Abstract
Background/Objective:
To study the effectiveness of knee-tendon to bladder artificial reflex arc in dogs.
Methods:
In 6 beagles, the proximal end of the right L5 anterior motor root and the distal end of the right S2 anterior root were anastomosed to build a knee-tendon to bladder reflex, whereas the right L5 posterior sensory root was kept intact. Action potential (AP) curves and electromyograms (EMGs) of the detrusor muscle, the intravesical pressure, horseradish peroxidase (HRP)-labeled neurons, and the passing rates of myelinic nerve fibers were calculated to evaluate its feasibility.
Results:
AP curves and EMG detected in all 6 dogs were similar to those of the control. Six and 18 months after surgery, the means for bladder contraction induced by percussion of the right knee-tendon were 38 ± 27% and 62 ± 5% that of the normal control, respectively. The mean duration times induced by percussion of the right knee-tendon at 6 and 18 months after surgery were 51± 37% and 84 ± 12% that of the normal control, respectively. HRP retrograde tracing and neurohistologic observation indicated the feasibility of the artificial reflex arc.
Conclusions:
Our data showed the effectiveness of bladder innervation below the level of spinal cord injury producing urination by knee-tendon to bladder reflex contractions, and therefore, might provide a new clinical approach for restoring bladder function in individuals with paraplegia.
Keywords: Spinal cord injuries, Neurogenic bladder, Reflex arc, Knee tendon reflex, Paraplegia, Dog
INTRODUCTION
Urinary dysfunction caused by spinal cord injury (SCI) from trauma or disease remains a clinical challenge. Because bladder dysfunction may cause severe urinary retention, urinary tract infection, or even chronic renal failure, it has become one of the main causes of death in patients with paraplegia (1–3). Nonsurgical interventions for bladder emptying with SCI include intermittent catheterization (IC), indwelling catheters, Credé/Valsalva maneuver, reflex voiding, and local or systemic pharmacologic therapy. Among them, IC is recommended as a primary supportive measure of bladder care, because IC provides complete bladder emptying and offers a practical means of obtaining a catheter-free state. Surgical interventions consist of rhizotomy, neurostimulator implantation, sphincterotomy, endourethral stents, and reconstruction of bladder innervation (4), which is a more proactive approach toward raising quality of life and reducing the mortality of patients with paraplegia.
Prior studies on reconstructing bladder function focused on using the normal somatic reflex above the injury level for reinnervation. In 1991, Chuang et al (5) anastomosed the spinal nerve anterior motor root above the SCI level with the sacral nerve anterior motor root below the SCI level intradurally in a rat model and successfully repaired the bladder dysfunction after low level SCI. In 1994, Xiao and Godec (6) anastomosed the central end of L4 spinal nerve anterior motor root with the peripheral end of the bladder-governing L6 spinal nerve anterior motor root intradurally in a rat model of L5 paraplegia. Electrophysiologic examination and horseradish peroxidase (HRP) tracing confirmed that an artificial “skin–CNS–bladder” reflex pathway was established successfully. The above studies made use of the normal somatic reflex above the paraplegic level to reconstruct bladder function. However, because the lower central circuit remains intact in SCI above the medullary cone, is it possible to make use of the healthy tendon reflexes below the level of injury such as knee-tendon reflex or heel-tendon reflex to reconstruct an artificial bladder reflex and rebuild urinary function? To test this hypothesis, a series of experiments with the knee-tendon to bladder reflex arc was performed to validate the effectiveness of the reflex pathway.
METHODS
Of the 6 dogs (beagles; weight, 10–12 kg), dogs 1–4 were used for observing the 6-month short-term postoperative outcome and dogs 5 and 6 were used for observing the 18-month long-term postoperative outcome.
