Abstract
Objective
To estimate the financial resources required to achieve the 2015 targets for global tuberculosis (TB) control, which have been set within the framework of the Millennium Development Goals (MDGs).
Methods
The Global Plan to Stop TB, 2006–2015 was developed by the Stop TB Partnership. It sets out what needs to be done to achieve the 2015 targets for global TB control, based on WHO’s Stop TB Strategy. Plan costs were estimated using spreadsheet models that included epidemiological, demographic, planning and unit cost data.
Findings
A total of US$ 56 billion is required during the period 2006–2015 (93% for TB-endemic countries, 7% for international technical agencies), increasing from US$ 3.5 billion in 2006 to US$ 6.7 billion in 2015. The single biggest cost (US$ 3 billion per year) is for the treatment of drug-susceptible cases in DOTS programmes. Other major costs are treatment of patients with multi- and extensively drug-resistant TB (MDR-TB and XDR-TB), collaborative TB/HIV activities, and advocacy, communication and social mobilization. Low-income countries account for 41% of total funding needs and 65% of funding needs for TB/HIV. Middle-income countries account for 72% of the funding needed for treatment of MDR-TB and XDR-TB. African countries require the largest increases in funding.
Conclusion
Achieving the 2015 global targets set for TB control requires a major increase in funding. To support resource mobilization, comprehensive and costed national plans that are in line with the Global Plan to Stop TB are needed, backed up by robust assessments of the funding that can be raised in each country from domestic sources and the balance that is needed from donors.
Résumé
Objectif
Estimer les ressources financières nécessaires pour atteindre les cibles 2015 concernant la lutte antituberculeuse mondiale, définies dans le cadre des objectifs du Millénaire pour le développement (OMD).
Méthodes
La Plan mondial Halte à la tuberculose, 2006-2015, a été développé par le Partenariat Halte à la tuberculose. Il définit ce qui doit être fait pour atteindre les cibles 2015 pour la lutte mondiale contre la tuberculose, sur la base de la Stratégie Halte à la TB de l’OMS. Les coûts de ce plan ont été estimés au moyen de modèles utilisant des feuilles de calcul et intégrant des données épidémiologiques, démographiques, prévisionnelles et relatives aux coûts unitaires.
Résultats
Au total, US $ 56 milliards sont nécessaires sur la période 2006-2015 (dont 93% pour les pays d’endémie de la tuberculose et 7% pour les agences techniques internationales), les besoins annuels passant d’US $ 3,5 milliards pour 2006 à US $ 6,7 milliards pour 2015. Le seul coût de très grande ampleur (US $ 3 milliards par an) concerne le traitement des cas de TB pharmacosensibles dans le cadre des programmes DOTS. D’autres coûts importants sont dus au traitement des patients atteints de TB multirésistante ou ultra résistante (TB-MR ou XDR-TB), aux activités anti-TB/VIH en collaboration et aux actions de sensibilisation, de communication et de mobilisation sociale. Les pays à faible revenu représentent 41% des besoins totaux de financement et 65% des besoins financiers pour la lutte anti TB/VIH. Les pays à revenu moyen représentent 72% des fonds nécessaires pour le traitement des TB-MR et des XDR-TB. Ce sont les pays africains dont le financement doit être le plus augmenté.
Conclusion
Atteindre les cibles mondiales 2015 fixées pour la lutte contre la TB requiert une forte augmentation du financement. Pour appuyer la mobilisation des ressources, il faudrait disposer de plans complets et nationaux chiffrés, cohérents avec le Plan mondial Halte à la tuberculose et étayés par des évaluations solides des fonds pouvant être levés dans chaque pays à partir de sources nationales et du complément à fournir par les donateurs.
Resumen
Objetivo
Estimar los recursos financieros necesarios para lograr las metas de 2015 establecidas en el marco de los Objetivos de Desarrollo del Milenio (ODM) para la lucha mundial contra la tuberculosis.
Métodos
La alianza Alto a la Tuberculosis trazó el Plan Mundial para Detener la Tuberculosis 2006–2015, en el que se exponen las medidas que deben adoptarse para alcanzar las metas de 2015 referentes al control mundial de esa enfermedad con arreglo a la estrategia de la OMS Alto a la Tuberculosis. El costo del plan se calculó utilizando hojas de cálculo con modelos que incorporaban datos epidemiológicos, demográficos, de planificación y sobre los costos unitarios.
Resultados
Se necesitan en total US$ 56 000 millones para el periodo 2006-2015 (93% para los países con tuberculosis endémica, 7% para los organismos técnicos internacionales), aumentando progresivamente desde US$ 3500 millones en 2006 hasta US$ 6700 millones en 2015. La mayor partida (US$ 3000 millones anuales) corresponde al tratamiento de los casos sensibles a los medicamentos en los programas de DOTS. Otros costos importantes son los asociados al tratamiento de los pacientes con tuberculosis multirresistente y extremadamente farmacorresistente (TB-MDR y TB-XDR), las actividades de colaboración en materia de tuberculosis/VIH, y la promoción, comunicación y movilización social. Los países de bajos ingresos representan un 41% de la financiación total necesaria y un 65% de las necesidades de financiación para combatir la coinfección tuberculosis/VIH. Los países de ingresos medios absorben el 72% de la financiación necesaria para tratar la TB-MDR y la TB-XDR. Los países africanos son los que necesitan un mayor esfuerzo de aumento de la financiación.
