Abstract
Background/Objective:
To develop and test a clinically relevant model for predicting the recovery of over ground walking speed after 36 sessions of progressive body weight–supported treadmill training (BWSTT) in individuals with motor incomplete spinal cord injury (SCI).
Design:
A retrospective review and stepwise regression analysis of a SCI clinical outcomes data set.
Setting:
Outpatient SCI laboratory.
Subjects:
Thirty individuals with a motor incomplete SCI who had participated in locomotor training with BWSTT. Eight individuals with similar diagnoses were used to prospectively test the prediction model.
Main Outcome Measures:
Over ground walking speed was assessed using the 10-m walking test.
Methods:
The locomotor training program consisted of 36 sessions of sequential comprehensive training comprised of robotic assisted BWSTT, followed by manual assisted BWSTT, and over ground walking. The dose of locomotor training was standardized throughout the protocol.
Results:
Clinical characteristics with predictive value for walking speed were time from injury onset, the presence or absence of voluntary bowel and bladder voiding, a functional spasticity assessment, and over ground walking speed before locomotor training. The model identified that these characteristics accounted for 78.3% of the variability in the actual final over ground walking speed after 36 sessions of locomotor training. The model was successful in prospectively predicting over ground walking speed in the 8 test participants within 4.15 ± 2.22 cm/s in their recovered walking speed.
Conclusions:
This prediction model can identify individuals who are most likely to experience success using locomotor training by determining an expected magnitude of training effect, thereby allowing individualized decisions regarding the use of this intensive approach to rehabilitation.
Keywords: Spinal cord injuries, Tetraparesis, Parapareses, Spasticity, Locomotor training, Lokomat, Physical therapy, Ambulation, Treadmill training, Body weight support, Robotic, Rehabilitation
INTRODUCTION
Spinal cord injury (SCI) affects approximately 11,000 people in the United States annually, and nearly 250,000 Americans deal with the consequences of the resulting disability on a daily basis (1). Although the prevalence of SCI is lower than that of many other disabilities, the reduction in quality of life and the associated costs of health care greatly exceeds those of other disorders. Furthermore, SCI occurs predominantly in young adults; therefore, the potential impact of improving functional recovery in that population in terms of quality of life and financial burden over the remainder of their lifetime is enormous. The National Spinal Cord Injury Statistical Center (NSCISC) reports that the United States would save as much as $400 billion on future direct and indirect lifetime costs by developing new therapies for individuals already injured and by preventing future injuries (1).
A major goal for many patients after SCI is to recover the ability to walk (2). Body weight–supported treadmill training (BWSTT) has been used in recent years to facilitate improvements in motor function and stepping ability in individuals with neurologic disabilities, including SCI and stroke (3). This intensive rehabilitation technique has improved recovery of locomotion in some but not all persons with motor incomplete SCI (4–7).
Currently, there is no consensus about what variables seen in this patient population predict successful walking after BWSTT. For example, Wirz et al (8) reported a decrease in extensor spasm scores after BWSTT using the Spinal Cord Assessment Tool for Spasticity (SCATS) (9); however, they did not find a decrease in Modified Ashworth Score (MAS), flexor spasms, or clonus. Furthermore, the changes observed in the extensor spasm scores did not correlate with any changes in gait speed or endurance. Evidence suggests that persons with chronic SCI are more likely to improve their over ground walking function if they are already walking when undergoing locomotor training (8,10). There has been, however, conflicting reports about whether persons with a chronic SCI can benefit from locomotor training (8,10,11). Lower extremity motor scores (LEMS) were used by Waters et al (12) to predict walking speed during over ground ambulation in people after SCI. A positive correlation has also been reported between initial LEMS and initial walking speed (13,14). However, LEMS has not been found to correlate with over ground walking speed after BWSTT (8,11,15). For individuals without voluntary motor function below the level of injury, the degree of sensory sparing has been shown to be an important predictor of walking recovery (12,16,17). However, it remains unclear whether sensory sparing is important in the recovery of locomotor function after BWSTT.
Because of the manpower and financial resources involved in delivering this intensive, repetitive form of therapy, a realistic estimation of the expected outcome and its magnitude for the patient is clinically relevant. Thus, the challenge for the rehabilitation team managing people with SCI is to decide which individuals will best benefit from this type of expensive rehabilitation approach. To our knowledge, no study has yet evaluated the independent or combined contributions of common clinical measures in predicting locomotor recovery of patients with motor incomplete SCI after BWSTT. Therefore, the purpose of this study was to develop and test a clinically relevant model for the predicting the recovery of locomotor speed after BWSTT in individuals with motor incomplete SCI.
