Abstract
We have generated RANK (receptor activator of NF-κB) nullizygous mice to determine the molecular genetic interactions between osteoprotegerin, osteoprotegerin ligand, and RANK during bone resorption and remodeling processes. RANK−/− mice lack osteoclasts and have a profound defect in bone resorption and remodeling and in the development of the cartilaginous growth plates of endochondral bone. The osteopetrosis observed in these mice can be reversed by transplantation of bone marrow from rag1−/− (recombinase activating gene 1) mice, indicating that RANK−/− mice have an intrinsic defect in osteoclast function. Calciotropic hormones and proresorptive cytokines that are known to induce bone resorption in mice and human were administered to RANK−/− mice without inducing hypercalcemia, although tumor necrosis factor α treatment leads to the rare appearance of osteoclast-like cells near the site of injection. Osteoclastogenesis can be initiated in RANK−/− mice by transfer of the RANK cDNA back into hematopoietic precursors, suggesting a means to critically evaluate RANK structural features required for bone resorption. Together these data indicate that RANK is the intrinsic cell surface determinant that mediates osteoprotegerin ligand effects on bone resorption and remodeling as well as the physiological and pathological effects of calciotropic hormones and proresorptive cytokines.
Bone remodeling and homeostasis is an essential function that regulates skeletal integrity throughout adult life in higher vertebrates and mammals. The maintenance of skeletal mass is controlled by the activities of specialized cells within the bone that have seemingly antagonistic activities: bone synthesis and bone resorption. Osteoblastic cells of mesenchymal origin synthesize and deposit bone matrix and increase bone mass. Osteoclastic cells are large, multinucleated phagocytes of hematopoietic origin that resorb both mature and newly synthesized bone upon activation. Bone synthesis and resorption processes are highly coordinated and are regulated by osteotropic and calciotropic hormones during physiological and pathological conditions (1, 2). Increased bone resorption and turnover mediated by activated osteoclasts is known to occur in various crippling diseases, such as osteoporosis and arthritis, and can lead to pathological decreases in bone mass and skeletal integrity.
Recently, two critical extracellular regulators of osteoclast differentiation and activation have been identified: osteoprotegerin (OPG) (3) and OPG ligand (OPGL) (4). OPGL is a tumor necrosis factor (TNF)-related cytokine that stimulates osteoclast differentiation from hematopoietic precursor cells and activation of mature osteoclasts in vitro and in vivo. Mice lacking OPGL also lack osteoclasts and have defects in bone remodeling processes that leads to severe osteopetrosis (5). OPG is a secreted TNF receptor (TNFR)-related protein that binds to and neutralizes OPGL bioactivity. Transgenic mice that overexpress OPG also have defects in osteoclastogenesis similar to OPGL−/− mice. In contrast, mice lacking OPG develop severe, early-onset osteoporosis and have brittle bones that spontaneously fracture (6). These findings indicate that both OPG and OPGL interact together as the key positive and negative regulators of osteoclastogenesis and bone resorption, and that modulation of their activities also modulates bone density.
OPGL, also known as ODF (7), RANKL (8), and TRANCE (9), has been shown to bind to a cell-associated TNFR-related protein known as RANK (receptor activator of NF-κB) (8, 10, 11). RANK is present on osteoclast precursors and is capable of initiating osteoclastogenic signal transduction after ligation with OPGL or anti-RANK agonist antibodies (10, 11). These data all indicate that RANK is an osteoclast receptor capable of mediating OPGL function during normal bone homeostasis and in disease. Although no other receptors for OPGL have been identified, it is not clear whether RANK is the sole receptor for OPGL/RANKL, or whether other TNFR-related proteins could compensate, particularly during disease processes. Dougall et al. (12) recently have generated RANK−/− mice and have determined an essential role in regulating bone mass and lymph node organogensis. We also have generated and analyzed RANK knockout mice to assess molecular genetic interactions between OPG, OPGL, and RANK. From this study we find that RANK serves as the sole osteoclast receptor for OPGL and that this interaction controls osteoclast development and activation and indirectly bone mass and calcium metabolism.
Materials and Methods
Generation of RANK−/− Mice.
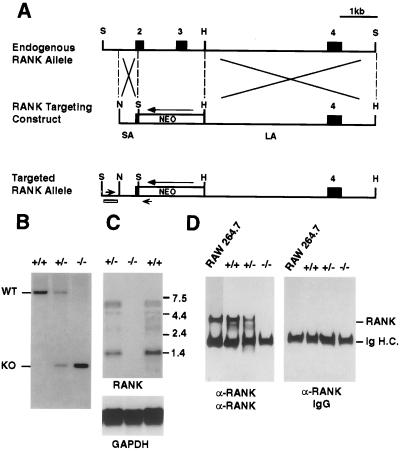
Murine RANK genomic clones were obtained from a 129 SVJ mouse bacterial artificial chromosome genomic library (Genome Systems, St. Louis) by using a probe corresponding to the 5′ end of murine RANK cDNA. Targeting vector was constructed with a 0.7-kb short arm and 5.0-kb long arm of homology flanking a PGK-neo cassette, introduced into RW4 embryonic stem (ES) cells (Genome Systems), and subjected to G418 selection (200 μg/ml). Recombinant ES clones with a targeted RANK allele were detected by PCR and Southern blotting and were injected into fertilized blastocysts from C57BL/6 female mice. Homozygous RANK knockout mice (RANK−/−) were identified and distinguished from heterozygotes (RANK+/−) and wild-type (RANK+/+) mice by Southern blot analysis of SpeI-digested genomic DNA hybridized to a 5′ flanking probe, and RANK mRNA and protein expression was confirmed by Northern and Western blotting as described (4).
