Abstract
Objective
To determine seroprotection and vaccine response rates produced by a diphtheria–tetanus–acellular pertussis–inactivated poliovirus–Haemophilus influenzae type-b conjugate (DTaP–IPV//PRP~T) vaccine containing a polyribosyl-ribitol-phosphate (PRP)–tetanus toxoid conjugate (Pentaxim™) and given with a hepatitis B vaccine.
Methods
In this multicentre open-label trial, 424 infants who received DTaP–IPV//PRP~T at 6, 10 and 14 weeks of age were also randomized to receive hepatitis B vaccine at either 6, 10 and 14 weeks or 0, 6 and 14 weeks of age. Antibody levels were determined at 6 and 18 weeks of age, and reactogenicity was monitored using parental reports.
Findings
Immunogenicity was high for all vaccine antigens and was similar to that in a historical control study. After primary vaccination, 98.7% of all infants had an anti-PRP antibody titre ≥ 0.15 μg/ml. Seroprotection against poliovirus type-1, -2 and -3 and tetanus was obtained in all infants, and against diphtheria, in 97.1%. Pertussis seroconversion, defined as a ≥ fourfold increase in antibody titre, occurred in 95.3% for anti-pertussis toxoid antibody and in 89.0% for anti-filamentous haemagglutinin antibody. The hepatitis B seroprotection rate was 99.5% with administration at 0, 6 and 14 weeks, and 97.8%, at 6, 10 and 14 weeks. However, the antibody titre was higher with the 0, 6 and 14-week schedule (601 mIU/ml versus 207 mIU/ml). The reactogenicity of both vaccines was low.
Conclusion
The DTaP–IPV//PRP~T vaccine was highly immunogenic. The anti-hepatitis B antibody response was seroprotective with both schedules, though the antibody titre was higher with the 0, 6 and 14-week schedule.
Résumé
Objectif
Déterminer les taux de séroprotection et de réponse vaccinale produits par un vaccin combiné, associant une composante antidiphtérique-antitétanique-anticoquelucheuse acellulaire et une composante conjuguée poliovirus inactivé/valence anti-Haemophilus influenzae type b (DTaP-IPV//PRP~T/Pentaxim™) et contenant du polyribosylribitol phosphate (PRP) conjugué à l’anatoxine tétanique, qui s’administre avec le vaccin contre l’hépatite B.
Méthodes
Dans le cadre d’un essai ouvert multicentrique, 424 nourrissons ayant reçu le vaccin DTaP-IPV//PRP~T à 6, 10 et 14 semaines ont également reçu de manière aléatoire l’un des deux schémas vaccinaux contre l’hépatite B suivants : administration à 6, 10 et 14 semaines ou à 0, 6 et 14 semaines. Les titres d’anticorps ont été déterminés à 6 et 18 semaines et la réactogénicité a été surveillée d’après les indications des parents.
Résultats
L’immunogénicité était élevée pour tous les antigènes vaccinaux et similaire à celle obtenue dans une étude témoin historique. Après la primo-vaccination, 98,7 % des nourrissons présentaient un titre d’anticorps anti-PRP ≥ 0,15 µg/ml. On obtenait une séroprotection contre les poliovirus de types 1, 2 et 3 et contre le tétanos chez tous les nourrissons et contre la diphtérie chez 97,1 % d’entre eux. La séroconversion pour la coqueluche, définie comme une augmentation d’un facteur supérieur à quatre du titre d’anticorps, s’est produite chez 95,3 % des nourrissons pour les anticorps dirigés contre l’anatoxine coquelucheuse et chez 89,0 % des nourrissons pour les anticorps dirigés contre l’hémagglutinine filamenteuse. Le taux de séroprotection contre l’hépatite B était de 99,5 % pour la série de doses administrées à 0, 6 et 14 semaines et de 97,8 % pour la série administrée à 6, 10 et 14 semaines. Néanmoins, les titres d’anticorps était plus élevés pour la première série (601 mlU/ml contre 207 mlU/ml). La réactogénicité des deux vaccins était faible.
Conclusion
Le vaccin DTaP-IPV//PRP~T est hautement immunogène. La réponse en anticorps contre l’hépatite B est séroprotectrice pour les deux schémas de vaccination, bien que le titre d’anticorps soit plus élevé pour l’administration à 0, 6 et 14 semaines.
Resumen
Objetivo
Determinar las tasas de seroprotección inducidas por una vacuna conjugada contra difteria-tétanos-tos ferina acelular-poliovirus inactivado-Haemophilus influenzae tipo B (DTaP–IPV//PRP~T) que contiene un conjugado de poliribosil-ribitol-fosfato (PRP) y anatoxina tetánica (Pentaxim™), administrada junto con otra vacuna contra la hepatitis B.
Métodos
En este ensayo multicéntrico al descubierto, 424 lactantes que recibieron DTaP–IPV//PRP~T a las 6, 10 y 14 semanas de edad fueron inmunizados también de forma aleatorizada con vacuna contra la hepatitis B, bien a las 6, 10 y 14 semanas, o bien a las 0, 6 y 14 semanas de edad. Se determinaron los niveles de anticuerpos a las 6 y las 18 semanas de edad, y se evaluó la reactogenicidad a partir de lo declarado por los padres.
Resultados
La inmunogenicidad de todos los antígenos vacunales fue elevada y similar a la observada en un estudio con grupo de referencia histórico. Tras la primovacunación, el 98,7% de los lactantes presentaron unos títulos de anticuerpos anti-PRP ≥ 0,15 μg/ml. Se indujo seroprotección contra los poliovirus de tipo 1, 2 y 3 y contra el tétanos en todos los lactantes, y contra la difteria en el 97,1% de los casos. La seroconversión contra la tos ferina, definida como un aumento de al menos el cuádruple de título de anticuerpos, se produjo en un 95,3% de los casos en lo que respecta a los anticuerpos contra el toxoide tosferínico, y en un 89,0% de los casos para el anticuerpo contra la hemaglutinina filamentosa. La tasa de seroprotección contra la hepatitis B fue del 99,5% con la pauta de administración a las 0, 6 y 14 semanas, y del 97,8% a las 6, 10 y 14 semanas. Sin embargo, el título de anticuerpos fue mayor con la pauta de las 0, 6 y 14 semanas (601 mUI/ml frente a 207 mUI/ml). La reactogenicidad de las dos vacunas fue baja.