MATERIALS AND METHODS
Study Design
This was a retrospective review and statistical modeling of a clinical SCI data set to identify which clinical variables were predictive of over ground walking speed in individuals with a motor incomplete SCI. Descriptive variables were collected to describe the population and to serve as possible explanatory variables in the stepwise regression analysis. Over ground walking speed after 36 sessions of locomotor training was the dependent variable. Walking speed was selected as the dependent variable for 3 reasons. First, it is a continuous variable that allows and fulfills the assumption of linearity; second, walking speed is an indicator of success in the ability to ambulate; and third, it is easily and accurately measured in most clinical settings.
Subjects
The study population consisted of 30 individuals who sustained a motor incomplete SCI and who met the inclusion criteria to be entered into the spinal cord locomotor study. The inclusion criteria included the following: age 14 to 65 years, ASIA impairment scale C or D, duration of injury ≤60 months, presence of lower extremity (LE) deep tendon reflexes, inability to walk or difficulty in walking, no history of long bone fractures secondary to osteoporosis, and range of motion of the hips, knees, and ankles sufficient to allow upright stance. Individuals were excluded if they were still wearing a cervical or spinal orthosis, had a cutaneous pressure area that interfered with harness support, weighed >115 kg, or had severe orthostatic hypotension (drops in systolic and diastolic blood pressure of >20 and 10 mmHg, respectively, when moving from sitting to standing). All participants signed an informed consent approved by the Institutional Review Board. All applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Clinical Assessment
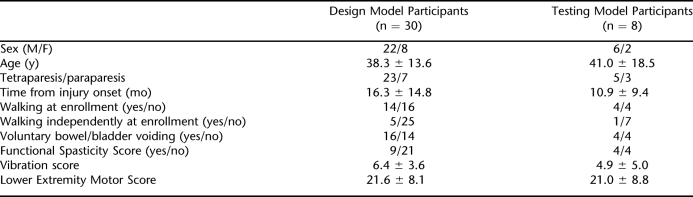
Physical therapists (JM, NF, PW) were trained and proven reliable on all clinical assessments initially and each subsequent year of the study. On admission into the study, the following demographic information was collected on each individual: age, sex, and time from onset of injury. Table 1 shows the clinical data that were collected before the initiation of locomotor treadmill training. Motor and sensory assessments adhered to the International Standards for Neurological Classification of Spinal Injury (18). Vibration was assessed as an indicator of dorsal column function (19). Walking Index for Spinal Cord Injury II (WISCI II) and 10-m walk test (10MWT) were used to assess ambulatory capacity and speed, respectively (20,21).
Table 1.
Clinical Measures
Locomotor Training Program.
All persons were trained at the Spinal Cord Injury Laboratory at The University of Texas Southwestern Medical Center at Dallas. Locomotor training program consisted of sequential comprehensive training comprised of robotic-assisted BWSTT, followed by manually assisted BWSTT and over ground walking. Each participant received locomotor training 3 times per week for 3 months. The duration of locomotor training was standardized throughout the protocol to equal 60 minutes including set-up time. During the 36 sessions of training, the individual did not receive any other physical or occupational therapy sessions at other facilities. Furthermore, all medications including antispasticity medications were maintained at their dosages at the time of entry into the study. At the end of the 36 sessions of training, locomotor performance was reassessed with WISCI II and walking speed with the 10MWT as previously described.
Robotic BWSTT.
