Abstract
Germinal matrix (GM) hemorrhage (GMH) is a major cause of mortality and of life-long morbidity from cerebral palsy (CP). GMH is typically preceded by hypoxic/ischemic events and is believed to arise from rupture of weakened veins in the GM. In the CNS, hypoxia/ischemia upregulate sulfonylurea receptor 1 (SUR1) -regulated NCCa-ATP channels in microvascular endothelium, with channel activation by depletion of ATP being responsible for progressive secondary hemorrhage. We hypothesized that this channel might be upregulated in the GM of preterm infants at risk for GMH. Here, we studied expression of the regulatory subunit of the channel, SUR1, and its transcriptional antecedent, hypoxia inducible factor 1 (HIF1), in postmortem tissues of premature infants who either were at risk for or who sustained GMH. We found regionally specific upregulation of HIF1 and of SUR1 protein and mRNA in GM tissues, compared to remote cortical tissues. Upregulation was prominent in most progenitor cells, whereas in veins, SUR1 was found predominantly in infants who had sustained GMH compared to those without hemorrhage. Our data suggest that the SUR1-regulaterd NCCa-ATP channel may be associated with GMH, and that pharmacological block of these channels could potentially reduce the incidence of this devastating complication of prematurity.
Keywords: cerebral palsy, germinal matrix hemorrhage, sulfonylurea receptor 1, hypoxia inducible factor 1, premature infant, intraventricular hemorrhage, glibenclamide
The neuropathology underlying cerebral palsy includes white matter injury, such as periventricular leukomalacia (PVL) and germinal matrix (GM) hemorrhage (GMH) (1, 2). Each has distinctive features, but both share important risk factors, including prematurity and hypoxia/ischemia, which may occur prenatally or may be due to post-natal ventilatory difficulties that are complicated by mild-to-moderate hypotension (1, 3, 4).
GMH is a common complication of prematurity, occurring in 15–45% of premature infants (3). GMH may range in severity from subependymal hemorrhage (grade 1) to intraventricular hemorrhage without (grade 2) or with (grade 3) ventricular dilatation, to periventricular venous infarction (grade 4). In survivors, neurological sequelae, particularly with higher grade GMH, include cerebral palsy, hydrocephalus requiring ventricular shunting, learning disabilities, and seizures (5, 6). Numerous factors are believed to contribute to GMH, including innate weakness of GM veins, autoregulatory dysfunction, hypoxic/ischemic tissue damage, damage due to post-ischemic reperfusion and increased venous pressure (4, 7–11). The incidence of GMH increases with the degree of prematurity (3), suggesting that advances in perinatal care that yield concomitant increases in the number of extremely premature infants will continue to be hampered by complications of GMH. At present, no effective prevention is available.
Hypoxia/ischemia in rodent and human CNS, both in utero (12) and in adults (13, 14), results in upregulation of sulfonylurea receptor 1 (SUR1). Under pathological conditions, SUR1 upregulation is associated with formation of SUR1-regulated NCCa-ATP channels, not KATP channels (13–15). Expression of SUR1-regulated NCCa-ATP channels in capillary endothelium has been causally implicated in progressive secondary hemorrhage in CNS, with block of these channels by infusion of low-dose (non-hypoglycemogenic) glibenclamide (glyburide) completely preventing secondary hemorrhage (15). Here, we postulated that this channel might be induced in the GM by hypoxia/ischemia, and thereby predispose to GMH. As an initial attempt to assess this hypothesis, we studied expression of the regulatory subunit of the channel, SUR1, and its transcriptional antecedent, hypoxia inducible factor 1 (HIF1) (S. Bhatta and J.M. Simard, unpublished observation), in postmortem tissues of premature infants who either were at risk for or who sustained GMH. We report findings consistent with the hypothesis that the SUR1-regulated NCCa-ATP channel may be causally linked to GMH.
METHODS
Specimens from premature infants without and with clinically diagnosed GMH were obtained from the Brain and Tissue Bank for Developmental Disorders, University of Maryland, Baltimore, with the collection protocol, including informed consent, reviewed and approved by the Institutional Review Board of the University of Maryland at Baltimore. The post-mortem interval was 3–24 hr. Cases were selected for study based either on: (i) the documented presence of GMH/IVH at autopsy or (ii) documented absence of GMH (used as “best-available” controls). Independent histological validation of presence or absence of GMH was made in all cases (see Table 1). In all but one case, the cause of prematurity was preterm rupture of membranes, with some cases also documenting chorioamnionitis by pathological examination of the placenta, and one case (without GMH) being induced for cardiac anomaly. The cause of death was extreme prematurity in all but two cases, with the others being listed as amniotic fluid aspiration syndrome or elective termination.