Conclusión
La vacuna DTaP–IPV//PRP~T tuvo un efecto inmunogénico muy intenso. La respuesta de anticuerpos contra la hepatitis B fue seroprotectora con las dos pautas empleadas, si bien el título de anticuerpos fue mayor con la pauta de 0, 6 y 14 semanas.
ملخص
الەدف
التعرف على معدلات الوقاية المصلية ومعدلات الاستجابة للقاح والتي تنجم عن لقاحات الخناق والكزاز واللقاح اللاخلوي للشاەوق واللقاح المعطَّل لشلل الأطفال واللقاح المقترن للمستدميات النزلية من النمط ب وتضم أيضاً ذوفان الكزاز المقترن ( بنتاكسيم) مع بولي ريبوزيل – ريبيتول – فوسفات (PRP) وتعطى مع لقاح التەاب الكبد البائي.
الطريقة
أجرى الباحثون دراسة متعددة المراكز مفتوحة اللصاقة شملت 242 طفلاً أعطوا اللقاحات المذكورة أعلاە في أعمار 6 و10 و14 أسبوعاً ثم اختيروا بصورة معشاة لتلقي لقاح التەاب الكبد أما في أعمار 6 و10 و14 أسبوعاً أو أعمار 0 و6 و14 أسبوعاً وقاس الباحثون مستوى الأضداد في الأسابيع 6 و18 من العمر لرصد مدى أثارە وردود الفعل باستخدام تقارير الوالدين.
الموجودات
كان الاستمناع مرتفعاً في جميع مستضدات اللقاحات، ومشابەاً لما لوحظ في دراسة مراقبة بالشواەد أجريت سابقاً. فبعد التلقيح البدئي كان لدى 98.7% من الأطفال أضداداً PRP تزيد أو تساوي عن 0.15 مكروغرام/ميلي لتر. كما حصل جميع الأطفال على وقاية مصلية ضد الأنماط 1 و2 و3 من فيروس شلل الأطفال ولدى 97.1% ضد الخناق، فيما حصل الانقلاب المصلي ضد الشاەوق لدى 95.3% من الأطفال. وقد عرف الانقلاب المصلي بأنە زيادة تساوي أو تزيد على أربعة أضعاف في عيار الأضداد، ولدى 89.0% بوجود أضداد التراص الدموي الخيطي. وقد بلغ معدل الوقاية المصلية 99.5% بإعطاء اللقاحات في أعمار 0 و6 و14 أسبوعاً و97.8% بإعطاء اللقاحات في أعمار 6 و10 و14 أسبوعاً، إلا أن عيار الأضداد كان أعلى لدى إعطاء اللقاحات في أعمار 0 و6 و14 أسبوعاً (فقد بلغ 601 ميكرو وحدة/ميلي لتر مقابل 207 ميكرو وحدة/ميلي لتر). وكانت الاستفعالية ضعيفة لدى الخطتين من اللقاحات.
الاستنتاج
للقاحات المذكورة فعالية استمناعية عالية، والاستجابة بأضداد التەاب الكبد البائي كانت ذات وقاية مصلية في كلا الخطتين، إلا أن عيار الأضداد كان في الخطة التي تعطى فيەا اللقاحات في أعمار 0 و6 و14 أسبوعاً أعلى مما كانت عليە في الخطة الأخرى.
Introduction
The original aim of the WHO Expanded Programme on Immunization (EPI) was to protect children against six childhood diseases: tuberculosis, diphtheria, neonatal tetanus, whooping cough, poliomyelitis and measles. Subsequently, others were added, including hepatitis B and Haemophilus influenzae type b. Combination vaccines that include several valences help ensure high vaccination coverage.
Concerns about the safety and reactogenicity of whole-cell pertussis (wP) vaccines led to the development of “acellular” pertussis (aP) vaccines, comprising purified Bordetella pertussis antigens, which are better tolerated than whole-cell vaccines and have similar immunogenicity.1–3 The protective efficacy of aP vaccines has been documented in controlled trials.4–6 Today, they are used in national immunization programmes in Canada, the United States of America (USA), and various European and Asian countries (e.g. Australia, Japan and the Republic of Korea).7 A recent WHO position paper on pertussis vaccines8 stated that: “The best aP vaccines have shown similar protective efficacy as the best wP vaccines” and that: “all licensed vaccines have proved to be highly effective in controlling pertussis in infants and young children”.
Sanofi pasteur has developed an aP vaccine that contains pertussis toxoid (PT) and filamentous haemagglutinin (FHA) that is used in paediatric combinations with not only diphtheria (D) and tetanus (T) proteins, but also inactivated poliovirus (IPV) vaccine and Haemophilus influenzae type-b capsular polyribosyl-ribitol-phosphate conjugated to tetanus toxoid (PRP~T). Both the PRP~T antigen and the IPV vaccine are WHO prequalified vaccines.9 The DTaP–IPV//PRP~T vaccine (known as Pentaxim™ or Pentavac™) used in this study has been evaluated clinically in various trials.10–14 The vaccine was first licensed in European countries in 1997 and is now licensed in approximately 67 countries worldwide.
Few data are available on the immunogenicity, reactogenicity and safety of aP combination vaccines in many countries that follow EPI immunization schedules. As a result, the present study was carried out to evaluate these characteristics when the DTaP–IPV//PRP~T vaccine was given to infants at 6, 10 and 14 weeks of age along with concomitant hepatitis B virus vaccination, administered on either of the two WHO-recommended vaccination schedules followed in the Philippines. The results were compared with those previously obtained in European infants given the same DTaP–IPV//PRP~T vaccine at 2, 3 and 4 months of age.
Methods
Study design
This randomized controlled open study enrolled infants at three medical centres in Manila, the Philippines: the Research Institute for Tropical Medicine (RITM), the University of the East Ramon Magsaysay Memorial Medical Center, and the Mary Chiles Hospital. The ethical review board at each centre approved the protocol. Written informed consent was obtained from infants’ parents or legal representatives before enrolment. In addition, mothers of potential study subjects also consented to clinical assessment and blood sampling during the third trimester of pregnancy to test for hepatitis B surface antigen (HBsAg). In those who were seropositive for HBsAg, the hepatitis B e antigen (HBeAg) level was also measured.
