Abstract
The kinetic pathway of CysM, a cysteine synthase from Mycobacterium tuberculosis, the expression of which is upregulated under conditions of oxidative stress, was studied by transient-state kinetic techniques. This enzyme exhibits extensive homology with the B-isozymes of the well-studied O-acetylserine sulfhydrylases and employs a similar chemical mechanism involving a stable α-aminoacrylate intermediate. However, we show that specificity of CysM for its amino acid substrate is more than 500-fold greater for O-phospho-L-serine than for O-acetyl-L-serine, suggesting that O-phospho-L-serine is the likely substrate in vivo. We also investigated the kinetics of the carbon-sulfur bond-forming reaction between the CysM-bound α-aminoacrylate intermediate and the thiocarboxylated sulfur-carrier protein, CysO-COSH. The specificity of CysM for this physiological sulfide equivalent is more than three orders of magnitude greater than that for bisulfide. Moreover, the kinetics of this latter reaction are limited by association of the proteins, whilst the reaction with bisulfide is consistent with a rapid equilibrium binding model. We interpret this finding to suggest that the CysM active site with the bound aminoacrylate intermediate is protected from solvent and that binding of CysO-COSH produces a conformational change allowing rapid sulfur transfer. This study represents the first detailed kinetic characterization of sulfide transfer from a sulfide carrier protein.
Mycobacterium tuberculosis is a widespread and dangerous pathogen which exerts significant deleterious effects on humans both from health and economic standpoints (1). An understanding of the metabolism of this bacterium is clearly advantageous in order to establish means for its control and treatment of the symptoms which infection produces in humans. This is particularly the case where the possibility of discovering new metabolic pathways specific to M. tuberculosis exists, as these may be exploited by the development of therapeutic agents.
The biosynthesis of L-cysteine is thought to occur via at least four pathways (Scheme 1). The first of these was reported by Kredich and Tompkins and involves two enzymatic steps (2) beginning with L-serine. The first step is activation as a leaving group of the serine hydroxyl by acetylation, catalyzed by the acetyl-CoA-dependent enzyme serine acetyltransferase (3). O-acetyl-L-serine then undergoes a β-replacement reaction catalyzed by a PLP-dependent cysteine synthase. In Salmonella typhimurium bisulfide (HS-) has been shown to be a nucleophilic substrate for the β-replacement reaction, and the kinetics of the S. typhimurium enzyme have been studied extensively. These studies have been reviewed by Tai and Cook (4). A variation on this pathway has been identified in the hyperthermophilic archaeon Aeropyrum pernix, where O-phospho-L-serine is the immediate biosynthetic precursor for L-cysteine (5, 6). The catabolism of L-cystathionine by L-cystathionine γ-lyase to produce L-cysteine, 2-oxobutyrate and ammonia is thought to be the predominant biosynthetic route for cysteine in eukaryotic and mammalian systems but also has been shown to take place in some bacteria (7). A fourth pathway in the methanogenic archaea was recently described which is tRNA-dependent and proceeds via the O-phosphoseryl-tRNACys intermediate which can be converted to cysteinyl-tRNACys and thence to cysteine (8).
Scheme 1.
Biosynthetic pathways for L-cysteine
Inspection of the sequenced M. tuberculosis genome reveals the presence of four loci assigned as coding for cysteine synthase enzymes. Locus Rv2334, coding for the cysK gene is contiguous with locus Rv2335 (cysE), proposed to code for serine acetyltransferase. A second open reading frame (Rv0848) contains the cysK1 gene but no serine acetyltransferase is present. Locus Rv3684 is also proposed to code for a cysteine synthase gene, but is also not clustered with the serine acetyltransferase, instead being neighbored by regions coding for a proposed phosphohydrolase enzyme (Rv3683) and prolyl tRNA (Rvnt40). The fourth and final open reading frame, Rv1336, codes for a cysteine synthase (CysM) which is found clustered with, amongst others, a gene coding for a small sulfur carrier protein (CysO).