Robotic BWSTT using the Lokomat Driven Gait Orthosis (DGO) has been previously described in the literature (8,22–24). Each participant was fitted with a weight-supporting harness that was placed around the hips and abdomen and fastened to fit snugly enough to minimize upward slipping of the harness when body weight suspension was applied. The participant was assisted to stand on the treadmill by the Lokolift body weight–support system using a motor driven winch capable of safely lifting the individual. For stepping, the Lokomat exoskeleton was aligned and secured to the participant's pelvis and legs by the use of Velcro straps and leg cuffs. Initially in the training program, the ankles were positioned in neutral dorsiflexion by use of an elastic foot strap. During robotic BWSTT, the amount of body weight support provided was set to allow maximum LE loading without allowing excessive knee flexion during stance or toe drag during swing. The amount of body weight supported was initially set at 40% and adjusted during the training period. These values ranged from 10% to 40% of the participant's body weight and were decreased as the individual showed improved stability during stance. When a participant demonstrated excessive tone that hindered normal walking in the exoskeleton of the Lokomat, manual assistance was given by one or more therapists to control the abnormal posturing during training. Speed of training was set initially from 1.8 to 2.0 km/h and was increased to 2.5 to 3.0 km/h as tolerated by the individual. Time for each training session was 1 hour including set-up time. Time for continuous walking was increased according to tolerance. Most participants could tolerate 40 to 45 min/session without difficulty by the second week of training.
During training in the Lokomat, sagittal plane forces would be decreased using the “Guidance Force” control to encourage voluntary movement of the lower extremities. In addition, real-time force generation from each of the load sensors in the motors was displayed graphically to the walking participant and physical therapist. The physical therapist used verbal cues to encourage the participant's volitional movement.
If the participant demonstrated adequate stance control of the trunk, hip, and knee, but lacked sufficient motor control for swing, functional electrical stimulation (FES) was incorporated into the training sessions. To assist in facilitating appropriate LE flexor muscle activity during the swing phase on the Lokomat, electrical stimulation to the peroneal nerve was used to activate pre-tibial muscles and/or elicit a flexor withdrawal reflex during swing. To ensure that the timing of the stimulation was appropriate at different training speeds, the hip and knee joint position was sampled from the Lokomat goniometric output. Stimulation onset was triggered at a predefined point in the hip position at pre-swing (25). Stimulation offset was set to allow controlled plantarflexion during loading response. Stimulation intensity and offset were controlled dynamically by the therapist according to participant's motor response and treadmill speed. During training with FES, the elastic foot straps were loosened but kept in place as a safety precaution when electrical stimulation was being used.
Robotic BWSTT was continued as long as the participant could not sustain an upright posture or assist in swing limb advancement. Participants were transitioned to therapist-assisted BWSTT when they were able to generate sufficient voluntary forces to partially accomplish swing limb advancement and maintain upright stance during a therapist-assisted BWSTT trial at a minimum treadmill speed of 2.0 km/h. If they required greater than minimal to moderate manual assistance of 2 to 3 physical therapists to assist with swing limb advancement and to facilitate pelvic kinematics, they remained training in the Lokomat.
Therapist-Assisted BWSTT.
As with Lokomat training, body weight was supported by a similar harness mechanism. Because the participants could now maintain stance stability, only 10% to 25% of body weight support was provided during therapist-assisted training. Although the participant had to be able to walk at a minimum treadmill speed of 2.0 km/h, treadmill speed during therapist-assisted BWSTT could be increased up to 5.5 km/h. Two or 3 therapists provided manual assistance to simulate normal LE kinematics and to assist with balance. Special attention was placed on the sensory cues related to locomotion (24,26). Participants were not allowed to use upper extremity support for balance but were encouraged to reciprocally swing their arms. Time for each training session was 1 hour including set-up time. Time for continuous walking was increased according to tolerance by the participant and therapists providing the manual assistance.
Over ground walking started when participants could maintain upright posture and walk with only minimal physical assistance of 1 therapist and assistive devices. Physical assistance was to aide in swing limb advancement or for balance assist. Once this goal was met, we continued with therapist assisted treadmill training for one half of the session and transitioned to over ground walking practice for the remaining 30 minutes.
Over Ground Walking.
Ankle-foot orthoses were used during over ground locomotor training if indicated to provide dorsiflexion during swing limb advancement and/or to assist tibial stability during single limb support phase of walking. The therapist chose the appropriate walking assistive device based on the participant's balance and walking endurance. During over ground walking, the participant was encouraged to walk as far and as fast as they could before requiring a rest.
Statistical Analysis
Unpaired t tests were used to evaluate differences between the participants used to build the model and between the 8 participants the model was tested on. Significance was set at P < 0.05. Variables that were considered for possible inclusion in the model included age, time from onset of injury, LEMS, MAS, functional spasticity score, pin prick score, vibration score, WISCI II, initial over ground walking speed, and whether the participant was capable of voluntary bowel and bladder voiding. To fulfill the assumption of linearity, bivariate scatter plots of all considered continuous variables were inspected. A curvilinear relationship was noted with time from onset of injury, so this variable was transformed using square root transformation.
