Abstract
Background/Objectives:
To examine nutrient intake and body mass index (BMI) in the spinal cord injury (SCI) population according to level of injury and sex.
Design:
Cross-sectional study conducted at 2 SCI treatment centers.
Participants/Methods:
Seventy-three community-dwelling individuals with C5-T12 ASIA Impairment Scale (AIS) A or B SCI. Subjects were divided into 4 groups: male tetraplegia (N = 24), male paraplegia (N = 37), female tetraplegia (N = 1), and female paraplegia (N = 11). Mean age was 38 years; 84% were male; 34% were white, 41% were African American, and 25% were Hispanic. Participants completed a 4-day food log examining habitual diet. Dietary composition was analyzed using Food Processor II v 7.6 software.
Results:
Excluding the 1 woman with tetraplegia, total calorie intake for the other 3 groups was below observed values for the general population. The female paraplegia group tended to have a lower total calorie intake than the other groups, although macronutrient intake was within the recommended range. The male tetraplegia group, male paraplegia group, and the 1 woman with tetraplegia all had higher than recommended fat intake. Intake of several vitamins, minerals, and macronutrients did not meet recommended levels or were excessively low, whereas sodium and alcohol intake were elevated. Using adjusted BMI tables, 74.0% of individuals with SCI were overweight or obese.
Conclusions:
Women with paraplegia tended to maintain healthier diets, reflected by lower caloric and fat intakes, fewer key nutrients falling outside recommended guidelines, and less overweight or obesity. Individuals with tetraplegia tended to take in more calories and had higher BMIs, and using adjusted BMI, the majority of the population was overweight or obese. The majority of people with SCI would benefit from nutritional counseling to prevent emerging secondary conditions as the population with SCI ages.
Keywords: Spinal cord injuries, Paraplegia, Tetraplegia, Nutrition, Body mass index, Obesity
INTRODUCTION
Individuals with spinal cord injury (SCI) are living longer (1), and as such, are developing chronic diseases, such as cardiovascular disease (CVD) (2–7) and diabetes mellitus (8) with greater frequency and potentially at younger ages than persons without SCI. CVD is now a frequent cause of death among persons surviving 30 years after injury (46% of deaths) and those older than 60 years (35% of deaths) (9,10). More recently, evidence on the apparent causes for accelerated CVD after SCI has been growing and now includes risks for dyslipidemia (11–14), insulin resistance (15), postprandial hyperlipidemia (16), and inflammation (17–20). The most consistent finding of the dyslipidemia profile is depressed blood plasma concentration of high-density lipoprotein cholesterol (HDL-C) (5,21,22), which provides cardiovascular protection through well-defined mechanisms. Prudent diet and physical activity are essential to minimizing these risks, thereby decreasing the chance of developing CVD and other chronic diseases. Despite this need, the diet of persons with SCI has received little research attention.
The paucity of literature on the nutritional status of individuals with SCI is surprising. Despite knowledge of accelerated aging, including the increased risk of CVD noted above, much of the available dietary research has been conducted on a small number of subjects during the acute phase of recovery (23,24) and far less after discharge from rehabilitation. In a group of individuals living at least 2 years after traumatic SCI, Moussavi et al (25) examined serum levels of vitamins A, C, and E and found that one quarter to one third of participants had serum levels below the reference range for each vitamin. Summarizing the few studies examining the diets of community-dwelling individuals with SCI, it has been shown that, although total calorie intake is often below recommended levels (26), proportion of fat intake is high, protein intake is adequate, and deficiencies have been noted for dietary fiber and selected vitamins and minerals (26,28), largely consistent with current dietary trends in the United States (27). At the same time, approximately one half of persons studied were overweight when assessed by traditional body mass indices (BMIs) (26).
To date, a detailed analysis of nutritional intake that also examines effects of sex and level of injury has yet to be undertaken within the SCI population. Stratification by sex is important because men and women have different nutrient requirements and recommended BMI guidelines. Moreover, dietary composition and caloric intakes have seen little comparison with body mass, despite the obvious cause-and-effect relationship. Therefore, in this study, we performed a detailed analysis of the sex-specific dietary and nutrient intake of individuals with SCI, analyzed the association of injury level on these intakes, and compared the intakes with authoritative guidelines for the general population. Because significant concern has been expressed for weight gain as individuals with SCI age with disability, we further examined BMI by sex and level of injury. We hypothesized that the overall intakes of individuals with tetraplegia would be different from those with paraplegia, in part because of hand impairment, greater mobility limitations, the need for assistance, and greater impact of barriers (such as transportation), and that the BMI would be universally high, based on national trends. We also sought to identify nutritional deficiencies that might be used to guide lifestyle interventions through dietary modification.
METHODS
This was a collaborative study of the National Rehabilitation Hospital (NRH) in Washington, DC, and Miller School of Medicine/Miami Project to Cure Paralysis at the University of Miami (UM) in Miami, FL. The study was approved by the Institutional Review Boards at both NRH and UM. Informed consent was obtained before entry into the study.