Subjects
The study involved healthy full-term infants of ≥ 37 weeks gestation who weighed ≥ 2.5 kg and whose mother was anti-HBsAg seronegative. All infants received the DTaP–IPV//PRP~T vaccine at 6, 10 and 14 weeks of age. In addition, they were randomized within 24 hours of birth, using a random numbers list, to receive recombinant hepatitis B vaccine at either 0, 6 and 14 weeks (Group A) or 6, 10 and 14 weeks of age (Group B). Infants whose mother was HBsAg-seropositive were not enrolled but were given hepatitis B immunoglobulins and the first dose of the hepatitis B vaccine within 24 hours of birth. Parents or guardians were informed of their child’s serological status and private physicians provided follow-up care.
Infants were excluded if they had immune system disease, including HIV infection, major congenital malformations or conditions, serious illness or malignancy, a history of neurological disorders or seizures, a mother with liver disease or an allergy to a vaccine component, or if they had received immunosuppressive therapy (other than inhaled and topical steroids), immunoglobulins or other blood products, previous immunization against DTP, H. influenzae type b or poliomyelitis or a vaccine other than bacille Calmette-Guérin (BCG), which is included in the national Philippine vaccination schedule.
Vaccines
The combined DTaP–IPV//PRP~T vaccine (Pentaxim™, batch W1538) was produced and supplied by sanofi pasteur, Lyon, France. Each 0.5-ml dose contained ≥ 30 IU [25 limit of flocculation (Lf) of diphtheria toxoid], ≥ 40 IU (10 Lf) of tetanus toxoid, 25 μg of PT, 25 μg of FHA, 40 D antigen units (DU) of IPV type-1 (Mahoney strain), 8 DU of IPV type-2 (MEF-1 strain), 32 DU of IPV type-3 (Saukett strain), and 10 μg of PRP~T. The lyophilized PRP~T component was reconstituted with the liquid DTaP–IPV vaccine immediately before injection. The antigen composition of the study vaccine was identical to that of the historical control vaccine. The recombinant hepatitis B vaccine (Recomvax B/Euvax B, batch UVA3002, LG Life Sciences, Seoul, the Republic of Korea) contained 10 μg of recombinant HBsAg. It is licensed and commercially available in the Philippines. The DTaP–IPV//PRP~T and hepatitis B vaccines were administered by intramuscular injection into the right and left anterior thigh, respectively.
Serology
Blood samples were taken for antibody determination when infants were 6 weeks of age, just before the first injection of the combination vaccine, and at approximately 18 weeks of age, one month after the third vaccination. Serological analyses were performed as described previously12 at a Sanofi pasteur central laboratory in Pennsylvania, USA. The levels of antibodies to HBsAg, PT, FHA and tetanus antitoxin were determined by enzyme-linked immunosorbent assay (ELISA). Anti-PRP antibodies were measured using a Farr-type radioimmunoassay.12 Anti-diphtheria antitoxin and anti-poliovirus type-1, -2 and -3 antibodies were measured by seroneutralization. The predefined antibody levels for seroprotection were: anti-PRP ≥ 0.15 µg/ml and ≥ 1.0 µg/ml, anti-poliovirus ≥ 8 reciprocal dilution (1/dil), anti-diphtheria ≥ 0.01 IU/ml, anti-tetanus ≥ 0.01 IU/ml and anti-HBsAg ≥ 0.01 mIU/ml. Since there are no accepted correlates of seroprotection for pertussis antibodies, the vaccine response for anti-pertussis antigens was defined as a ≥ fourfold or a ≥ twofold increase in antibody concentration after primary vaccination.
Reactogenicity and safety
Infants were monitored for 30 minutes after each injection for immediate local or systemic reactions. Parents or legal guardians recorded solicited local reactions (i.e. injection site redness, swelling and pain or tenderness) and solicited systemic events (i.e. rectal temperature ≥ 38 °C, drowsiness, unusual fussiness, unusual crying, loss of appetite, vomiting and diarrhoea) daily for 8 days following vaccination. Pain or tenderness was scored as severe if the infant cried when the limb was moved or if the pain appeared to prevent normal activity. To be severe, crying or irritability had to continue for more than 3 hours. Fever was graded severe if the rectal temperature was ≥ 40 °C. The date of onset, intensity and resolution of any unsolicited events were recorded for 30 days after each vaccination.
Statistical analysis
The mean seroprotection or vaccine response rate and the antibody geometric mean titre (GMT) were calculated for each vaccine antigen, along with the 95% confidence interval (CI), for each study group and for combined data from the two study groups. Reverse cumulative distribution curves (RCDC) for antibody titres were also derived to illustrate immune responses.
The primary objective of the study was to show that seroprotection rates for diphtheria, tetanus, different poliovirus types and PRP, and the vaccine response rate for pertussis antigens obtained with the combination vaccine are noninferior to those observed in the French historical control study in which the same DTaP–IPV//PRP~T vaccine was given at 2, 3 and 4 months of age. This control study was chosen because no data were available for use of the study vaccine at 6, 10 and 14 weeks of age.
The vaccine was considered noninferior if the 95% CI of the seroprotection or vaccine response rate lay entirely above the historical reference value minus a predefined clinically acceptable difference of 10%. Given the expected seroprotection and seroconversion rates derived from the historical control study, a predefined clinically relevant difference of 10%, and a power of 90%, it was found that a sample size of 180 subjects per group was required to show noninferiority. In addition, on the assumption that 15% of subjects would be lost to evaluation, the intention was to enrol 212 in each group. The statistical analysis was performed using SAS software (SAS Institute, Cary, NC, USA). All subjects who received at least one dose of the combination vaccine were included in the safety assessment.
