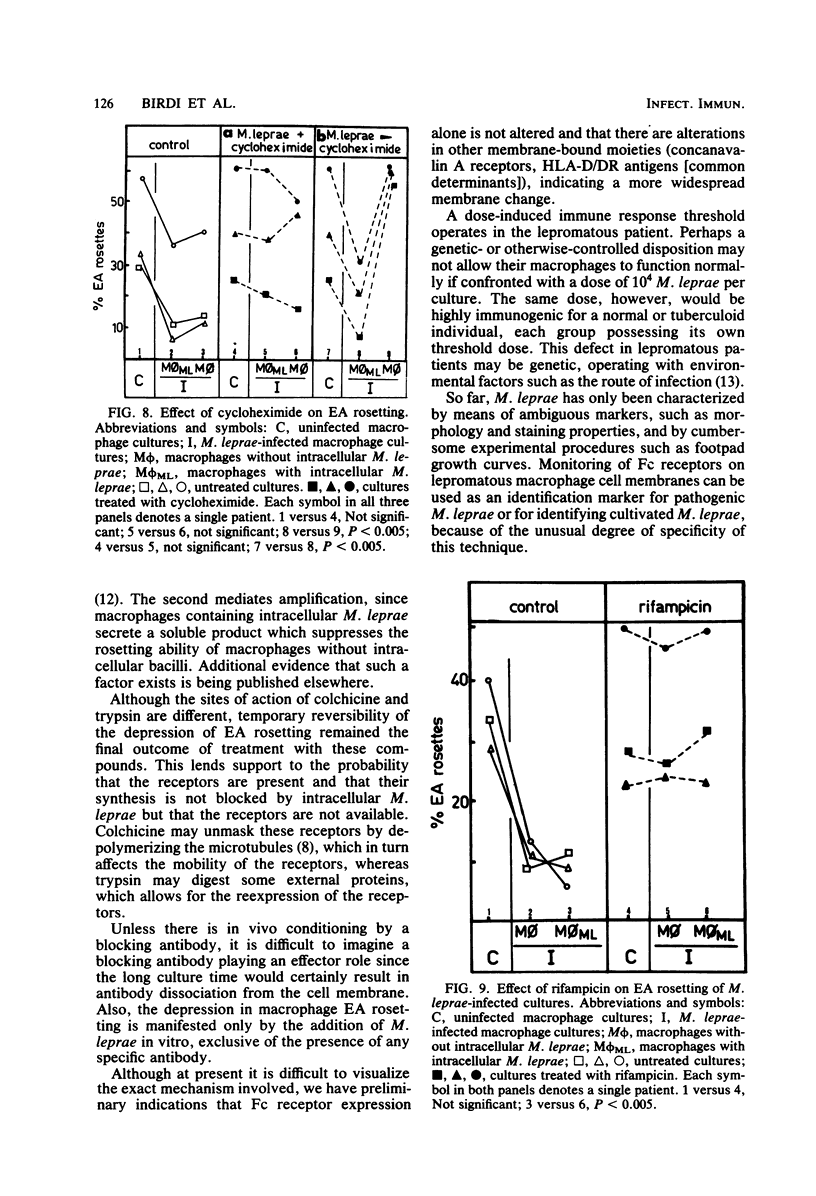
Abstract
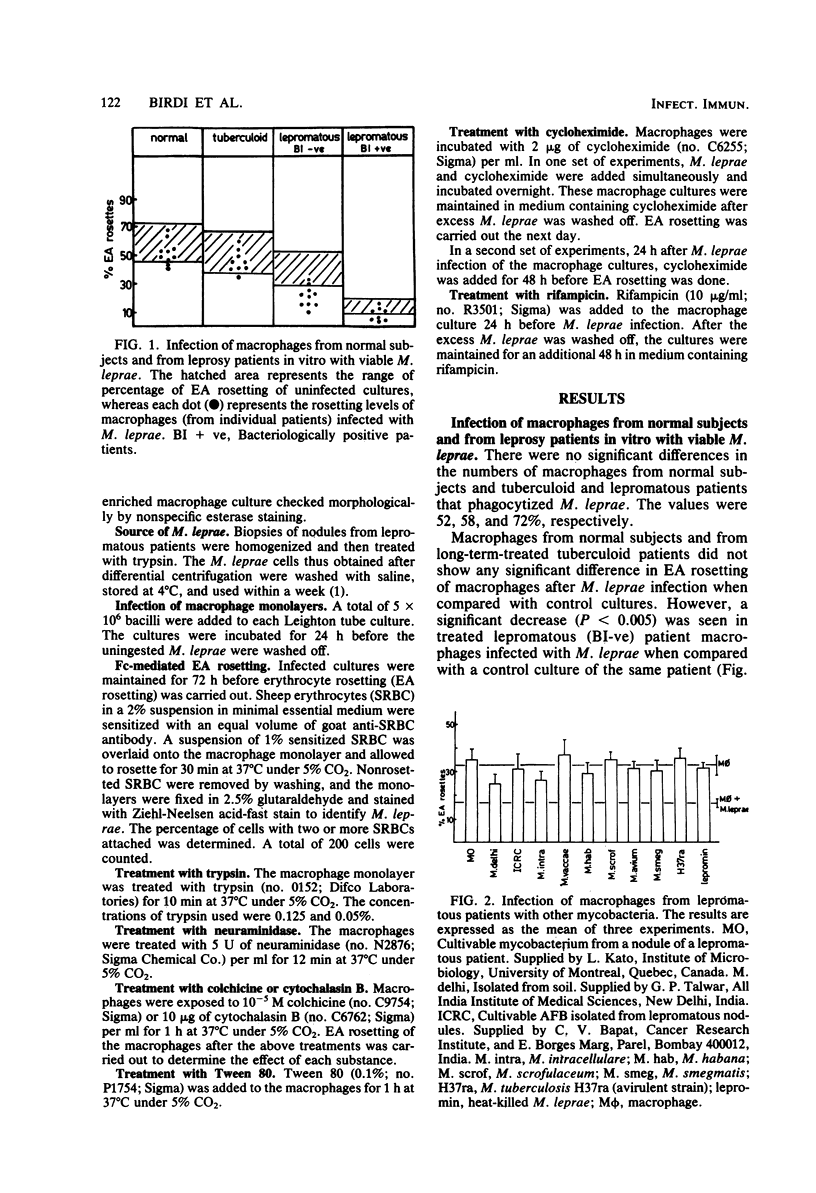
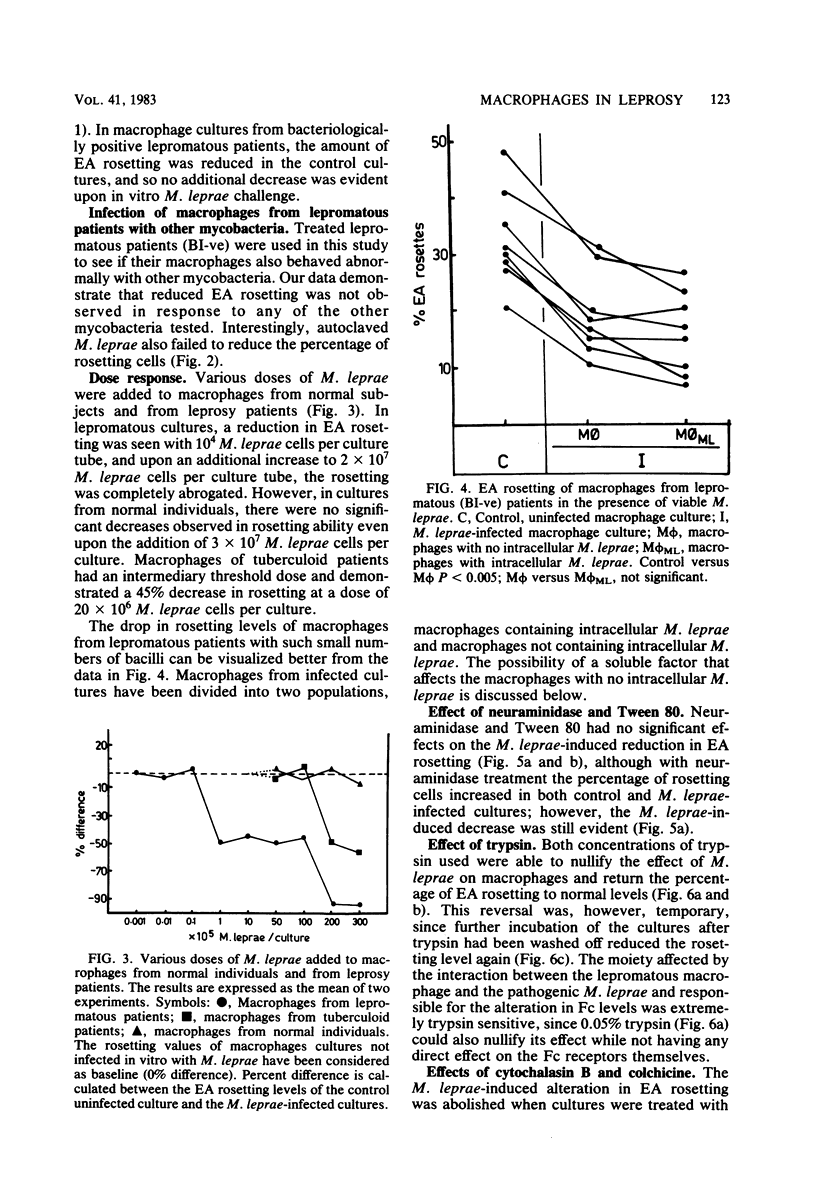
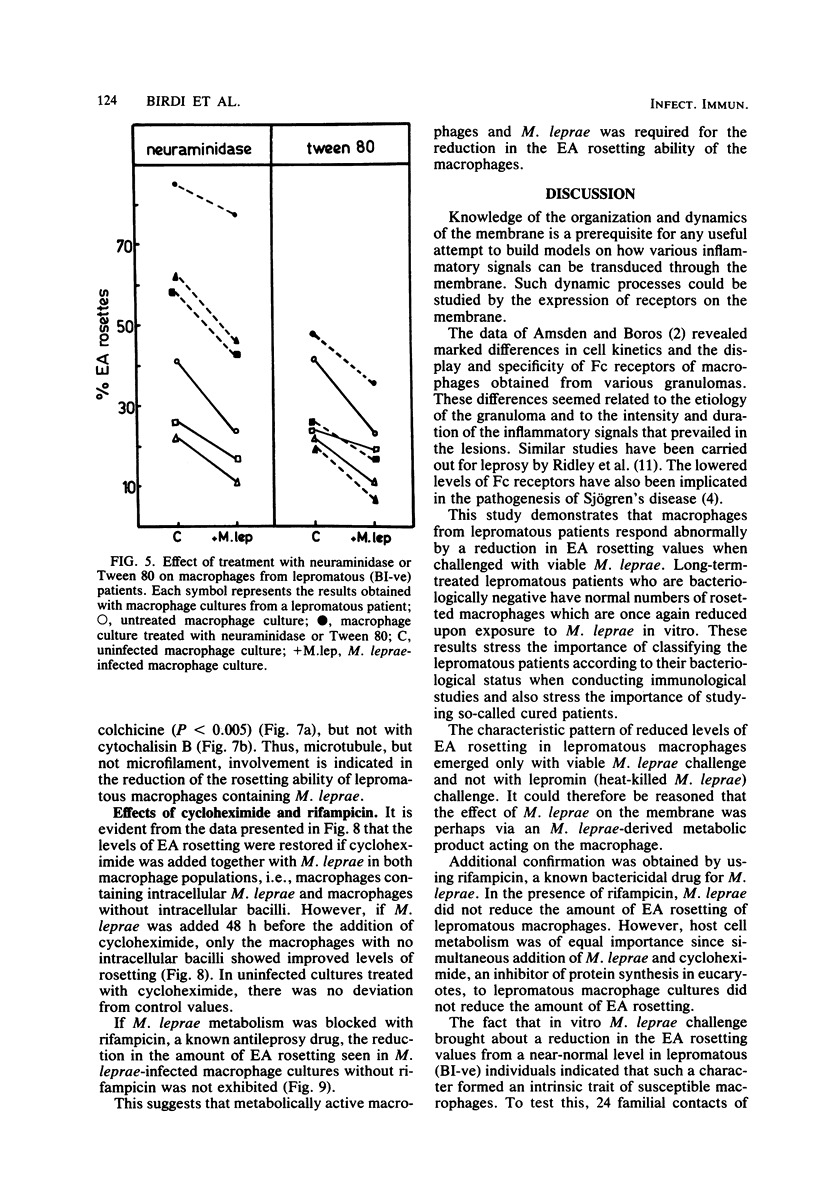
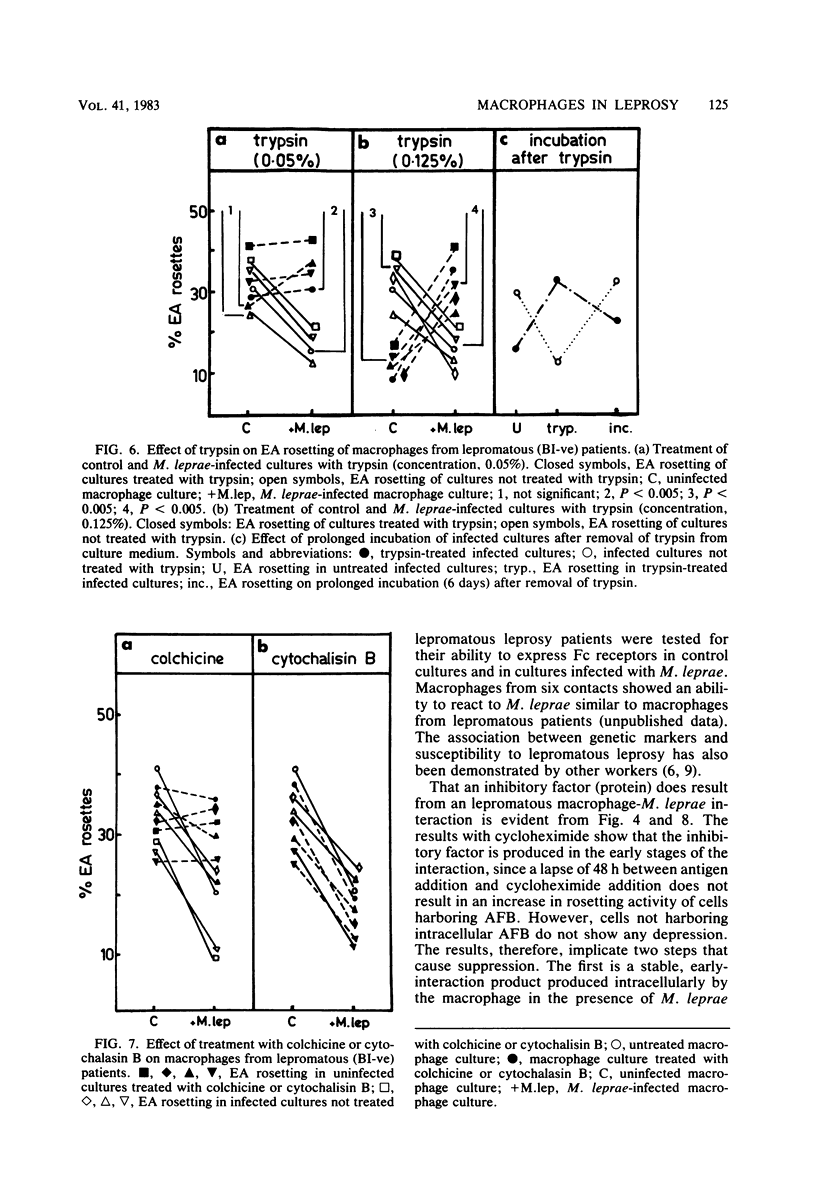
Macrophage cultures pulsed with viable Mycobacterium leprae were assessed for erythrocyte rosetting in three groups of individuals, i.e., normal subjects, and tuberculoid and lepromatous patients. Of these, only the lepromatous group showed a reduction in rosetting ability after infection with M. leprae. The specificity of such a reduction pattern was confirmed by using various mycobacteria to infect the macrophages. A threshold effect was noted in all three groups. Although a reduction was obtained in the amount of rosetting of macrophages from lepromatous patients with 10(4) acid-fast bacilli per culture, tuberculoid and normal macrophages resisted such an effect with as large a dose as 20 X 10(6) to 30 X 10(6) and 30 X 10(6) bacilli per