TABLE 1.
Summary of cases examined.
| Case # | Gestational Age @ birth (weeks) | Hemorrhage clinically | Hemorrhage* in H&E section | HIF1 protein in cells** | SUR1 protein in cells§ | SUR1 protein in veins¶ |
|---|---|---|---|---|---|---|
| 1 | 19 | none | none | + | +++ | 0 |
| 2 | 19 | none | none | ++ | + | 0/+ |
| 3 | 22 | none | none | ++ | +++ | + |
| 4 | 22 | none | none | +++ | ++ | + |
| 5 | 22 | none | none | +++ | + | +/++ |
| 6 | 23 | none | microscopic | ++ | + | +/++ |
| 7 | 24 | none | microscopic | +++ | +++ | +/++ |
| 8 | 24 | grade 1 | grade 1 | +++ | +++ | ++++ |
| 9 | 22 | grade 1 | grade 1 | ++++ | ++++ | ++++ |
| 10 | 24 | grade 2/3 | none | ++++ | ++++ | ++++ |
| 11 | 30 | grade 2/3 | grade 1 | +++++ | +++ | ++++ |
| 12 | 23 | grade 2/3 | grade 2/3 | +++++ | ++ | +++ |
clinical information was available only on “intraventricular hemorrhage” without differentiating further into grade, hence the designation, grade 2/3; some discrepancies in clinical vs. histological evaluation of hemorrhage may be due to histological evaluation of the GM contralateral to the side of hemorrhage, which available data were insufficient to resolve
scale for HIF1 immunolabeling in progenitor cells within the GM: +, present in most cells, similar in intensity to some distant neurons; ++, present in most cells, somewhat more intense than in neurons; +++, present in most cells, definitely more intense than in neurons; ++++, present in all cells, more intense than in neurons; +++++, present in all cells, many with very intense labeling
scale for SUR1 immunolabeling in progenitor cells within the GM: +, present in few single cells; ++, present in a moderate number of scattered cells; +++, present in patches or groups of cells; ++++ present in most cells
scale for SUR1 immunolabeling in veins within the GM: 0, none; +, in 1–2 veins; ++, in a few veins; +++, in many veins; ++++, in nearly all veins.
GM tissues and associated hemorrhages, when present, were dissected from coronal slices of formalin-fixed cerebral hemispheres. Cryosections and paraffin-embedded sections were prepared. Sections were stained with hematoxylin and eosin (H&E) or were immunolabeled using primary antibodies directed against SUR1 (C-16; Santa Cruz Biotechnology Inc.; diluted 1:200; 1 hr at room temperature (RT), 48 hr at 4° C), or HIF-1α (SC-10790; Santa Cruz; 1:100), or von Willebrand factor (F-3520; Sigma; 1:200). CY-3 or FITC conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA) were used. Slides were cover slipped with ProLong Gold antifade reagent containing 4',6-diamino-2-phenylindole (DAPI) for nuclear staining (P36935, Invitrogen, Carlsbad, CA). For in situ hybridization, digoxigenin-labeled probes (antisense, 5’-TGCAGGGGTCAGGGTCAGGGcGCTGTCGGTCCACTTGGCCAGCCAGTA-3’), designed to hybridize to nucleotides 3217–3264 located within coding sequence of human Abcc8 gene (NM_00352), were supplied by GeneDetect (Brandenton, FL). Hybridization was performed according to the manufacturer’s protocol, as previously described (13).
RESULTS
The germinal matrix appeared as a dense collection of small cells located periventricularly (Fig. 1A). In some cases, evidence of a parenchymal hemorrhage was found (Fig. 1A, arrow).