Results
Study population
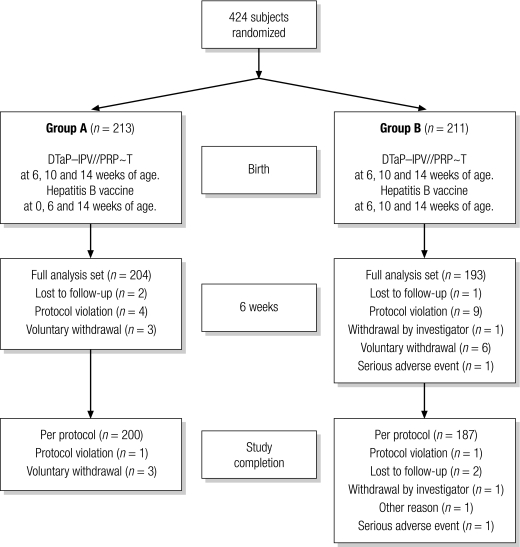
The study included 424 infants: 264 at the RITM, 75 at the University of the East Ramon Magsaysay Memorial Medical Center, and 85 at the Mary Chiles Hospital. Of these, 213 were randomized to Group A and 211, to Group B. There were more male than female infants (57% versus 43%). Their mean (±standard deviation) age at enrolment was 1.0 (±0.6) days. Their mean age at the first combination vaccine dose was 6.4 (±0.5) weeks. Of the 424 enrolled infants, 397 (204 in Group A and 193 in Group B) received at least one dose of the combination vaccine (i.e. the full analysis set) and 387 (200 in Group A and 187 in Group B) received three doses (i.e. the per-protocol set). Fig. 1. Twenty-seven subjects (6.7%, nine in Group A and 18 in Group B) discontinued the study or were excluded from the full analysis set before receiving a first dose of the combination vaccine. Of those who received a first dose, 10 did not complete the study: four in Group A and six in Group B. All but one of the protocol violations reported (Fig. 1) involved the receipt of DTP and oral polio vaccine. One subject underwent a blood transfusion before the first combination vaccine dose. Two subjects withdrew because of severe adverse events: one had sepsis neonatorum and the other, cardiopulmonary arrest secondary to a ventricular septal defect. Neither was related to vaccination.
Fig. 1.
Study flowchart showing the number of infants enrolled in the study, their random allocations, and the numbers who did or did not complete the vaccination schedules
DTaP–IPV//PRP~T, diphtheria–tetanus–acellular pertussis–inactivated poliovirus–Haemophilus influenzae type-b conjugate.
Immunogenicity
The observed seroprotection and vaccine response rates 1 month after the third combined DTaP–IPV//PRP~T vaccine dose were high for each vaccine antigen, similar in both study groups, and similar to the corresponding rates observed in the historical control study (Table 1). In both study groups, and for each antigen included in the combination vaccine, the 95% CI of the seroprotection or vaccine response rate lay entirely above the rate for the historical control study minus the predefined 10% limit for noninferiority. Pooled data from the two groups showed that 98.7% had an anti-PRP level ≥ 0.15 μg/ml and 67.2% had a level ≥ 1.0 μg/ml. The seroprotection rate was 100% for tetanus and poliovirus type-1, -2 and -3, and 97.1% for diphtheria. The vaccine response rates for anti-PT and anti-FHA, defined as at least a fourfold increase in antibody titre, were 95.1% and 88.8%, respectively. The corresponding rates for a twofold increase were 98.8% and 95.5%, respectively. An anti-PT antibody titre ≥ 25 equivalent international units (EU)/ml was observed in 99.5% and 99.0% in Groups A and B respectively, and an anti-FHA antibody titre ≥ 25 EU/ml was observed in 98.5% and 99.4% respectively.
Table 1. Seroprotection and vaccine response ratesa 1 month after the third combined vaccine dose.
| Seroprotection level or vaccine response | Historical control group rate (%)b,c | Group A rate (%)b,d | Group B rate (%)b,e | Pooled data for groups A and B rate (%)b,d,e |
|---|---|---|---|---|
| Anti-diphtheria ≥ 0.01 IU/ml | 100 (95.9–100) | 98.0 (94.9–99.4) | 96.2 (92.4–98.5) | 97.1 (94.9–98.6) |
| Anti-tetanus ≥ 0.01 IU/ml | 100 (95.9–100) | 100 (98.2–100) | 100 (98.0–100) | 100 (99.0–100) |
| Anti-PT ≥ fourfold increase | 89.6 (81.7–94.9) | 94.3 (89.7–97.2) | 96.1 (91.7–98.5) | 95.1 (92.2–97.2) |
| Anti-FHA ≥ fourfold increase | 89.5 (81.5–94.8) | 90.9 (85.8–94.6) | 86.4 (80.3–91.2) | 88.8 (85.0–91.9) |
| Anti-poliovirus type-1 ≥ 8 1/dil | 97.0 (91.5–99.4) | 100 (98.2–100) | 100 (98.0–100) | 100 (99.0–100) |
| Anti-poliovirus type-2 ≥ 8 1/dil | 100 (96.4–100) | 100 (98.2–100) | 100 (98.0–100) | 100 (99.0–100) |
| Anti-poliovirus type-3 ≥ 8 1/dil | 99.0 (94.6–100) | 100 (98.1–100) | 100 (98.0–100) | 100 (99.0–100) |
| Anti-PRP ≥ 0.15 µg/ml | 98.0 (93.0–99.8) | 99.0 (96.4–99.9) | 98.3 (95.2–99.7) | 98.7 (97.0–99.6) |
| Anti-HBsAgf ≥ 10 mIU/ml | NA | 99.5 (97.2–100) | 97.8 (94.6–99.4) | – |
DTaP–IPV//PRP~T, diphtheria–tetanus–acellular pertussis–inactivated poliovirus–Haemophilus influenzae type-b conjugate; FHA, filamentous haemagglutinin; HBsAg, hepatitis B surface antigen; NA, not available; PRP, polyribosyl-ribitol-phosphate; PT, pertussis toxoid. a The rate (%) listed is the percentage of infants with an immune response (i.e. with seroprotection, seroconversion or a vaccine response). b Values in parentheses are 95% confidence intervals. c The historical control group received the DTaP–IPV//PRP~T combination vaccine at 2, 3 and 4 months of age. d Group A received the DTaP–IPV//PRP~T combination vaccine at 6, 10, 14 weeks of age and the recombinant hepatitis B vaccine at 0, 6 and 14 weeks of age. e Group B received the DTaP–IPV//PRP~T vaccine and the recombinant hepatitis B vaccine at 6, 10 and 14 weeks of age. f The anti-HBsAg antibody level was determined when infants were 6 and 18 weeks of age.