Conclusión
El logro de las metas mundiales de control de la tuberculosis establecidas para 2015 exige un incremento considerable de la financiación. En apoyo de la movilización de recursos, se requieren planes nacionales amplios y presupuestados que estén en consonancia con el Plan Mundial para Detener la Tuberculosis, y que cuenten con el respaldo de estudios sólidos sobre los fondos que puedan obtenerse en cada país a partir de fuentes nacionales y de la aportación adicional requerida de los donantes.
ملخص
الەدف
تقدير الموارد المالية المطلوبة لتحقيق الأەداف العالمية لمكافحة السل عام 2015، وەي الأەداف التي وضعت ضمن إطار المرامي الإنمائية للألفية.
الطريقة
أعدت الخطة العالمية لمكافحة السل 2006 – 2015 من قِبَل شراكة دحر السل، وفيەا تبيان للاحتياجات اللازمة لتحقيق الأەداف العالمية لمكافحة السل عام 2015 استناداً إلى استراتيجية منظمة الصحة العالمية لدحر السل. وقد وضعت تقديرات للتكاليف باستخدام نماذج الصحائف الممتدة التي تتضمن معطيات حول تكاليف الوحدة وحول التخطيط والمعطيات الوبائية والديموغرافية.
الموجودات
يبلغ إجمالي المطلوب 56 بليون دولار أمريكي خلال الفترة 2006 – 2015 (93% منە للبلدان التي يتوطن فيەا السل، 7% منەا للوكالات التقنية الدولية)، مما يمثل زيادة تبلغ عام 2015 6.7 بليون دولار عما كانت عليە عام 2006 وەي 3.5 بليون دولار. وتعود أكبر التكاليف المفردة (ومقدارەا 3 بلايين دولار أمريكي سنوياً) لمعالجة حالات السل المستجيبة للأدوية ضمن برامج المعالجة القصيرة الأمد تحت الإشراف المباشر (دوتس)، أما التكاليف الكبرى الأخرى فتعود للمرضى بالسل المقاوم لأدوية متعددة، والسل المستعصي على الأدوية، والأنشطة التعاونية لمكافحة السل والإيدز معاً، والحملات الإعلامية والتواصل واستنەاض المجتمع. وتمثل البلدان المنخفضة الدخل 41% من مجمل احتياجات التمويل و65% من احتياجات التمويل إلى الأنشطة المشتركة لمكافحة السل وفيروس الإيدز معاً. أما البلدان المتوسطة الدخل فتمثل 72% من مجمل احتياجات التمويل لمعالجة السل المقاوم لأدوية متعددة والسل المستعصي على الأدوية. وتحتاج البلدان الأفريقية إلى أكبر زيادة في التمويل.
الاستنتاج
يتطلب تحقيق الأەداف العالمية لمكافحة السل في عام 2015 زيادة كبيرة في التمويل ولتعزيز استجلاب الموارد ينبغي وضع خطط وطنية شاملة وواضحة التكاليف وتتماشى مع الخطة العالمية لدحر السل، مع تعزيزەا بتقييمات سليمة للتمويل الذي يمكن الحصول عليە في كل بلد من مواردە المحلية، والمبالغ المطلوبة من المانحين.
Introduction
Tuberculosis (TB) is a major global health problem, with an estimated 8.8 million new cases and 1.6 million deaths from TB in 2005.1 It is a leading cause of adult mortality in low- and middle-income countries, ranking third after HIV and ischaemic heart disease as a cause of death among those aged 15–59 years.2 Following the identification of extensively drug-resistant TB (XDR-TB) in more than 30 countries since 2006, multidrug-resistant TB (MDR-TB) and XDR-TB have recently become a particular focus of international concern.3
Alongside child mortality, maternal and infant mortality, HIV and malaria, TB is one of five global health issues included within the eight Millennium Development Goals (MDGs) defined by the UN in 2000, and for which targets for 2015 were set (available at: http://www.un.org/millenniumgoals). Targets and indicators for TB are included under MDG 6, which is to “combat HIV/AIDS, malaria and other diseases”. Using the framework provided by the MDGs, WHO and the Stop TB Partnership have set targets for global TB control for 2015 as well as for 2050.4–6 The MDG, WHO and the Stop TB Partnership targets for 2015 are as follows: (i) to halt and reverse incidence; (ii) to halve prevalence and mortality rates compared to their level in 1990; (iii) to detect at least 70% of infectious cases; and (iv) to successfully treat 85% of the infectious cases that are detected.