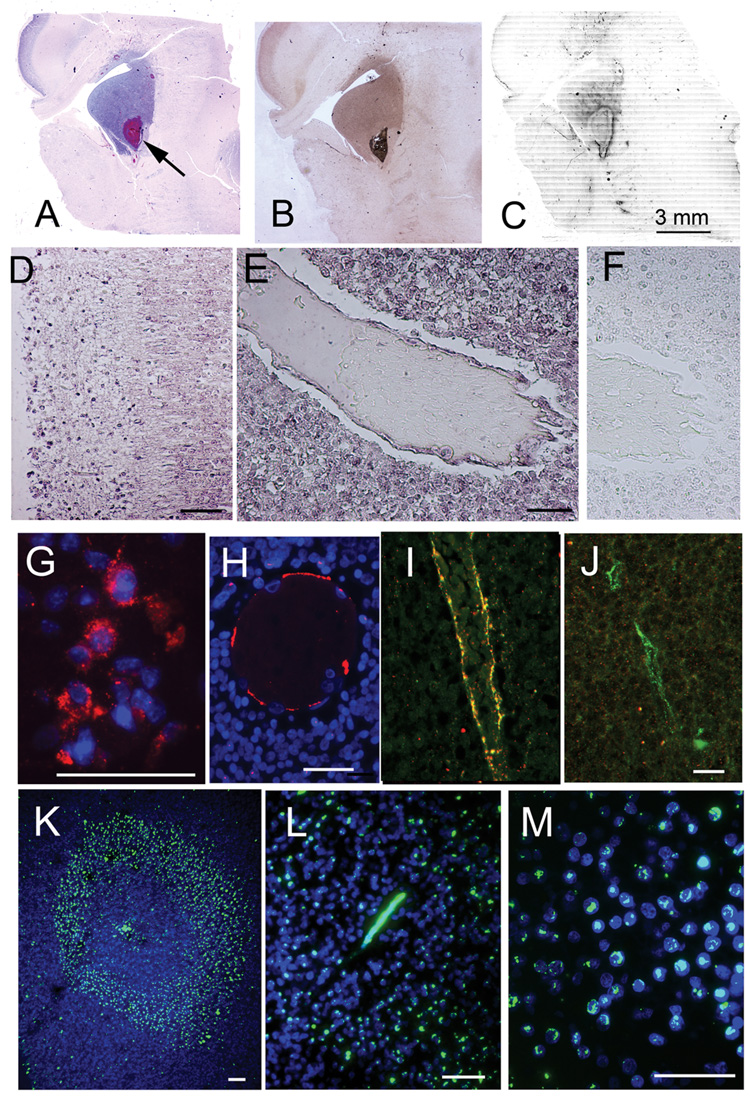
Figure 1. SUR1 and HIF1 are upregulated in the germinal matrix of premature infants.
A–C: Low power micrographs (A,B) or montage of micrographs (C) of periventricular tissue stained with H&E (A), showing densely packed neural progenitor cells of the GM, with an arrow pointing to a small intraparenchymal hematoma, or labeled for mRNA for Abcc8, which encodes SUR1, using in situ hybridization (B), or immunolabeled for SUR1 (C); the latter two demonstrate regionally-specific labeling for SUR1 mRNA and protein in the GM; the montage in (C) shows positive immunolabeling in black pseudocolor; case #9 in Table 1: premature infant of 22 wk gestation who lived ~12 hr and was hypoxic prior to death, necessitating intubation and mechanical ventilation; post-mortem interval, 3 hr. D–F: Micrographs of cortical tissues (D) or GM tissues (E,F) processed for in situ hybridization for mRNA for Abcc8, using antisense probe (D,E) or sense probe (F). G–J: Micrographs of GM tissues immunolabeled for SUR1 (red, CY3 for SUR1, and blue, DAPI for nuclei), and double-labeled for von Willebrand factor (green; panels I and J only); co-labeling is indicated by yellow color; SUR1 was identified in neural progenitor cells (G), and in thin-walled veins from infants with GMH (panel H, red and panel I, yellow) but not in an infant without GMH (panel J, green); panels H, I, J are from cases #11, 10, 1 in Table 1, respectively. K–M: Low (K) and high (L,M) power micrographs of sections immunolabeled for HIF1α (green, FITC for HIF1α, and blue, DAPI for nuclei), showing HIF1α in a microvessel (L) and in neural progenitor cells (M). In panels D–M, the bars represent 50 µm.