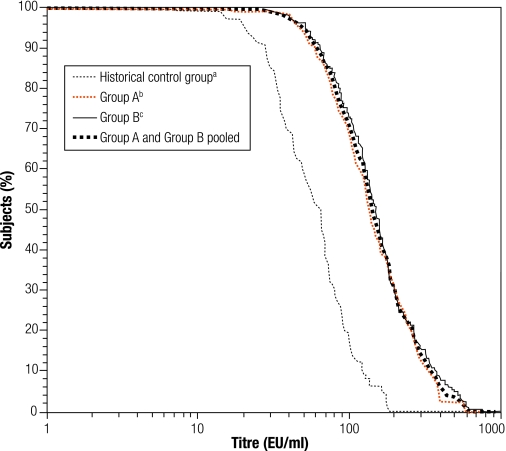
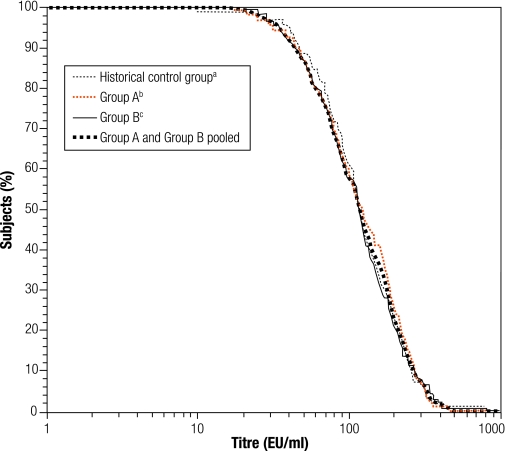
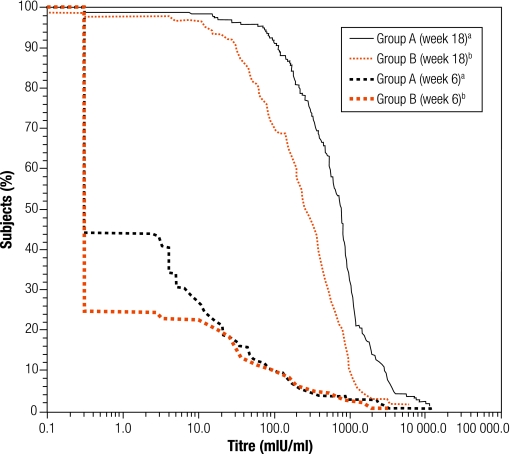
There was no significant difference in the antibody GMT for any vaccine antigen between Groups A and B, which enabled the data to be pooled (Table 2). Antibody GMTs increased significantly from before to after primary vaccination for each vaccine antigen. The pooled anti-PRP GMT increased from 0.2 to 2.0 µg/ml. The anti-poliovirus type-1 GMT increased from 9.3 to 569 1/dil, the type-2 GMT increased from 16.5 to 769 1/dil, and the type-3 GMT, from 10.3 to 1788 1/dil. The anti-PT and anti-FHA GMTs increased from 3.14 to 142 EU/ml and from 5.23 to 115 EU/ml, respectively. The reverse cumulative distribution curves (RCDCs) in Fig. 2 and Fig. 3 show anti-PT antibody and anti-FHA antibody titres, respectively, in subjects from Group A, Group B, from both groups pooled together and from the historical control study. Immune responses to PT and FHA were similar in the two study groups. The RCDCs for anti-PT antibody show that responses in Groups A and B were stronger than that in the historical control group. A seroprotective anti-HBsAg titre ≥ 10 mIU/ml was observed in at least 97.8% of all infants (Table 1), but the GMT was higher in Group A than Group B, at 601 mIU/ml versus 207 mIU/ml, respectively (Table 2). The RCDCs in Fig. 4 show anti-HBsAg responses in Groups A and B in sera obtained at 6 and 18 weeks of age.
Table 2. GMT for all vaccine antigens before and after primary vaccination.
| Antibody | Group Aa GMT |
Group Bb GMT |
Pooled data for
Groups A and Ba,b GMT |
|||||
|---|---|---|---|---|---|---|---|---|
| before dose 1 | after dose 3 | before dose 1 | after dose 3 | before dose 1 | after dose 3 | |||
| Anti-diphtheria (IU/ml)c | 0.019 (0.016–0.024) | 0.051 (0.044–0.060) | 0.018 (0.015–0.023) | 0.051 (0.043–0.060) | 0.019 (0.016–0.022) | 0.051 (0.045–0.057) | ||
| Anti-tetanus (IU/ml)c | 0.676 (0.513–0.890) | 2.23 (2.04–2.44) | 0.637 (0.480–0.846) | 2.13 (1.93–2.34) | 0.657 (0.540–0.800) | 2.18 (2.04–2.33) | ||
| Anti-PT (EU/ml)c | 3.31 (2.80–3.91) | 136 (122–150) | 2.96 (2.47–3.54) | 150 (136–165) | 3.14 (2.78–3.54) | 142 (132–153) | ||
| Anti-FHA (EU/ml)c | 5.29 (4.52–6.20) | 117 (106–129) | 5.16 (4.39–6.06) | 113 (102–125) | 5.23 (4.67–5.85) | 115 (107–123) | ||
| Anti-poliovirus type-1 (1/dil)c | 9.67 (7.89–11.8) | 545 (448–663) | 8.91 (7.32–10.8) | 595 (487–727) | 9.29 (8.07–10.7) | 569 (495–654) | ||
| Anti-poliovirus type-2 (1/dil)c | 14.4 (11.6–17.8) | 800 (651–983) | 19.5 (15.4–23.9) | 738 (599–909) | 16.5 (14.2–19.3) | 769 (664–890) | ||
| Anti-poliovirus type-3 (1/dil)c | 10.3 (8.68–12.1) | 1988 (1697–2328) | 10.4 (8.59–12.5) | 1 594 (1356–1873) | 10.3 (9.10–11.7) | 1788 (1597–2002) | ||
| Anti-PRP (µg/ml)c | 0.208 (0.166–0.259) | 1.94 (1.63–2.29) | 0.228 (0.183–0.284) | 2.09 (1.75–2.51) | 0.217 (0.186–0.254) | 2.01 (1.78–2.27) | ||
| Anti-HBsAgd (mIU/ml)c | 2.15 (1.51–3.08) | 601.0 (508–712) | 1.16 (0.808–1.66) | 207.0 (166–257) | – | – | ||
DTaP–IPV//PRP~T, diphtheria–tetanus–acellular pertussis–inactivated poliovirus–Haemophilus influenzae type-b conjugate; GMT, geometric mean titre; HBsAg, hepatitis B surface antigen; FHA, filamentous haemagglutinin; PRP, polyribosyl-ribitol-phosphate; PT, pertussis toxoid. a Group A received the DTaP–IPV//PRP~T combination vaccine at 6, 10, 14 weeks of age and the recombinant hepatitis B vaccine at 0, 6 and 14 weeks of age. b Group B received the DTaP–IPV//PRP~T vaccine and the recombinant hepatitis B vaccine at 6, 10 and 14 weeks of age. c Values in parentheses are 95% confidence intervals. d The anti-HBsAg antibody level was determined when infants were 6 and 18 weeks of age.