Individuals with SCI for at least 1 year and residing in the community were recruited for this study. Eligible participants included men and women with American Spinal Injury Association (ASIA) Impairment Scale A or B SCI from C5 to T12. Participants were recruited through multiple announcements, including database/medical record query, posted announcements, newsletter advertisement, and website announcements through the Rehabilitation Research and Training Center (RRTC; www.sci-health.org) and the National Spinal Cord Injury Association (NSCIA, www.spinalcord.org). Interested individuals were scheduled for appointments at NRH or UM.
Medical records at NRH and UM were reviewed to confirm neurologic status, and abbreviated AIS (ASIA Impairment Scale) neurologic assessments were performed as an additional confirmation of level and completeness of injury. Demographic information was obtained by face-to-face interview during the first appointment. Participant height was obtained by self-report, and weight was obtained using an accessible scale that was appropriately calibrated for use with wheelchairs.
A previously published 4-day food log examining habitual diet after SCI was used to assess the subjects' nutrient intake (28). This log includes weekend food consumption, which often differs from that of the work week. An orientation session, typically lasting 2 hours, was completed with each participant, during which the participant was provided a food scale with measuring cup, pen, notebook, and intake form. Written and oral guidelines for weighing, measuring, and recording foods were reviewed. Participants were instructed to complete and return a sample 2-day dietary record, which was reviewed and immediately returned to study participants. When necessary, additional training was provided. Habitual food and drink consumption was recorded for 2 additional days, and logs were returned to study personnel. Subjects did not include supplement intake as part of the food log. Dietary composition was analyzed using a widely used nutritional software package (Food Processor II Windows v. 7.6; ESHA Research, Salem, OR).
Participants' nutrient intakes were compared with those of the general population derived from the National Health and Nutrition Examination Survey (NHANES) data set (29). NHANES is a periodic survey conducted by the National Center for Health Statistics (NCHS) that provides a nationally representative sample of the US population. The survey was designed to provide national estimates of the health and nutritional status of the US civilian, noninstitutionalized population (30). The diet component includes a 24-hour dietary recall and dietary food frequency, occurring at mobile examination centers.
Whenever possible, comparison was made with US population values of dietary reference intakes (DRI) and the acceptable macronutrient distribution range (AMDR). If the DRI and/or AMDR have not been determined for a given nutrient, the recommended daily allowance (RDA) was used for comparison.
Statistical analyses included the use of a 2-tailed Mann-Whitney test to compare continuous data and the Fisher exact test for categorical data. To assess the differences in nutrition intake between the groups mixed by sex but varying by level of injury (paraplegia vs tetraplegia) and mixed by severity of injury but varying by sex (male vs female), independent sample t tests were performed for 4 variables: intake of fat, carbohydrate, and protein, and total number of calories. For these comparisons, analyses were performed twice, with and without the outlier (female tetraplegia). Within-female group comparisons did not allow for a calculation of P values, because there was only 1 woman with tetraplegia. Statistical significance was defined a priori to be at the 0.05 level. Microsoft Office Excel and SPSS (Statistical Package for the Social Sciences) were used for data management and analysis, respectively.
RESULTS
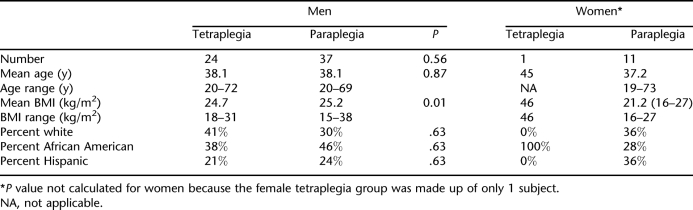
Participants included 61 men (84%) and 12 women (16%) with a mean age of 38 years (range = 19–73 years). Sex distribution was consistent with that expected for traumatic SCI (80% men; 20% women). The groups were relatively equally divided by race and ethnicity. Mean BMI for the group was 25.2 kg/m2 (range = 15–38 kg/m2). Table 1 summarizes the demographic information of the participants.
Table 1.
Demographic Distribution of Participants
Energy Intake
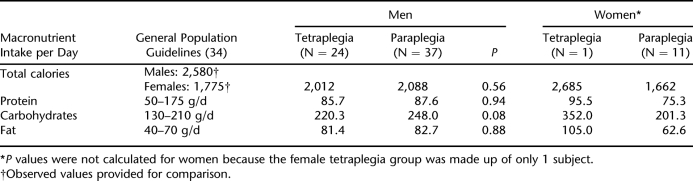
Protein, carbohydrate, and fat intakes were calculated for each group according to level of injury and sex. Table 2 summarizes this information and provides a comparison to observed US population values for calorie intake and recommended values for protein, carbohydrate, and fat intake. When male and female groups were combined to allow for severity of injury comparisons, there were no statistically significant differences between the groups in intake of protein (paraplegia, 84.7 g/d vs tetraplegia, 85.7 g/d; P = 0.86), carbohydrates (paraplegia 237.3 g/d vs tetraplegia 220.3; P = 0.60), or fats (paraplegia 78.1 g/d vs tetraplegia 81.4 g/d; P = 0.58). Similarly, there were no significant differences in total calorie, protein, carbohydrate, or fat intake between the male tetraplegia and male paraplegia groups. When men and women were compared, total calorie intake was significantly greater for men than women (2,049.0 vs 1,662.5 kcal/d; P = 0.04), and there was a trend toward greater fat intake in the male groups (82.3 vs 62.6 g/d; P = 0.06). For all subjects, protein intake (g/d) was within recommended ranges, but fat and carbohydrate intakes (g/d) were higher than recommended for all but the female paraplegia group.