Recently work from our laboratory reported the reconstitution of a new cysteine biosynthetic pathway in M. tuberculosis (9) which involves CysM. This enzyme has extensive sequence homology to the O-acetylserine sulfhydrylases and is predicted on this basis to belong to the O-acetylserine sulfhydrylase B family of enzymes. The new pathway utilizes the 93-amino acid sulfur carrier protein (CysO), which clusters with CysM in the M. tuberculosis H37Rv genome. Similar sulfur carrier proteins have been identified in the biosynthetic pathways for thiamin (10) and molybdopterin (11) and quinolobactin (12, 13). These proteins also show homology to components of the system which targets doomed proteins by ubiquitination for degradation by the proteasome (14). A key feature of these proteins is a flexible C-terminal Gly-Gly tail which can insert into the active site of their partner enzymes to facilitate sulfur transfer. It has been shown that to form the C-terminal thiocarboxylate, the proteins are first activated by adenylation of their C-termini and then undergo nucleophilic addition-elimination chemistry with a sulfide equivalent, the production of which involves enzymes such as cysteine desulfurases or rhodanese homology domain proteins. The M. tuberculosis moeZ (Rv3206) gene product (15) contains such a rhodanese homology domain as well as a ThiF-like domain and was shown to catalyze the formation of the CysO thiocarboxylate using an unidentified sulfur source in cell-free extract. ThiF is the enzyme responsible for the adenylation of ThiS-COOH in the thiamin biosynthetic pathway in prokaryotes (16). The expression of MoeZ is upregulated under the same conditions that produce upregulation of CysO and CysM expression. A zinc-dependent hydrolase Mec+ (17) was shown to catalyze the selective hydrolysis of the CysO-cysteine adduct formed after attack of thiocarboxylated CysO (CysO-COSH) at the α-aminoacrylate intermediate formed at the CysM active site. The mec+ gene (Rv1334) clusters with the cysO and cysM genes.
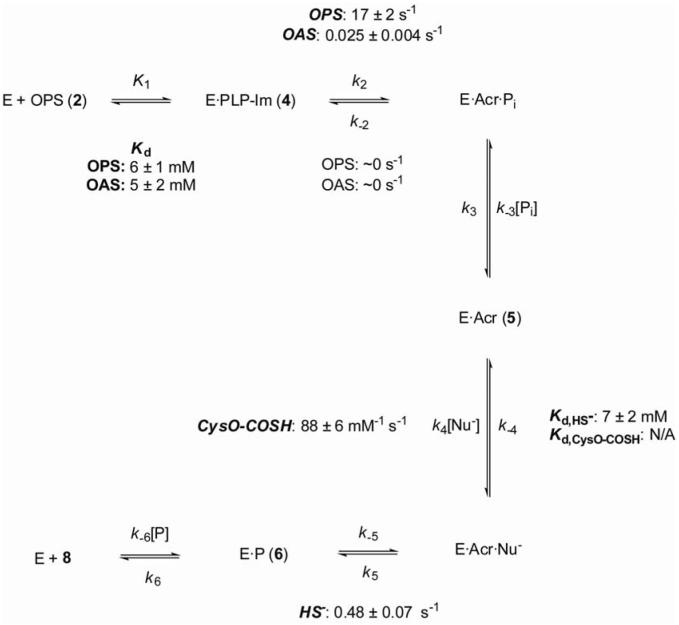
Given that no acetyltransferase gene is found clustered with the cysM and cysO genes, there is no reason to assume a priori that O-acetyl-L-serine is the physiological amino acid substrate for CysM. In order to test this hypothesis, and to investigate the reaction of CysO-COSH as the nucleophilic substrate for CysM, we have characterized the CysM kinetic pathway under transient-state and single-turnover conditions using both O-acetyl- and O-phospho-L-serine as the amino acid substrates and CysO-COSH and bisulfide as the nucleophilic substrates. The proposed chemical mechanism for CysM is shown in Scheme 2. In brief, this mechanism involves formation of an imine (4) between CysM-bound pyridoxal 5′-phosphate and the amino acid substrate via a transient geminal diamine intermediate (3), followed by elimination across the α,β bond of the substrate to form a relatively stable α-aminoacrylate intermediate (5). 1,4-Addition of a suitable nucleophile to this intermediate affords the β-substituted amino acid imine (6), which undergoes transimination with an active site lysine residue to release the product (8) and regenerate the active site for catalysis.