Fig. 2.
RCDCs for anti-pertussis toxoid antibody titres after the third combined DTaP–IPV//PRP~T vaccine dose
DTaP–IPV//PRP~T, diphtheria–tetanus–acellular pertussis–inactivated poliovirus–Haemophilus influenzae type-b conjugate; RCDC, reverse cumulative distribution curve.
a The historical control group received the DTaP–IPV//PRP~T combination vaccine at 2, 3 and 4 months of age.
b Group A received the DTaP–IPV//PRP~T combination vaccine at 6, 10, 14 weeks of age and the recombinant hepatitis B vaccine at 0, 6 and 14 weeks of age.
c Group B received the DTaP–IPV//PRP~T vaccine and the recombinant hepatitis B vaccine at 6, 10 and 14 weeks of age.
Fig. 3.
RCDCs for anti-pertussis filamentous haemagglutinin titres after the third combined DTaP–IPV//PRP~T vaccine dose
DTaP–IPV//PRP~T, diphtheria–tetanus–acellular pertussis–inactivated poliovirus–Haemophilus influenzae type-b conjugate; RCDC, reverse cumulative distribution curve.
a The historical control group received the DTaP–IPV//PRP~T combination vaccine at 2, 3 and 4 months of age.
b Group A received the DTaP–IPV//PRP~T combination vaccine at 6, 10, 14 weeks of age and the recombinant hepatitis B vaccine at 0, 6 and 14 weeks of age.
c Group B received the DTaP–IPV//PRP~T vaccine and the recombinant hepatitis B vaccine at 6, 10 and 14 weeks of age.
Fig. 4.
RCDCs for anti-hepatitis B surface antigen titres in sera obtained at 6 and 18 weeks of age from infants in Groups A and B
DTaP–IPV//PRP~T, diphtheria–tetanus–acellular pertussis–inactivated poliovirus–Haemophilus influenzae type-b conjugate; RCDC, reverse cumulative distribution curve.
a Group A received the DTaP–IPV//PRP~T combination vaccine at 6, 10, 14 weeks of age and the recombinant hepatitis B vaccine at 0, 6 and 14 weeks of age.
b Group B received the DTaP–IPV//PRP~T vaccine and the recombinant hepatitis B vaccine at 6, 10 and 14 weeks of age.
Reactogenicity
Injection site pain was the most frequently recorded solicited local reaction. It was observed after 12.0% and 21.2% of combined DTaP–IPV//PRP~T vaccine doses in Groups A and B, respectively, and after 13.7% and 18.9% of hepatitis B vaccine doses in the two groups, respectively. Severe pain was observed in no more than 0.7% (Table 3). Redness, swelling and induration were reported after no more than 3% of any dose.
Table 3. Incidence of local and systemic adverse reactions solicited from parents and guardians that occurred within 8 days of vaccination.
| Adverse reactions | Group A vaccine dosesa (n = 606) |
Group B vaccine dosesb (n = 579) |
||||
|---|---|---|---|---|---|---|
| DTaP–IPV//PRP~T (% of doses) | Hepatitis B (% of doses) | DTaP–IPV//PRP~T (% of doses) | Hepatitis B (% of doses) | |||
| Local adverse reactions | any | 12.9 | 13.9 | 22.1 | 19.1 | |
| severe | 0.3 | 0.2 | 0.7 | 0.5 | ||
| Pain | Any | 12.0 | 13.7 | 21.2 | 18.9 | |
| severe | 0.3 | 0.2 | 0.7 | 0.5 | ||
| Redness | Any | 0.5 | 1.2 | 0.4 | 0.2 | |
| severe | 0 | 0 | 0 | 0 | ||
| Swelling | Any | 1.0 | 0.7 | 2.8 | 1.8 | |
| severe | 0 | 0 | 0 | 0 | ||
| Induration | Any | 2.0 | 0.7 | 3.0 | 1.2 | |
| severe | 0 | 0 | 0 | 0 | ||
| Systemic adverse reactions | any | 22.9 | 25.4 | |||
| severe | 0.5 | 0.7 | ||||
| Fever | Any | 12.2 | 11.0 | |||
| severe | 0 | 0.5 | ||||
| Drowsiness | Any | 5.4 | 5.4 | |||
| severe | 0 | 0 | ||||
| Irritability | Any | 11.6 | 16.6 | |||
| severe | 0.5 | 0.2 | ||||
| Loss of appetite | Any | 6.3 | 6.5 | |||
| severe | 0 | 0 | ||||
DTaP–IPV//PRP~T, diphtheria–tetanus–acellular pertussis–inactivated poliovirus–Haemophilus influenzae type-b conjugate. a Group A received the DTaP–IPV//PRP~T combination vaccine at 6, 10, 14 weeks of age and the recombinant hepatitis B vaccine at 0, 6 and 14 weeks of age. b Group B received the DTaP–IPV//PRP~T vaccine and the recombinant hepatitis B vaccine at 6, 10 and 14 weeks of age.
Fever and irritability were the most common solicited systemic events, with fever observed after 12.2% and 11.0% of doses in Groups A and B, respectively, and irritability after 11.6% to 16.6%, respectively. Severe fever or irritability was seen after 0.2% to 0.5%. Drowsiness or loss of appetite was reported after 5.4% to 6.5%, with no severe cases.
Of the 397 subjects who received one dose or more of the combination vaccine, 213 (53.7%) reported at least one unsolicited adverse event within 30 days. Only two of these events, both of which involved fever after the third dose in infants in Group B, were interpreted by the investigator as related to vaccination. The most frequently reported unsolicited systemic adverse events in Group A were upper respiratory tract infection (42 infants), nasopharyngitis (31), pyrexia (30) and cough (26). In Group B, they were upper respiratory tract infection (40 infants), nasopharyngitis (34), pyrexia (26) and cough (20). No serious adverse event was regarded as being related to vaccination.
