Abstract
Objective
To analyse the early loss of patients to antiretroviral therapy (ART) programmes in resource-limited settings.
Methods
Using data on 5491 adult patients starting ART (median age 35 years, 46% female) in 15 treatment programmes in Africa, Asia and South America with ≥ 12 months of follow-up, we investigated risk factors for no follow-up after treatment initiation, and loss to follow-up or death in the first 6 months.
Findings
Overall, 211 patients (3.8%) had no follow-up, 880 (16.0%) were lost to follow-up and 141 (2.6%) were known to have died in the first 6 months. The probability of no follow-up was higher in 2003–2004 than in 2000 or earlier (odds ratio, OR: 5.06; 95% confidence interval, CI: 1.28–20.0), as was loss to follow-up (hazard ratio, HR: 7.62; 95% CI: 4.55–12.8) but not recorded death (HR: 1.02; 95% CI: 0.44–2.36). Compared with a baseline CD4-cell count ≥ 50 cells/µl, a count < 25 cells/µl was associated with a higher probability of no follow-up (OR: 2.49; 95% CI: 1.43–4.33), loss to follow-up (HR: 1.48; 95% CI: 1.23–1.77) and death (HR: 3.34; 95% CI: 2.10–5.30). Compared to free treatment, fee-for-service programmes were associated with a higher probability of no follow-up (OR: 3.71; 95% CI: 0.97–16.05) and higher mortality (HR: 4.64; 95% CI: 1.11–19.41).
Conclusion
Early patient losses were increasingly common when programmes were scaled up and were associated with a fee for service and advanced immunodeficiency at baseline. Measures to maximize ART programme retention are required in resource-poor countries.
Résumé
Objectif
Analyser la perte précoce de patients par les programmes de traitement antirétroviral (ART) dans les pays à ressources limitées.
Méthodes
A partir des données concernant 5491 patients adultes débutant un traitement ART (âge médian : 35 ans, 46% de femmes), dans le cadre de 15 programmes de traitement en Afrique, en Asie et en Amérique du Sud comprenant un suivi de 12 mois et plus, nous avons étudié les facteurs d’absence de suivi après le début du traitement et les nombres de perdus pour le suivi et de décès au cours des 6 premiers mois.
Résultats
Globalement, 211 patients (3,8%) n’ont pas eu de suivi, 880 (16,0%) ont été perdus pour le suivi et 141 (2,6%) sont décédés, à la connaissance du programme, dans les 6 premiers mois. La probabilité d’une absence de suivi était plus élevée pendant la période 2003-2004 qu’en 2000 ou dans les années antérieures (odds ratio, OR : 5,06 ; intervalle de confiance à 95%, IC : 1,28-20,0) et il en était de même pour les perdus pour le suivi (ratio de danger, RD : 7,62 ; IC à 95% : 4,55-12,8), mais pas pour le nombre de décès enregistrés (RD : 1,02 ; IC à 95% : 0,44-2,36). Par rapport à une numération des CD4 de référence ≥ 50 cellules/µl, une numération < 25 cellules/µl était associée à une plus forte probabilité d’absence de suivi (OR : 2,49 ; IC à 95% : 1,43-4,33), de perte pour le suivi (RD : 1,48 ; IC à 95% : 1,23-1,77) et de décès (RD : 3,34 ; IC à 95% : 2,10-5,30). Par rapport à un traitement gratuit, les programmes fonctionnant avec une tarification à l’acte était associés à une plus grande probabilité d’absence de suivi (OR : 3,71 ; IC à 95% : 0,97-16,05) et à une plus forte mortalité (RD : 4,64 ; IC à 95% : 1,11-19,41).
Conclusion
Les pertes précoces de patients sont devenues de plus en plus courantes avec l’élargissement des programmes et sont associées à la tarification à l’acte et à une immunodéficience avancée au départ du traitement. Des mesures pour augmenter au maximum la rétention des patients dans les programmes ART sont nécessaires dans les pays à faibles ressources.
Resumen
Objetivo
Analizar la pérdida temprana de pacientes para el seguimiento en los programas de tratamiento antirretroviral (TAR) aplicados en entornos con recursos limitados.
Métodos
A partir de los datos de 5491 pacientes adultos que comenzaron el TAR (mediana de la edad: 35 años, mujeres en el 46% de los casos) en 15 programas de tratamiento en África, Asia y América del Sur con al menos 12 meses de seguimiento, investigamos los factores de riesgo de seguimiento nulo tras el inicio del tratamiento, y de pérdida para el seguimiento odefunción durante los 6 primeros meses.
Resultados
Globalmente, 211 pacientes (3,8%) no fueron objeto de seguimiento alguno, 880 (16,0%) fueron perdidos para el seguimiento y 141 (2,6%) murieron antes de transcurridos seis meses. La probabilidad de seguimiento nulo fue mayor en 2003–2004 que en 2000 o en años anteriores (razón de posibilidades [odds ratio, OR]: 5,06; intervalo de confianza (IC) del 95%, IC95%: 1,28–20,0), al igual que la pérdida para el seguimiento (cociente de riesgos instantáneos [hazard ratio, HR]: 7,62; IC95%: 4,55–12,8), pero no así la mortalidad registrada (HR: 1,02; IC95%: 0,44–2,36). En comparación con un recuento de CD4 ≥ 50 células/µl al comienzo del estudio, un recuento < 25 células/µl se asoció a una probabilidad mayor de seguimiento nulo (OR: 2,49; IC95%: 1,43–4,33), de pérdida para el seguimiento (HR: 1,48; IC95%: 1,23–1,77) y de defunción (HR: 3,34; IC95%: 2,10–5,30). Comparados con el tratamiento gratuito, los programas con cobro de honorarios por servicios prestados se asociaron a un aumento de la probabilidad de seguimiento nulo (OR: 3,71; IC95%: 0,97–16,05) y a una mayor mortalidad (HR: 4,64; IC95%: 1,11–19,41).