The Stop TB Partnership was founded in 2000 and, by 2006, consisted of over 400 partners including international and bilateral agencies, high TB-burden countries and nongovernmental organizations, with a secretariat housed in WHO. One of the first major initiatives of the partnership was to develop a Global Plan to Stop TB for the period 2001–2005.7 This plan was to provide a framework for national and international efforts to make progress in global TB control, and its major objectives were to achieve the two global targets set by the World Health Assembly for 2005, i.e. to detect 70% of sputum smear-positive cases of TB and to successfully treat 85% of such cases. Estimates of the financial resources required for the period 2001–2005, totalling US dollars (US$) 6 billion, were produced.8 With accelerated progress notable from 2003 onwards, the 2005 targets were almost achieved globally, with a 60% case detection rate and an 84% successful treatment rate.1
Building on the first Global Plan for TB Control, the coordinating board of the Stop TB Partnership called for the development of a second global plan in October 2004, this time to cover the 10-year period leading up to the MDG target year of 2015. The plan was to be designed to achieve the MDG, WHO and Stop TB Partnership targets set for 2015. A Global Plan study team consisting of a core team of experts from WHO and the secretariats of the seven working groups that make up the Stop TB Partnership was established to develop the plan, to assess the funding required to implement it and to forecast its likely epidemiological impact.9 As part of a series on the costs of achieving the health-related MDGs,10 this paper presents the estimates of the financial resources required for TB control that were prepared for the Global Plan to Stop TB, 2006–2015, including an update that was made to the MDR-TB and XDR-TB component of the plan in mid-2007.3,6
Methods
Full details of the methods used to produce the cost estimates included in the Global Plan to Stop TB, 2006–2015 have been published elsewhere.1,6,9,11 Here, we provide an abbreviated explanation of the key methods.
Consistency with cost estimates for other health-related MDGs
High priority was given to ensuring consistency with cost estimates for other health-related MDGs. At the outset, discussions were held with the coordinator of the WHO-CHOICE (CHOosing Interventions that are Cost-Effective) team. WHO-CHOICE comprises a set of methods for conducting cost and cost-effectiveness analyses, supported by: (i) a generic (not disease-specific) epidemiological model; and (ii) databases of generic (not disease-specific) unit prices and expected resource use for 14 epidemiological subregions (and, in some instances, individual countries). The WHO epidemiological subregions are listed at: http://www.who.int/choice/demography/regions. We made extensive use of the generic WHO-CHOICE databases (e.g. for unit prices of hospital bed-days and clinic visits). We replaced WHO-CHOICE data only when we had access to what we considered to be better data, i.e. data that were TB-specific and country-specific. Similarly, we used an epidemiological model developed specifically for TB,12 in preference to the generic population model developed for WHO-CHOICE. For TB/HIV-related interventions, we worked closely with the Joint United Nations Programme on HIV/AIDS (UNAIDS) and the WHO-CHOICE team that contributed to the UNAIDS estimates to ensure consistency in the methods and data used. This was particularly the case for antiretroviral treatment (ART), for which UNAIDS provided country-specific estimates of the costs of ART and projections of the number of TB patients that would be started on ART to achieve the target of universal access to treatment by 2010. All costs associated with TB/HIV interventions (e.g. antiretroviral drugs and use of general health-system staff and infrastructure required for ART) were included. Importantly, however, the costs of ART for HIV-positive TB patients were considered for six months only, i.e. the maximum period during which TB and ART overlap. For this reason, the costs presented in this paper may seem surprisingly low compared to those estimated for HIV prevention, care and treatment as a whole. Finally, we excluded the resources required for research and development related to new TB drugs, diagnostics and vaccines, which have been estimated at US$ 11 billion globally for the 10-year period 2006–2015.3,6
Countries considered
A total of 171 countries that collectively accounted for 98% of global TB cases in 2004 were considered. These countries were grouped into seven epidemiological regions.6 Countries classified by the World Bank as established market economies and countries in central Europe were excluded, due to their relatively low burden of TB and the assumption that TB control can be fully funded from domestic resources.
Interventions considered: the Stop TB Strategy
From the mid-1990s until 2005, the internationally recommended strategy for TB control was known as DOTS.13 To guide TB control efforts during the period 2006–2015, WHO developed the more broad-based Stop TB Strategy during 2005 and formally launched it in March 2006.5 The Stop TB Strategy includes six major components, with implementation of DOTS at its foundation as the first component. The Global Plan to Stop TB, 2006–2015 was based on the Stop TB Strategy, with interventions grouped in five major categories: (i) DOTS expansion; (ii) collaborative TB/HIV activities; (iii) treatment of MDR-TB and XDR-TB; (iv) advocacy, communication and social mobilization (ACSM); and (v) technical assistance provided to national TB programmes (NTPs) by international agencies. Table 1 describes these interventions in further detail.
Table 1. The five major interventions of the Global Plan to Stop TB, 2006–2015.