Scheme 2.
Proposed chemical mechanism for CysM
MATERIALS and METHODS
All reagents and chemicals were of the highest purity commercially available. Sodium sulfide (90 % dry, balance water) was obtained from Acros Organics. Stopped-flow experiments were performed using a KinTek stopped-flow spectrophotometer (KinTek Corporation). All reactions were carried out in 50 mM Tris-HCl at pH 8. Protein concentrations were determined by the method of Bradford (18) and the concentrations thus-determined for CysM were in agreement with those calculated from the absorbance (19) of the enzyme-PLP internal imine (1, ε412 = 7600 M-1 cm-1) and the α-aminoacrylate intermediate (5, ε465 ∼ 9800 M-1 cm-1).
Overexpression and purification of proteins
CysM was overexpressed in Escherichia coli BL21(DE3) using a pET16b vector and purified by nickel affinity chromatography. The thiocarboxylated sulfur carrier protein CysO-COSH was overexpressed as a fusion with an intein protein possessing a chitin binding domain using a modification of the method of Kinsland et al. (20). The cysO gene was expressed in pTYB1 in E. coli grown at 24 °C. The protein was purified using chitin affinity chromatography. The C-terminal thiocarboxylate was generated by soaking the chitin resin-bound protein obtained from clarified cell lysate in a cleavage buffer containing 25 mM (NH4)2S, 50 mM NaCl and 1 mM EDTA at pH 8 and at 4 °C for 40 h before elution. All proteins were buffer-exchanged into 50 mM Tris-HCl, pH 8 and concentrated prior to use.
Data analysis
Single-wavelength stopped-flow data for formation and decay of the α-aminoacrylate were fit to exponential functions with the general form of equation (1):
| (1) |
where At is the absorbance at time t, A∞ is the absorbance at time ∞, Ai is the amplitude of the ith transient and ki is the phenomenological observed first order rate constant (kobs) for the ith transient. The data for formation and quenching of the α-aminoacrylate intermediate which showed a hyperbolic dependence of the phenomenological first order rate constants for formation on the concentration of substrates were fit to equation (2) which describes a rapid equilibrium binding model. Details of this model are treated in the discussion. The kinetic and thermodynamic constants K1 = 1/Kd, k2 and k-2 are defined in Scheme 2. [S] is the substrate concentration.
| (2) |
Linear data for dependence of the first order rate constants for decay of the aminoacrylate were fit to equation (3):
| (3) |
where kqapp is the apparent second order rate constant for quenching and kb is a complex function of mechanistic rate constants contributing to re-formation of the aminoacrylate. Linear data for formation of the aminoacrylate were fit to equation (4):
| (4) |
where kfapp is the apparent second order rate constant for quenching and kc is a complex function of mechanistic rate constants contributing to the reverse reaction.
Ultraviolet-visible spectroscopy of formation of the α-aminoacrylate intermediate
CysM (0.76 mg/mL) was treated with excess O-acetyl-L-serine (5 mM) in 50 mM Tris at pH 8 and at room temperature. Absorbance spectra in the region 300 – 550 nm were taken manually at intervals of approximately 34 s until no further change in the spectra was observed. In the case of O-phospho-L-serine, the enzyme (14 μM) was mixed with OPS (10 μM) and the absorbance spectra were recorded in the 380-500 nm region, with individual traces recorded at intervals of approximately 30 s.