Discussion
This study evaluated the immunogenicity and safety of a DTaP–IPV//PRP~T combination vaccine given to infants as a primary series at 6, 10 and 14 weeks of age. The results were compared with a historical control group given the same vaccine at 2, 3 and 4 months of age in a clinical trial conducted in France, where the vaccine has been in routine use. Infants were randomized at birth, before administration of the combination vaccine, to receive a commercially available recombinant hepatitis B vaccine at either 0, 6 and 14 weeks or 6, 10 and 14 weeks of age. The study provided essential data on an aP combination vaccine that is given at 6, 10 and 14 weeks in many EPI schedules. To our knowledge, this is also the first clinical report on an aP combination vaccine being given together with a monovalent hepatitis B vaccine on a schedule starting either at birth or 6 weeks of age.
The immunogenicity of the combination vaccine was high for all antigens, was similar in the present two study groups, and was similar to that in the historical control group. The seroprotection rates and antibody GMTs associated with the IPV vaccine were of particular interest because of the schedule used. Indeed, seroprotection against all three polioviruses was present in all infants after vaccination. Large increases in anti-polio neutralizing antibody GMTs occurred: from 9.2 to 569 1/dil for type-1 poliovirus, from 16.5 to 769 1/dil for type 2, and from 10.3 to 1788 1/dil for type 3. The response to IPV vaccine antigens reported here provides additional data on the immunogenicity of the IPV vaccine in tropical countries such as the Philippines, and is consistent with the findings of previous clinical studies in temperate countries.10–17
After the third combination vaccine dose, the anti-PRP titre was ≥ 0.15 μg/ml in 98.7% of infants, and the anti-PRP GMT increased from 0.2 to 2.0 µg/ml. This result, obtained in our study with vaccinations given at 6, 10 and 14 weeks of age, is consistent with those of previous studies that used the same schedule and with those of studies that used the same PRP~T antigen but administered doses at 2, 3 and 4 months of age or 2, 4 and 6 months of age.11,12,14,15
As there are no recognized serological correlates for protection against pertussis, a fourfold increase in the antibody titre from before to after vaccination was used as the criterion for a vaccine response to pertussis antigens. A fourfold increase in anti-PT and anti-FHA antibody titre was obtained in 95.1% and 88.8% of infants, respectively, and a twofold increase was obtained in 98.8% and 95.5%, respectively. Anti-PT and anti-FHA antibody GMTs were similar in the two study groups before vaccination, at 3.1 EU/ml for PT and 5.2 EU/ml for FHA. After vaccination, over 99% of infants had an anti-PT or anti-FHA antibody titre ≥ 25 EU/ml. The distributions observed on the RCDCs showed that the FHA responses induced in infants in Groups A and B were similar and comparable to that seen in the historical control group (Fig. 2). Both groups in this study also had similar anti-PT antibody distributions but the RCDC was shifted to the right relative to that of the historical control group, indicating a somewhat stronger immune response in the present study. The immunogenicity observed for PT and FHA, as measured by the vaccine response and GMT, is also consistent with that seen in studies in Europe.11,12,14 Previous studies have demonstrated that the aP vaccine used in this study is effective.4,6 Additional evidence has also been provided by surveillance in Sweden following several years of routine use in the national vaccination programme.18,19
The hepatitis B vaccine dose given at birth ensures protection in populations where hepatitis B is endemic and the risk of perinatal transmission is increased. Administering the vaccine at birth is an important public health measure for infants born to mothers who are HBsAg-seropositive or whose hepatitis B status is unknown and when screening errors occur.20,21 This study’s results confirm that the combination vaccine used is compatible with hepatitis B vaccination schedules recommended by the WHO EPI. After the third hepatitis B vaccine dose, the anti-HBsAg GMT was higher in Group A than Group B. The magnitude of anti-HBsAg response is known to be schedule-dependent. This study also confirms the observation made in previous studies that lengthening the interval between the last two doses increases the final anti-HBsAg titre.20–22 However, despite the difference in anti-HBsAg antibody titre seen here, there was no difference in the seroprotection rate between the groups.
In this study population, vaccination was associated with a low incidence of solicited local and systemic adverse reactions. Severe pain was recorded after only 0.2–0.7% of doses, and no other severe local reaction was reported. A similar pattern was observed for severe fever and irritability, which occurred after no more than 0.5% of doses.
In summary, this study demonstrated that the DTaP–IPV//PRP~T combination vaccine was highly immunogenic for all antigens, well tolerated and safe when given to infants at 6, 10 and 14 weeks of age, a schedule used frequently in the WHO EPI. Moreover, it could be given concomitantly with monovalent hepatitis B vaccine at either 0, 6 and 14 weeks of age or 6, 10 and 14 weeks of age. However, the magnitude of the hepatitis B immune response was greater when the first dose was given at birth. The immunogenicity and safety of the DTaP–IPV//PRP~T combination vaccine in healthy children in the Philippines were similar to those observed in a European historical control study that administered the same vaccine at 2, 3 and 4 months of age. ■
Acknowledgements
We thank Agnès Garinga and Pascale Chavand for study monitoring, Christèle Deroche and Valèrie Bosch-Castells for the analysis of study data, and Clement Weinberger for assistance in preparing the manuscript. The above are employees of sanofi pasteur in the Philippines or in France.
Footnotes
Funding: This study (http://www.clinicaltrials.gov/ct/show/NCT00254917?order=3) was conducted with the support of sanofi pasteur, Lyon, France.
Competing interests: Esteban Ortiz, and Valèrie Bosch are employed by Sanofi Pasteur, Lyon, France. Maria Rosario Capeding has received fees from Sanofi Pasteur for speaking at medical congresses.