To identify predictors of over ground walking speed, a stepwise regression analysis was performed using the probability to enter = 0.05 and probability to remove = 0.10. Residual values were examined to determine whether outliers existed that biased the regression coefficients. To confirm the assumption of normality of the error term for the final model, a histogram of the standardized residuals and normal probability plots was plotted to compare the distribution of standardized residuals to a normal distribution. All statistical analyses were performed with the SPSS program version 12.0 (SPSS, Chicago, IL). The model was subsequently validated prospectively on 8 new individuals who entered and completed the 36-session locomotor training program.
RESULTS
The sample used to build the model consisted of 30 participants. Table 2 shows the characteristics of the individuals used for developing the model and the 8 participants used for testing the model. There was no statistical difference in age, time from injury onset, LEMS, or vibration score from the 30 individuals used to build the model and the 8 individuals the model was tested on.
Table 2.
Characteristics of Participants for the Design and Testing Models
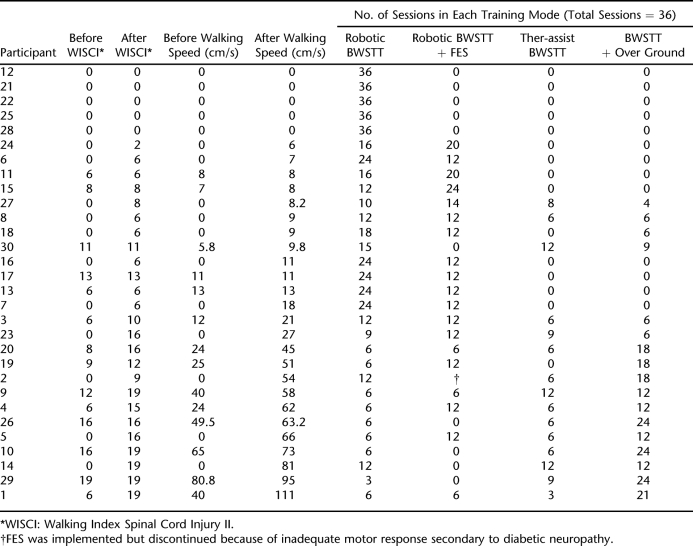
Table 3 provides the locomotor outcome measures and the treatment progression throughout the training protocol on the 30 participants. The outcome measures (WISCI II and walking speed) have been sorted in ascending order by final walking speed. Sixteen participants entered the study initially unable to walk as defined by their WISCI II scores. Of those 16, 5 (31.3%) individuals remained unable to ambulate, 7 (44%) recovered ambulation but needed physical assistance (WISCI = 2–8), and 4 (25%) recovered independent ambulation (WISCI = 9–19). The 5 individuals who did not obtain any ability to ambulate never progressed beyond exclusive robotic training. Those who achieved independent ambulation over ground progressed beyond exclusive robotic training within 18 sessions of the training protocol.
Table 3.
Locomotor Outcomes Before and After Intervention for Participants in the Design Model
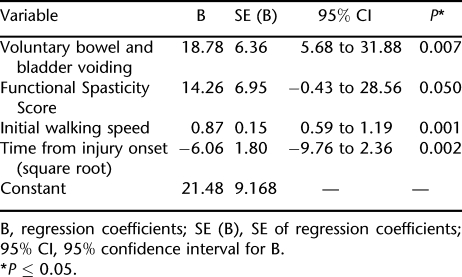
Using the data from these 30 participants, stepwise regression analyses identified the following 4 independent predictors of final over ground walking speed: square root of time from injury onset, voluntary bowel and bladder voiding, functional spasticity score, and walking speed before locomotor training. The regression coefficients of the final model are given in Table 4. The final prediction formula for over ground walking speed is calculated as follows: final over ground walking speed = 21.48 + (voluntary bowel and bladder voiding × 18.78) + (functional spasticity score × 14.3) + (initial walking speed × 0.87) – (square root time from injury onset × 6.06).
Table 4.
Independent Predictive Variables for Final Over Ground Gait Speed Based on Results of Multivariate Regression Analyses
Figure 1 is a scattergram with line of identity for the final over ground walking speed predicted by the model vs the actual achieved final walking speed after 36 sessions of locomotor training. This model was able to predict 78.3% of the variance of final walking speed measured at the end of locomotor training.