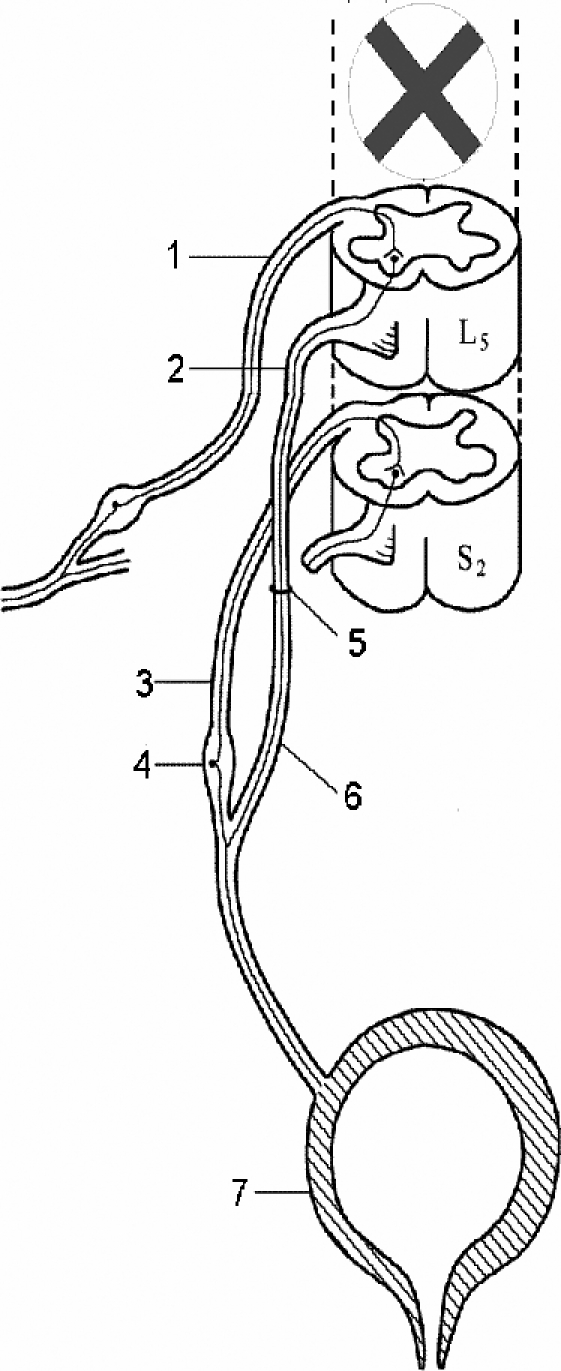
All dogs were anesthetized intravenously with 3% sodium pentobarbital (25 mg/kg). The knee-tendon reflex center of the dog is located at the L4 to L6 spinal segment, and the spinal nerve center governing the bladder is located at the S1 to S3 segment. A posterior median incision was made at L3 to S2; the bilateral laminas were removed, and the dura was incised to expose the cauda equina. The nerve root was localized by anatomy and electrostimulation. The right L5 anterior motor root coming from the dura and the right S2 anterior motor root on the same level were cut off. The proximal end of the right L5 anterior motor root and the distal end of the right S2 anterior motor root were anastomosed with 11–0 monofilament nylon under a 10-power microscope with 4 stitches. The right L5 posterior sensory root was kept intact (Figure 1). The anastomosis was labeled with a silk thread, and absorbable sutures were used in the internal wounds; 1,000 mL of intra- and postoperative fluid was replaced routinely. Penicillin (1.6 million U/d) was administered intramuscularly for 3 consecutive days postoperatively. The intact left S2 root was used as a normal control.
Figure 1. Illustration of “tendon-spinal cord-bladder” artificial reflex arc in canines. (1) Right L5 posterior sensory root. (2) Right L5 anterior motor root. (3) Right S2 posterior sensory root. (4) Spinal ganglia. (5) Anastomotic stoma. (6) Right S2 anterior motor root. (7) Bladder.