References
- 1.Pines E, Barrand P, Fabre P, Salomon H, Blondeau C, Wood SC, et al. New acellular pertussis-containing paediatric combined vaccines. Vaccine. 1999;17:1650–6. doi: 10.1016/S0264-410X(98)00422-8. [DOI] [PubMed] [Google Scholar]
- 2.Decker MD, Edwards KM, Steinhoff MC, Rennels MB, Pichichero ME, Englund JA, et al. Comparison of 13 acellular pertussis vaccines: adverse reactions. Pediatrics. 1995;96:557–66. [PubMed] [Google Scholar]
- 3.Edwards KM, Meade BD, Decker MD, Reed GF, Rennels MB, Steinhoff MC, et al. Comparison of 13 acellular pertussis vaccines: overview and serologic response. Pediatrics. 1995;96:548–57. [PubMed] [Google Scholar]
- 4.Cherry JD. Comparative efficacy of acellular pertussis vaccines: an analysis of recent trials. Pediatr Infect Dis J. 1997;16(Suppl 4):S90–6. doi: 10.1097/00006454-199704001-00004. [DOI] [PubMed] [Google Scholar]
- 5.Edwards KM, Decker M. Pertussis vaccines. In: Plotkin SA, Orenstein WA, eds. Vaccines 4th edn. Philadelphia, PA: Saunders Co.; 2004. 21:471-528. [Google Scholar]
- 6.Simondon F, Preziosi MP, Yam A, Kane CT, Chabirand L, Iteman I, et al. A randomized double-blind trial comparing a two-component acellular to a whole-cell pertussis vaccine in Senegal. Vaccine. 1997;15:1606–12. doi: 10.1016/S0264-410X(97)00100-X. [DOI] [PubMed] [Google Scholar]
- 7.Therre H, Baron S. Pertussis immunisation in Europe - the situation in late 1999. Euro Surveill. 2000;5:6–10. doi: 10.2807/esm.05.01.00001-en. [DOI] [PubMed] [Google Scholar]
- 8.Pertussis vaccines. Wkly Epidemiol Rec. 2005;80:29–40. [Google Scholar]
- 9.United Nations prequalified vaccines (WHO list of vaccines for purchase by UN agencies as of April 2008). Available from: http://www.who.int/immunization_standards/vaccine_quality/pq_suppliers/en/index.html [accessed on 5 May 2008].
- 10.Lagos R, Kotloff KL, Hoffenbach A, San Martin O, Abrego P, Ureta AM, et al. Clinical acceptability and immunogenicity of a pentavalent parenteral combination vaccine containing diphtheria, tetanus, acellular pertussis, inactivated poliomyelitis and Haemophilus influenzae type b conjugate antigens in two-, four- and six month-old Chilean infants. Pediatr Infect Dis J. 1998;17:294–304. doi: 10.1097/00006454-199809001-00010. [DOI] [PubMed] [Google Scholar]
- 11.Carlsson RM, Claesson BA, Selstam U, Fagerlund E, Granstrom M, Blondeau C, et al. Safety and immunogenicity of a combined diphtheria, tetanus, acellular pertussis-inactivated polio vaccine-Haemophilus influenzae type b vaccine administered at 2–4–6–13 or 3–5–12 months of age. Pediatr Infect Dis J. 1998;17:1026–33. doi: 10.1097/00006454-199811000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Mallet E, Fabre P, Pines E, Salomon H, Staub T, Schödel F, et al. Immunogenicity and safety of a new liquid hexavalent combined vaccine compared with separate administration of reference licensed vaccines in infants. Pediatr Infect Dis J. 2000;19:1119–27. doi: 10.1097/00006454-200012000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Carlsson RM, Claesson BA, Fagerlund UE, Knutsson N, Laudin C. Antibody persistence in five-year old children who received a pentavalent combination vaccine in infancy. Pediatr Infect Dis J. 2002;21:535–41. doi: 10.1097/00006454-200206000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Kanra G, Selier T, Yurdakök K, Yavuz T, Baskan S, Ulukol B, et al. Immunogenicity study of a combined diphtheria, tetanus, acellular pertussis, inactivated poliomyelitis vaccine used to reconstitute a freeze-dried Haemophilus influenzae type b vaccine (DTacP-IPV//PRP-T) administered simultaneously with a hepatitis B vaccine at two, three and four months of life. Vaccine. 1999;18:947–54. doi: 10.1016/S0264-410X(99)00331-X. [DOI] [PubMed] [Google Scholar]
- 15.Gylca R, Gylca V, Benes O, Melnic A, Chicu V, Weisbecker C, et al. A new DTPa-HBV-IPV vaccine co-administered with Hib, compared to a commercially available DTPw-IPV/Hib vaccine co-administered with HBV, given at 6, 10 and 14 weeks following HBV at birth. Vaccine. 2000;19:825–33. doi: 10.1016/S0264-410X(00)00231-0. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan R, Jadhav M, John TJ. Efficacy of inactivated poliovirus vaccine in India. Bull World Health Organ. 1983;61:689–92. [PMC free article] [PubMed] [Google Scholar]
- 17.Plotkin SA, Vidor E. Poliovirus vaccine—inactivated. In: Plotkin SA, Orenstein WA, eds. Vaccines 4th ed. Philadelphia, PA: Saunders, 2004. pp. 625–49. [Google Scholar]
- 18.Pertussis surveillance in Sweden with enhanced follow-up of cohorts immunized with acellular pertussis vaccines [Appendix 2 SP-MSD]. Swedish Institute for Infectious Disease Control; 2006. Available from: http://www.smittskyddsinstitutet.se/upload/PDF-filer/seven-year-report-app-2-SP-MSD.pdf [accessed on 5 May 2008].
- 19.Olin P, Gustafsson L, Barreto L, Hessel L, Mast TC, Rie AV, et al. Declining pertussis incidence in Sweden following the introduction of acellular pertussis vaccine. Vaccine. 2003;21:2015–21. doi: 10.1016/S0264-410X(02)00777-6. [DOI] [PubMed] [Google Scholar]
- 20.Hepatitis B vaccines. Wkly Epidemiol Rec. 2004;79:255–64. [PubMed] [Google Scholar]
- 21.Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HA. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol. 2005;34:1329–39. doi: 10.1093/ije/dyi206. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg DP, Wong VK, Partridge S, Howe BJ, Ward JI. Safety and immunogenicity of a combination diphtheria-tetanus toxoids-acellular pertussis-hepatitis B vaccine administered at two, four and six months of age compared with monovalent hepatitis B vaccine administered at birth, one month and six months of age. Pediatr Infect Dis J. 2002;21:769–76. doi: 10.1097/00006454-200208000-00014. [DOI] [PubMed] [Google Scholar]