Conclusión
Las pérdidas tempranas de pacientes para el seguimiento fueron más frecuentes cuando los programas se expandieron, y se asociaron al cobro de honorarios por servicios prestados y a una inmunodeficiencia avanzada al inicio del estudio. Se deben tomar medidas para optimizar la retención de los pacientes en los programas de TAR en los países con recursos escasos.
ملخص
الەدف
تحليل أسباب الفقدان المبكر للمرضى المدرجين في برامج المعالجة بمضادات الفيروسات القەقرية في الأماكن المحدودة الموارد.
الطريقة
لقد قمنا بدراسة عوامل الاختطار الناجمة عن توقف المتابعة بعد بدء المعالجة، وافتقاد المتابعة أو الوفيات في الأشەر الستة الأولى، وذلك بالاعتماد على البيانات الخاصة بنحو 5491 مريضاً بالغاً ممن تلقوا مضادات الفيروسات القەقرية (العمر الوسطي 35 عاماً، و46% منەم من الإناث) من خلال 15 برنامج معالجة في أفريقيا، وآسيا، وجنوب أمريكا، وتمت متابعتەم لمدة اثني عشر شەراً أو أكثر.
الموجودات
بشكل عام لم يحصل 211 مريضاً (3.8%) على متابعة، وفقد 880 مريضاً (16.0%) من المتابعة، في حين علمنا بوفاة 141 مريضاً (2.6%) في الأشەر الستة الأولى. فاحتمال عدم المتابعة في عامي 2003 – 2004 كان أكبر منە في عام 2000 أو ما قبل ذلك (نسبة الأرجحية 5.06 بفاصل ثقة 95% إذ تراوح معدل الأرجحية بين 1.28 و20.0)، شأنە شأن التسرب من المعالجة (نسبة المخاطر 7.62 بفاصل ثقة 95% إذ تراوح معدل الأرجحية بين 12.8 و4.55) على عكس الوفيات غير المسجلة (نسبة المخاطرة 1.02 بفاصل ثقة 95%، إذ تراوح معدل الأرجحية بين 0.44 و2.36). فبالمقارنة بالعدد الأساسي لخلايا CD4 أكبر من أو يساوي 50 خلية لكل مكرولتر، كان العدد أقل من 25 خلية مصحوب بدرجة أعلى من احتمالية عدم المتابعة (نسبة الأرجحية 2.49 بفاصل ثقة 95% إذ تراوح معدل الأرجحية بين 1.43 و4.33)، أما التسرب من المعالجة (نسبة المخاطرة 1.48 بفاصل ثقة 95% إذ تراوح معدل الأرجحية بين 1.23 و1.77) والوفيات (نسبة المخاطرة 3.34 بفاصل ثقة 95%، إذ تراوح معدل الأرجحية بين 2.10 و5.30). وبالمقارنة بالمعالجة المجانية، نجد أن برامج الحصول على الخدمات بمقابل يصاحبەا احتمال أعلى بعدم المتابعة (نسبة المخاطرة 3.71 بفاصل ثقة 95% إذ تراوح معدل الأرجحية بين0.97 و16.05) والوفيات (نسبة المخاطرة 4.64 بفاصل ثقة 95%، إذ تراوح معدل الأرجحية بين 1.11 و19.41).
الاستنتاج
يشيع فقدان المرضى مبكراً وبشكل متزايد، في حالة توسيع نطاق البرامج، وفرض رسوم على الخدمات، وتقدم القيمة القاعدة للعوز المناعي. ومن ثم ينبغي اتخاذ تدابير لتحقيق أقصى معدل استبقاء للمرضى في برامج المعالجة بمضادات الفيروسات القەقرية في البلدان الشحيحة الموارد.
Introduction
The increasingly widespread use of potent combination antiretroviral therapy (ART) since 1996 has substantially improved the prognosis of patients infected with HIV in industrialized countries.1–3 In resource-constrained settings in Africa, Asia and South America, where 90% of people with HIV/AIDS live, access to ART has improved substantially: according to WHO estimates, two million people with HIV/AIDS were receiving treatment in low- and middle-income countries in December 2006, which represents 28% of the estimated 7.1 million people in urgent need of treatment.4
The administration of ART to individual patients and the monitoring and evaluation of HIV/AIDS treatment programmes critically depend on regular and complete patient follow-up. Individual treatment decisions can then be made in the light of clinical and laboratory results, and treatment response, complication and mortality rates can be accurately estimated at the programme level.
Using data from a network of treatment programmes in Africa, Asia and South America, we examined the early loss of patients starting ART programmes in low-income countries; this included no follow-up after the initial visit, and loss to follow-up and death in the first 6 months.
Methods
Study population
The Antiretroviral Therapy in Lower-Income Countries (ART-LINC) collaboration5 of the International epidemiological Databases to Evaluate AIDS (IeDEA)6 is a network of HIV/AIDS treatment programmes in Africa, Asia and South America that has been described elsewhere.7,8 Of the 23 treatment programmes in low- and middle-income countries approached, 19 agreed to participate and 15 contributed data to the present analysis. All eligible programmes systematically collected prospective data on adolescents and adults aged 16 years or older starting ART, and institutional review boards or ethics committees approved data collection.