In situ hybridization for mRNA for Abcc8, which encodes SUR1, showed regionally specific upregulation in the GM (Fig. 1B,E) that was noticeably more prominent than in surrounding tissues or in remote cortical tissues (Fig. 1D). Immunolabeling confirmed regionally specific upregulation of SUR1 protein in the GM (Fig. 1C), with SUR1 protein located in neural progenitor cells in all GM specimens examined (Fig. 1G). SUR1 protein was also identified in veins from infants with GMH (Fig. 1H,I), but was less likely to be found in veins from infants without GMH (Fig. 1J). Negative controls, including omission of primary antibody and use of a blocking peptide, showed no immunolabeling for SUR1 (not shown).
An important molecular antecedent of SUR1 is the transcription factor, HIF1 (S. Bhatta and J.M. Simard, unpublished observation), which is upregulated by hypoxia (16), a common condition associated with prematurity. Immunolabeling for HIF1α showed that this ubiquitous marker of hypoxia was prominently upregulated, with characteristic nuclear localization, in all GM specimens examined (Fig. 1K–M).
We performed a semi-quantitative assessment of HIF1αand SUR1 expression in specimens from 12 premature infants, some of whom had either clinical or histological evidence of GMH (Table 1). All specimens showed HIF1α expression, with all but one showing more prominent expression in progenitor cells than in remote neurons in the same tissue sections, supporting the hypothesis that physiologically meaningful hypoxia was present in the GM of all of these cases. The most prominent expression of HIF1α was found in specimens from infants with frank GMH. All specimens showed SUR1 expression in progenitor cells. In 3 specimens, SUR1 was identified only in scattered cells, whereas in most specimens, SUR1 expression was evident in contiguous sheets of cells or in some cases, in nearly all cells. The clearest distinction in SUR1 expression vis-à-vis GMH was in the veins of the GM. In specimens without GMH, the veins typically exhibited little to moderate SUR1 expression, as in Fig. 1J, whereas in all specimens with frank GMH, all or nearly all veins exhibited strong SUR1 expression, as in Fig. 1H,I.
DISCUSSION
We provide evidence that expression of SUR1 is increased in neural progenitor cells and in vascular endothelium of the GM of premature infants who either are at risk for or who sustained GMH. Immunohistochemical analysis of post-mortem tissues can sometimes be complicated by non-specific binding of antibodies, especially if necrosis is present. However, the specimens we studied showed intact cellular structures with H&E staining, as well as regionally-specific immunolabeling of cellular and vascular structures for SUR1 in the GM. Most importantly, we used in situ hybridization to confirm that SUR1 was upregulated at the mRNA level. Together, the two independent techniques using molecularly distinct probes provide important corroborative evidence that SUR1 was upregulated in GM tissues of premature infants. Additional work will be required to demonstrate concomitant upregulation of the pore-forming subunit of the channel (14).
Pathophysiology
The pathophysiological antecedents of GMH have been extensively discussed, but no fully encompassing theory has been put forth to explain it. Considerable attention has been focused on the structural weakness of GM microvessels (8,9). However, it is evident that any innate weakness of these vessels, by itself, would be insufficient to cause GMH, since the same weakness exists during every gestation, and most gestations are not complicated by GMH. Thus, an event must transpire to weaken these vessels further and increase the likelihood of their structural failure. In the premature brain, the GM is at the terminal end of its afferent arteriolar supply (“ventriculopetal” vascular pattern) (17) and therefore GM tissues and the vessels contained therein are highly susceptible to global hypoxic/ischemic events. Apart from hypoxia due to ventilatory abnormalities, one or more hypotensive episodes may contribute to the overall hypoxic/ischemic burden that adversely affects GM tissues. In addition, it is likely that yet another hemodynamic stress must be applied to structurally compromised vessels to cause an actual GMH. Because GMH most frequently arises from veins (7, 11), it is thought that episodes of increased venous pressure, as can occur with mechanical ventilation or airway suctioning, may be important for triggering the actual structural failure of weakened vessels that results in GMH.
Despite the important role of hypoxia/ischemia in producing vascular changes that predispose to GMH, there is little experimental evidence to elucidate the molecular mechanism involved, either in animal models or in humans. To our knowledge, ours is the first report to show that the transcription factor, HIF1, is upregulated in the GM of infants at risk. In many organs including the CNS, hypoxia results in activation of HIF1, which in turn stimulates the transcription of genes that are essential for adaptation to hypoxia/ischemia, including genes important for erythropoiesis, glycolysis and angiogenesis (16). HIF1 plays a critical role in expression of the angiogenic factor, vascular endothelial growth factor (VEGF), which is prominently upregulated in the GM of infants at risk (18). Conversely, HIF1 also causes transcription of genes with seemingly maladaptive effects (19) and, in some settings, may promote ischemia-induced neuronal death (20). HIF1 has not been extensively studied in the premature infant brain, and a role for HIF1 has not previously been suggested in the context of GMH. However, the localization of HIF1 with two of its important transcriptional targets, VEGF (18) and SUR1, in the GM of infants at risk reaffirms the importance of this molecular response to hypoxia.