| Intervention categories | Component of Stop TB Strategy/ Partnership Working Group | Description | Costs included | Definition of unit cost | Baseline (2005) level of intervention coverage and scale-up anticipated by 2015 | Main sources of cost data |
|---|---|---|---|---|---|---|
| DOTS expansion | Component 1 Component 3 Component 4 Component 5.1 Working Group: DOTS Expansion | Case-finding and treatment according to the DOTS strategy as implemented up to 2005, plus much more emphasis on four newer approaches to TB control: PPM; PAL;a community TB care; and diagnosis based on culture and DST as well as sputum smear microscopy | First-line drugs, NTP staff, NTP management and supervision activities, training, NTP buildings and vehicles, laboratory equipment and supplies for smears, cultures and DST, consultants, X-rays for diagnosis of smear-negative TB, days in hospital and outpatient visits to health facilities during treatment | Cost per patient treated, except for PPM start-up and organizational costs, community TB care, PAL and laboratory inputs required for culture and DST, for which costs were estimated per 500 000 population | Case detection rate of 60% globally in 2005. By 2015, case detection rate 84% globally (range 80% in Africa to 98% in Eastern Europe),b with total of 50 million patients treated under DOTS 2006–2015. Increased case detection facilitated by large increase in percentage of population living in areas where PPM and PALa are implemented (10–100% and 20–60% respectively, range reflects variation among regions), and increase in availability of community TB care (to cover all of Africa and 20–30% population in other regions), all versus low/negligible coverage in 2005 | WHO Global Financial Monitoring Project (complete data for around 90 countries with 90% global cases), disease control priorities in developing countries project (DCPP), WHO-CHOICE database, PPM costing studies |
| MDR-TB and XDR-TB diagnosis and treatment | Component 2 Working Group: MDR-TB | Treatment of patients with MDR-TB and XDR-TB using first- and second-line drugs according to WHO guidelines | Second-line drugs, hospitalization, outpatient visits, incentives and enablers, management of side-effects, training, laboratory tests, programme/data management | Cost per patient treated | Negligible number of patients with MDR-TB treated according to WHO guidelines before 2006 (cumulative total about 10 000). By 2010, 100% of diagnosed cases of MDR-TB and XDR-TB treated according to WHO guidelines. Number of patients treated: 1.6 million over 10 years | Published costing studies from Estonia, Peru, the Philippines, and the Russian Federation |
| Collaborative TB/HIV activities | Component 2 Working Group: TB/HIV | Activities recommended by WHO, which fall into three categories: (1) mechanisms for collaboration between TB and HIV programmes (four activities); (2) activities to reduce burden of HIV in TB patients – HIV testing and counselling, HIV prevention services, ART, CPT, HIV care and support (five activities); (3) activities to reduce burden of TB in people living with HIV – IPT, infection control, intensified TB case finding (three activities) | Coordinating bodies at different administrative levels, staff to coordinate TB and HIV programme activities, six months of ART and six months of CPT for eligible TB patients, HIV tests and counsellors, clinical staff and questionnaires for screening of TB, palliative care and treatment of opportunistic infections, six months of IPT for those eligible | Cost per patient treated (ART, CPT, HIV care and support). Cost per person tested (HIV testing and counselling, HIV prevention services). Cost per person treated (IPT) | Negligible or limited coverage in 2005. Universal access to ART by 2010, with other activities scaled-up accordingly. Total of 3 million HIV+ TB patients enrolled on ART 2006–2015 | UNAIDS and WHO-CHOICE (ART, other HIV care and support, HIV prevention); costing of projects in Malawi, South Africa and Zambia, for all other activities |
| ACSM | Component 5 Working Group: ACSM | Activities aimed at placing TB high on the political agenda, improving knowledge about TB among general public, mobilizing communities | Mass media campaigns, press conferences, training, information, education and communication, community outreach, promotion of patients’ charter | Cost per 500 000 population covered | Negligible or limited coverage in 2005. All countries implementing ACSM activities countrywide by 2015 | Global Fund Round 5 proposals for five countries |
| Technical assistance | All components and working groups | Assistance provided in NTPs by staff from international agencies with expertise in TB control | Staff and activities such as country missions, workshops, guideline development | Cost per region | Staff and activities approximately doubled from baseline (2005) levels | DOTS Expansion Working Group secretariat |
ACSM, advocacy, communication and social mobilization; ART, antiretroviral treatment; CHOICE, CHOosing Interventions that are Cost-Effective; CPT, co-trimoxazole preventive therapy; DOTS, a strategy for TB control; DST, drug susceptibility testing; IPT, isoniazid preventive therapy; MDR-TB, multidrug-resistant tuberculosis; NTP, national tuberculosis programme; PAL, Practical Approach to Lung Health; PPM, public–public and public–private mix; TB, tuberculosis; UNAIDS, the Joint United Nations Programme on HIV/AIDS; XDR-TB, extensively drug-resistant tuberculosis. a PAL (Practical Approach to Lung Health) is designed to improve the management of patients with respiratory symptoms by training general health care workers, nurses, doctors and managers working in primary health care settings. b Epidemiological regions are given in reference 6.
Anticipated coverage and scale-up
Baseline (2005) levels of intervention coverage and the scaling-up anticipated by 2015 are also described in Table 1 (column 6). The major source of data for the 2005 level of intervention coverage was the latest WHO annual report on global TB control.14 For each intervention, scale-up factors were defined with two considerations in mind: (i) taken together, across all seven epidemiological regions,6 scale-up factors should be sufficient to achieve the MDG, Stop TB Partnership and WHO targets at global level; and (ii) the feasibility of scaling up interventions in particular regions and countries.
Type of costs considered
Costs were considered from a provider perspective in US dollars and covered: (i) costs incurred at the level of direct patient care (e.g. drugs, laboratory tests, days spent in hospital); and (ii) costs associated with activities undertaken at subnational (i.e. district, province) or national level that are not directly linked to individual patients (e.g. management and supervision activities, training, recording and reporting of data). These costs are termed “patient” and “programme” costs respectively in WHO-CHOICE.15 Costs were assessed from a financial rather than an economic perspective, i.e. the focus was on costs that need to be paid for, with the full cost of any capital items (e.g. laboratory equipment) allocated to their year of purchase. Inflation was assumed to be 3% per year.16 Details regarding the costs considered are provided in Table 1 (column 4).