Information obtained on patients included age, sex, date of starting ART, type of treatment initiated, date of last contact with the programme, date of death and, when available, CD4-cell count, WHO HIV clinical stage, total lymphocyte count, haemoglobin level and HIV-1 ribonucleic acid (RNA) plasma level at baseline and during follow-up. The most common ART regimens were: stavudine, lamivudine and nevirapine; zidovudine, lamivudine and efavirenz; zidovudine, lamivudine and nevirapine; and stavudine, lamivudine and efavirenz. These four combinations accounted for 66% of all regimens used.7,8 The type of ART regimen was classified as either protease inhibitor (PI)-based [i.e. two nucleoside reverse transcriptase inhibitors (NRTIs) plus one PI, including ritonavir-boosted PI], non-nucleoside reverse transcriptase inhibitor (NNRTI)-based (i.e. two NRTIs plus one NNRTI), or another combination, which included triple NRTI regimens and any other regimen containing a minimum of three drugs. The characteristics of the treatment programmes were also recorded, including procedures in place for tracing patients lost to follow-up. We included all patients who had not previously received antiretrovirals, except for the prevention of mother-to-child HIV transmission, who were aged 16 years or older and whose date of starting ART was documented.
Outcomes
We considered three endpoints that characterized the loss of patients to a programme: no follow-up, loss to follow-up and death in the first 6 months after starting ART. No follow-up was when a patient did not return to the clinic after the ART initiation visit, although the database remained open for an additional 12 months or more. A patient was considered lost to follow-up if the last follow-up visit occurred during the first 6 months after starting ART and the database remained open for an additional 6 months or more. The 6-month interval was chosen to accommodate the longest interval between visits in participating programmes. The database closing date was defined as the date of the most recent follow-up visit in a given patient cohort.
Statistical analysis
Logistic regression with a random effect for the cohorts was used to examine factors associated with no follow-up. We used competing risk models9 to analyse the time to loss to follow-up and the time to death, as measured from the start of ART (i.e. baseline). Competing risk analysis assumes that each individual is exposed to two risks, namely loss to follow-up and death, and accounts for the fact that these risks may not be independent. The competing risk data set was prepared by stacking the data and generating separate strata for death and loss to follow-up, with each patient appearing in both strata. The effect of prognostic factors on outcome was analysed using a Weibull proportional hazard model, controlling for cohort and stratifying by event, thus allowing the baseline hazard to differ between competing risks. Robust variance adjustment was used to allow for the fact that each individual contributed two data points. P-values for the contribution of prognostic factors to the explained variance were derived using a Wald test. The effect of programme factors was evaluated by controlling for individual patient factors.
For some patients, data on CD4-cell count or clinical stage at baseline were missing. We, therefore, created dummy variables that indicated whether or not CD4 cells and clinical stage had been assessed. In sensitivity analyses, we used multiple imputations of CD4-cell counts and clinical stage, as described previously.8 The following variables were considered for inclusion in logistic and competing risk models: sex, age, CD4-cell count (< 25 cells/µl, 25–50 cells/µl, > 50 cells/µl, and not measured), clinical stage, initial ART regimen, and calendar period of ART initiation (< 2001, 2001–2002 and 2003–2004). The choice of CD4 categories reflects the fact that there was little variation in the rate of loss to follow-up for different cell-count subdivisions above 50 cells/µl. The clinical disease stage was categorized as less advanced [i.e. United States Centers for Disease Control (CDC) stage A/B, WHO stage I/II], more advanced (i.e. CDC stage C, WHO stage III/IV) and not assessed. Two programme variables, namely free access to treatment with no cost to patients and type of follow-up (i.e. active tracing versus passive follow-up), were also included in the models. All analyses were carried out using Stata version 9.2 (Stata Corp. LP, College Station, TX, United States of America). The results are presented as odds ratios (ORs) or hazard ratios (HRs) with a 95% confidence interval (CI).
Results
The IeDEA ART-LINC database included a total of 7651 patients who started ART in 15 treatment programmes in Africa, Asia and South America. Of these, 5491 were eligible for the present analysis. The characteristics of programmes contributing data are shown in Table 1. The number of patients on ART increased rapidly between 2001 and 2005, particularly in some African programmes and at the site in India. Eleven sites actively followed patients using telephone calls (often to mobile phones), letters or home visits and 11 provided free access to treatment. The number of patients included in the analysis ranged from 36 in Thailand to 1219 in Malawi and the median baseline CD4-cell count ranged from 45 cells/µl in the township of Khayelitsha to 241 cells/µl in the Cape Town AIDS Cohort (CTAC), both in South Africa. Patient characteristics at baseline are summarized in Table 2. The patients’ median age was 35 years, 2519 (46%) were women and the median CD4-cell count was 105 cells/µl in the 4087 patients for whom data were available. Most patients (1727 or 69%) were at an advanced clinical disease stage when starting ART. A total of 2498 patients (45%) were treated in programmes with active follow-up and 3298 (60%) had free access to ART.