Events in the GM
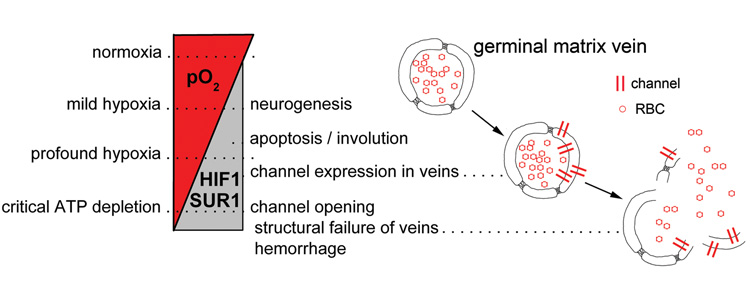
Mild hypoxia activates quiescent neural progenitor cells, resulting in their activation and differentiation into neurons and glia, whereas severe hypoxia induces apoptotic death in developing brain neurons (21). Thus, mild-to-moderate hypoxia, resulting from the position of the GM as the distant-most tissue fed by a ventriculopetal blood supply (17), may be involved not only in stimulating neurogenesis from GM progenitor cells, but also in the normal involution of the GM (Fig. 2). HIF1, the ubiquitous sensor of hypoxia, may be a key molecular participant in both. Notably, the same hypoxic signal working via HIF1 also leads to transcriptional upregulation of SUR1 (S. Bhatta and J.M. Simard, unpublished observation) and of SUR1-regulated NCCa-ATP channels (15). In all of the 12 cases studied, most of the progenitor cells exhibited both HIF1 and SUR1, suggesting that mild hypoxia may be a normal state in germinal matrix parenchyma, and that this tissue may be normally “primed” with SUR1. When the NCCa-ATP channel is expressed in response to an hypoxic stimulus, no adverse functional consequence is expected, as long as intracellular ATP is maintained at sufficient levels (>30 µM) to keep the channel from opening (14).
Figure 2. Events in the germinal matrix of premature infants.
Scheme depicting the reciprocal relationship between O2 tension on the one hand, and HIF1 activation and SUR1 expression on the other hand. Mild hypoxia, which may be the norm due to the ventriculopetal blood supply, promotes neurogenesis, whereas moderate hypoxia may promote apoptosis resulting in involution of the GM. More severe hypoxia may promote expression of SUR1-regulated NCCa-ATP channels, which remain inactive until critical ATP depletion is reached (~30 µM), at which point the channels open, leading to oncotic death of cells, including endothelial cells, thereby compromising the structural integrity of veins and predisposing to GMH during episodes of venous hypertension.
Under conditions of extreme duress, a normal hypoxic signal may be magnified by one or more ischemic events, leading to more profound hypoxia. Under such conditions, HIF1 activation and SUR1 expression would become more likely, especially in veins (Fig. 2). Normally, cells of the vascular tree are less likely than parenchymal cells to experience hypoxia, but under conditions of extreme duress, when maximum extraction of O2 has already occurred from hypoxic blood, venular cells will experience the strongest hypoxic challenge. In the cases we studied, veins generally were less likely to exhibit SUR1 than parenchymal cells, but in cases with GMH, SUR1 expression was reliably found in most veins – the very structures that are believed to be the source of hemorrhage (7, 11). When ATP is depleted to critical levels, SUR1-regulated NCCa-ATP channels open, leading to oncotic cell death (13) not only of progenitor cells but of vascular endothelial cells, thereby further weakening thin walled, structurally compromised veins. In this setting, increased venous pressure would almost certainly cause extravasation of blood from damaged veins. Petechial hemorrhages may enlarge to microhemorrhages or grade 1 GMH, or worse, depending on the severity and extent of GM tissues involved. We believe that this sequence (Fig. 2), postulating critical involvement of HIF1 and SUR1, accounts for numerous observations and encompasses numerous hypotheses that have been put forth to explain GMH.