Osteoclast-Forming Assay.
In vitro osteoclast-forming assays were performed as described (4). A total of 1 × 106 purified nonadherent cells were cultured with various concentrations (0.16–500 ng/ml) of OPGL [158–316] in the presence of colony-stimulating factor 1 (CSF-1) (30 ng/ml) for 4–5 days or with 1 × 105 ST2 bone marrow stromal cells in media supplemented with 10 nM 1α,25-dihydroxy vitamin D3 [1α,25-(OH)2D3], 100 nM dexamethasone, and 250 nM prostaglandin E2. Cocultures were incubated for 7–10 days, and new media containing fresh supplements were added every 3–4 days.
Adoptive Bone Marrow Transfer.
To generate bone marrow chimeras, we isolated bone marrow cells from 8-week-old rag1−/− (recombinase activating gene 1) mice and transplanted 1 × 107 bone marrow cells by tail vein injection into 5-week-old γ-irradiated (8.0 Gy, Cs137, single dose) RANK−/− and RANK+/+ host mice. Bone marrow chimeras were examined 4 weeks after transplantation for bone histology and in vitro osteoclastogenesis.
Retroviral Reconstitution of RANK−/− Mice.
Full-length murine RANK cDNA was cloned into a retroviral expression vector murine stem cell virus (MSCV) 2.1. Retrovirus was transiently produced by transfecting Bosc 23 cell line with pRANK-MSCV or pMSCV. Spleen cells were harvested from 5- to 7-week-old RANK−/− mice and preincubated in 10% FBS-Iscove's Modified Dulbecco's Media (IMDM) containing 5 ng/ml rmIL-3, 100 ng/ml recombinant rat stem cell factor, 10 ng/ml recombinant human megakaryocyte growth and differentiation factor, and 100 ng/ml rhIL-6 for approximately 36–40 hr before viral infection. At the end of preincubation, 1.5 × 107 preincubated spleen cells were seeded into a 100-mm retronectin-coated Petri dish (3 μg/cm2) containing 13.5 ml of viral supernatant and 1.5 ml of growth factors as described (13). At the end of infection, spleen cells were harvested and plated (1–5 × 105/ml) in 10% FBS-IMDM, 30 ng/ml rCSF-1 (R & D Systems), and 100 ng/ml murine OPGL. Osteoclast function was measured by using a resorption pit forming assay as described (4). For in vivo reconstitution, 10-week-old RANK−/− mice were used as donors and recipients. Retrovirally transduced RANK−/− spleen cells were harvested and transplanted into γ-irradiated (8.0 Gy, Cs137, single dose) RANK−/− mice. Each recipient received 1.0 × 106 viral-infected cells.
Calciotropic Hormone and Cytokine Challenge.
Age- and sex-matched RANK−/− and RANK+/+ mice (n = 5) were injected daily for 4.5 days. Calvarial and femur bone morphology was analyzed 3 hr after the final injection. IL-1β (5 μg/day), TNF-α (1.0 mg/kg body weight per day), parathyroid hormone-related protein (PTHrP) (Bachem) (20 μg/day), or vehicle alone (PBS) were injected s.c. as reported (14) over the left side of the calvaria twice daily in a volume of 10 μl. Additional RANK−/− and RANK+/+ mice were injected once daily with either OPGL (15 mg/kg) or vehicle alone (PBS), s.c. on the flank, or 1α,25-(OH)2D3 (0.025 mg/kg) (Sigma) or vehicle alone (corn oil) s.c. over the nape of the neck. Whole blood ionized calcium levels were determined as described (15) at 3 hr posttreatment (day 0) and on days 3 and 5 to determine the level of hypercalcemia. On day 5, after final ionized calcium levels were determined, the calvariae and femurs were removed and processed for tartrate-resistant acid phosphatase (TRAP) staining and cathepsin-K fluorescent immuno-histochemistry as described (15).
Results
Profound Osteopetrosis in RANK-Deficient Mice.
Mice that lack a functional RANK protein were generated by targeted disruption of the RANK genomic locus in ES cells. Homologous recombination of the targeting vector resulted in the loss of exons encoding the first 1½ N-terminal TNFR-like cysteine-rich domains (amino acids 34–96) (Fig. 1A). Two independently derived heterozygous RANK mutant ES clones were microinjected into C57BL/6 blastocysts, and the resulting male chimeras were bred to Black Swiss females to generate heterozygous RANK+/− mice. These heterozygous mice, which have no obvious abnormalities, then were intercrossed to generate homologous RANK−/− mice (Fig. 1B). Both mutant ES cell lines achieved germ-line transmission, and mice derived from either line were indistinguishable in all subsequent experiments. The null mutation of RANK was demonstrated by the absence of RANK mRNA expression in Northern blot analysis, using cDNA probe encompassing the deleted exons of RANK (Fig. 1C). Western blot analysis of immunoprecipitates obtained from intestinal lysates using polyclonal antibody against extracellular domain of RANK indicates a complete absence of the RANK polypeptide in the RANK−/− mice (Fig. 1D).