Table 1. Characteristics of the ART programmes that contributed data to the analysis.
| Region and programme name | Country | Number of patients starting ART by 2004a | Number of patients eligible for analysis | Median CD4-cell count (cells/µl) | Free access to treatment | Tracing method for patients lost to follow-up | Status 6 months after
the start of ART |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No
follow-up |
Initially followed up, then lost |
Known
to have died |
|||||||||||
| (n) | (%)b | (n) | (%)b | (n) | (%)b | ||||||||
| Northern Africa | |||||||||||||
| Morocco ART Cohort | Morocco | 423 (127; 270) | 412 | 108 | Yes | Phone or letter | 0 | 0 | 47 | 11 | 15 | 3.6 | |
| Southern Africa | |||||||||||||
| Gaborone Independent | Botswana | 288 (228; 279) | 213 | 185 | No | Phone or letter | 1 | 0.5 | 10 | 4.7 | 2 | 0.9 | |
| Lighthouse | Malawi | 1520 (0; 732) | 1219 | 56 | No | None | 109 | 8.9 | 441 | 36 | 36 | 3.0 | |
| CTAC | South Africa | 313 (275; 307) | 305 | 241 | Yes | Home visits | 0 | 0 | 10 | 3.3 | 1 | 0.3 | |
| Khayelitsha | South Africa | 287 (0; 287) | 273 | 45 | Yes | Home visits | 0 | 0 | 0 | 0 | 34 | 13 | |
| OPERA | South Africa | 63 (1; 54) | 46 | 87 | Yes | Phone or letter | 0 | 0 | 0 | 0 | 0 | 0 | |
| Eastern Africa | |||||||||||||
| Eldoret | Kenya | 1138 (0; 223) | 839 | 94 | Yes | Home visits | 33 | 3.9 | 142 | 17 | 6 | 0.7 | |
| Central and western Africa | |||||||||||||
| Cotrame ANRS 1203 | Côte d’Ivoire | 137 (43; 128) | 123 | 133 | Yes | Home visits | 0 | 0 | 0 | 0 | 8 | 6.5 | |
| Nigeria HAART | Nigeria | 115 (2; 36) | 44 | 213 | No | Phone or letter | 0 | 0 | 0 | 0 | 3 | 6.8 | |
| ISAARV | Senegal | 153 (140; 153) | 148 | 125 | Yes | Home visits | 0 | 0 | 6 | 4.1 | 6 | 4.1 | |
| HIMS | Various | 104 (0; 61) | 59 | 142 | Yes | Phone or letter | 2 | 3.4 | 2 | 3.4 | 0 | 0 | |
| South America | |||||||||||||
| Rio de Janeiro HIV | Brazil | 789 (378; 654) | 541 | 166 | Yes | None | 0 | 0 | 28 | 5.2 | 2 | 0.4 | |
| SobrHIV | Brazil | 854 (640; 800) | 516 | 161 | Yes | None | 3 | 0.6 | 25 | 4.8 | 1 | 0.2 | |
| Asia | |||||||||||||
| YRG CARE | India | 1367 (84; 744) | 717 | 83 | No | None | 63 | 8.8 | 169 | 24 | 27 | 3.8 | |
| HIV NAT | Thailand | 100 (76; 85) | 36 | 121 | Yes | Phone or letter | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | – | 7651 (1994; 4813) | 5491 | – | – | – | 211 | 3.8 | 880 | 16 | 141 | 2.6 | |
ANRS, Agence Nationale de Recherches sur le Sida et les hépatites virales; ART, antiretroviral therapy; CTAC, Cape Town AIDS Cohort; HAART, highly active antiretroviral therapy; HIMS, Heineken International Medical Services; ISAARV, Senegalese Initiative on Access to Antiretroviral Drugs; NAT, Netherlands–Australia–Thailand Research Collaboration; OPERA, Operational Research on Antiretrovirals; SobrHIV, South Brazil HIV cohort; YRG CARE, YR Gaitonde Centre for AIDS Research and Education. a Values in parentheses are: number of patients starting ART < 2001; number of patients starting ART < 2003. b The percentages given are for the individual programmes.
Table 2. Baseline characteristics of the 5491 patients starting ART who were included in the data analysis.
| Characteristics | Number of patients |
|
|---|---|---|
| (n) | (%) | |
| Age (years) | ||
| 16–29 | 1312 | 24 |
| 30–39 | 2491 | 45 |
| 40–49 | 1232 | 23 |
| ≥ 50 | 456 | 8 |
| Median | 35 | 30–41a |
| Sex | ||
| Female | 2519 | 46 |
| Male | 2972 | 54 |
| Baseline CD4-cell count (cells/µl) | ||
| ≤ 25 | 810 | 20b |
| 25–49 | 482 | 12b |
| 50–99 | 680 | 17b |
| 100–199 | 1001 | 24b |
| 200–349 | 767 | 19b |
| ≥ 350 | 347 | 8b |
| Median | 105 | 35–210a |
| Not measured | 1404 | 26 |
| Clinical stage | ||
| CDC stage A/B, WHO stage I/II | 760 | 14 |
| CDC stage C, WHO stage III/IV | 1727 | 31 |
| Not recorded | 3004 | 55 |
| Initial ART regimen | ||
| NNRTI-based | 4031 | 73 |
| PI-based | 988 | 18 |
| Unknown or other combination | 472 | 9 |
| Year of starting ART | ||
| ≤ 2000 | 1463 | 27 |
| 2001–2002 | 2432 | 44 |
| 2003–2004 | 1596 | 29 |
ART, antiretroviral therapy; CDC, Centers for Disease Control and Prevention; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor. a Value is the interquartile range. b Percentage of the 4087 patients whose CD4-cell count was available.
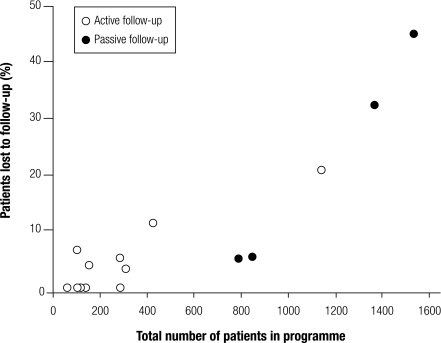
Overall 211 patients (3.8%) were not seen after the ART initiation visit, 880 (16.0%) were lost to follow-up later on and 141 (2.6%) were known to have died in the first 6 months. There was considerable variation across treatment sites (Table 1). Sites with larger numbers of patients were less likely to actively trace patients who did not return to the clinic, and these sites also had higher percentages of patients lost to follow-up (Fig. 1).