Muscular Electrophysiologic Examination
After anesthesia, in dogs 1–4 (6 months postoperative) and dogs 5 and 6 (18 months postoperative), the skin was incised along the original incision; the scar outside dura was stripped off, and the dura was incised to separate the cauda equine carefully. The anastomosis was identified by means of the label, ensuring that the nerve root was well protected (1). The recording electrode was placed at the distal end of the anastomosis in the right left S2 anterior motor root, and the stimulation electrode was placed at the right L5 posterior sensory root, which were connected to the EDJ-V Biosignal system (Shanghai Teaching Equipment Co., Shanghai, China) and stimulated at 115 mV and 1.0 ms with unidirectional rectangular pulses. Action potential (AP) curves were recorded (2). The recording electrode was placed at the left S2 anterior motor root and the stimulation electrode was placed at the left S2 posterior sensory root, which were stimulated with the same parameters as control. (3) The spinal cord was transected acutely at T10 canal level to construct a complete paraplegia model, and observation was made 48 hours after paraplegia was induced (1).
A median incision was made in the lower abdomen in dogs 5 and 6. The muscular electrophysiology electrodes were placed in the anterior wall of bladder detrusor muscle close to the top of the bladder and connected to the Cantata 2000 muscular electrograph (Dantec Medical, Copenhagen, Denmark) to stimulate the right L5 posterior sensory root and the right femoral nerve, which was the afferent nerve of the knee-tendon reflex, at 3.8 mA and 1.0 Hz. Electromyograms (EMGs) of the detrusor muscle were recorded. The left S2 posterior sensory root was stimulated with the same parameters as control.
Measurement of Intravesical Pressure
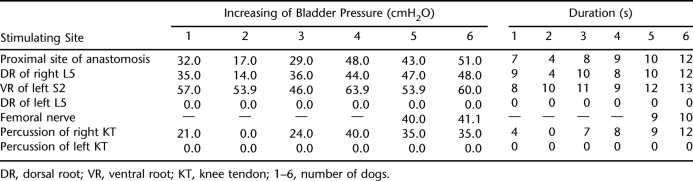
After anesthesia, the spinal cord was transected acutely at T10 canal level to construct a paraplegia model. The paralyzed dogs were kept alive for 48 hours. A median incision was made in the lower abdomen to expose the bladder cervix and posterior urethra after anesthesia. A 12-F urinary catheter was inserted to the bladder through a small incision on the posterior urethra, and the incision was sutured. The urinary catheter was connected to a self-designed urodynamic 3-lead simplified pressure machine (7). Approximately 150 mL normal saline was injected into the bladder through the fluid inlet, ensuring that the water column of the pressure test tube was at the 10-cm baseline to observe easily. The fluid inlet was closed. The cauda equina was exposed through the original median approach to find the anastomosis of right L5 anterior motor root and S2 anterior motor root. In dogs 5 and 6, a right femoral anterior lateral incision was made to expose the femoral nerve, which was the afferent nerve of the knee-tendon reflex. A series stimulation current of 1,000 mV and 10 Hz at 2 seconds from an EJD-V Biosignal system was emitted to stimulate the proximal end of the right L5 anterior motor root anastomosis, right L5 posterior sensory root, left S2 anterior motor root, left L5 posterior sensory root, and right femoral nerve. A medical percussion hammer was used to percuss the right knee-tendon and the left knee-tendon for 10 seconds at a frequency of 2 times per second. The increased value of bladder pressure (cmH2O) and duration time (T) were recorded. The left S2 anterior motor root was used as a normal control, and the left L5 posterior sensory root and left knee-tendon were used as negative controls.
HRP Retrograde Tracing
Parallel to paraplegic model construction, 30 to 100 μL 10% HRP was injected at multiple points of the bladder wall. The animals that had survived 48 hours were fixed with neutral formaldehyde. The spinal cord below L2 was excised, embedded with protein gelatin, frozen, and sliced into 40-μm sections, which were dehydrated, hyalinized, and mounted with neutral resin to observe distribution of HRP-positive cells under an optic microscope.
The total number of HRP-labeled neurons (N) under the microscope was calculated, where A is the thickness of the section and D is the diameter of the neuron, according to the following formula: total N = N × A/(A + D). The density of HRP-labeled neurons was calculated, where S represents the cross area of the spinal cord and H represents the height of the spinal cord sectioned, according to the following formula: D = N/(S × H).