Table 2.
Energy Intake According to Sex and Level of Injury
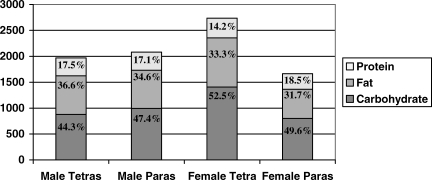
Figure 1 summarizes the proportion of energy intake derived from protein, fat, and carbohydrates according to injury level and sex. Depending on sex and level of injury, protein intake varied from 14.2% to 18.5% of total intake, carbohydrates from 44.3% to 52.5% of total intake, and fat from 31.7% to 36.6% of total intake.
Figure 1. Source of calories by sex and level of injury. Proportions do not always add up to 100% because of the additional contribution of alcohol in the calculation of total caloric intake.
Saturated Fat Intake
For all groups, the proportion of saturated fat intake was higher than the maximum of 7% recommended by the American Heart Association. Saturated fat intake was greatest for the male tetraplegia group (11.9%), followed by the male paraplegia group (10.9%), female paraplegia group (9.9%), and the 1 woman with tetraplegia (9.6%). Of note, although the woman with tetraplegia had the lowest proportion of energy derived from saturated fat, she also had the highest absolute intake of saturated fat (29.8 g/d compared with 27.2, 25.6, and 19.4 g/d for the male tetraplegia, male paraplegia, and female paraplegia groups, respectively; P = 0.52).
Macronutrient, Vitamin, and Mineral Intake
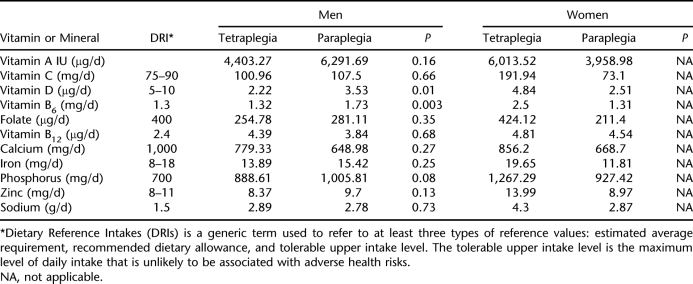
Key macronutrients, vitamins, and minerals were examined and compared with recommended values (Table 3). All groups had adequate intake of vitamins C, B6, and B12, phosphorus, iron (excluding the female paraplegia group) and zinc, and lower than recommended intake of vitamin D, calcium, and folate (excluding the woman with tetraplegia). Additionally, all groups had lower than recommended intake of vitamin E, vitamin K, pantothenic acid, biotin, chromium, iodine, molybdenum, potassium, and chloride. The male tetraplegia group had significantly lower intake of vitamin D (P = 0.01), vitamin B6 (P < 0.01), and potassium (P < 0.01) compared with the male paraplegia group.
Table 3.
Key Vitamin and Mineral Intake by Gender and Level of Injury
Fiber intake across the groups was universally lower than the recommended values of 38 and 25 g/d for men and women, respectively (male tetraplegia and male paraplegia groups: 12.7 and 14.5 g/d, respectively, P = 0.14; female paraplegia group: 13.4 g/d). All men and the women with paraplegia had lower than recommended intakes of linoleic acid (recommended 17 and 11 g/d for men and women, respectively; men with SCI: 8.7–9.7 g/d [P = 0.47]; women with paraplegia: 7.3 g/d). Women exceeded or approached the recommended intake of linolenic acid (1.1 g/d recommended: intake 1.0–1.6 for the female paraplegia group and the woman with tetraplegia), whereas all men with SCI had lower than the recommended intake (recommended 1.6 g/d; men with SCI, 0.8 g/d). Only the woman with tetraplegia met RDA or AMDR requirements for fiber and essential fatty acid intake. There were no significant differences by level of injury.
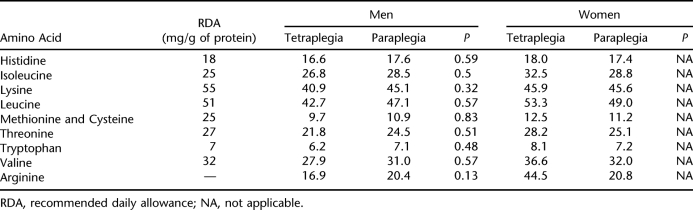
Table 4 summarizes amino acid intake by sex and level of injury. Subjects approached or met dietary recommendations for amino acid intake with the exception of lysine, leucine, threonine, methionine, and cysteine. There were no significant differences in amino acid intake by level of injury, although men with paraplegia consumed a greater amount of every amino acid compared with men with tetraplegia.