Figure 1. Line of identity for the predicted walking speed vs the actual final over ground walking speed achieved after 36 sessions of locomotor training in 30 subjects with motor incomplete SCI. R2 = 0.783.
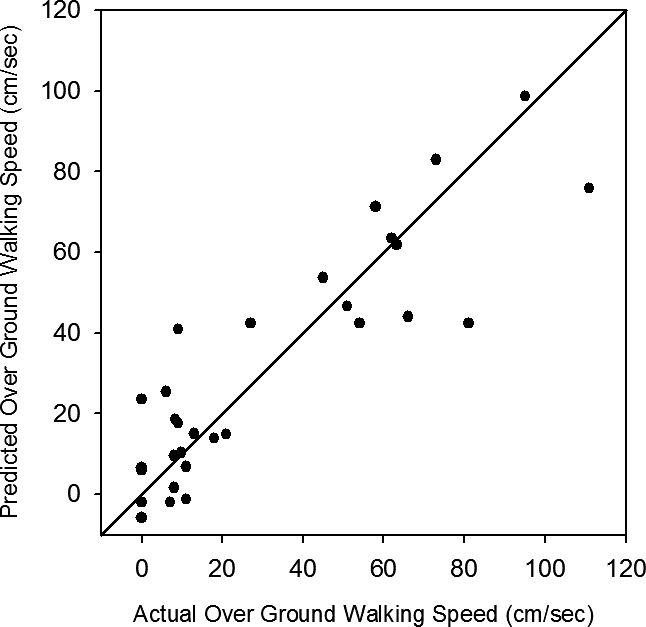
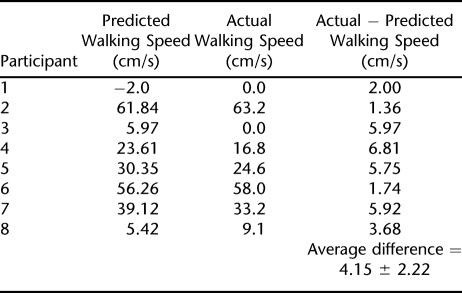
Table 5 shows the actual and predicted values for final over ground walking speed for 8 test participants. The final predicted walking speeds were calculated using the final prediction formula. For example, Participant 1 did not have voluntary bowel and bladder control (score = 0), demonstrated a functional spasticity score of 0, was unable to walk at the time of entrance into the study so walking speed was 0 cm/s, and was 15 months postonset from injury. The formula used to predict Participant 1′s final walking speed was 21.48 + (0 × 18.78) + (0 × 14.3) + (0 × 0.87) − (3.87 × 6.06), which predicted a final walking speed of −2.0 cm/s. Participant 1's final walking speed was 0 cm/s, indicating no improvement or treatment progression over 36 sessions of locomotor training. In comparison, Participant 6 had voluntary bowel and bladder control (score = 1), demonstrated a functional spasticity score of 1, had a walking speed of 14 cm/s at the time of entrance into the study, and was 3 months postonset of injury. The formula used to predict Participant 6's final walking speed was 21.48 + (1 × 18.78) + (1 × 14.3) + (14 × 0.87) − (1.73 × 6.06). Participant 6's final walking speed was 58 cm/s compared with a predicted final walking speed of 56.3 cm/s after 36 sessions of progressive locomotor training.
Table 5.
Predicted and Actual Values for Final Over Ground Walking Speed for Participants in the Test Model
When tested a priori in this group of 8 participants, the model was able to predict within 4.15 ± 2.22 cm/s of the actual change observed in walking speed. It is important to note that this represents the absolute difference in predicted vs actual walking speed.
DISCUSSION
This study developed a model that predicted locomotor recovery after 36 sessions of locomotor training, as measured by over ground walking speed, in persons with motor incomplete SCI. The 4 clinical variables identified as predictors of final walking speed are commonly assessed and easily acquired during an initial evaluation: voluntary bowel and bladder voiding, time from injury onset, walking speed if they are ambulatory, and whether spasticity interferes with standing. Furthermore, the model has been validated in a group of 8 study participants who experienced identical treadmill training. Final over ground walking speed was predicted within a mean of <5 cm/s (range, 1.4–6.8 cm/s). Considering that normal walking speed is approximately 133 cm/s (27), 5 cm/s represents less than 4% of normal speed and is not considered a clinically relevant difference.