Figure 1.
Gene targeting at the RANK locus. (A) Restriction maps of part of RANK locus being targeted (Top) and the targeting construct (Middle). In the RANK targeting construct, the PGK-neo cassette was placed in reverse orientation to replace coding exons 2 and 3A. Exons are shown as boxes. (Bottom) The small open box and arrowheads represent the 5′ flanking probe and PCR primers used for genotyping. SA, short arm; LA, long arm. Restriction sites: S, SpeI; H, HincII; N, NotI. (B) Southern blot of SpeI-digested genomic DNA using the 5′ flanking probe as indicated in A. Positions of wild-type allele (WT, 8 kb) and targeted allele (KO, 1 kb) also are indicated. (C) Northern blot of intestine poly(A)+ RNA using cDNA probe encompassing the deleted RANK exons. Positions of the RNA size markers are indicated, and the same blot was reprobed with internal control glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA (Bottom). (D) Immunoprecipitation and Western blot analysis of RANK protein. RANK was immunoprecipitated from total intestine lysate by using polyclonal antibody raised against the extracellular region of RANK protein. Cell lysate from 1 × 107 RAW264.7 cells also was immunoprecipitated as positive control.
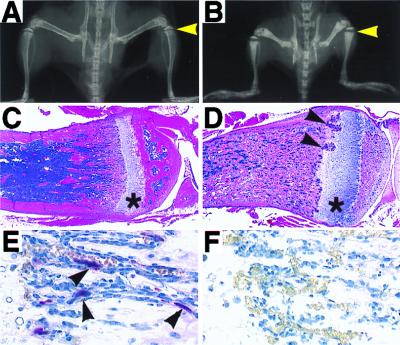
RANK−/− mice were born alive and at the expected Mendelian frequency. However, compared with control littermates, RANK−/− mice are clearly runted upon weaning at 3 weeks of age and lacked teeth, a similar situation to that observed in OPGL−/− mice (5). The incisors and molars of all of the RANK−/− mice fail to erupt during development (data not shown), a typical finding in rodent osteopetrosis models. Radiographic analysis of RANK−/− mice reveals profound osteopetrosis in these animals (Fig. 2 A and B). The long bones, vertebrae, and cranium appear dense radiologically, and radiolucent marrow spaces normally observed in control mice are absent in RANK−/− mice. The long bones are shortened and club shaped and have a broader epiphysis and narrower diaphysis (Fig. 2B). Osteopetrosis is also evident in the axial skeleton, such as in the vertebral bodies (data not shown).
Figure 2.
Severe osteopetrosis in RANK−/− mice. (A and B) Radiographs of the leg, pelvis, and vertebrae regions of RANK+/+ (A) and RANK−/− (B) littermates. Arrowheads indicate the growth plate area. (C and D) Hematoxylin/eosin staining of tibia from RANK+/+ (C) and RANK−/− (D) littermates demonstrates severe osteopetrosis in RANK knockout mice. Note in RANK−/− mice the significantly widened growth plate (*) and the highly vascularized invasive growth (arrowheads) within the growth plate. (E and F) TRAP staining of bone sections from RANK+/+ (E) and RANK−/− (F) littermates shows the absence of TRAP+ osteoclasts (arrowheads in E) in RANK−/− mice. (Original magnifications: A–D, ×4; E and F, ×60.)
Histological analysis of bone sections confirmed the severe osteopetrotic phenotype in RANK−/− mice. The marrow spaces of long bones from RANK−/− mice were filled with solid matrix composed of cartilage encased in mineralized bone matrix (Fig. 2D). Examination of osteoclasts by TRAP staining of bone sections from RANK−/− mice revealed a total absence of TRAP-positive multinucleated osteoclasts in any of the bone sections studied (Fig. 2F), in sharp contrast to what is observed in control littermate mice (Fig. 2E). These results suggest that the severe osteopetrotic phenotype in RANK−/− mice is the result of a defect in bone resorption caused by the absence of osteoclasts. Recently, Dougall et al. (12) also have generated mice deficient of RANK and reported similar osteopetrotic phenotype caused by the lack of osteoclast formation. In addition, they show that RANK−/− mice have a developmental defect in lymph node and B cell formation, as seen in OPGL−/− mice (5). Analysis of these RANK−/− mice (data not shown) confirms this earlier finding (12).
Alteration of Cartilaginous Growth Plates in RANK−/− Endochondral Bone.
In RANK−/− mice, the proximal tibial cartilaginous growth plates appear significantly wider than the corresponding control region (Fig. 2 A–D), as are the growth plate regions of other long bones (data not shown). Histologically, the general columnar structure of the hypertrophic cartilage in the growth plates of RANK−/− mice is well preserved with only an occasional disorganized column (Fig. 2D and data not shown). However, the widened hypertrophic chondrocyte layer in most knockout mice is focally altered by the invasions of highly vascularized proliferating tissue (arrowheads in Fig. 2D). The identity of these proliferating cells in the invading foci currently is unknown.