culture, respectively. The M. leprae-caused alterations in macrophages from lepromatous patients were reversible by treatment with trypsin and colchicine. Cytochalasin B and Tween 80 were unable to alter the pattern. Treatment of cells with neuraminidase was inconclusive since it enhanced rosetting values of both control and infected cultures. These manipulations were significant in elucidating the target point of the host (macrophage) and parasite (M. leprae) interaction and in delineation of the external and internal effects upon the macrophages. Both M. leprae and macrophages were participants in Fc reduction, as treatment of the former with rifampicin and of the latter with cyclocheximide significantly augmented the rosetting ability. In conclusion, it appears that M. leprae, upon entering a lepromatous macrophage, initiates the production of a protein which acts via the microtubules to alter membrane topography. It is possible that the altered membrane prevents effective macrophage-lymphocyte interaction. This could be one of the mechanisms by which cell-mediated immunity is suppressed in lepromatous leprosy.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrose E. J., Khanolkar S. R., Chulawalla R. G. A rapid test for bacillary resistance to dapsone. Lepr India. 1978 Apr;50(2):131–143. [PubMed] [Google Scholar]
- Amsden A. F., Boros D. L. Fc-receptor-bearing macrophages isolated from hypersensitivity and foreign-body granulomas. Delineation of macrophage dynamics, fc receptor density/avidity and specificity. Am J Pathol. 1979 Aug;96(2):457–476. [PMC free article] [PubMed] [Google Scholar]
- Birdi T. J., Salgame P. R., Antia N. H. The role of macrophages in leprosy as studied by protein synthesis of macrophages from resistant and susceptible hosts--a mouse and human study. Lepr India. 1979 Jan;51(1):23–42. [PubMed] [Google Scholar]