Neurohistologic Observation
A nerve specimen was taken from the place 0.5-cm proximal to the anastomosis and 1.0-cm distal to the anastomosis and hematoxylin and eosin (HP) stained for counting the number of fibers containing medullar nerves with Leica FW4000 Image Analysis System (Leica, Solms, Germany). Dividing the number of fibers in the distal end with that in the proximal end equaled the passing rate of myelinic nerve fibers. Data were expressed as mean ± SD, analyzed with SPSS12.0, and tested with the Student t test. P < 0.05 was considered statistically significant.
We certify that this animal experiment was conducted in accordance with the Helsinki Declaration.
RESULTS
Muscular Electrophysiologic Examination
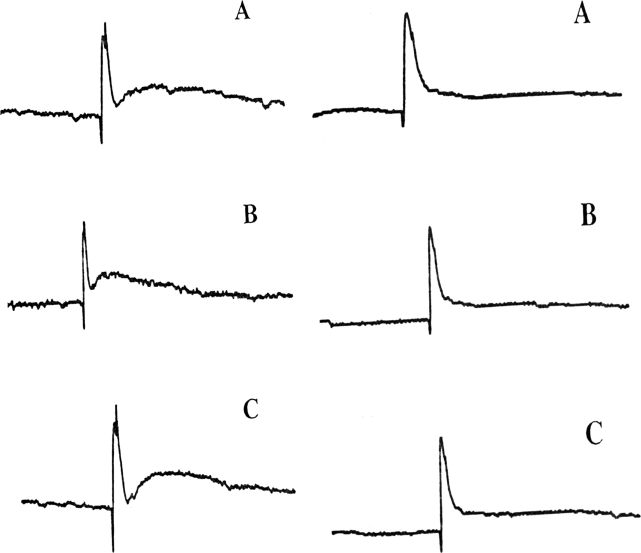
Using the same current to stimulate L5 posterior sensory nerve root before paraplegia and 48 hours after complete paraplegia at the T10 canal level, AP curves were detected in S2 anterior motor root distal to the anastomosis in all 6 dogs whose morphology and amplitude were similar to those of the control (Figure 2).
Figure 2. AP curves of dogs 5 (left) and 6 (right), which were detected by stimulating at the left S2 anterior motor root and recording at the left S2 posterior sensory root (A), stimulating at the right L5 posterior sensory root before (B) and 48 hours after a complete paraplegia and recording at the distal ends of the anastomosis at 115 mV and 1.0 ms with unidirectional rectangular pulses (C).
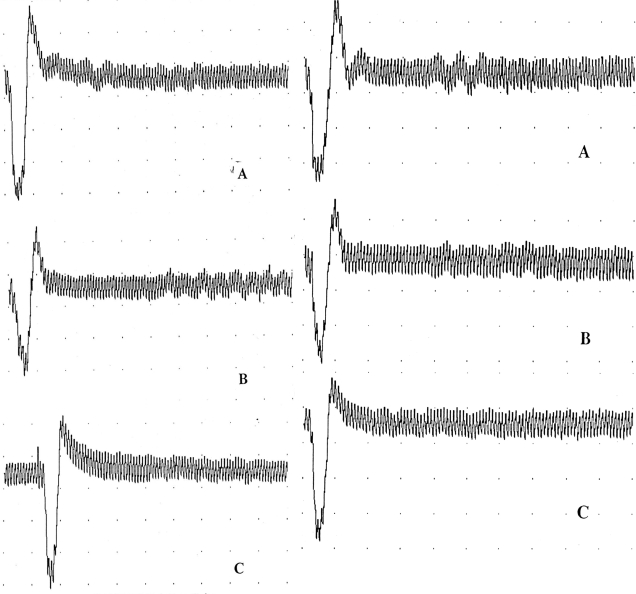
At 48 hours after complete paraplegia at the T10 canal level, EMG of the detrusor muscle was detected at the right L5 posterior sensory root and right femoral nerve, which was the afferent nerve of the knee-tendon reflex, whose morphology and amplitude were similar to those of the left S2 posterior sensory root of the control, whereas stimulation of the left L5 posterior sensory root failed to elicit an EMG response of the detrusor muscle (Figure 3).