Analysis of total costs by year
The total cost of each intervention was calculated for each year during the period 2006–2015 using the ingredients approach,17 i.e. total costs were calculated as the quantity of an intervention that is required in each year multiplied by its unit price.
The quantities of each intervention required (e.g. number of patients treated, number of units of 500 000 population covered) were estimated within Excel (Microsoft Corporation, Seattle, WA, United States of America) spreadsheet models.9 These models included epidemiological, demographic, planning and unit cost data as inputs. In combination with an in-built epidemiological model of TB12 that was adapted for the purposes of the Global Plan,18 these inputs were converted into five major kinds of output or impact indicators: (i) the total number of TB patients to be treated; (ii) the total number of individuals benefiting from an intervention; (iii) the total size of population covered by an intervention; (iv) the total costs for each major intervention and all interventions combined; and (v) the total number of TB cases and deaths. Demographic inputs including projections up to 2015 were based on UN Population Division data.
If we expected costs to be directly related to the number of patients treated, unit costs were defined on a per patient basis. For costs incurred at subnational level that were not expected to vary in direct proportion to the number of patients treated, unit costs were defined for a population of 500 000 people, which corresponds to the size of the basic administrative or planning unit in many countries. For costs incurred only at national level that are not expected to vary with the number of patients treated, unit costs were defined per country. Gross national income per capita was used as the basis for extrapolating costs from countries for which unit cost data were available, to countries for which unit cost data were not available. The definition of unit costs for each intervention, as well as sources of data,19–26 are shown in Table 1 (columns 5 and 7).
Incremental costs
Incremental costs were calculated as the financial resources required for TB control in each year during the period 2006–2015 minus the estimated financial resources used for TB control in 2005. Costs in 2005 were based on the latest data reported to WHO.1 Total and incremental costs for TB control were also converted into per capita amounts and compared with annual government health expenditure per capita.27
Findings
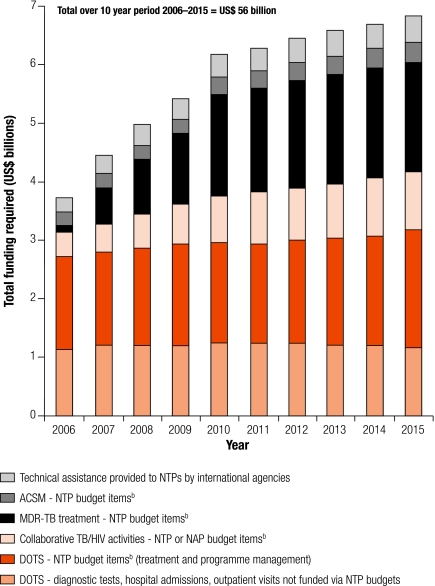
The total funding estimated to be required for TB control during the period 2006–2015, both in total and for major interventions, is shown in Fig. 1. The total funding required over 10 years is estimated at US$ 56 billion, increasing from US$ 3.5 billion in 2006 to US$ 6.7 billion in 2015. Almost all of this funding – US$ 52 billion – is required for implementation of recommended interventions at country level. The remainder of almost US$ 3.8 billion (7%) is needed for international agencies to provide technical assistance to national TB programmes. The single biggest cost is for diagnosis and treatment of new cases of drug-susceptible TB in DOTS programmes, which amounts to around US$ 3 billion each year (first two parts of the bars in Fig. 1). Of this US$ 3 billion, about US$ 2 billion is for funding that would be channelled through NTP budgets, while the remainder represents funding required for diagnostic tests, hospitalization and outpatient visits provided within the general health care system and funded via general health care budgets. The other major costs are for the newer elements of TB control: (i) diagnosis and treatment of MDR-TB and XDR-TB; (ii) collaborative TB/HIV activities; and (iii) advocacy, communication and social mobilization. Unlike the costs for DOTS implementation, the costs of these interventions increase steadily over time, reflecting low levels of implementation before 2006.
Fig. 1.
Total funding required for TB control by intervention, 2006–2015a
ACSM, advocacy, communication and social mobilization; DOTS, a strategy for TB control; NAP, national AIDS programme; NTP, national TB programme; TB, tuberculosis.
a Amounts include allowance for inflation at 3% per year. In real terms, total costs over 10 years would amount to US$ 49 billion, rather than US$ 56 billion. Annual costs rise in real terms from about US$ 3.5 billion per year in 2006 to around US$ 5.2 billion in 2015.
b NTP budget items – all costs described in the row for DOTS expansion in Table 1, excluding diagnostic tests, hospital admissions and outpatient visits in general (multipurpose) primary health care services.