Single-wavelength kinetics of formation of the α-aminoacrylate intermediate
The instrumentation used varied with the timescale for aminoacrylate formation. Solutions of O-acetyl-L-serine were prepared freshly immediately before each experiment. All concentrations are final after mixing. In the case of O-acetyl-L-serine, CysM (20 μM) was treated with solutions of O-acetyl-L-serine (500 μM — 10 mM). The absorbance at 465 nm was recorded for 900 s after mixing. The concentration of OAS was not corrected for its first order conversion to N-acetyl-L-serine, which occurs at ∼1% min-1 (21). The reaction conditions to form the aminoacrylate intermediate were still pseudo-first-order in OAS even taking a conversion of ∼15% to N-acetyl-l-serine into account. No evidence suggesting inhibition of formation of the aminoacrylate intermediate by N-acetyl-L-serine was observed. The absorbance as a function of time after mixing was fit in each case to a single exponential function to give the rates of formation of the aminoacrylate intermediate at the various concentrations of OAS. These rates were plotted as a function of the concentration of OAS and the resulting data were fit to a hyperbolic function. In the case of O-phospho-L-serine, where formation of the aminoacrylate intermediate was found to occur on a much faster timescale, the mixing was carried out using a stopped-flow device. CysM (4.5 μM) was rapidly mixed with OPS (1 mM — 10 mM) and the absorbance at 465 nm was recorded after mixing. The data from this experiment were analyzed as described above for OAS.
Single-wavelength kinetics of formation and decay of the α-aminoacrylate intermediate with L-cysteine
CysM (31 μM) was treated with L-cysteine (1 mM — 10 mM). The absorbance at 465 nm was monitored as a function of time (900 s) after mixing. The resulting trace showed an increase and subsequent decrease in absorbance over the course of the experiment and this was best fit to a double exponential to obtain the two first order rate constants for formation and decay of the absorbance at 465 nm. These were plotted as a function of L-cysteine concentration and in the case of both sets of rate constants fitted to linear functions.
Effect of phosphate on the α-aminoacrylate intermediate
CysM (19 μM), in 50 mM Tris-HCl at pH 8 was treated with potassium phosphate (10 mM — 100 mM) prepared from a 1.0 M stock solution, the pH of which was adjusted to 8 before dilution.
Single-wavelength kinetics of quenching of the α-aminoacrylate intermediate
The instrumentation in this case also varied with the nucleophilic substrate. In both the case of CysO-COSH and sodium sulfide, the aminoacrylate intermediate was pre-formed by treatment of CysM (14 μM) with a solution of O-phospho-L-serine (11 μM) under single turnover conditions. This was then mixed with solutions of CysO-COSH (25 – 250 μM) or sodium sulfide (100 μM — 15 mM). In the case of CysO-COSH, the lowest concentration used (25 μM) was not under pseudo-first order conditions. For CysO-COSH, the aminoacrylate and CysO-COSH solutions were mixed rapidly in a stopped-flow device and the decay of absorbance at 465 nm due to reaction of the aminoacrylate intermediate was observed. In the case of sodium sulfide, the aminoacrylate and bisulfide-containing solutions were mixed manually and the decay of absorbance was recorded in the presence of the reductant TCEP (2 mM). In both cases these decays were fit by non-linear regression to single exponential functions to yield the first order rate constants for quenching of the aminoacrylate under the varying conditions of nucleophile concentration. These rate constants were then plotted as a function of nucleophile concentration. In the case of CysO-COSH the data thus obtained were fit to a line, whereas in the case of bisulfide the dependence of the first order rate constants on the nucleophile concentration was fit to a hyperbola.
RESULTS
Formation of the α-aminoacrylate intermediate from O-acetyl-L-serine
The aminoacrylate intermediate could be formed from O-acetyl-L-serine (Figure 1A) and the increase in absorbance at 465 nm due to its formation on treatment of CysM with OAS could be best fit using a single exponential function at each of the various concentrations of OAS employed, to give the first order rate constants for formation. The dependence of these rate constants on the OAS concentration was best described by a hyperbolic function. A dissociation constant (Kd = 1/K1) for OAS of 5 ± 2 mM and a first order rate constant of 0.025 ± 0.004 s-1 were determined for formation of the aminoacrylate intermediate. This intermediate was stable for more than 30 minutes (data not shown). Additionally, the hyperbolic function was found to pass through the origin. The limiting initial slope of the hyperbola, determined as kmax/Kd provides an estimate of the second order rate constant for formation of the aminoacrylate intermediate, and was found to be 0.005 ± 0.002 mM-1 s-1. This estimate is model-dependent and applies only in the case of rapid-equilibrium binding followed by a single rate-limiting step, consistent with the proposed chemical mechanism. No evidence for reversibility of the formation of the aminoacrylate was found under the conditions of its formation.