Fig. 1.
Effect of ART programme size and type of follow-up on the percentage of patients lost to follow-upa
ART, antiretroviral therapy.
a The number of patients in each programme includes all those starting ART during the study period.
Individual patient factors associated with the three outcomes are shown in Table 3. The probability that a patient would be lost to the programme, due to either no follow-up after the initial visit or subsequent loss to follow-up, was greater for those starting ART in more recent calendar years. There was a striking increase after the year 2000: the OR for no follow-up in 2003–2004 compared with 2000 or earlier was 5.06 (95% CI: 1.28–20.0) and the corresponding HR for a loss to follow-up in the first 6 months was 7.62 (95% CI: 4.55–12.8). A low baseline CD4-cell count and the absence of a CD4-cell measurement were also associated with a loss to the programme, with particularly strong associations with no follow-up and death. More advanced HIV disease and the absence of clinical stage assessment were strongly associated with the risk of death, but not with no follow-up or a loss to follow-up in the first 6 months. Patients aged 50 years or over were more likely to have no follow-up after the initial visit. Finally, there was no association between the type of ART regimen and any of the three outcomes.
Table 3. Individual patient factors associated with the three study outcomes in the first 6 months after starting ART.
| Patient factors | No follow-up |
Initially followed-up, then lost |
Death |
|||||
|---|---|---|---|---|---|---|---|---|
| Odds ratioa (95% CI) | P-valueb | Hazard ratioa (95% CI) | P-valueb | Hazard ratioa (95% CI) | P-valueb | |||
| Age (years) | < 0.0001 | 0.0040 | 0.061 | |||||
| 16–29 | 0.83 (0.61–1.14) | 1.38 (1.05–1.83) | 0.84 (0.38–1.83) | |||||
| 30–39 | 0.72 (0.56–0.94) | 1.05 (0.82–1.36) | 1.36 (0.67–2.78) | |||||
| 40–49 | 0.73 (0.64–0.83) | 1.28 (0.98–1.66) | 0.84 (0.38–1.84) | |||||
| ≥ 50 | 1 | 1 | 1 | |||||
| Sex | 0.25 | 0.93 | 0.31 | |||||
| Male | 1 | 1 | 1 | |||||
| Female | 0.76 (0.49–1.20) | 0.99 (0.86–1.15) | 0.83 (0.58–1.18) | |||||
| Year of starting ART | 0.055 | < 0.0001 | 0.35 | |||||
| ≤ 2000 | 1 | 1 | 1 | |||||
| 2001–2002 | 4.54 (1.28–16.1) | 2.70 (1.64–4.46) | 1.38 (0.69–2.78) | |||||
| 2003–2004 | 5.06 (1.28–20.0) | 7.62 (4.55–12.8) | 1.02 (0.44–2.36) | |||||
| Initial ART regimen | 0.31 | 0.35 | 0.58 | |||||
| NNRTI-based | 1 | 1 | 1 | |||||
| PI-based | 0.30 (0.06–1.45) | 0.79 (0.50–1.23) | 1.38 (0.63–3.02) | |||||
| Unknown or other combination | 1.24 (0.44–3.44) | 1.21 (0.77–1.92) | 1.75 (0.40–7.72) | |||||
| Baseline CD4-cell count (cells/µl)c | < 0.0001 | < 0.001 | < 0.0001 | |||||
| ≥ 50 | 1 | 1 | 1 | |||||
| 25–50 | 2.76 (1.69–4.51) | 1.03 (0.81–1.32) | 1.52 (0.79–2.93) | |||||
| < 25 | 2.49 (1.43–4.33) | 1.48 (1.23–1.77) | 3.34 (2.10–5.30) | |||||
| Not measured | 2.88 (1.43–5.77) | 1.16 (0.96–1.40) | 1.81 (0.97–3.40) | |||||
| Clinical staged | 0.26 | 0.83 | 0.036 | |||||
| CDC stage A/B, WHO stage I/II | 1 | 1 | 1 | |||||
| CDC stage C, WHO stage III/IV | 0.85 (0.43–1.69) | 0.96 (0.69–1.35) | 5.35 (1.50–19.1) | |||||
| Not assessed | 3.73 (0.77–18.1) | 1.07 (0.76–1.51) | 4.36 (0.93–20.5) | |||||
ART, antiretroviral therapy; CDC, Centers for Disease Control and Prevention; CI, confidence interval; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor. a Odds ratios were calculated using multivariable random-effects logistic regression, and hazard ratios were calculated using multivariable Weibull proportional hazard models incorporating the competing risks of death and loss to follow-up. Estimates were adjusted for all variables listed in Table 3. b P-values were calculated using the Wald test. c Not measured in 1404 patients. d Not assessed in 3004 patients.
As shown in Table 4, the programme factor of receiving treatment in a fee-for-service programme was associated with an increased probability of no follow-up or death. Furthermore, programmes in which there was no active tracing of patients were more likely to experience losses after the initial visit and less likely to record deaths. In the programmes with active follow-up, the death rates were higher because deaths were more reliably identified.