Figure 3. EMGs of the detrusor muscle of dogs 5 (left) and 6 (right), which were detected by stimulating at the left S2 posterior sensory root (A), the right L5 posterior sensory root (B), and the right femoral nerve (C), which was the afferent nerve of the knee-tendon reflex, at 3.8 mA and 1.0 Hz.
Intravesical Pressure Measurement
Intravesical pressure (IVP) measurements 48 hours after the experimental paraplegia are shown in Table 1. Serial stimulation (1,000 mV, 10 Hz, 2 s) of the right L5 posterior sensory root was conducted 48 hours after paraplegia in dogs 1–4 (6 months postoperative). Results showed that mean bladder contraction and mean duration time induced by the newly established artificial reflex arc were 59 ± 23% and 83 ± 31% of those of the control group with the same stimulation of the left S2 anterior motor root. Mean bladder contraction and mean duration time induced by percussion of the right knee-tendon were 38 ± 27% and 51 ± 37% of those of the control group. In the remaining 2 dogs, mean bladder contraction and mean duration times induced by the same current 18 months after operation were 84 ± 5% and 88 ± 6% of the values in the control group, and 62 ± 5% and 84 ± 12% by percussion of the knee-tendon, respectively. Mean duration times induced by percussion of the right knee-tendon were 88 ± 6% of those of the control group, respectively. Mean duration times induced by percussion of the right knee-tendon were 84 ± 12% of those of the control group, respectively. The increase in bladder pressure was significant between the short-term and the long-term groups (P < 0.05). Duration time of urination was significantly prolonged with stimulation of the right L5 posterior sensory root between the short-term and long-term groups (P < 0.05), whereas no significant prolongation was seen with percussion of the right knee-tendon (P > 0.05; Table 2).
Table 1.
Bladder Pressure and Duration in Experimental Canines After 48 Hours of Paraplegia
Table 2.
Bladder Pressure–Increasing Rate and Duration–Prolonging Rate in Canines
HRP Retrograde Tracing
After injection of HRP in the bladder wall, numerous positive HRP-labeled neurons were seen at the L5 segment of the experimental side, the plasma of which was dark blue and the morphology of which was polygonal, with multiple projections and group arrangement. Most of them were distributed in the anterior horn of spinal cord and as individual cells scattered around the horn. No positive cells were seen on the contralateral side. Computer image system analysis showed that the mean cell area was 247.2 ± 105.4 μm2 and diameter was 8.9 ± 2.0 μm, which are similar to values for α motor neurons. There were also some small neurons, whose area was 81.3 ± 22.8 μm2 and diameter was 5.1 ± 1.4 μm, similar to γ motor neurons. The total number of positive HRP-labeled neurons was 338 ± 131, L5 spinal segment height was 0.8 ± 0.2 mm, the cross-section area of the spinal cord was 5.9 ± 0.6 mm2, and the cell density was 70 ± 11/mm3.
Neurohistologic Observation
HE stain showed that numerous nerve fiber growths passed through the anastomoses on the experimental sides, the longitudinal section of which showed that the regenerated nerve fibers were well arranged and ran in the same direction. The cross-section showed that the regenerated nerve fibers presented as a typical myelinated nerve fiber structure (ie, myelin sheath in the periphery and nerve axon in the center). The numbers of nerve fibers distal and proximal to the anastomosis on the experimental side were 574 ± 261 and 988 ± 124, respectively, and the passing rate of myelinated nerve fibers was 58.1 ± 13.4%, whereas the number of fibers of the left S2 anterior root was 867 ± 349. The difference between the two sides was statistically significant (P < 0.05).