Table 4.
Intake of Amino Acids by Sex and Level of Injury
Sodium Intake
Intake of sodium for all groups exceeded the recommended value of 2,400 mg/d, with the male tetraplegia and male paraplegia groups having average intakes of 2,892 and 2,786 mg/d, respectively (P = 0.72) and the female tetraplegia and female paraplegia groups having average intakes of 4,299 and 2,875 mg/d, respectively. When men and women were collapsed into groups, there was no significant difference between the groups with tetraplegia and those with paraplegia (P = 0.64).
Caffeine Intake
Caffeine intake was greatest for the male paraplegia group (38.8 mg/d), followed by the female paraplegia group (37.1 mg/d), the woman with tetraplegia (36.7 mg/d), and the male tetraplegia group (17.1 mg/d). Within the male group, the difference between tetraplegia and paraplegia was statistically significant (P = 0.01).
Alcohol Intake
Alcohol intake was greatest for all men compared with women (6.43 vs 2.24 g/d, respectively; P < 0.01). The male paraplegia group had the greatest intake (9.53 g/d), followed by the male tetraplegia group (4.56 g/d), the female paraplegia group (2.45 g/d), and the woman with tetraplegia (0 g/d). The difference between the male tetraplegia and male paraplegia groups approached statistical significance (P = 0.08).
Body Mass Index
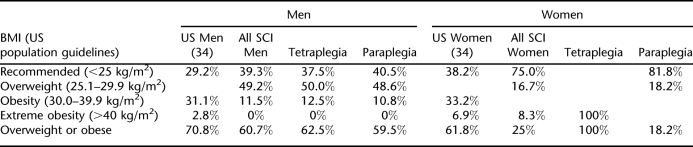
BMI of the male paraplegia group was slightly greater than that of the male tetraplegia group (25.2 vs 24.7 kg/m2; P < 0.01), whereas the female paraplegic group had a lower BMI than all other groups (21.2 kg/m2; P = 0.01). Table 5 shows a comparison of the general population and distribution of SCI subjects according to BMI, sex, and level of injury.
Table 5.
Distribution of Subjects by BMI, Sex, and Level of Injury and Comparison With Recent General Population Data
Proportion of overweight or obese was comparable between men with SCI and that observed in men in the US general population. The majority of women with paraplegia (81.8%) fell into the recommended BMI category (compared with 38.2% observed from observed US population data), with only 18.2% classified as overweight or obese. Distribution of BMI by level of injury was similar with 37.5% and 40.5% of the male tetraplegia and male paraplegia groups, respectively, falling into the recommended BMI range. Approximately 50% in each male group were overweight by BMI, and 12.5% and 10.8%, respectively, were classified as obese. Overall, when compared with the general population-observed distribution by BMI, a greater proportion of men with SCI and women with paraplegia fell into the desirable BMI range and fewer fell into the obese category.
DISCUSSION
This study contributes to our understanding of nutrition and body habitus in individuals with chronic SCI by providing data on a diverse sample of individuals with SCI and for the first time comparing both level of injury and sex relationships. The study also used a dietary log to quantify intake, made comparisons to current guidelines for intake, and examined a varied population of persons with SCI, all of which enhance externally validity.
This study collected data using a dietary log as opposed to a food frequency questionnaire (FFQ). This provides the advantage of objective computer analysis of nutrient components and more accurate data acquisition, the latter because study participants are asked to enter data immediately after the food is consumed. The likelihood of inaccurate estimation of food quantities was also minimized with the training and utilization of food scales and measuring cups (although some element of bias may exist because of the possibility for under-reporting of intake). Although a FFQ allows identification of inadequate intake of a food group (and hence identification of dietary and nutrient deficiencies), it nonetheless carries the disadvantages of possible errors in filling out the questionnaire, difficulty in determining how foods are prepared, and over- or underestimation of food quantities.
For this study, we presented current population guidelines for nutrient intake, including DRI and AMDR, in addition to RDAs. For more than 50 years, RDAs were the nutrient and energy standards used in the United States. The RDA concept is defined as the amount of a given nutrient needed to prevent deficiency and was primarily developed to plan and evaluate the diets of various populations. By contrast, DRIs are the amounts of nutrient intake to be used for planning and assessing diets for healthy people and are made up of sets of reference values (both recommended intakes and tolerable upper intake levels) designed to prevent nutrient deficiencies and reduce the risk of chronic disease. DRIs are replacing RDAs and represent a shift in emphasis from preventing deficiency to decreasing the risk of chronic disease through nutrition. DRIs express the distribution of macronutrients in the diet (or percent of calories coming from protein, carbohydrate, and fat) as the AMDR. The AMDR for adults recommends percentage caloric intake from protein (10–35%), fat (20–35%), and carbohydrate (45–65%), which represents the ranges associated with a reduced risk for chronic disease while providing essential nutrients such as vitamins and minerals. People whose diet is outside the AMDR have the potential of increasing their risk of developing chronic disease.