Table 4. Programme factors associated with the three study outcomes in the first 6 months after starting ART.
| Programme factors | No follow-up |
Initially followed-up, then lost |
Death |
|||||
|---|---|---|---|---|---|---|---|---|
| Odds ratioa (95% CI) | P-valueb | Hazard ratioa (95% CI) | P-valueb | Hazard ratioa (95% CI) | P-valueb | |||
| Treatment access | 0.055 | 0.46 | 0.031 | |||||
| Free of charge | 1 | 1 | 1 | |||||
| Fee for service | 3.71 (0.97–16.05) | 1.69 (0.42–6.77) | 4.64 (1.11–19.41) | |||||
| Follow-up | 0.58 | 0.21 | 0.035 | |||||
| Passive | 1 | 1 | 1 | |||||
| Active | 0.66 (0.15–2.92) | 0.41 (0.10–1.66) | 5.15 (1.16–22.79) | |||||
ART, antiretroviral therapy; CI, confidence interval. a Odds ratios were calculated using multivariable random-effects logistic regression, and hazard ratios were calculated using multivariable Weibull proportional hazard models incorporating the competing risks of death and loss to follow-up. Models were adjusted for the variables listed in Table 4 and for age, sex, year of starting ART, initial ART regimen, baseline CD4-cell count and clinical stage. b P-values were calculated using the Wald test.
The results of sensitivity analyses based on multiply imputed data for baseline CD4-cell count and clinical stage were similar to those presented here. Additional tables with these results are available at: http://www.art-linc.org/fileadmin/IeDEA/Publications/ARTLINC_BullWHO2008_WebTables.pdf. Of note, 81% of cases in which disease stage was unknown were classified as having advanced disease following multiple imputations and, in these analyses, advanced stage was associated with no follow-up after the initial visit.
Discussion
Main findings
Using data from a large collaborative network of ART treatment programmes in resource-limited settings, we investigated three important early outcomes: failure to return to the clinic after the first ART prescription, and loss to follow-up and death in the first 6 months after starting ART. We found that only 3% of patients were known to have died by 6 months, but on average 21% of patients had been lost to programmes by that time, including about 4% who had not been seen since receiving their first ART prescription. The percentage of patients lost to programmes was substantially greater in more recent calendar periods than in the period before the year 2000. This suggests that many sites find it increasingly difficult to follow-up the growing population of patients and to trace those not returning to the clinic. Treating the maximum number of new patients possible has been the top priority for many public sector programmes, with the possible consequence that documenting and tracing patients lost to follow-up has become increasingly inadequate.10
Strengths and limitations
An important strength of our study is the inclusion of a diverse group of clinics and programmes. This made it possible to examine factors associated with both individual patients and programmes that could influence the probability of patient retention. However, we stress that the reasons patients were lost to follow-up were not collected systematically for individuals. Our study has several other limitations. While the ART-LINC cohorts are broadly representative of the types of ART services operating across resource-limited settings, the generalizability of these results requires careful consideration. One limitation of large collaborative databases is that a relatively small number of variables is available for analysis: the standardization and harmonization of data collection across many different sites is challenging. The result is that factors that are more difficult to assess, for example adherence, cannot be examined.
Another limitation is that we were unable to explore fully the relationships between patients lost to programmes and patient numbers, the approach to tracing patients, staffing and infrastructure. Detailed standardized up-to-date information was not available and the size of a treatment programme was closely related to whether or not patients were actively traced. It was therefore not possible to link unequivocally time trends in the loss to follow-up to changes in specific programme characteristics over time. Programme size and other features of ART services that affect patient outcomes require further investigation to inform decisions on how best to deliver ART to large numbers.
In the context of other studies
We focused on the first 6 months of treatment, which is a crucial period for the long-term success of ART. The initial response to ART has long-term prognostic significance, and optimizing adherence in early months is important for ensuring long-term immunological and virological success.11,12 Data from the ART-LINC collaboration8 and other treatment programmes, for example the Médecins Sans Frontières (MSF) programme in Malawi, show that loss to follow-up and death mostly occur in the first 6 months after ART initiation and that community support improves outcomes.13,14 Not all studies confirm this, however. Data from a South African ART programme demonstrate that while mortality decreased rapidly after ART initiation, the rate of loss to follow-up remained fairly constant during the first 2 years.15
Other studies of treatment programmes in resource-limited settings also found high rates of loss to follow-up. For example, in the urban primary health care setting of Lusaka, Uganda, 3406 (21%) of 16 199 patients starting ART in 2004–2005 were more than 30 days late for a scheduled pharmacy appointment.16 An evaluation of the HIV Drug Access Initiative of the Ministry of Health of Uganda and the Joint United Nations Programme on HIV/AIDS (UNAIDS), in which patients paid reduced prices for their medications, found that 114 (24%) of 476 patients were lost to follow-up in the first year.17 Other studies found lower rates of loss to follow-up: in Port-au-Prince, Haiti, for example, only 71 (7%) of 910 patients were lost over a median follow-up period of 13 months.18 In a large observational cohort of MSF HIV/AIDS programmes, 4.8% of patients were lost to follow-up over a median period of 4.1 months.19 Unfortunately, often study results are not directly comparable because of differences in the definition of a loss to follow-up, or because no clear definition is reported. Some cohorts were established in a clinical research context, and included relatively small numbers of patients who were closely monitored.20–23 These studies reported low rates of loss to follow-up that will not reflect the realities of scaling up ART programmes.
