respiratory health of preschool children in South Africa. Southern African Journal of Epidemiology & Infection 2006. pp. 2-54.
- 24.Saksena S, Thompson L, Smith KR. Indoor air pollution and exposure database: household measurements in developing countries. 2004 Available from: http://ehs.sph.berkeley.edu/hem/page.asp?id=33 [accessed on 25 March 2008].
- 25.Smith KR, Dutta K, Chengappa C, Gusain PPS, Masera O, Berrueta V, et al. Monitoring and evaluation of improved biomass cookstove programs for indoor air quality and stove performance: conclusions from the Household Energy and Health Project. Energy for Sustainable Development. 2007;11:5–18. [Google Scholar]
- 26.Albalak R, Bruce NG, McCracken JP, Smith KR. Indoor respirable particulate matter concentrations from an open fire, improved cookstove, and LPG/open fire combination in a rural Guatemalan community. Environ Sci Technol. 2001;35:2650–5. doi: 10.1021/es001940m. [DOI] [PubMed] [Google Scholar]
- 27.Bruce N, McCracken JP, Albalak R, Schei M, Smith KR, Lopez V, et al. The impact of improved stoves, house construction and child location on levels of indoor air pollution and exposure in young Guatemalan children. J Expo Anal Environ Epidemiol. 2004;14(Suppl 1):S26–33. doi: 10.1038/sj.jea.7500355. [DOI] [PubMed] [Google Scholar]
- 28.Ramakrishna J, Durgaprasad MB, Smith KR. Cooking in India: the impact of improved stoves on indoor air quality. Environ Int. 1989;15:341–52. doi: 10.1016/0160-4120(89)90047-0. [DOI] [Google Scholar]
- 29.Mishra V. Indoor air pollution from biomass combustion and acute respiratory illness in preschool age children in Zimbabwe. Int J Epidemiol. 2003;32:847–53. doi: 10.1093/ije/dyg240. [DOI] [PubMed] [Google Scholar]
- 30.Mishra V, Smith KR, Retherford RD. Effects of cooking smoke and environmental tobacco smoke on acute respiratory infections in young Indian children. Popul Environ. 2006;26:375. doi: 10.1007/s11111-005-0005-y. [DOI] [Google Scholar]
- 31.Armstrong JR, Campbell H. Indoor air pollution exposure and lower respiratory infections in young Gambian children. Int J Epidemiol. 1991;20:424–9. doi: 10.1093/ije/20.2.424. [DOI] [PubMed] [Google Scholar]
- 32.Pandey MR, Neupane RP, Gautam A, Shrestha IB. Domestic smoke pollution and acute respiratory infections in a rural community of the hill region of Nepal. Environ Int. 1989;15:337. doi: 10.1016/0160-4120(89)90046-9. [DOI] [Google Scholar]
- 33.Kossove D. Smoke-filled rooms and lower respiratory disease in infants. S Afr Med J. 1982;61:622–4. [PubMed] [Google Scholar]
- 34.O’Dempsey TJD, McArdle TF, Morris J, Lloyd-Evans N, Baldeh I, Laurence BE, et al. A study of risk factors for pneumococcal disease among children in a rural area of West Africa. Int J Epidemiol. 1996;25:885–93. doi: 10.1093/ije/25.4.885. [DOI] [PubMed] [Google Scholar]
- 35.Jin C, Rossignol AM. Effects of passive smoking on respiratory illness from birth to age eighteen months, in Shanghai, People’s Republic of China. J Pediatr. 1993;123:553–8. doi: 10.1016/S0022-3476(05)80949-7. [DOI] [PubMed] [Google Scholar]
- 36.Lozano JM. Epidemiology of hypoxia in children with acute lower respiratory infection. Int J Tuberc Lung Dis. 2001;5:496–504. [PubMed] [Google Scholar]
- 37.O’Dempsey TJ, McArdle TF, Morris J, Lloyd-Evans N, Baldeh I, Laurence BE, et al. A study of risk factors for pneumococcal disease among children in a rural area of west Africa. Int J Epidemiol. 1996;25:885–93. doi: 10.1093/ije/25.4.885. [DOI] [PubMed] [Google Scholar]
- 38.Johnson AW, Aderele WI. The association of household pollutants and socio-economic risk factors with the short-term outcome of acute lower respiratory infections in hospitalized pre-school Nigerian children. Ann Trop Paediatr. 1992;12:421–32. doi: 10.1080/02724936.1992.11747609. [DOI] [PubMed] [Google Scholar]
- 39.Bruce NG, Rehfuess E, Mehta S, Hutton G, Smith KR. Indoor air pollution. In: Jamison DT, Breman J, Measham AR, Alleyne G, Claeson M, Evans DB et al., eds. Disease control priorities in developing countries 2nd edn. New York: Oxford University Press/World Bank; 2006. pp. 793-815. [Google Scholar]
- 40.Rollin HB, Mathee A, Bruce N, Levin J, Von Schirnding YER. Comparison of indoor air quality in electrified and un-electrified dwellings in rural South African villages. Indoor Air. 2004;14:208. doi: 10.1111/j.1600-0668.2004.00238.x. [DOI] [PubMed] [Google Scholar]
- 41.Campbell H, Armstrong JR, Byass P. Indoor air pollution in developing countries and acute respiratory infection in children. Lancet. 1989;333:1012. doi: 10.1016/S0140-6736(89)92647-0. [DOI] [PubMed] [Google Scholar]
- 42.Shah N, Ramankutty V, Premila PG, Sathy N. Risk factors for severe pneumonia in children in south Kerala: a hospital-based case-control study. J Trop Pediatr. 1994;40:201–6. doi: 10.1093/tropej/40.4.201. [DOI] [PubMed] [Google Scholar]
- 43.Collings DA, Sithole SD, Martin KS. Indoor woodsmoke pollution causing lower respiratory disease in children. Trop Doct. 1990;20:151–5. doi: 10.1177/004947559002000403. [DOI] [PubMed] [Google Scholar]
- 44.Integrated management of childhood illness Geneva: WHO; 2006. Available from: http://www.who.int/child-adolescent-health/publications/IMCI/WHO_CHS_CAH_98.1.htm [accessed on 19 March 2008].
- 45.Broor S, Pandey RM, Ghosh M, Maitreyi RS, Lodha R, Singhal T, et al. Risk factors for severe acute lower respiratory tract infection in under-five children. Indian Pediatr. 2001;38:1361–9. [PubMed] [Google Scholar]
- 46.Kumar S, Awasthi S, Jain A, Srivastava RC. Blood zinc levels in children hospitalized with severe pheumonia: A case control study. Indian Pediatr. 2004;41:486. [PubMed] [Google Scholar]
- 47.Mahalanabis D, Gupta S, Paul D, Gupta A, Lahiri M, Khaled MA. Risk factors for pneumonia in infants and young children and the role of solid fuel for cooking: a case-control study. Epidemiol Infect. 2002;129:65–71. doi: 10.1017/S0950268802006817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58:563–7. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]