- Hamburger M. I., Moutsopoulos H. M., Lawley T. J., Frank M. M. Sjögren's syndrome: a defect in reticuloendothelial system Fc-receptor-specific clearance. Ann Intern Med. 1979 Oct;91(4):534–538. doi: 10.7326/0003-4819-91-4-534. [DOI] [PubMed] [Google Scholar]
- Hirschberg H. The role of macrophages in the lymphoproliferative response to Mycobacterium leprae in vitro. Clin Exp Immunol. 1978 Oct;34(1):46–51. [PMC free article] [PubMed] [Google Scholar]
- Kidson C. Population studies: potential and limitations for analysis of genetic and non-genetic factors in leprosy. Lepr Rev. 1981 Dec;52 (Suppl 1):177–185. doi: 10.5935/0305-7518.19810068. [DOI] [PubMed] [Google Scholar]
- Mahadevan P. R., Antia N. H. Biochemical alteration in cells following phagocytosis of M. leprae--the consequence--a basic concept. Int J Lepr Other Mycobact Dis. 1980 Jun;48(2):167–171. [PubMed] [Google Scholar]
- Patarroyo M. E., Molina E., Londoño F., Bernal D., Caro L., Velasques A., Silva Y., Moya R., Guevara J., Meness A. Identification of a particular B cell alloantigen associated with susceptibility to lepromatous leprosy. Lepr Rev. 1981 Dec;52 (Suppl 1):121–135. doi: 10.5935/0305-7518.19810064. [DOI] [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Ridley M. J., Ridley D. S., Turk J. L. Surface markers on lymphocytes and cells of the mononuclear phagocyte series in skin sections in leprosy. J Pathol. 1978 Jun;125(2):91–98. doi: 10.1002/path.1711250204. [DOI] [PubMed] [Google Scholar]
- Salgame P. R., Birdi T. J., Mahadevan P. R., Antia N. H. Role of macrophages in defective cell mediated immunity in lepromatous leprosy. I. Factor(s) from macrophages affecting protein synthesis and lymphocyte transformation. Int J Lepr Other Mycobact Dis. 1980 Jun;48(2):172–177. [PubMed] [Google Scholar]
- Stoner G. L. Importance of the neural predilection of Mycobacterium leprae in leprosy. Lancet. 1979 Nov 10;2(8150):994–996. doi: 10.1016/s0140-6736(79)92564-9. [DOI] [PubMed] [Google Scholar]