Over ground walking speed was selected as our dependent variable to serve as an index of locomotor recovery after training. Walking speed is often used to predict whether an individual will be able to walk in the home or community setting or whether they will require a wheelchair for mobility-related activities of daily living (ADLs). Walking speed as an indicator of over ground walking ability is easily measured and is commonly measured using the 10MWT (21,28). Holden et al (29) have shown good interrater reliability (r = 0.99) and test–retest reliability (r = 0.90) for the 10MWT in patients with neurologic involvement.
The major conceptual limitation of all regression techniques is that one can only ascertain relationships but never be sure about underlying causal mechanisms. For example, we found a strong positive relationship (correlation) between voluntary bowel and bladder voiding and final over ground walking speed. It would be wrong to conclude that the neural circuitry controlling bowel and bladder function is the same circuitry that generates stepping, but it may be that both sets of circuits share modulation by common local interneuronal circuits or supraspinal centers or that both are similarly sensitive to the neuromodulatory milieu of the partially injured spinal cord. It is a common observation that recovery of spontaneous locomotion and bladder voiding occurs simultaneously in rodent models of incomplete SCI (30). Clinical experience has shown that both walking and voiding ability are related to the severity of injury. Patients that have maintained or recovered voiding may have mild enough injuries that their lumbosacral neural circuitry for stepping is amenable to training with afferent input during BWSTT.
Limited evidence studying the efficacy of BWSTT in SCI suggests that a person with a chronic SCI is more likely to improve their over ground walking function if they are already walking when undergoing locomotor training (8,10). Our model supports these initial observations by indicating a negative correlation to time since onset and a positive correlation to initial walking speed. In other words, our model supports that greater recovery of locomotion is seen in patients who are still in the acute to subacute stages of their injury and the greatest improvement in walking speed seen in patients with chronic SCI tends to be within subgroups that can ambulate before their participation in locomotor rehabilitation programs. For example, only 1 of the 5 participants who had an onset that was longer than 24 months and was not walking at the time of the study showed an increase in their final over ground walking speed. His change in walking speed was not clinically significant, and he remained dependent on a wheelchair for mobility-related ADLs.
Our model showed a positive relationship between final over ground walking speed and our functional spasticity measure. Consistent with other studies, no significant relationship was found between final over ground walking speed and MAS scores (8,11). Spasticity after SCI is more complex than resistance to single joint movement assessed using the MAS and includes 3 distinct types of spasms; clonus, multijoint flexor spasms, and multijoint extensor spasms (31). Although the functional clinical assessment of spasticity used in this model does not characterize the various types of spastic behavior observed in individuals after SCI, it was useful in identifying who may not be able to benefit from aggressive locomotor training.
Of course, if this model is to have relevance to other facilities offering locomotor rehabilitation programs, it is imperative that the sample population characteristics used to build the model can be generalized to the population of patients who are potential candidates for a program of locomotor training similar to ours. This program used a progressive locomotor training protocol incorporating robotic BWSTT, robotic BWSTT with FES, therapist-assisted BWSTT, and over ground training. Participants were required to show defined functional tasks essential for successful over ground ambulation to progress to more posturally challenging training interventions. Although the 5 participants who never achieved the task of stance stability were unable to progress beyond robotic training, the participants who obtained the fastest walking speed were advanced to the interventions requiring progressively increasing demands with decreasing external assistance.
Our sample population reflected the demographics typically reported for sex and age in the SCI population. The sample included only individuals with motor incomplete SCI (22 ASIA C and 8 ASIA D). The larger number of participants with ASIA C further strengthened the prediction model because persons with ASIA D classification typically will not require this aggressive locomotor retraining to achieve independence in walking.
CONCLUSION
The results of this study have economic implications because the costs of administering an aggressive program of locomotor training are expensive (32). The challenge for clinicians involved in the rehabilitation of individuals after SCI is to decide which patients are appropriate candidates for expensive, time-consuming interventions such as BWSTT. Establishing a model for predicting success after locomotor training (ie, over ground walking speed) should enhance the delivery of appropriate rehabilitation interventions to those individuals who have the potential to maximize their function after SCI.