Figure 1.
Ultraviolet-visible spectroscopy of formation of the CysM-bound α-aminoacrylate intermediate. (A) Mixing of CysM (14 μM) with O-acetyl-L-serine (5 mM) produces a decrease in the absorbance due to enzyme-bound pyridoxal-5′-phosphate (1) at 412 nm and an increase in the absorbance at 458 nm due to the formation of an α-aminoacrylate intermediate (5). The traces were recorded at intervals of approximately 34 s after mixing, in 50 mM Tris-HCl, pH 8.0 and at room temperature. (B) Absorbance changes due to mixing of O-phospho-L-serine (10 μM) with CysM (14 μM) in 50 mM Tris-HCl at pH 8.0 and at room temperature. Individual traces were recorded at intervals of approximately 30 s. The changes reflect conversion of the enzyme-bound PLP imine (1, λmax = ∼412 nm) to a stable α-aminoacrylate intermediate (5, λmax = ∼462 nm).
Formation of the α-aminoacrylate intermediate from O-phospho-L-serine
The α-aminoacrylate intermediate could also be formed from O-phospho-L-serine (Figure 1B) and the increase in absorbance at 465 nm due to its formation on treatment of CysM with OPS could also be fit using a single exponential function at the various concentrations of OPS examined (Figure 2). The data for this substrate was treated as described for O-acetyl-L-serine to give a first order rate constant of 17 ± 2 s-1 for formation of the aminoacrylate and a Kd of 6 ± 1 mM. The second order rate constant for formation of the aminoacrylate intermediate was thus estimated at 2.8 ± 0.7 mM-1 s-1. The intermediate could be partially quenched by addition of high concentrations of potassium phosphate (> 10 mM, data not shown).
Figure 2.

Kinetics of formation of α-aminoacrylate intermediate from O-phospho-L-serine. (A) Changes in absorbance at 465 nm following rapid mixing of O-phospho-L-serine (OPS; 0.5, 1, 2, 5 and 10 mM in order of increasing rate) with CysM (4.5 μM) in 50 mM Tris-HCl at pH 8 and at ∼22 °C. The resulting data were fit to single exponential functions to give the first order rates at the various concentrations of O-phospho-L-serine. (B) Plot of the rates of acrylate formation as a function of O-phospho-L-serine concentration, fit to a hyperbolic function describing a rapid equilibrium binding model. From this fit, the Kd for OPS was found to be 6 ± 1 mM and the second order rate constant for formation of the aminoacrylate intermediate was 2.8 ± 0.7 mM-1 s-1.
Formation and decay of the α-aminoacrylate intermediate with L-cysteine
When CysM was treated with L-cysteine, an increase in the absorbance at 465 nm was detected consistent with formation of the α-aminoacrylate intermediate. However, this was found to subsequently decay on a longer timescale. The resulting traces at the various concentrations of L-cysteine examined were best fit by double exponential functions to give two sets of first order rate constants at the various cysteine concentrations, one for formation and one for decay of the aminoacrylate intermediate (Figure 3). The first order rate constants for formation of the aminoacrylate were plotted as a function of the concentration of L-cysteine and the resulting data were best fit by a line with a slope of 0.0044 ± 0.0004 mM-1 s-1. The rate of decay of the aminoacrylate intermediate was independent of the concentration of L-cysteine within experimental error, being of the order of 0.001 s-1 (data not shown).
Figure 3.