DISCUSSION
Spinal cord injury above the medullary cone may lead to spasmodic bladder. The approach carried out in cauda equina or sacral nerve root is the main neurologic procedure for reconstructing neural function of the bladder (8–10). Enlightened by the somatic nerve-autonomic nerve artificial bladder reflex arc of Xiao et al, we used the existing healthy somatic reflex and constructed a knee-tendon to bladder reflex arc by nerve anastomosis. After the L5 anterior motor root was anastomosed with the S1 anterior motor root to reconstruct the bladder reflex, the bladder volume enlarged and urine output increased, so bladder function improved. In the early postoperative period, although the artificial bladder reflex arc was not established, breaking of the dominant nerve root governing the bladder might play a role of improving the urine storage. With the regeneration of nerve roots and reconstruction of the artificial reflex arc in the later stage, reinnervation was built up. Furthermore, the knee-tendon reflex became a new trigger for inducing bladder urination.
The purpose of this study was to investigate the effects of a new knee-tendon to bladder reflex arc in dogs, which was shown by electrophysiology, IVP measurement, HRP tracing, and neurohistologic study. Electrical stimulation in 6 dogs showed that bladder contraction was induced by the new reflex arc, whereas dog 2 showed that bladder contraction was induced by the percussion of the knee-tendon. The reason might be caused by poor quality of anastomosis—not enough axons had passed through the stoma in a timely fashion—or because of mismatch of the nerve fibers. The bladder contraction at 18 months was significantly stronger than that at 6 months after surgery, indicating that the long-term axon regeneration, regovernment of the bladder nerve, and recovery of the bladder function were better than in the early stage. HRP tracing showed the positive neurons appeared only in the anterior horn of the L5 spinal cord, which indicated that the somatic motor nerve fibers of L5 anterior motor root could grow into the myelin sheath of the parasympathetic nerve fibers of S2 anterior motor root through the anastomosis and built a new nonphysiologic nerve connection with its target organ—the detrusor muscle. These findings were consistent with those of Vorstman et al (11) and Xiao and Godec (6). Neurohistologic study showed that numerous nerve fiber growths passed through the anastomosis at the experimental sides. This also showed that the autonomic nerve fibers of S2 can be reinnervated by the somatic nerve fibers of L5. Above all, reconstructed bladder reinnervation below the level of SCI could produce urination by knee-tendon, whereby motor impulses of the somatic reflex produced by percussion of the knee-tendon were transmitted to the bladder through the motor efferent branch, inducing spontaneous contraction of the bladder. This new approach provided theoretical references for clinical applications.
Our anastomosis site differs markedly from the reports by Xiao and Godec (6) and Chuang et al (5), who used the normal somatic reflex above the injury level for reinnervation of the bladder. However, in our experiments, a healthy tendon reflex below the injury level was used to reconstruct an artificial bladder reflex. The results of our study are similar to the clinical report of Xiao et al (12) in 15 patients with spasmodic bladder, who underwent microanastomosis of the proximal end of L5 anterior with the distal end of the S2 to S3 anterior root in the dura to reconstruct urination function of the bladder through the artificial somatic–central nervous system–autonomic reflex pathway. Of the 15 patients, 10 (67%) achieved controllable urination.
The “knee tendon-spinal cord-bladder” reflex arc has the following advantages. Compared with the prior approaches, urine storage is improved by breaking of the dominant nerve root governing the bladder. In addition, the approach makes it possible to achieve stressless nerve anastomosis without the need for nerve transplantation because of L5 overlapping the S1 nerve root in the cauda equina and to shorten the nerve regeneration time because the anastomotic stoma is relatively low. Furthermore, the “waste” nerves below the injury level are used to reconstruct voiding with no further functional losses of lower limbs caused by SCI. As the unilateral sacral anterior nerve root is severed and another lateral anterior nerve root, the posterior nerve root, and sacral cord are kept intact, bowel and sexual functions will not be affected. Finally, anastomosis of simple motor nerves avoids mismatching of axons, so that nerve function can recover smoothly. However, further study should be performed to find the mechanisms of the interactions between the somatic and autonomic motor nerves.