Energy and Nutrient Intake After SCI
In this study, we found that individuals with SCI (excluding the 1 woman with tetraplegia) had total calorie intakes lower than those observed in the general population, the difference being more substantial for men (∼500 kcal/d, depending on injury level). Protein intake was generally within recommended ranges, but fat and carbohydrate intake was generally high, with the exception of the female paraplegia group.
These results are comparable to those of Tomey et al (26), although in that study the population was restricted to community-dwelling urban men with paraplegia. Our male paraplegia population is similar in age, but with a greater proportion of Latinos and African Americans. This subsample consumed less total calories (2,088 kcal/d in our sample vs 2,268 kcal/d), and mean BMI was lower (25.2 vs 26.2 kg/m2, respectively) than that reported by Tomey et al (26). This is reflected in the higher proportion of overweight or obese in that population (75.4%) compared with our population (59.4%). Other factors that may have contributed to the differences between the populations include racial/ethnic differences, geographic location, and that ours may have been a more health- and diet-conscious group.
In a 1992 study of nutritional intake (recorded by a 7-day diet record and food frequency chart) of 33 individuals with chronic SCI (two thirds with tetraplegia), mean daily energy intake was 1,682 and 1,282 kcal, respectively, for men and women (28), which is substantially lower than both our study and the study of Tomey et al. Proportion of energy intake was fairly similar, however, with 37.9% of diet derived from fat, compared with 36.2% and 33.9% for our male and female participants, respectively. Similar to this study, women tended to consume proportionally less fat than men did. Of note, individuals in this study tended to be younger, have tetraplegia, and were described as “quite active.” Overall, taking into account population differences, energy intake of our sample is relatively consistent with those reported by Tomey et al (26) and Levine et al (28) and reflects the higher-fat diet observed in the United States.
There were few clinically relevant differences in nutrient intake by injury level. Of importance was that fiber intake was universally low across groups and sexes (range, 12.7–28.6 g/d), and levels were notably consistent with those reported by Levine et al (28) (12.2 and 14.3 g/d for males and females, respectively), as was intake of linoleic and linolenic acids. Critical for bone health, vitamin D and calcium intakes were low for all groups, with the male tetraplegia group having significantly lower vitamin D intake than their counterparts with paraplegia. This is similar to deficiencies reported by Levine et al (28) and clinically relevant given the profound osteoporosis and subsequent fracture risk observed soon after SCI. Intake of vitamin K, folate, chromium, iodine, molybdenum, and potassium generally did not meet recommended levels as well. Sodium intake was high across all groups, and alcohol intake was significantly higher among men with paraplegia, both of which are potential areas for intervention.
Energy Expenditure and the Impact of Body Composition
Muscle atrophy is relatively universal after SCI and is related to severity and level of injury. Associated with this is a subsequent increase in fat mass and imposed immobility caused by neurologic impairment. These factors are significant when one considers the impact on resting energy expenditure (REE). In 2004, Bauman et al (31) compared REE in 13 pairs of monozygotic twins discordant for SCI. REE was 1,682 kcal/d for the SCI group compared with 1,854 kcal/d for the able-bodied twins. Similarly, Buchholz et al (32) found that individuals with complete paraplegia had total daily energy expenditures (TDEE) of 2,072 kcal/d compared with the recommended TDEE of 2,582 kcal/d in adults without paralysis. Energy expenditure is multifactorial, but in addition to genetic factors, resting muscle metabolism has been shown to be a major determinant in the interindividual variation observed in REE, which may also be dependent on a number of factors such as muscle tone, sympathetic innervation, muscle fiber type, and thyroid hormone levels, all potentially altered after SCI (33).
One factor key to muscle metabolism is body composition, specifically lean mass. In the clinic environment, calculated BMI (based on a height vs weight calculation) is often used to quickly and indirectly quantify body composition and is used as an estimate of risk for developing malnutrition or chronic disease. BMI guidelines are as follows (34): underweight, BMI <18.5 kg/m2; recommended, BMI = 18.5 to 24.9 kg/m2; overweight, BMI = 25.0 to 29.9 kg/m2; obesity, BMI >30.0 kg/m2.
Despite its pervasive use in the medical community and because it is an indirect calculation of body composition, over- or underestimation of body fat occurs in certain populations, such as overestimation of body fat in athletes and others with a muscular build and underestimation of body fat in the elderly or others with decreased muscle mass (34–37).
There have been numerous studies that have examined the prevalence of overweight and obesity by BMI in the SCI population (38–45). In a study using DXA to assess body composition, it was found that for every BMI level, individuals with SCI (paraplegia/tetraplegia) had a greater proportion of body fat and less lean body mass than nondisabled controls (45). This is substantiated in a recent evidence-based guideline requested by the Spinal Cord Medicine Consortium, Paralyzed Veterans of America, and published by the Agency for Healthcare Research and Quality (AHRQ), in which it is concluded that “BMI is unlikely to be an accurate measure of obesity in the SCI population” and that “when obesity is measured as percent body fat, individuals with SCI may be at elevated risk” (46). As with many systematic reviews in SCI, caution is recommended in drawing conclusions because of the lack of “high-quality” studies.