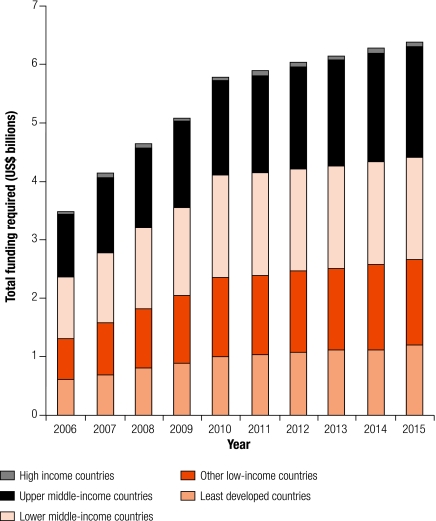
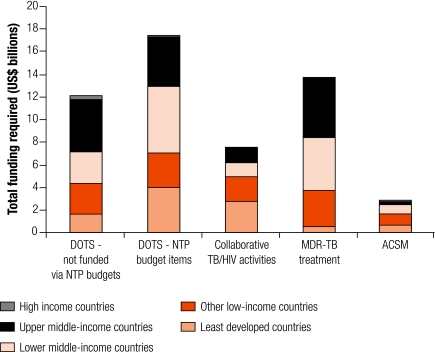
The breakdown of costs at country level (i.e. excluding technical assistance) according to the income classifications used by the World Bank is shown in Fig. 2 and Fig. 3. Overall, across 10 years, the least-developed and low-income countries account for US$ 22 billion (41%) of total costs (Fig. 2) and for most of the costs associated with collaborative TB/HIV activities (Fig. 3). Costs increase steadily over time, rising from US$ 0.6 to US$ 1.2 billion per year in the least-developed countries and from US$ 0.7 to US$ 1.5 billion per year in other low-income countries (Fig. 2). Lower and upper middle-income countries account for more than 50% of total costs (US$ 15 billion for both sets of countries over 10 years); these countries also account for 72% of the funding required for the treatment of MDR-TB. Results are similar when countries are classified according to the scheme used by the Commission on Macroeconomics and Health (data not shown).
Fig. 2.
Total funding required for TB control by country income levela
TB, tuberculosis.
a Income level as defined by the World Bank.
Fig. 3.
Total funding required for TB control by intervention category and income levela
ACSM, advocacy, communication and social mobilization; DOTS, a strategy for TB control; MDR-TB, multidrug-resistant tuberculosis; NTP, national TB programme; TB, tuberculosis.
a Income level as defined by the World Bank.
Africa is the epidemiological region with the largest funding needs and where the biggest increase in funding is required, followed by South-East Asia (Table 2). On a per capita basis, the increase in funding needed for TB control in Africa is equivalent to an 8% increase in existing government health care expenditure, a 4% increase in South-East Asia, and an increase of around 1% in Eastern Europe. In contrast, the per capita increases in government health expenditure that would be needed in Latin America, the Eastern Mediterranean and the Western Pacific are fractions of 1%.
Table 2. Total and incremental costs for TB control by epidemiological region6 compared to annual government health expenditure per capita.
| Epidemiological region | Total costs, 2006–2015 (US$ billions) | Cumulative incremental costs, 2006–2015a (US$ billions) | Regional share of cumulative incremental costs, 2006–2015 (%) | Total cost per capita per year, 2006–2015 (US$) | Incremental cost per capita per year (US$) | Government health expenditure per capita in 2004b (US$) | Incremental cost as proportion of government health expenditure (%) |
|---|---|---|---|---|---|---|---|
| Africa | 20 | 15 | 46 | 2.4 | 1.8 | 22 | 8.4 |
| Eastern Mediterranean | 3.1 | 2.3 | 7 | 0.5 | 0.4 | 50 | 0.8 |
| Eastern Europe | 13 | 5.9 | 18 | 3.3 | 1.5 | 127 | 1.2 |
| Latin America | 1.7 | 0.6 | 2 | 0.3 | 0.1 | 141 | 0.1 |
| South-East Asia | 8.3 | 5.7 | 18 | 0.5 | 0.3 | 8 | 4.0 |
| Western Pacific | 6 | 3 | 9 | 0.4 | 0.2 | 38 | 0.5 |
| Total | 52c | 32 | NA | 0.9 | 0.5 | 45 | 1.2 |
NHA, national health accounts; NA, not applicable; TB, tuberculosis. a Calculated as total costs 2006–2015 minus total costs that would apply if the financial resources available for TB control in 2005 increased only in line with inflation. Cost of TB control in 2005 estimated at US$ 1.8 billion. b Latest available NHA data is for 2004. c Total excludes technical assistance of US$ 3.8 billion over 10 years. Technical assistance was estimated globally rather than by region.
Discussion
Summary of major findings
The financial estimates prepared for the Global Plan to Stop TB 2006–2015 identify a funding need for TB control totalling US$ 56 billion during the 10-year period 2006–2015, rising from US$ 3.5 billion in 2006 to US$ 6.7 billion in 2015. Almost all (93%) of the required funding is for implementation of interventions recommended in WHO’s Stop TB Strategy at country level, with a relatively small fraction (7%) for technical assistance provided by international agencies with expertise in TB control. Of the interventions to be implemented, diagnosis and treatment of new cases of drug-susceptible TB in DOTS programmes accounts for the largest costs (almost US$ 30 billion), but it is the newer components of TB control, such as collaborative TB/HIV activities and in particular treatment of drug-resistant TB, that are responsible for the big growth in costs over time. Around US$ 22 billion of the required funding is for low-income countries, while Africa is the region in which the largest absolute and relative increases in funding are needed.