The disadvantages of this approach are that it is more invasive, and the risk for skin breakdown is higher compared with IC, indwelling catheterization, and Credé/Valsalva maneuvers. Another obvious deficit of this approach is that the artificial reflex pathway thus constructed is not a complete reflex arc; rather, it is a new trigger of urination. The patient may still lack the sensation of urination even after the reconstruction of the reflex arc, so that he/she is unable to void timely and flexibly, which may still affect quality of life. After reconstruction of the bladder innervation with tendon reflex, a potential problem could be that stretching the knee or ankle joint while standing could produce reflex urination.
CONCLUSION
In contrast with previous studies (5,6), we used healthy tendon reflexes below the injury level, such as the knee-tendon reflex, to successfully reconstruct urinary function after SCI in dogs. The most important implication of our data is that this may provide a new clinical approach for reconstructing bladder function.
REFERENCES
- Benevento BT, Sipski ML. Neurogenic bladder, neurogenic bowel, and sexual dysfunction in people with spinal cord injury. Phys Ther. 2002;82:601–612. [PubMed] [Google Scholar]
- Frankel HL, Coll JR, Charlifue SW, et al. Long-term survival in spinal cord injury: a fifty year investigation. Spinal Cord. 1999;36:266–274. doi: 10.1038/sj.sc.3100638. [DOI] [PubMed] [Google Scholar]
- Potter PJ. Disordered control of the urinary bladder after human spinal cord injury: what are the problems. Prog Brain Res. 2005;152:51–57. doi: 10.1016/S0079-6123(05)52004-1. [DOI] [PubMed] [Google Scholar]
- Consortium for Spinal Cord Medicine. Bladder management for adults with spinal cord injury: a clinical practice guideline for health-care providers. J Spinal Cord Med. 2006;29:527–573. [PMC free article] [PubMed] [Google Scholar]
- Chuang DC, Chang PL, Cheng SY. Root reconstruction for bladder reinnervation: an experimental study in rats. Microsurgery. 1991;12:237–245. doi: 10.1002/micr.1920120403. [DOI] [PubMed] [Google Scholar]
- Xiao CG, Godec CJ. A possible new reflex pathway for micturition after spinal cord injury. Paraplegia. 1994;32:300–307. doi: 10.1038/sc.1994.52. [DOI] [PubMed] [Google Scholar]
- Liu M, Hou C, Bao J. A new simple uroflometer. Acad J Second Military Med Univers. 1997;18:392–393. [Google Scholar]
- Xiao CG, Du MX, Li B, et al. An artificial somatic-autonomic reflex pathway procedure for bladder control in children with spina bifida. J Urol. 2005;173:2112–2116. doi: 10.1097/01.ju.0000158072.31086.af. [DOI] [PubMed] [Google Scholar]
- Wang J, Hou C, Jiang J, et al. Selection of the sacral nerve posterior roots to establish skin-CNS-bladder reflex pathway: an experimental study in rats. Microsurgery. 2007;27:118–124. doi: 10.1002/micr.20316. [DOI] [PubMed] [Google Scholar]
- Sievert KD, Xiao CG, Hennenlotter J, et al. Voluntary micturition after intradural nerve anastomosis. Urologe A. 2005;44:756–761. doi: 10.1007/s00120-005-0849-x. [DOI] [PubMed] [Google Scholar]
- Vorstman B, Schlossberg S, Kass L. Investigations on urinary bladder reinnervation. Historical perspective and review. Urology. 1987;30:89–96. doi: 10.1016/0090-4295(87)90168-3. [DOI] [PubMed] [Google Scholar]
- Xiao CG, Du MX, Dai C, et al. An artificial somatic-central nervous system-autonomic reflex pathway for controllable micturition after spinal cord injury: preliminary results in 15 patients. J Urol. 2003;170:1237–1241. doi: 10.1097/01.ju.0000080710.32964.d0. [DOI] [PubMed] [Google Scholar]