Specifically addressing body fat percentage, Gater (47) recently summarized available evidence and found that a (“recommended”) BMI in the 22- to 25-kg/m2 range among individuals with SCI correlated with 22% to 36% body fat, which varied somewhat according to method of body composition determination and population characteristics (sex, age, duration of injury, level of injury). In concert, these data indicate that, for people with SCI, BMI in the “recommended” range may often correlate with a fat mass in the overweight (or obese) categories.
As a feasible alternative in the clinic setting, clinicians should consider adjusting BMI categories to the SCI population to better determine risk for comorbid conditions related to increased body fat, as suggested by Gater (47). Using our study population and based solely on traditional BMI tables, it seems that the majority of the SCI population should not be at risk for developing obesity-related comorbid conditions. However, when our study population was re-examined using the following guidelines (47) (recommended, BMI <22 kg/m2; overweight, BMI = 22–25 kg/m2; obese, BMI >25 kg/m2), we found that 29.7% of the male paraplegia group, 37.5% of the male tetraplegia group, and 36.4% of the female paraplegia group were overweight, and 48.6%, 37.5%, and 18.2%, of the groups, respectively, satisfied the criteria for obesity. While the female paraplegia group tended toward more favorable energy intake and BMI, in total, 74.0% of the entire population studied fell into either the overweight or obese categories using these BMI criteria adjusted for SCI, indicating a need for further study and intervention.
Although this study contributes to the evidence base in SCI research, several limitations should be considered when drawing conclusions from these data. There exists the potential for under-reporting of intake despite mechanisms in place to attempt to increase the accuracy. Potential inaccuracies exist in the calculation of BMI because of self-reporting of height. When compared with other published evidence, these data are fairly consistent, especially regarding the larger issues of energy intake, BMI, and selected nutrient deficiencies. Last, conclusions must be interpreted in the context of the study design (exploratory, cross-sectional) nature and sample size (adequate in total, but with low subgroup samples, particularly in the female tetraplegia subgroup).
CONCLUSIONS
The population with SCI likely has a very narrow range of optimal fat mass below or above which is associated with a variety of health risks. Although heightened fat mass is associated with a number of comorbid conditions, some proportion of fat and muscle is needed to protect from other conditions, such as pressure ulcers. In the majority of individuals with SCI in this study, BMI fell into the recommended (BMI < 25 kg/m2) stratum, yet applying an adjustment for SCI showed the majority of subjects to be overweight or obese, thereby heightening disease risk. Because there is not a readily available and feasible means to measure fat mass in the clinical environment, clinicians should give consideration to adjusting existing BMI tables as outlined above (at least until research is better able to show the relationships among levels of fat, muscle mass, and development of comorbid conditions). This will allow a simple initial estimation of disease risk.
A critical question that arises is how to best proceed programmatically to promote optimal body weight and composition to reduce disease risk. Typical management options include dietary change, physical activity, and pharmacologic options. First-line self-management is often dietary changes for improved weight control and disease prevention. To achieve benefits, many individuals with SCI would have to follow stringent diets with lower total energy intake and an emphasis on nutrient-rich foods. Even with a substantial dietary modification, the beneficial impact is not clear. This is exemplified by data in the able-bodied population, which show a modest improvement in lipid and carbohydrate parameters among selected highly motivated individuals. In the same report, it is stated that “translation of these findings to community settings of adults with SCI has not been demonstrated, and even the effectiveness in the general able-bodied population in unclear” (46). Anecdotally, however, in our studies, we observed a very high level of interest on proper nutrition from individuals with chronic SCI living in the community.
Given this, it is imperative for clinicians to encourage even simple changes, such as increasing fruit and vegetable intake, which would improve fiber and vitamin and mineral intake (especially vitamin D and calcium), and decreasing overall fat and refined carbohydrate intakes. Last, this study indicates a need (and desire) for nutrition counseling and education in all SCI populations and perhaps a heightened awareness on the part of clinicians regarding body composition and nutrition.
Footnotes
This study was funded by NIDRR Grant H133B031114, and the Rehabilitation Research and Training Center on SCI: Promoting Health and Preventing Complications through Exercise and the University of Miami, General Clinical Research Center Grant M01RR16587-03.
This article is based on a platform presentation given at the 2006 combined annual meetings of the American Spinal Injury Association (ASIA) and the International Spinal Cord Society (ISCoS), Boston, MA, June 25-28, 2006.
REFERENCES
- National Spinal Cord Injury Statistical Center. Spinal cord injury: facts and figures at a glance. Available at: http://www.spinalcord.uab.edu/show.asp?durki=21446. Accessed May 4, 2006.