Analysis of blood serum chemistry data obtained from RANK−/− mice shows that they develop hypocalcemia [8.4 ± 0.18 mg/dL (RANK−/−, n = 14) vs. 9.6 ± 0.13 mg/dL (RANK+/+, n = 14)] and hypophosphatemia (data not shown). Serum parathyroid hormone (PTH) levels in the RANK−/− mice are significantly elevated over wild-type mice [118.8 ± 26.7 pg/ml (RANK−/−, n = 14) vs. 39.5 + 3.1 pg/ml (RANK+/+, n = 14)] with an apparent inverse relationship between serum calcium and PTH levels.
Intrinsic Defect of Osteoclast Formation in RANK−/− Mice.
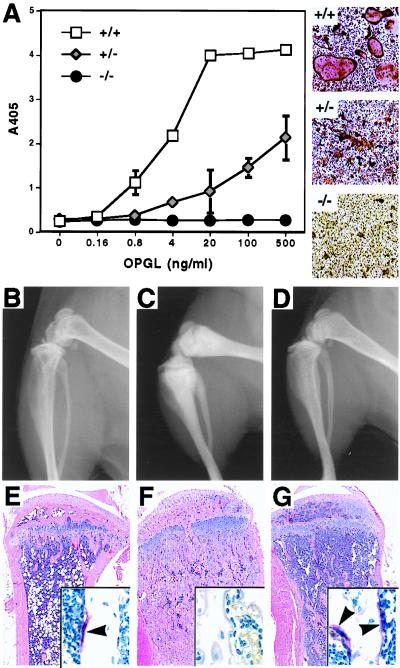
To investigate the cellular mechanism underlying the failure of osteoclast formation in the RANK−/− mice, we performed an in vitro osteoclast differentiation assay. Hematopoietic progenitor cells were isolated from spleen and cultured with CSF-1 and various concentrations of recombinant OPGL for 4–5 days. In RANK+/+ mice, splenic progenitors can differentiate into numerous mature, TRAP-positive, multinucleated osteoclasts in a dose-dependent manner when exposed to varying concentrations of OPGL (Fig. 3A). In contrast, no TRAP+ cells can be detected by using RANK−/− splenic progenitors, even with high concentrations of OPGL. This finding also is confirmed in coculture assay of splenic progenitors and ST2 stromal cells in the presence of dexamethasone, 1α,25-(OH)2D3, and prostaglandin E2 (data not shown). These results indicate that there is an intrinsic defect in osteoclast progenitors in RANK−/− mice, and osteoclast development is arrested at a stage before the acquisition of TRAP expression.
Figure 3.
RANK−/− mice have an intrinsic defect in osteoclast development. (A) Osteoclast differentiation from RANK+/+, RANK+/−, and RANK−/− splenic hematopoietic progenitors in the presence of CSF-1 (30 ng/ml) and various concentrations of recombinant murine OPGL as measured by TRAP solution assay. (Right) Representative photographs (×20) of TRAP-stained culture treated with CSF-1 (30 ng/ml) and OPGL (500 ng/ml). (B, C, and D) Radiographs of femur and tibia/fibula 4 weeks after bone marrow adoptive transfer from rag1−/− mice. Note the massive decrease of bone radiodensity in rag1−/−RANK−/− mice (D) compared to nontransferred RANK−/− littermate (C), to a level similar to that of rag1−/−RANK+/+ mice (B). (E–G) Hematoxylin/eosin and TRAP (Insets) staining of tibia 4 weeks after bone marrow adoptive transfer from rag1−/− mice. Overall bone morphology of rag1−/−RANK−/− (H) mice is much closer to that of the rag1−/−RANK+/+ littermate (F) than to the nontransplanted RANK−/− littermate (G). Note the obvious increase of marrow space and the appearance of many TRAP+ multinucleated osteoclasts (arrowheads). (Original magnifications: ×4; Insets, ×60.)
To confirm the intrinsic defect of osteoclast precursors in RANK−/− mice, we have used adoptive transfer of bone marrow cells in an attempt to reconstitute bone resorption in RANK−/− mice. Bone marrow cells isolated from rag1−/− mice, which have normal osteoclast precursors, were transplanted into irradiated young adult RANK+/+ and RANK−/− mice. Four weeks after the transplantation, radiographs of rag1−/−RANK−/− chimera showed a marked reduction in bone density throughout the skeleton compared with nontransplanted RANK−/− mice, and the tibia and femur of rag1−/−RANK−/− chimeras have become less club shaped (Fig. 3 D and C). Histological analysis of femur and tibia sections of rag1−/−RANK−/− chimeras indicates that bone resorption processes have re-engaged and the normal trabecular morphology seen in wild-type controls has been restored (Fig. 3 E–G). Furthermore, rag1−/−RANK−/− chimeras have large numbers of multinucleated TRAP+ osteoclasts attached to the surfaces of trabecular bone (Inset in Fig. 3G). In addition, the width of the growth plate in the rag1−/−RANK−/− chimeras also is reduced. This phenotypic reversal of osteopetrosis was accompanied by the appearance of functional osteoclast precursors in the spleens of rag1−/−RANK−/− chimera mice detected by using in vitro osteoclast-forming assays (data not shown). Thus, bone marrow cells from rag1−/− mice are sufficient for in vivo functional reconstitution of osteoclasts in the RANK−/− mice.