Acknowledgments
We thank all of the study participants for their dedication to this research, Emily Proctor for assistance in training, and Alan Elliot in Biostatistics for advice and assistance on statistical analysis.
REFERENCES
- University of Alabama. Spinal Cord Injury Information Network. National SCI Statistical Center; Spinal Cord Injury Facts & Figures, 2006 update. Available at: http://www.spinalcord.uab.edu/show.asp?durki=19775. Accessed February 1, 2008. [Google Scholar]
- Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Fung J. The role of rehabilitation in the recovery of walking in the neurological population. Curr Opin Neurol. 2001;14:735–740. doi: 10.1097/00019052-200112000-00009. [DOI] [PubMed] [Google Scholar]
- Wernig A, Muller S, Nanassy A, Cagol E. Laufband therapy based on ‘rules of spinal locomotion' is effective in spinal cord injured persons. Eur J Neurosci. 1995;7:823–829. doi: 10.1111/j.1460-9568.1995.tb00686.x. [DOI] [PubMed] [Google Scholar]
- Wernig A, Muller S. Treadmill locomotion with body weight support in persons with severe spinal cord injury. Paraplegia. 1992;30:229–238. doi: 10.1038/sc.1992.61. [DOI] [PubMed] [Google Scholar]
- Colombo G, Wirz M, Dietz V. Effect of locomotor training related to clinical and electrophysiological examinations in spinal cord injured humans. Ann N Y Acad Sci. 1998;860:536–538. doi: 10.1111/j.1749-6632.1998.tb09097.x. [DOI] [PubMed] [Google Scholar]
- Barbeau H. Locomotor training in neurorehabilitation: emerging rehabilitation concepts. Neurorehabil Neural Repair. 2003;17:3–11. doi: 10.1177/0888439002250442. [DOI] [PubMed] [Google Scholar]
- Wirz M, Zemon DH, Rupp R, et al. Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: a multicenter trial. Arch Phys Med Rehabil. 2005;86:672–680. doi: 10.1016/j.apmr.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Benz EN, Hornsby G, Bode RK, Scheidt RA, Schmidt BD. A physiologically based clinical measure for spastic reflexes in spinal cord injury. Arch Phys Med Rehabil. 2005;86:52–59. doi: 10.1016/j.apmr.2004.01.033. [DOI] [PubMed] [Google Scholar]
- Hicks AL, Adams MM, Ginis KM, et al. Long-term body-weight-supported treadmill training and subsequent follow-up in persons with chronic SCI: effects on functional walking ability and measures of subjective well-being. Spinal Cord. 2005;43:291–298. doi: 10.1038/sj.sc.3101710. [DOI] [PubMed] [Google Scholar]
- Protas EJ, Holmes SA, Qureshy H, Johnson A, Lee D, Sherwood AM. Supported treadmill ambulation training after spinal cord injury: a pilot study. Arch Phys Med Rehabil. 2001;82:825–831. doi: 10.1053/apmr.2001.23198. [DOI] [PubMed] [Google Scholar]
- Waters RL, Adkins R, Yakura J, Vigil D. Prediction of ambulatory performance based on motor scores derived from standards of the American Spinal Injury Association. Arch Phys Med Rehabil. 1994;75:756–760. [PubMed] [Google Scholar]
- Field-Fote EC, Lindley SD, Sherman AL. Locomotor training approaches for individuals with spinal cord injury: a preliminary report of walking related outcomes. J Neuro Phys Ther. 2005;29:127–137. doi: 10.1097/01.npt.0000282245.31158.09. [DOI] [PubMed] [Google Scholar]
- Dobkin B, Barbeau H, Deforge D, et al. The evolution of walking-related outcomes over the first 12 weeks of rehabilitation for incomplete traumatic spinal cord injury: the multicenter randomized Spinal Cord Injury Locomotor Trial. Neurorehabil Neural Repair. 2007;21:25–35. doi: 10.1177/1545968306295556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field-Fote EC. Combined use of body weight support, functional electric stimulation, and treadmill training to improve walking ability in individuals with chronic incomplete spinal cord injury. Arch Phys Med Rehabil. 2001;82:818–824. doi: 10.1053/apmr.2001.23752. [DOI] [PubMed] [Google Scholar]