Preventing GMH
Available strategies for preventing GMH are limited. Currently, the most effective measures are those that target the respiratory system (22, 23). Vitamin E, phenobarbital, morphine, ibuprofen, indomethacin, agents that target coagulation, and magnesium/aminophylline have been tried, but are either ineffective or their use remains controversial. In an animal model of GMH, prenatal treatment with angiogenic inhibitors reduces the incidence of GMH (18), but angiogenic suppression in premature infants would be undesirable, since it could impair lung maturation (24). Novel treatment strategies are desperately needed to combat GMH. Block of SUR1 using glibenclamide may be such a treatment. Glibenclamide pretreatment in humans is associated with significantly better outcomes from stroke (14, 25), and constant infusion of drug at doses below those that give hypoglycemia is highly effective in preventing progressive secondary hemorrhage in the CNS (15). The findings reported here are consistent with the hypothesis that the SUR1-regulated NCCa-ATP channel may be causally linked to GMH. If corroborated, involvement of the channel would suggest that glibenclamide might be useful in reducing the incidence of this devastating complication of prematurity.
Acknowledgments
Financial Support: This work was supported by grants to JMS from the National Heart, Lung and Blood Institute (HL082517), the National Institute of Neurological Disorders and Stroke (NS048260), the Department of Veterans Affairs (Baltimore, MD), and the Christopher and Dana Reeve Foundation.
Abbreviations
- DAPI
4',6-diamino-2-phenylindole
- GM
germinal matrix
- GMH
germinal matrix hemorrhage
- H&E
hematoxylin and eosin
- HIF1
hypoxia inducible factor 1
- SUR1
sulfonylurea receptor 1
Footnotes
Conflict of interest: JMS holds a US patent (# 7,285,574), "A novel non-selective cation channel in neural cells and methods for treating brain swelling".
Publisher's Disclaimer: Pediatric Research Articles Ahead of Print contains articles in unedited manuscript form that have been peer-reviewed and accepted for publication. As a service to our readers, we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting and review of the resulting proof before it is published in its final definitive form. Please note that during the production process errors may be discovered, which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vergani P, Locatelli A, Doria V, Assi F, Paterlini G, Pezzullo JC, Ghidini A. Intraventricular hemorrhage and periventricular leukomalacia in preterm infants. Obstet Gynecol. 2004;104:225–231. doi: 10.1097/01.AOG.0000130838.02410.b7. [DOI] [PubMed] [Google Scholar]
- 2.Folkerth RD. Neuropathologic substrate of cerebral palsy. J Child Neurol. 2005;20:940–949. doi: 10.1177/08830738050200120301. [DOI] [PubMed] [Google Scholar]
- 3.Kadri H, Mawla AA, Kazah J. The incidence, timing, and predisposing factors of germinal matrix and intraventricular hemorrhage (GMH/IVH) in preterm neonates. Childs Nerv Syst. 2006;22:1086–1090. doi: 10.1007/s00381-006-0050-6. [DOI] [PubMed] [Google Scholar]
- 4.Lou HC. On the pathogenesis of germinal layer hemorrhage in the neonate. APMIS. 1993;40 Suppl:97–102. [PubMed] [Google Scholar]
- 5.Levy ML, Masri LS, McComb JG. Outcome for preterm infants with germinal matrix hemorrhage and progressive hydrocephalus. Neurosurgery. 1997;41:1111–1117. doi: 10.1097/00006123-199711000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Pikus HJ, Levy ML, Gans W, Mendel E, McComb JG. Outcome, cost analysis, and long-term follow-up in preterm infants with massive grade IV germinal matrix hemorrhage and progressive hydrocephalus. Neurosurgery. 1997;40:983–988. doi: 10.1097/00006123-199705000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura Y, Okudera T, Fukuda S, Hashimoto T. Germinal matrix hemorrhage of venous origin in preterm neonates. Hum Pathol. 1990;21:1059–1062. doi: 10.1016/0046-8177(90)90256-5. [DOI] [PubMed] [Google Scholar]