Reconstitution of Osteoclastogenesis by RANK Gene Transfer.
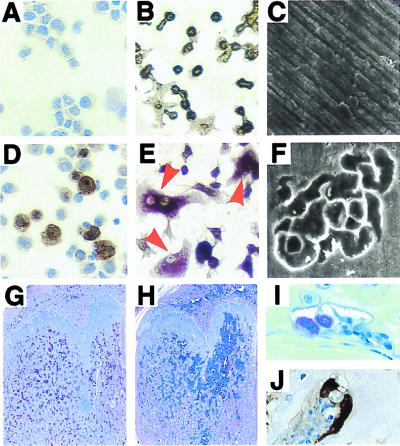
We have attempted to restore RANK function in RANK−/− splenic precursors in vitro by using a retroviral-mediated gene transfer strategy. Recombinant retrovirus carrying full-length RANK cDNA was produced transiently in Bosc 23 cells. Spleen cells from RANK−/− mice were infected with either MSCV-RANK retrovirus or the parental vector MSCV in vitro. After viral infection, cultures transduced with control and RANK vector resulted in 97 ± 3% (n = 3) and 93 ± 3% (n = 3) G418-resistant colony-forming cells, respectively. Immunostaining of the harvested cells with anti-RANK antibodies detects RANK protein surface expression only in MSCV-RANK-infected cells (Fig. 4D). When infected spleen cells were cultured with OPGL and CSF-1, multinucleated TRAP-positive osteoclasts were obtained again only in MSCV-RANK infected cells (Fig. 4E). In cultures where osteoclasts are formed on bone slices, osteoclast activity occurred only in RANK-transduced cultures grown in the presence of CSF-1 and OPGL, as demonstrated by the formation of characteristic resorption pits (Fig. 4F). These results demonstrate that RANK expression alone ensures the osteoclastogenic potential of hematopoietic progenitors in vitro. We subsequently transplanted the retrovirus-infected RANK−/− spleen cells back into other osteopetrotic RANK−/− mice and were able to induce bone resorption in vivo. Six weeks after transplantation, RANK−/− mice who received MSCV-RANK-infected spleen cells had significantly reduced trabecular bone mass, particularly in the regions below the growth plates (Fig. 4 G and H). Reconstitution of bone resorption was demonstrated by the development of TRAP+ (Fig. 4I) and cathepsin K+ (Fig. 4J) osteoclasts lining the newly formed marrow spaces.
Figure 4.
Retroviral reconstitution of osteoclastogenic potential in RANK−/− mice. RANK−/− splenic hematopoietic cells were transduced with control vector MSCV (A–C), or MSCV-RANK retroviruses (D–F). After viral infection, cytocentrifuge preparations of cells transduced with MSCV (A) or MSCV-RANK retrovirus (D) were stained with anti-RANK antibodies. Virus-transduced hematopoietic cells were cultured for an additional 7 days in the presence of CSF-1 and OPGL. Multinucleated TRAP+ osteoclasts are demonstrated only in cultures transduced with MSCV-RANK retrovirus (E), not in cultures transduced with MSCV (B). Scanning electron microscopic images of bone slices exposed to MSCV- (C) or MSCV-RANK- (F) transduced hematopoietic cells show resorption lacunae only in MSCV-RANK-transduced cultures (F). Hematoxylin/eosin staining of femurs from RANK−/− mice 6 weeks after they received transplantation of RANK−/− splenic hematopoietic cells transduced with either MSCV (G) or MSCV-RANK (H) retroviruses. Note the significantly increased marrow space in the metaphysis region of H. Osteoclasts that are positively stained with TRAP (red in I) or cathepsin K (brown in J) can be detected only in adjacent sections of H. (Original magnifications: A, B, D, and E, ×40; G and H, ×4; C and F, ×350, I and J, ×60.)
RANK Mediates Proresorptive Function of Calciotropic Hormones and Cytokines.
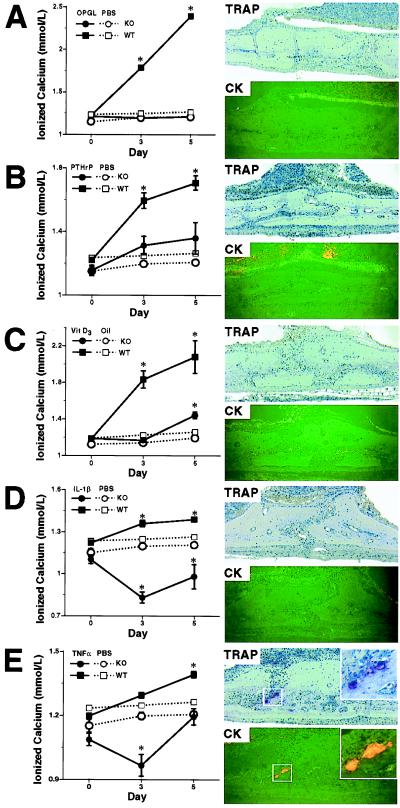
To address the question of whether other receptors can mediate the osteoclastogenic effects of OPGL in the absence of RANK, RANK−/− and control mice were challenged with recombinant OPGL. OPGL treatment of control mice leads to significant increases in whole blood-ionized calcium levels compared with PBS-treated mice at both days 3 and 5 of treatment (Fig. 5A) and a marked increase in the numbers of TRAP+ and cathepsin K+ osteoclasts within the calvaria and femur (data not shown). In contrast, RANK−/− mice are completely resistant to the hypercalcemic effects of OPGL (Fig. 5A), and there were no TRAP+ and cathepsin K+ osteoclasts detectable in either calvaria or femur. These data therefore confirm that RANK is the sole osteoclast receptor for OPGL in vivo.