Reasons for losses to follow-up
Few programmes in resource-limited settings systematically assess the reasons patients are lost to follow-up, but surveys found that a substantial proportion had died. For example, among 727 patients lost to follow-up at the Lighthouse Clinic in Lilongwe, Malawi, 30% had died, 19% had transferred to another facility, 22% were alive and on treatment, 3% had stopped treatment and 26% could not be found. The last group had a low median CD4-cell count, suggesting that some may also have died.24 A smaller study from northern Malawi investigated the fate of 253 patients lost to follow-up and reported that 50% had died, 23% were alive and 27% could not be traced.25 Similarly, among 801 patients traced after they missed scheduled visits in Lusaka, Zambia, 46% had died.26 Taken together, these data suggest that about 50% of patients lost to follow-up in lower-income settings may have died.
The present analysis extends our previous study of mortality in high- and low-income countries8 and shows that free access to treatment is a critical determinant of programme retention and mortality. This association was particularly strong for patients who had no follow-up after ART was started, but less strong for those lost to follow-up later on. Interestingly, in the UNAIDS–Ugandan Ministry of Health HIV Drug Access Initiative, a fee-for-service programme, the majority of patients (65%) who were lost did not return after their initial visit.17 As discussed previously,8 paying for initial antiretroviral treatment does not mean that households can pay later on. Associations were also found between providing ART free of charge and a higher probability of sustained suppression of viral replication and better adherence to therapy.27
In individual patients, older age and profound immune suppression were associated with early losses to treatment. Some older people may fail to return because they do not want to burden their families, who may contribute to costs. Patients with advanced HIV infections may not return because they are too weak. Access to transport is also important. For example, ownership of a bicycle was associated with a reduced loss to follow-up in one programme, but patients’ access to transport was not consistently assessed. In the Lusaka programme, some patients stopped treatment because of high transport costs.25 In the large Academic Model for the Prevention and Treatment of HIV/AIDS (AMPATH) programme in Eldoret, Kenya, men were more likely to be lost to follow-up than women.28 In our study, there was some evidence that men were less likely to return to the clinic than women.
Conclusion
Our results support the notion that evaluations of the scale-up of ART in resource-limited settings should consider not only the number of new patients starting ART but also the number remaining in long-term care: the number lost to follow-up is an important indicator of programme effectiveness.15 Our results indicate that better ART outcomes, including higher programme retention rates, may be obtained in services that have smaller numbers of patients and, therefore, that population coverage should be achieved with smaller decentralized facilities rather than a few large programmes. In general, given the large numbers of patients and the limited resources facing health services, developing strategies that prevent patients from missing appointments may be more cost-effective than tracking those who do not return.10 Future studies should address the causes of the late initiation of ART15 as well as the barriers preventing patients from returning to clinics, record transfers to other programmes and assess mortality in patients lost to programmes. In conclusion, early patient losses to ART programmes are increasingly common in resource-limited countries. This should not detract from the fact that many patients benefit from the introduction and scaling-up of ART or from the need to continue efforts to improve access to therapy. ■
Acknowledgements
We thank Jack Whitescarver and Paolo Miotti (NIH/OAR), Carolyn Williams (NIH/NIAID), Michel Kazatchkine, Jean-François Delfraissy, Brigitte Bazin and Séverine Blesson (ANRS) for encouraging and supporting this collaborative work.
The organization of the Antiretroviral Therapy in Lower-Income Countries (ART-LINC) Collaboration of the International epidemiological Databases to Evaluate AIDS (IeDEA) is given at: http://www.art-linc.org
Footnotes
Funding: The ART-LINC Collaboration is funded by the United States National Institute of Health (Office of AIDS Research) and the French Agence Nationale de Recherches sur le Sida et les hépatites virales (ANRS).
Competing interests: None declared.
References
- 1.Egger M, Hirschel B, Francioli P, Sudre P, Wirz M, Flepp M, et al. Impact of new antiretroviral combination therapies in HIV infected patients in Switzerland: prospective multicentre study. BMJ. 1997;315:1194–9. doi: 10.1136/bmj.315.7117.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogg RS, Yip B, Kully C, Craib KJ, O’Shaughnessy MV, Schechter MT, et al. Improved survival among HIV-infected patients after initiation of triple-drug antiretroviral regimens. CMAJ. 1999;160:659–65. [PMC free article] [PubMed] [Google Scholar]
- 3.Mocroft A, Vella S, Benfield TL, Chiesi A, Miller VT, Gargalianos P, et al. Changing patterns of mortality across Europe in patients infected with HIV-1. Lancet. 1998;352:1725–30. doi: 10.1016/S0140-6736(98)03201-2. [DOI] [PubMed] [Google Scholar]
- 4.Towards universal access by 2010: How WHO is working with countries to scale-up HIV prevention, treatment, care and support Geneva: WHO; 2007. Available from: https://www.who.int/hiv/mediacentre/universal_access_progress_report_en.pdf [accessed on 22 April 2008].
- 5.Dabis F, Balestre E, Braitstein P, Miotti P, Brinkhof WGM, Schneider M, et al. Cohort profile: Antiretroviral Therapy in Lower Income Countries (ART-LINC): International collaboration of treatment cohorts. Int J Epidemiol. 2005;34:979–86. doi: 10.1093/ije/dyi164. [DOI] [PubMed] [Google Scholar]
- 6.Antiretroviral Treatment in Lower Income Countries (ART-LINC) Collaboration. Homepage. Available from: www.art-linc.org [accessed on 22 April 2008].
- 7.International epidemiological Databases to Evaluate AIDS (IeDEA). Homepage. Available from: http://www.iedea-hiv.org [accessed on 22 April 2008].