Strengths
The analytical work behind these numbers has several strengths. It used financial data that are routinely reported to WHO on an annual basis by around 90 countries that collectively account for 90% of global TB cases,1,19 and the WHO-CHOICE database, which includes unit cost estimates for hospital bed-days and outpatient visits for all countries based on standardized methods and a thorough review of the available evidence.20 It benefited from the input of a wide range of people via the secretariats of the Stop TB Partnership11 and made use of a well-established model of the epidemiology of TB.12 The interventions considered were based on a widely endorsed and up-to-date strategy. There was close collaboration with UNAIDS on the TB/HIV estimates and with other departments in WHO to ensure consistency with the costing methods used to produce price tags for other health-related MDGs.11
Limitations
As with all estimates of this kind, several limitations need to be acknowledged. The epidemiological projections, which indicate 5–6% per year reductions in TB incidence in some regions, may be overoptimistic. Recent data for Asia suggest that reductions in practice may be more modest, for reasons that are not fully understood.28 This means that costs may have been underestimated, particularly those for treatment in DOTS programmes (many of which are directly related to trends in TB incidence). If incidence rates remain stable and do not decline as projected in the Global Plan, costs for DOTS implementation could be US$ 6 billion higher over 10 years. The evidence on unit costs for collaborative TB/HIV activities, MDR-TB and XDR-TB treatment and ACSM was limited to a few countries. Costs for strengthening health systems as a whole, such as training costs for adding to the existing stock of health workers, improved salaries or staff incentives, and building new infrastructure, all of which need to be budgeted for an entire system rather than for TB control specifically, could not be included. Related to this, it remains to be seen whether the scaling-up envisaged in the Global Plan can be realized should full funding become available, given health-system and non-financial barriers to progress, especially in the context of the ambitious targets set for TB/HIV and diagnosis and treatment of MDR-TB and XDR-TB. Information collected by WHO on an annual basis about implementation of different components of TB control will enable periodic revision of the cost estimates to allow for progress in practice. New information on costs and revisions to estimates that were used as inputs in the original Global Plan analyses (e.g. demographic projections or HIV prevalence rates among TB cases) will also necessitate periodic revision of the cost estimates.
This paper has focused on the funding required for TB control, without including an analysis of what funding is available, the size of the funding gap, and how funding gaps could be closed. The Global Plan itself does include projections of funding, using the assumption that funding from domestic and donor (excluding the Global Fund) sources will be sustained at 2005 levels in real terms (in financial terms, this means funding will increase only in line with inflation) and then adding on funding commitments announced by the Global Fund in rounds 1–5.11 The resulting financial gap of US$ 33 billion is, not surprisingly, almost identical to the cumulative incremental costs of US$ 32 billion presented in this paper. What remains to be done is an assessment of how the funding gap can be closed. Based on the Commission on Macroeconomics and Health’s analysis of resource mobilization potential (middle-income countries could fund all, or almost all, of their health care from domestic sources while the least-developed countries would need donor support to fund about 50% of their resource requirements),29 a rough indicative figure for the funding needed from donors would be about US$ 1 billion per year.
Implications and next steps
Given the limitations we have identified, the figures presented in this paper should be seen as approximate estimates. To produce more accurate figures, the Global Plan to Stop TB needs to be converted into concrete country-specific plans and budgets. WHO has developed a tool that is designed to help planning and budgeting at country level in line with the Global Plan and the Stop TB Strategy (available at: http://www.who.int/tb/dots/planning_budgeting_tool/en/index.html) and this is now being used in several countries, notably in the African region. Assessment of how the necessary resources can be mobilized should be done alongside plan development, to produce better estimates of the financing that can be raised from domestic sources and the balance needed from external donors. This will vary according to a country’s income level, existing government health care expenditure per capita and the burden of TB relative to the size of the population. The data we have presented suggest that donor funding is likely to be most needed for DOTS implementation and collaborative TB/HIV activities in low-income African countries.
Conclusion
Achieving the MDG, WHO and Stop TB Partnership targets set for TB control for 2015 requires a major increase in funding for TB control. To assist resource mobilization efforts, it is now essential to produce more precise country-by-country assessments of resource requirements, backed up by analysis of the share of funding that can be raised in each country from domestic sources and the balance that is needed from donors. ■
Acknowledgements
A large study team developed the Global Plan to Stop TB. The members of this study team and all individuals who contributed to the plan are listed in the plan document, available at http://www.stoptb.org. Individuals who contributed to the costing analysis are listed in Annex 1 of the plan.11 We particularly thank Ben Johns and Tessa Tan Torres Edejer for their support in the production of this paper.
Footnotes
Competing interests: None declared.
References
- 1.Global tuberculosis control: surveillance, planning, financing Geneva: WHO; 2007 (WHO/HTM/TB/2007.376).
- 2.Lopez AD, Mathers CD, Ezzati M, Murray CJL, Jamison DT. Global burden of disease and risk factors New York: Oxford University Press and World Bank; 2006. [DOI] [PubMed] [Google Scholar]
- 3.The global MDR-TB and XDR-TB response plan 2007-2008 Geneva: WHO; 2007 (WHO/HTM/STB/2007.387).