Figure 5.
Effects of calciotropic hormones and cytokines challenge in RANK−/− mice. WT, wild type; KO, knockout. RANK+/+ and RANK−/− mice were challenged with (A) murine OPGL, (B) human PTHrP, (C) 1α,25-(OH)2D3, (D) human IL-1β, and (E) murine TNF-α, using PBS (A, B, D, and E) or corn oil (C) as control. (Left) Whole blood ionized calcium levels are shown. (Right) TRAP staining and cathepsin K fluorescent immuno-histochemistry on sections of calvaria from challenged RANK−/− mice are shown. Note the complete absence of any TRAP and cathepsin K-positive osteoclast in OPGL (A), PTHrP (B), 1α,25-(OH)2D3 (C), or IL-1β (D) challenged RANK−/− mice, and the appearance of TRAP and cathepsin K-positive osteoclasts (Inset in E) in TNF-α-challenged RANK−/− mice. *, Statistically different from control group, P < 0.001 (group n = 5, mean ± SEM). (Original magnification: ×10; Inset in E, ×20.)
To determine whether RANK is the osteoclast receptor that mediates the proresorptive function of calciotropic factors, we have challenged RANK−/− and control mice with PTHrP, 1α,25-(OH)2D3, IL-1β, and TNF-α. As expected, administration of each of these agents to normal mice leads to significant, time-dependent elevation in blood-ionized calcium levels (Fig. 5 B–E) and bone resorption (data not shown). In contrast, no bone resorption is induced in PTHrP-, 1α,25-(OH)2D3-, and IL-1β-treated RANK−/− mice, as shown by the complete absence of TRAP+ and cathepsin K+ osteoclasts in their calvaria (Fig. 5 B–D). The hypercalcemic response normally induced by these treatments also has mostly been suppressed. Therefore, the proresorptive activities of PTHrP, 1α,25-(OH)2D3, and IL-1β are indeed mediated exclusively through RANK. Surprisingly in the calvaria of TNF-α-treated RANK−/− mice, we find the rare appearance of TRAP+ and cathepsin K+ osteoclasts on bone surfaces (Fig. 5E). However, TNF-α treatment (1.0 mg/kg body wt per day) fails to induce hypercalcemia in RANK−/− mice (Fig. 5E), and osteoclast-like cells are found only within the calvaria near the site of administration. In addition, no radiographic signs of bone resorption are detected (data not shown). These results suggest that TNF-α may represent an alternative compensatory pathway for the local formation of TRAP- and cathepsin K-positive osteoclasts in RANK−/− mice.
Discussion
RANK knockout mice have severe osteopetrosis characterized by the accumulation of newly synthesized bone and a defect in bone resorption and remodeling. Hematopoiesis in these mice is unaffected with the exception that osteoclasts, which generally are believed to stem from monocyte/macrophage precursors, are absent throughout the skeleton. In addition, lymph node formation is disrupted in the RANK−/− mice (data not shown) (12), much like in OPGL−/− mice (5). In agreement with a recent report by Dougall et al. (12), we find that dendritic cell development and function in the RANK−/− mice is apparently normal (data not shown). Therefore, the principle nondevelopmental function of RANK in vivo is to regulate osteoclastogenesis, and we have focused on further defining its precise role in this current report.
Hematopoietic precursors isolated from RANK−/− mice are unable to form osteoclasts in vitro in the presence of OPGL and CSF-1, suggesting an intrinsic defect in the osteoclast progenitors. Furthermore, RANK−/− mice are also resistant to the effects of OPGL treatment in vivo, indicating that RANK is the sole receptor that mediates the biological activities of OPGL. Osteoclastogenesis and bone resorption can be reinitiated in these mice by transplanting hematopoietic precursors from rag1−/− mice, indicating that the osteoclast precursors lacking in RANK−/− mice are restricted to the erythro-myeloid lineage. As definitive proof that RANK is the key receptor that mediates osteoclast differentiation and activation, we have shown that retroviral delivery of the RANK cDNA into RANK−/− hematopoietic precursors can restore osteoclastogenesis. When RANK-infected hematopoietic cells are transferred back into RANK−/− mice, partial restoration of osteoclastogenesis and bone resorption is achieved. Together these data unequivocally identify RANK as the hematopoietic cell surface determinant that mediates OPGL-induced osteoclastogenesis.