- Crozier KS, Graziani V, Ditunno JF, Herbison GJ. Spinal cord injury: prognosis for ambulation based on sensory examination in patients who are initially motor complete. Arch Phys Med Rehabil. 1991;72:119–121. [PubMed] [Google Scholar]
- Folman Y, el Masri W. Spinal cord injury: prognostic indicators. Injury. 1989;20:92–93. doi: 10.1016/0020-1383(89)90148-4. [DOI] [PubMed] [Google Scholar]
- ASIA Neurological Standards Committee 2002: Marino RJ, Barros T, Biering-Sorensen F, Burns SP, Donovan WH, Graves D, Haak M, Hudson L, Priebe M.International Standards for Neurological Classification of Spinal Injury. Revision 2002 J Spinal Cord Med. 200326 (Suppl 1) S50–S56. [DOI] [PubMed] [Google Scholar]
- Gilman S. Joint position sense and vibration sense: anatomical organisation and assessment. J Neurol Neurosurg Psychiatry. 2002;73:473–477. doi: 10.1136/jnnp.73.5.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditunno JF, Ditunno PL. Walking index for spinal cord injury (WISCI II): scale revision. Spinal Cord. 2001;39:654–656. doi: 10.1038/sj.sc.3101223. [DOI] [PubMed] [Google Scholar]
- Wade DT, Wood VA, Heller A, Maggs J, Langton Hewer R. Walking after stroke: measurement and recovery over the first 3 months. Scand J Rehabil Med. 1987;19:25–30. [PubMed] [Google Scholar]
- Colombo G, Joerg M, Schreier R, Dietz V. Treadmill training of paraplegic patients using a robotic orthosis. J Rehabil Res Dev. 2000;37:693–700. [PubMed] [Google Scholar]
- Colombo G, Wirz M, Dietz V. Driven gait orthosis for improvement of locomotor training in paraplegic patients. Spinal Cord. 2001;39:252–255. doi: 10.1038/sj.sc.3101154. [DOI] [PubMed] [Google Scholar]
- Hornby TG, Zemon DH, Campbell D. Robotic-assisted, body-weight-supported treadmill training in individuals following motor incomplete spinal cord injury. Phys Ther. 2005;85:52–66. [PubMed] [Google Scholar]
- Querry RG, Pacheco F, Annaswamy T, Goetz L, Winchester PK, Tansey KE. Synchronous stimulation and monitoring of the soleus H-reflex during robotic body weight supported ambulation in subjects with spinal cord injury. J Rehabil Res Dev. 2008;45:175–186. doi: 10.1682/jrrd.2007.02.0028. [DOI] [PubMed] [Google Scholar]
- Behrman AL, Bowden MG, Nair PM. Neuroplasticity after spinal cord injury and training: an emerging paradigm shift in rehabilitation and walking recovery. Phys Ther. 2006;86:1406–1425. doi: 10.2522/ptj.20050212. [DOI] [PubMed] [Google Scholar]
- Perry J. Gait Analysis. Normal and Pathological Function. Thorofare, NJ: Slack; 1992. [Google Scholar]
- Rossier P, Wade DT. Validity and reliability comparison of 4 mobility measures in patients presenting with neurologic impairment. Arch Phys Med Rehabil. 2001;82:9–13. doi: 10.1053/apmr.2001.9396. [DOI] [PubMed] [Google Scholar]
- Holden MK, Gill KM, Magliozzi MR, Nathan J, Piehl-Baker L. Clinical gait assessment in the neurologically impaired. Reliability and meaningfulness. Phys Ther. 1984;64:35–40. doi: 10.1093/ptj/64.1.35. [DOI] [PubMed] [Google Scholar]
- Pikov V, Gillis RA, Jasmin L, Wrathall JR. Assessment of lower urinary tract functional deficit in rats with contusive spinal cord injury. J Neurotrauma. 1998;15:375–386. doi: 10.1089/neu.1998.15.375. [DOI] [PubMed] [Google Scholar]
- Little JW, Micklessen P, Umlauf R, Brittel C. Lower extremity manifestations of spasticity in chronic spinal cord injury. Am J Phys Med Rehabil. 1989;68:32–36. doi: 10.1097/00002060-198902000-00009. [DOI] [PubMed] [Google Scholar]
- Morrison SA, Backus D. Locomotor training: is translating evidence into practice financially feasible. J Neurol Phys Ther. 2007;31:50–54. doi: 10.1097/NPT.0b013e3180690679. [DOI] [PubMed] [Google Scholar]