- 4.Dye C, Maher D, Weil D, Espinal M, Raviglione M. Targets for global tuberculosis control. Int J Tuberc Lung Dis. 2006;10:460–2. [PubMed] [Google Scholar]
- 5.Raviglione MC, Uplekar MW. WHO’s new Stop TB Strategy. Lancet. 2006;367:952–5. doi: 10.1016/S0140-6736(06)68392-X. [DOI] [PubMed] [Google Scholar]
- 6.The global plan to stop TB, 2006-2015 Geneva: WHO and Stop TB Partnership; 2006.
- 7.The global plan to stop TB, 2001-2005 Geneva: WHO and Stop TB Partnership; 2001 (WHO/CDS/STB/2001.16).
- 8.Floyd K, Blanc L, Raviglione M, Lee JW. Resources required for global tuberculosis control. Science. 2002;295:2040–1. doi: 10.1126/science.1069771. [DOI] [PubMed] [Google Scholar]
- 9.Maher D, Dye C, Floyd K, Pantoja A, Lonnroth K, Reid A, et al. Planning to improve global health: the next decade of tuberculosis control. Bull World Health Organ. 2007;85:341–7. doi: 10.2471/BLT.06.037820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nordström A, Tan-Torres Edejer T, Evans D. What will it cost to attain the health MDGs? Bull World Health Organ. 2007;85:246. doi: 10.2471/BLT.07.041467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The global plan to stop TB, 2006-2015 [Annex 1: methods used to estimate costs, funding and funding gaps]. Geneva: WHO and Stop TB Partnership; 2006.
- 12.Dye C, Garnett GP, Sleeman K, Williams BG. Prospects for worldwide tuberculosis control under the WHO DOTS strategy. Directly observed short-course therapy. Lancet. 1998;352:1886–91. doi: 10.1016/S0140-6736(98)03199-7. [DOI] [PubMed] [Google Scholar]
- 13.An expanded DOTS framework for effective tuberculosis control Geneva: WHO; 2002 (WHO/CDS/TB/2002.297). pp. 1-20 [PubMed]
- 14.Global tuberculosis control: surveillance, planning, financing Geneva: WHO; 2005 (WHO/HTM/TB/2005.349).
- 15.Johns B, Baltussen R, Hutubessy R. Programme costs in the economic evaluation of health care interventions. Cost Eff Resour Alloc. 2003;1:1. doi: 10.1186/1478-7547-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stenberg KJB, Scherpbier RW, Tan-Torres Edejer T. A financial roadmap to scaling up essential child health interventions in 75 countries. Bull World Health Organ. 2007;85:305–14. doi: 10.2471/BLT.06.032052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drummond MF, O’Brien B, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes [Oxford Medical Publications]. Oxford: Oxford University Press; 2005. [Google Scholar]
- 18.Laxminarayan R, Klein E, Dye C, Floyd K, Darley S, Odeji O. Economic benefit of tuberculosis control [Policy research working paper 4295]. Washington, DC: World Bank; 2007. [Google Scholar]
- 19.Floyd K, Pantoja A, Dye C. Financing tuberculosis control: the role of a global financial monitoring system. Bull World Health Organ. 2007;85:334–40. doi: 10.2471/BLT.06.034942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choosing interventions that are cost-effective (WHO-CHOICE) Geneva: WHO; 2000. Available from: http://www.who.int/choice [accessed on 16 June 2008].
- 21.Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, et al. Disease control priorities in developing countries, 2nd edn. New York: Oxford University Press and World Bank; 2006. [PubMed] [Google Scholar]
- 22.Tupasi TE, Gupta R, Quelapio MI, Orillaza RB, Mira NR, Mangubat NV, et al. Feasibility and cost-effectiveness of treating multidrug-resistant tuberculosis: a cohort study in the Philippines. PLoS Med. 2006;3:e352. doi: 10.1371/journal.pmed.0030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suárez PG, Floyd K, Portocarrero J, Alarcón E, Rapiti E, Ramos G, et al. Feasibility and cost-effectiveness of standardised second-line drug treatment for chronic tuberculosis patients: a national cohort study in Peru. Lancet. 2002;359:1980–9. doi: 10.1016/S0140-6736(02)08830-X. [DOI] [PubMed] [Google Scholar]
- 24.The feasibility and efficiency of controlling MDR-TB using the DOTS-Plus strategy in the Russian Federation Geneva: WHO; 2005 (WHO/HTM/TB/2005.357c).
- 25.Hausler HP, Sinanovic E, Kumaranayake L, Naidoo P, Schoeman H, Karpakis B, et al. Costs of measures to control tuberculosis/HIV in public primary care facilities in Cape Town, South Africa. Bull World Health Organ. 2006;84:528–36. doi: 10.2471/BLT.04.018606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terris-Prestholt F, Kumaranayake L. Cost analysis of the Zambian ProTEST project: a package to reduce the impact of tuberculosis and other HIV-related diseases London: London School of Hygiene and Tropical Medicine; 2003 (unpublished report).
- 27.National health accounts Geneva: WHO; 2007. Available from: http://www.who.int/nha [accessed on 16 June 2008].
- 28.Vree M, Hoa NB, Bui DD, Sy DN, Borgdorff MW, Cobelens FG. Tuberculosis trends, Vietnam. Emerg Infect Dis. 2007;13:796–7. doi: 10.3201/eid1305.060904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Report of the Commission on Macroeconomics and Health Geneva: WHO; 2001.