Most, if not all, calciotropic hormones and proresorptive cytokines recently have been shown to up-regulate mRNA expression of OPGL in cell lines and primary cell cultures (16). OPG, which blocks osteoclastogenesis induced by OPGL in rodents, also can inhibit osteoclast formation and bone resorption induced by treatment with calciotropic factors (15), suggesting that the RANK signaling pathway is the ultimate common mediator of humoral signals that regulate bone resorption and calcium metabolism. We have tested this hypothesis by challenging RANK−/− and RANK+/+ mice with TNF-α, IL-1β, 1α,25-(OH)2D3, and PTHrP, which, excluding PTH, are the major calciotropic factors that are known to induce increases in bone resorption and serum hypercalcemia. PTHrP acts via the same receptor as PTH and so can be expected to have similar activity as PTHrP. The absence of RANK in these knockout mice prevents the hypercalcemic response normally induced by these factors. Interestingly, both TNF-α and IL-1β administration leads to transient hypocalcemia in RANK−/− mice, suggesting that they modulate other mechanisms of calcium homeostasis such as calcium absorption and/or excretion that normally are masked by effects of these cytokines on bone resorption. Surprisingly, challenge of RANK−/− mice with TNF-α (1.0 mg/kg body weight per day) leads to the rare occurrence of osteoclast formation near the site of injection, although no significant radiographic or histologic signs of bone resorption are detected. This finding suggests that TNF can trigger an alternative pathway leading to osteoclast formation in the RANK knockout mice, presumably by activation of either TNFR1 and/or TNFR2.
RANK−/− mice suffer from hypocalcemia and hypophosphatemia. We find that these mice also have hyperparathyroidism characterized by increased circulating PTH levels that are inversely proportional to serum calcium. The observed increases in PTH can be interpreted as a physiologic response to lowered serum calcium levels. RANK−/− mice also have gross alterations in the cartilaginous growth plates of endochondral bone, characterized by a widened hypertrophic cartilage zone. PTHrP is a related polypeptide to PTH that acts as a paracrine regulator of chondrocyte differentiation in the growth plate of endochondral bone (17, 18). It is known that PTHrP, the PTH/PTHrP receptor, and Indian hedgehog function together to form a negative feedback loop that down-regulates chondrocyte differentiation, and PTH is also capable of stimulating the PTHrP receptor on chondrocytes (17). Thus, hyperparathyroidism observed in these mice may contribute to the alterations in the growth plate physiology in RANK−/− mice by altering the normal chondrocyte development and survival. In support of this hypothesis, reconstitution of bone resorption by adoptive transfer of rag1−/− bone marrow restores calcium metabolism and also restores the normal appearance of growth plate cartilage.
RANK, like other TNFR-related proteins, is known to activate a cascade of intracellular signaling events, including interactions with various TNFR-associated factor (TRAF) adaptor molecules, activation of transcription factor NF-κB, stimulation of c-Jun N-terminal kinase (JNK) activity, and induction of gene expression programs (8, 10, 19–21). TRAF6 previously has been shown to interact with RANK during OPGL-induced signaling in osteoclast precursors in vitro (10). TRAF6-deficient mice develop osteopetrosis because of impaired function of mature osteoclast (22). However, mice deficient in other individual TRAF family members, such as TRAF2, TRAF3, and TRAF5, have not shown an essential role for these proteins during osteoclastogenesis (23–25). Mice deficient in both the p50 and p52 subunits of NF-κB develop severe osteopetrosis because of an intrinsic defect in osteoclast development (26, 27). In addition, mice deficient in c-fos, a transcription factor and component of AP-1, also have osteopetrosis because of a defect in osteoclast differentiation (28, 29). Treatment of osteoclast precursors with OPGL leads to a rapid induction in JNK activity and presumably the activation of AP-1-responsive gene expression (10). Although the precise events that are required for osteoclastogenesis still remain to be elucidated, the retroviral reconstitution system we have established by using RANK−/− mice should allow unambiguous resolution of the critical mechanism(s) that are downstream of RANK in vivo.