- 8.Wei W, Xin-Ya S, Cai-Dong L, Zhong-Han K, Chun-Peng C. Relationship between extracellular matrix both in choroid plexus and the wall of lateral ventricles and intraventricular hemorrhage in preterm neonates. Clin Anat. 2000;13:422–428. doi: 10.1002/1098-2353(2000)13:6<422::AID-CA5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 9.Anstrom JA, Brown WR, Moody DM, Thore CR, Challa VR, Block SM. Subependymal veins in premature neonates: implications for hemorrhage. Pediatr Neurol. 2004;30:46–53. doi: 10.1016/s0887-8994(03)00404-1. [DOI] [PubMed] [Google Scholar]
- 10.Berger R, Garnier Y, Jensen A. Perinatal brain damage: underlying mechanisms and neuroprotective strategies. J Soc Gynecol Investig. 2002;9:319–328. [PubMed] [Google Scholar]
- 11.Ghazi-Birry HS, Brown WR, Moody DM, Challa VR, Block SM, Reboussin DM. Human germinal matrix: venous origin of hemorrhage and vascular characteristics. AJNR Am J Neuroradiol. 1997;18:219–229. [PMC free article] [PubMed] [Google Scholar]
- 12.Xia Y, Eisenman D, Haddad GG. Sulfonylurea receptor expression in rat brain: effect of chronic hypoxia during development. Pediatr Res. 1993;34:634–641. doi: 10.1203/00006450-199311000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Simard JM, Chen M, Tarasov KV, Bhatta S, Ivanova S, Melnitchenko L, Tsymbalyuk N, West GA, Gerzanich V. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med. 2006;12:433–440. doi: 10.1038/nm1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simard JM, Woo SK, Bhatta S, Gerzanich V. Drugs acting on SUR1 to treat CNS ischemia and trauma. Curr Opin Pharmacol. 2008;8:42–49. doi: 10.1016/j.coph.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simard JM, Tsymbalyuk O, Ivanov A, Ivanova S, Bhatta S, Geng Z, Woo SK, Gerzanich V. Endothelial sulfonylurea receptor 1-regulated NC Ca-ATP channels mediate progressive hemorrhagic necrosis following spinal cord injury. J Clin Invest. 2007;117:2105–2113. doi: 10.1172/JCI32041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE 2005. 2005:re12. doi: 10.1126/stke.3062005re12. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura Y, Okudera T, Hashimoto T. Vascular architecture in white matter of neonates: its relationship to periventricular leukomalacia. J Neuropathol Exp Neurol. 1994;53:582–589. doi: 10.1097/00005072-199411000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Ballabh P, Xu H, Hu F, Braun A, Smith K, Rivera A, Lou N, Ungvari Z, Goldman SA, Csiszar A, Nedergaard M. Angiogenic inhibition reduces germinal matrix hemorrhage. Nat Med. 2007;13:477–485. doi: 10.1038/nm1558. [DOI] [PubMed] [Google Scholar]
- 19.Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–268. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang YC, Huang CC. Perinatal brain injury and regulation of transcription. Curr Opin Neurol. 2006;19:141–147. doi: 10.1097/01.wco.0000218229.73678.a8. [DOI] [PubMed] [Google Scholar]
- 21.Pourie G, Blaise S, Trabalon M, Nedelec E, Gueant JL, Daval JL. Mild, non-lesioning transient hypoxia in the newborn rat induces delayed brain neurogenesis associated with improved memory scores. Neuroscience. 2006;140:1369–1379. doi: 10.1016/j.neuroscience.2006.02.083. [DOI] [PubMed] [Google Scholar]
- 22.Cools F, Offringa M. Neuromuscular paralysis for newborn infants receiving mechanical ventilation. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD002773.pub2. CD002773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright LL, Horbar JD, Gunkel H, Verter J, Younes N, Andrews EB, Long W. Evidence from multicenter networks on the current use and effectiveness of antenatal corticosteroids in low birth weight infants. Am J Obstet Gynecol. 1995;173:263–269. doi: 10.1016/0002-9378(95)90211-2. [DOI] [PubMed] [Google Scholar]
- 24.Thebaud B. Angiogenesis in lung development, injury and repair: implications for chronic lung disease of prematurity. Neonatology. 2007;91:291–297. doi: 10.1159/000101344. [DOI] [PubMed] [Google Scholar]
- 25.Kunte H, Schmidt S, Eliasziw M, del Zoppo GJ, Simard JM, Masuhr F, Weih M, Dirnagl U. Sulfonylureas improve outcome in patients with type 2 diabetes and acute ischemic stroke. Stroke. 2007;38:2526–2530. doi: 10.1161/STROKEAHA.107.482216. [DOI] [PMC free article] [PubMed] [Google Scholar]