- Krum H, Howes LG, Brown DJ, et al. Risk factors for cardiovascular disease in chronic spinal cord injury patients Paraplegia. 199230 (6) 381–388. [DOI] [PubMed] [Google Scholar]
- Yekutiel M, Brooks ME, Ohry A, Yarom J, Carel R.The prevalence of hypertension, ischaemic heart disease and diabetes in traumatic spinal cord injured patients and amputees Paraplegia. 198927 (1) 58–62. [DOI] [PubMed] [Google Scholar]
- Cardus D, Ribas-Cardus F, McTaggart WG.Coronary risk in spinal cord injury: assessment following a multivariate approach Arch Phys Med Rehabil. 199273 (10) 930–933. [PubMed] [Google Scholar]
- Bauman WA, Spungen AM, Raza M, Rothstein J, Zhang R, Zhang V.Coronary artery disease: metabolic risk factors and latent disease in individuals with paraplegia Mt Sinai J Med. 199259 (2) 163–168. [PubMed] [Google Scholar]
- Bauman WA, Raza M, Spungen AM, Machac J.Cardiac stress testing with thallium-201 imaging reveals silent ischemia in individuals with paraplegia Arch Phys Med Rehabil. 199475 (9) 946–950. [PubMed] [Google Scholar]
- Bauman WA, Raza M, Chayes Z, Machac J.Tomographic thallium-201 myocardial perfusion imaging after intravenous dipyridamole in asymptomatic subjects with quadriplegia Arch Phys Med Rehabil. 199374 (7) 740–744. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Kahn NN, Grimm DR, Spungen AM.Risk factors for atherogenesis and cardiovascular autonomic function in persons with spinal cord injury Spinal Cord. 199937 (9) 601–616. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM. Metabolic changes in persons after spinal cord injury. Phys Med Rehab Clin North Am. 2000;11:102–140. [PubMed] [Google Scholar]
- Bauman WA, Spungen AM, Adkins RH, Kemp BJ.Metabolic and endocrine changes in persons aging with spinal cord injury Assist Technol. 199911 (2) 88–96. [DOI] [PubMed] [Google Scholar]
- Moussavi RM, Ribas-Cardus F, Rintala DH, Rodriguez GP.Dietary and serum lipids in individuals with spinal cord injury living in the community J Rehabil Res Dev. 200138 (2) 225–233. [PubMed] [Google Scholar]
- Demirel S, Demirel G, Tukek T, Erk O, Yilmaz H.Risk factors for coronary heart disease in patients with spinal cord injury in Turkey Spinal Cord. 200139 (3) 134–138. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Adkins RH, Spungen AM, et al. Is immobilization associated with an abnormal lipoprotein profile? Observations from a diverse cohort Spinal Cord. 199937 (7) 485–493. [DOI] [PubMed] [Google Scholar]
- Dallmaijer AJ, van der Woude LH, van Kamp GJ, Hollander AP.Changes in lipid, lipoprotein and apolipoprotein profiles in persons with spinal cord injuries during the first 2 years post-injury Spinal Cord. 199937 (2) 96–102. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM.Carbohydrate and lipid metabolism in chronic spinal cord injury J Spinal Cord Med. 200124 (4) 266–277. [DOI] [PubMed] [Google Scholar]
- Nash MS, DeGroot J, Martinez-Arizala A, Mendez AJ.Evidence for an exaggerated postprandial lipemia in chronic paraplegia J Spinal Cord Med. 200528 (4) 320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MY, Myers J, Abella J, Froelicher VF, Perkash I, Kiratli BJ.Homocysteine and hypertension in persons with spinal cord injury Spinal Cord. 200644 (8) 474–479. [DOI] [PubMed] [Google Scholar]
- Lee MY, Myers J, Hayes A, et al. C-reactive protein, metabolic syndrome, and insulin resistance in individuals with spinal cord injury J Spinal Cord Med. 200528 (1) 20–25. [DOI] [PubMed] [Google Scholar]
- Frost F, Roach MJ, Kushner I, Schreiber P.Inflammatory C-reactive protein and cytokine levels in asymptomatic people with chronic spinal cord injury Arch Phys Med Rehabil. 200586 (2) 312–317. [DOI] [PubMed] [Google Scholar]
- Manns PJ, McCubbin JA, Williams DP.Fitness, inflammation, and the metabolic syndrome in men with paraplegia Arch Phys Med Rehabil. 200586 (6) 1176–1181. [DOI] [PubMed] [Google Scholar]
- Washburn RA, Fiboni SF.High-density lipoprotein cholesterol in individuals with spinal cord injury: the potential role of physical activity Spinal Cord. 199937 (10) 685–695. [DOI] [PubMed] [Google Scholar]
- Nash MS, Jacobs PL, Mendez AJ, Goldberg RB.Circuit resistance training improves the atherogenic lipid profiles of persons with chronic paraplegia J Spinal Cord Med. 200124 (1) 2–9. [DOI] [PubMed] [Google Scholar]
- Laven GT, Huang CT, DeVivo MJ, Stover SL, Kuhlemeier KV, Fine PR.Nutritional status during the acute state of spinal cord injury Arch Phys Med Rehabil. 198970 (4) 277–281. [PubMed] [Google Scholar]
- Huang CT, DeVivo MJ, Stover SL. Anemia in acute phase of spinal cord injury. Arch Phys Med Rehabil. 1990;71:3–7. [PubMed] [Google Scholar]