- 8.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 9.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. doi: 10.2307/2670170. [DOI] [Google Scholar]
- 10.Myer L, el Sadr W. Expanding access to antiretroviral therapy through the public sector — the challenge of retaining patients in long-term primary care. S Afr Med J. 2004;94:273–4. [PubMed] [Google Scholar]
- 11.Chêne G, Sterne JA, May M, Costagliola D, Ledergerber B, Phillips AN, et al. Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: analysis of prospective studies. Lancet. 2003;362:679–86. doi: 10.1016/S0140-6736(03)14229-8. [DOI] [PubMed] [Google Scholar]
- 12.Carrieri MP, Raffi F, Lewden C, Sobel A, Michelet C, Cailleton V, et al. Impact of early versus late adherence to highly active antiretroviral therapy on immuno-virological response: a 3-year follow-up study. Antivir Ther. 2003;8:585–94. doi: 10.1177/135965350300800606. [DOI] [PubMed] [Google Scholar]
- 13.Ferradini L, Jeannin A, Pinoges L, Izopet J, Odhiambo D, Mankhambo L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367:1335–42. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- 14.Zachariah R, Teck R, Buhendwa L, Fitzerland M, Labana S, Chinji C, et al. Community support is associated with better antiretroviral treatment outcomes in a resource-limited rural district in Malawi. Trans R Soc Trop Med Hyg. 2007;101:79–84. doi: 10.1016/j.trstmh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Lawn SD, Myer L, Harling G, Orrell C, Bekker LG, Wood R. Determinants of mortality and nondeath losses from an antiretroviral treatment service in South Africa: implications for program evaluation. Clin Infect Dis. 2006;43:770–6. doi: 10.1086/507095. [DOI] [PubMed] [Google Scholar]
- 16.Stringer JS, Zulu I, Levy J, Stringer EM, Mwango A, Chi BH, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–93. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 17.Weidle PJ, Malamba S, Mwebaze R, Sozi C, Rukundo G, Downing R, et al. Assessment of a pilot antiretroviral drug therapy programme in Uganda: patients’ response, survival, and drug resistance. Lancet. 2002;360:34–40. doi: 10.1016/S0140-6736(02)09330-3. [DOI] [PubMed] [Google Scholar]
- 18.Severe P, Leger P, Charles M, Noel F, Bonhomme G, Bois G, et al. Antiretroviral therapy in a thousand patients with AIDS in Haiti. N Engl J Med. 2005;353:2325–34. doi: 10.1056/NEJMoa051908. [DOI] [PubMed] [Google Scholar]
- 19.Calmy A, Pinoges L, Szumilin E, Zachariah R, Ford N, Ferradini L. Generic fixed-dose combination antiretroviral treatment in resource-poor settings: multicentric observational cohort. AIDS. 2006;20:1163–9. doi: 10.1097/01.aids.0000226957.79847.d6. [DOI] [PubMed] [Google Scholar]
- 20.Landman R, Schiemann R, Thiam S, Vray M, Canestri A, Mboup S, et al. Once-a-day highly active antiretroviral therapy in treatment-naive HIV-1-infected adults in Senegal. AIDS. 2003;17:1017–22. doi: 10.1097/00002030-200305020-00010. [DOI] [PubMed] [Google Scholar]
- 21.Laurent C, Diakhate N, Gueye NF, Toure MA, Sow PS, Faye MA, et al. The Senegalese government’s highly active antiretroviral therapy initiative: an 18-month follow-up study. AIDS. 2002;16:1363–70. doi: 10.1097/00002030-200207050-00008. [DOI] [PubMed] [Google Scholar]
- 22.Danel C, Moh R, Anzian A, Abo Y, Chenal H, Guehi C, et al. Tolerance and acceptability of an efavirenz-based regimen in 740 adults (predominantly women) in West Africa. J Acquir Immune Defic Syndr. 2006;42:29–35. doi: 10.1097/01.qai.0000219777.04927.50. [DOI] [PubMed] [Google Scholar]
- 23.Orrell C, Bangsberg DR, Badri M, Wood R. Adherence is not a barrier to successful antiretroviral therapy in South Africa. AIDS. 2003;17:1369–75. doi: 10.1097/00002030-200306130-00011. [DOI] [PubMed] [Google Scholar]
- 24.Hochgesang M, Kuyenda A, Hosseinipour M, Phiri S, Weigel R, Mhango E, et al. Active tracing of ART patients lost to follow-up at Lighthouse shows that few stopped treatment for their own reasons, but many have died [Abstract TUPE0119]. In: XVI International AIDS Conference, Toronto, 13-18August2006 [Google Scholar]
- 25.Yu JK, Chen SC, Wang KY, Chang CS, Makombe SD, Schouten EJ, et al. True outcomes for patients on antiretroviral therapy who are “lost to follow-up” in Malawi. Bull World Health Organ. 2007;85:550–4. doi: 10.2471/BLT.06.037739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krebs D, Chi B, Mulenga Y, Cantrell R, Levy J. A community-based contact tracing program for patients enrolled in a district-wide program for antiretroviral therapy (ART) [Abstract TUPE0143]. In: XVI International AIDS Conference, Toronto, 13-18August2006 [Google Scholar]
- 27.Ivers LC, Kendrick D, Doucette K. Efficacy of antiretroviral therapy programs in resource-poor settings: a meta-analysis of the published literature. Clin Infect Dis. 2005;41:217–24. doi: 10.1086/431199. [DOI] [PubMed] [Google Scholar]
- 28.Wools-Kaloustian K, Kimaiyo S, Diero L, Siika A, Sidle J, Yiannoutsos CT, et al. Viability and effectiveness of large-scale HIV treatment initiatives in sub-Saharan Africa: experience from western Kenya. AIDS. 2006;20:41–8. doi: 10.1097/01.aids.0000196177.65551.ea. [DOI] [PubMed] [Google Scholar]