Abbreviations
- RANK
receptor activator of NF-κB
- OPG
osteoprotegerin
- OPGL
OPG ligand
- CSF
colony-stimulating factor
- TRAP
tartrate-resistant acid phosphatase
- rag1
recombinase activating gene 1
- TNF
tumor necrosis factor
- TNFR
TNF receptor
- TRAF
TNFR-associated factor
- PTH
parathyroid hormone
- PTHrP
parathyroid hormone-related protein
- 1α,25-(OH)2D3
1α,25-dihydroxy vitamin D3
- ES
embryonic stem
- MSCV
murine stem cell virus
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Rodan G A, Martin T J. Calcif Tissue Int. 1981;33:349–351. doi: 10.1007/BF02409454. [DOI] [PubMed] [Google Scholar]
- 2.Suda T, Takahashi N, Martin T J. Endocr Rev. 1992;13:66–80. doi: 10.1210/edrv-13-1-66. [DOI] [PubMed] [Google Scholar]
- 3.Simonet W S, Lacey D L, Dunstan C R, Kelley M, Chang M S, Luthy R, Nguyen H Q, Wooden S, Bennett L, Boone T, et al. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 4.Lacey D L, Timms E, Tan H L, Kelley M J, Dunstan C R, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 5.Kong Y Y, Yoshida H, Sarosi I, Tan H L, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos A J, Van G, Itie A, et al. Nature (London) 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 6.Bucay N, Sarosi I, Dunstan C R, Morony S, Tarpley J, Capparelli C, Scully S, Tan H L, Xu W, Lacey D L, et al. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, et al. Proc Natl Acad Sci USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson D M, Maraskovsky E, Billingsley W L, Dougall W C, Tometsko M E, Roux E R, Teepe M C, DuBose R F, Cosman D, Galibert L. Nature (London) 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 9.Wong B R, Rho J, Arron J, Robinson E, Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett F S, 3rd, Frankel W N, et al. J Biol Chem. 1997;272:25190–25194. doi: 10.1074/jbc.272.40.25190. [DOI] [PubMed] [Google Scholar]
- 10.Hsu H, Lacey D L, Dunstan C R, Solovyev I, Colombero A, Timms E, Tan H L, Elliott G, Kelley M J, Sarosi I, et al. Proc Natl Acad Sci USA. 1999;96:3540–3545. doi: 10.1073/pnas.96.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Yano K, Morinaga T, Higashio K. Biochem Biophys Res Commun. 1998;253:395–400. doi: 10.1006/bbrc.1998.9788. [DOI] [PubMed] [Google Scholar]
- 12.Dougall W C, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko M E, Maliszewski C R, et al. Genes Dev. 1999;13:2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanenberg H, Xiao X L, Dilloo D, Hashino K, Kato I, Williams D A. Nat Med. 1996;2:876–882. doi: 10.1038/nm0896-876. [DOI] [PubMed] [Google Scholar]
- 14.Boyce B F, Aufdemorte T B, Garrett I R, Yates A J, Mundy G R. Endocrinology. 1989;125:1142–1150. doi: 10.1210/endo-125-3-1142. [DOI] [PubMed] [Google Scholar]
- 15.Morony S, Capparelli C, Lee R, Shimamoto G, Boone T, Lacey D L, Dunstan C R. J Bone Miner Res. 1999;14:1478–1485. doi: 10.1359/jbmr.1999.14.9.1478. [DOI] [PubMed] [Google Scholar]
- 16.Hofbauer L C, Khosla S, Dunstan C R, Lacey D L, Boyle W J, Riggs B L. J Bone Miner Res. 2000;15:2–12. doi: 10.1359/jbmr.2000.15.1.2. [DOI] [PubMed] [Google Scholar]
- 17.Kronenberg H M, Lanske B, Kovacs C S, Chung U I, Lee K, Segre G V, Schipani E, Juppner H. Recent Prog Horm Res. 1998;53:283–301. [PubMed] [Google Scholar]
- 18.Chung U I, Lanske B, Lee K, Li E, Kronenberg H. Proc Natl Acad Sci USA. 1998;95:13030–13035. doi: 10.1073/pnas.95.22.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darnay B G, Ni J, Moore P A, Aggarwal B B. J Biol Chem. 1999;274:7724–7731. doi: 10.1074/jbc.274.12.7724. [DOI] [PubMed] [Google Scholar]
- 20.Galibert L, Tometsko M E, Anderson D M, Cosman D, Dougall W C. J Biol Chem. 1998;273:34120–34127. doi: 10.1074/jbc.273.51.34120. [DOI] [PubMed] [Google Scholar]
- 21.Wong B R, Josien R, Lee S Y, Vologodskaia M, Steinman R M, Choi Y. J Biol Chem. 1998;273:28355–28359. doi: 10.1074/jbc.273.43.28355. [DOI] [PubMed] [Google Scholar]
- 22.Lomaga M A, Yeh W C, Sarosi I, Duncan G S, Furlonger C, Ho A, Morony S, Capparelli C, Van G, Kaufman S, et al. Genes Dev. 1999;13:1015–1024. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh W C, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeham A, de la Pompa J L, Ferrick D, Hum B, Iscove N, et al. Immunity. 1997;7:715–725. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- 24.Xu Y, Cheng G, Baltimore D. Immunity. 1996;5:407–415. doi: 10.1016/s1074-7613(00)80497-5. [DOI] [PubMed] [Google Scholar]
- 25.Nakano H, Sakon S, Koseki H, Takemori T, Tada K, Matsumoto M, Munechika E, Sakai T, Shirasawa T, Akiba H, et al. Proc Natl Acad Sci USA. 1999;96:9803–9808. doi: 10.1073/pnas.96.17.9803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franzoso G, Carlson L, Xing L, Poljak L, Shores E W, Brown K D, Leonardi A, Tran T, Boyce B F, Siebenlist U. Genes Dev. 1997;11:3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iotsova V, Caamano J, Loy J, Yang Y, Lewin A, Bravo R. Nat Med. 1997;3:1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- 28.Grigoriadis A E, Wang Z Q, Cecchini M G, Hofstetter W, Felix R, Fleisch H A, Wagner E F. Science. 1994;266:443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z Q, Ovitt C, Grigoriadis A E, Mohle-Steinlein U, Ruther U, Wagner E F. Nature (London) 1992;360:741–745. doi: 10.1038/360741a0. [DOI] [PubMed] [Google Scholar]