- Moussavi RM, Garza HM, Eisele SG, Rodriquez G, Rintala DH.Serum levels of vitamins A, C, and E in persons with chronic spinal cord injury living in the community Arch Phys Med Rehabil. 200384 (7) 1061–1067. [DOI] [PubMed] [Google Scholar]
- Tomey KM, Chen DM, Wang X, Braunschweig CL.Dietary intake and nutritional status of urban community-dwelling men with paraplegia Arch Phys Med Rehabil. 200586 (4) 664–671. [DOI] [PubMed] [Google Scholar]
- Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. Ann Rev Nutr. 2004;24:401–431. doi: 10.1146/annurev.nutr.23.011702.073349. [DOI] [PubMed] [Google Scholar]
- Levine AM, Nash MS, Green BA, Shea JD, Aronica MJ.An examination of dietary intakes and nutritional status of chronic health spinal cord injured individuals Paraplegia. 199230 (12) 880–889. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM.Prevalence of overweight and obesity in the United States, 1999–2004 JAMA. 2006295 (13) 1549–1555. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services (DHHS). National Center for Health Statistics. Third National Health and Nutrition Examination Survey, 1988\–1994, NHANES III Dietary Record. Hyattsville, MD: Centers for Disease Control and Prevention; 2004. [Google Scholar]
- Bauman WA, Spungen AM, Wang J, Pierson RN.The relationship between energy expenditure and lean tissue in monozygotic twins discordant for spinal cord injury J Rehabil Res Dev. 200441 (1) 1–8. [DOI] [PubMed] [Google Scholar]
- Buchholz AC, McGillivray CF, Pencharz PB.Physical activity levels are low in free-living adults with chronic paraplegia Obes Res. 200311 (4) 563–570. [DOI] [PubMed] [Google Scholar]
- Zurlo F, Larson K, Bogardus C Ravussin.Skeletal muscle metabolism is a major determinant of resting energy expenditure J Clin Invest. 199086 (5) 1423–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. About BMI for adults. Available at: http://www.cdc.gov/nccdphp/dnpa/BMI/adult_BMI/about_adult_BMI.htm. Accessed April 17, 2008.
- Ode JJ, Pivarnik JM, Reeves MJ, Knous JL.Body mass index as a predictor of percent fat in college athletes and nonathletes Med Sci Sports Exerc. 200739 (3) 403–409. [DOI] [PubMed] [Google Scholar]
- Dudeja V, Misra A, Pandey M, Devina G, Kumar G, Vikram NK.BMI does not accurately predict overweight in Asian Indians in northern India Br J Nutr. 200186 (1) 105–112. [DOI] [PubMed] [Google Scholar]
- Nevill AM, Stewart AD, Olds T, Holder R.Relationship between adiposity and body size reveals limitations of BMI Am J Phys Anthropol. 2006129 (1) 151–156. [DOI] [PubMed] [Google Scholar]
- Weaver FM, Collins EG, Kurichi J, et al. Prevalence of obesity and high blood pressure in veterans with spinal cord injuries and disorders: a retrospective review Am J Phys Med Rehabil. 200786 (1) 22–29. [DOI] [PubMed] [Google Scholar]
- Gupta N, White KT, Sandford PR. Body mass index in spinal cord injury—a retrospective study. Spinal Cord. 2006;44:92–94. doi: 10.1038/sj.sc.3101790. [DOI] [PubMed] [Google Scholar]
- Garshick E, Kelley A, Cohen SA, et al. A prospective assessment of mortality in chronic spinal cord injury Spinal Cord. 200543 (7) 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MV, Diab ME, Chu BC, Kirshblum S.Preventive services and health behaviors among people with spinal cord injury J Spinal Cord Med. 200528 (1) 43–54. [DOI] [PubMed] [Google Scholar]
- Zhong YG, Levy E, Bauman WA.The relationships among serum uric acid, plasma insulin, and serum lipoprotein levels in subjects with spinal cord injury Horm Metab Res. 199527 (6) 283–286. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Adkins RH, Spungen AM, Waters RL.The effect of residual neurological deficit on oral glucose tolerance in persons with chronic spinal cord injury Spinal Cord. 199937 (11) 765–771. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM, Zhong YG, Rothstein JL, Petry C, Gordon SK.Depressed serum high density lipoprotein cholesterol levels in veterans with spinal cord injury Paraplegia. 199230 (10) 697–703. [DOI] [PubMed] [Google Scholar]
- Spungen AM, Adkins RH, Stewart CA, et al. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study J Appl Physiol. 200395 (6) 2398–2407. [DOI] [PubMed] [Google Scholar]
- Wilt TJ, Carlson FK, Goldish GD, et al. Carbohydrate & Lipid Disorders & Relevant Considerations in Persons with Spinal Cord Injury. Evidence Report/Technology Assessment No. 163. Rockville, MD: Agency for Healthcare Research and Quality; 2008. [PMC free article] [PubMed] [Google Scholar]
- Gater DR.Obesity after spinal cord injury Phys Med Rehabil Clin North Am. 200718 (2) 333–351. [DOI] [PubMed] [Google Scholar]