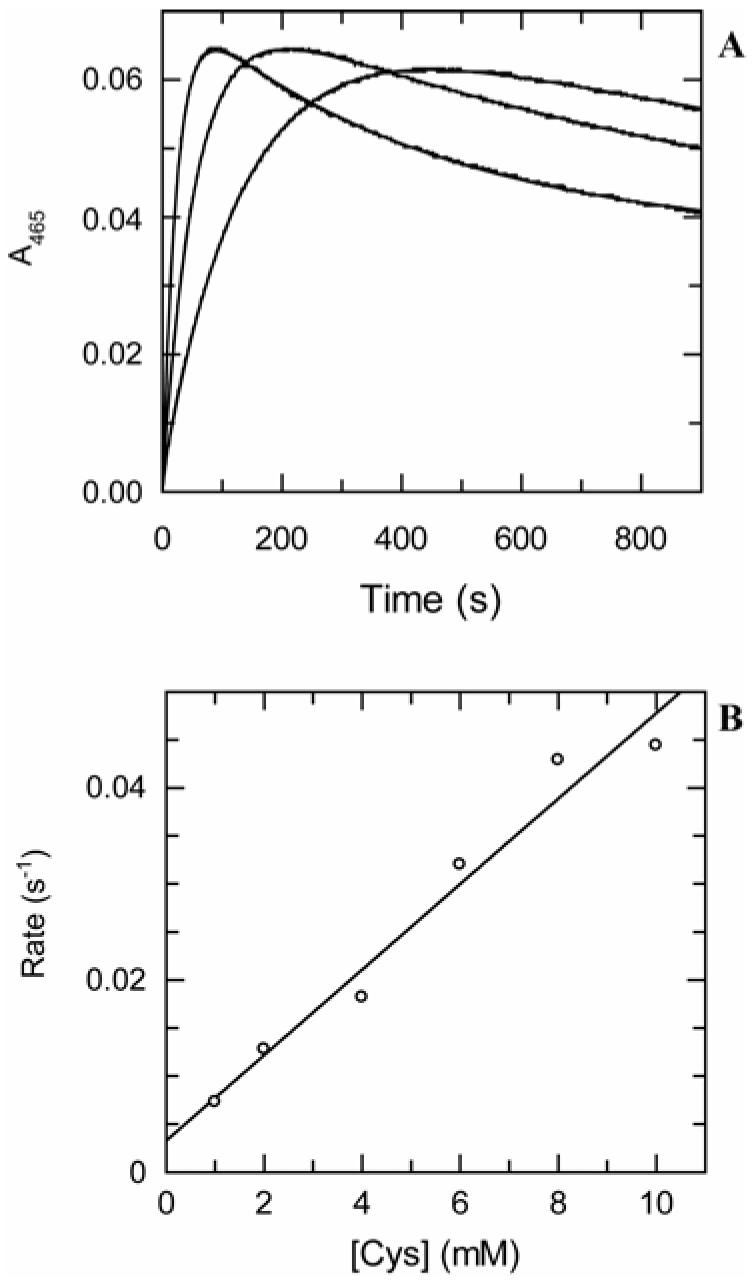
Kinetics of formation and decay of the α-aminoacrylate intermediate in the presence of cysteine. (A) CysM (31 μM) was mixed with cysteine (1, 4 and 8 mM in order of increasing rate) in 50 mM Tris-HCl, pH 8.0 and at ∼22 °C and the change in absorbance at 465 nm was monitored. The data are shown fit to functions of the form y = Ae-kt + Be-kt + C. (B) Dependence of the rate of formation of the aminoacrylate intermediate on the concentration of cysteine. The slope of the line fitting the data is a measure of the specificity of the enzyme for this amino acid substrate and was found to be 0.0044 ± 0.0004 mM-1 s-1. The rate of decay of the aminoacrylate intermediate was independent of the concentration of L-cysteine within experimental error, being of the order of 0.001 s-1.
Quenching of the α-aminoacrylate intermediate by bisulfide
The α-aminoacrylate intermediate was pre-formed under single turnover conditions by treatment of CysM (25 μM) with O-phospho-L-serine (20 μM) in 50 mM Tris-HCl at pH 8. Addition of sodium sulfide (0.1 – 15 mM final bisulfide concentration) caused a decrease in the absorbance at 465 nm (Figure 4). This decrease was fit to a single exponential function to give the observed rates for decay at the various bisulfide concentrations employed. The rate constants exhibited the hyperbolic dependence on the concentration of bisulfide consistent with a rapid-equilibrium binding model, with a predicted rate constant for quenching of 0.48 ± 0.07 s-1 and a Kd for bisulfide of 7 ± 2 mM, yielding an apparent second order rate constant for quenching of 0.07 ± 0.02 mM-1 s-1.
Figure 4.
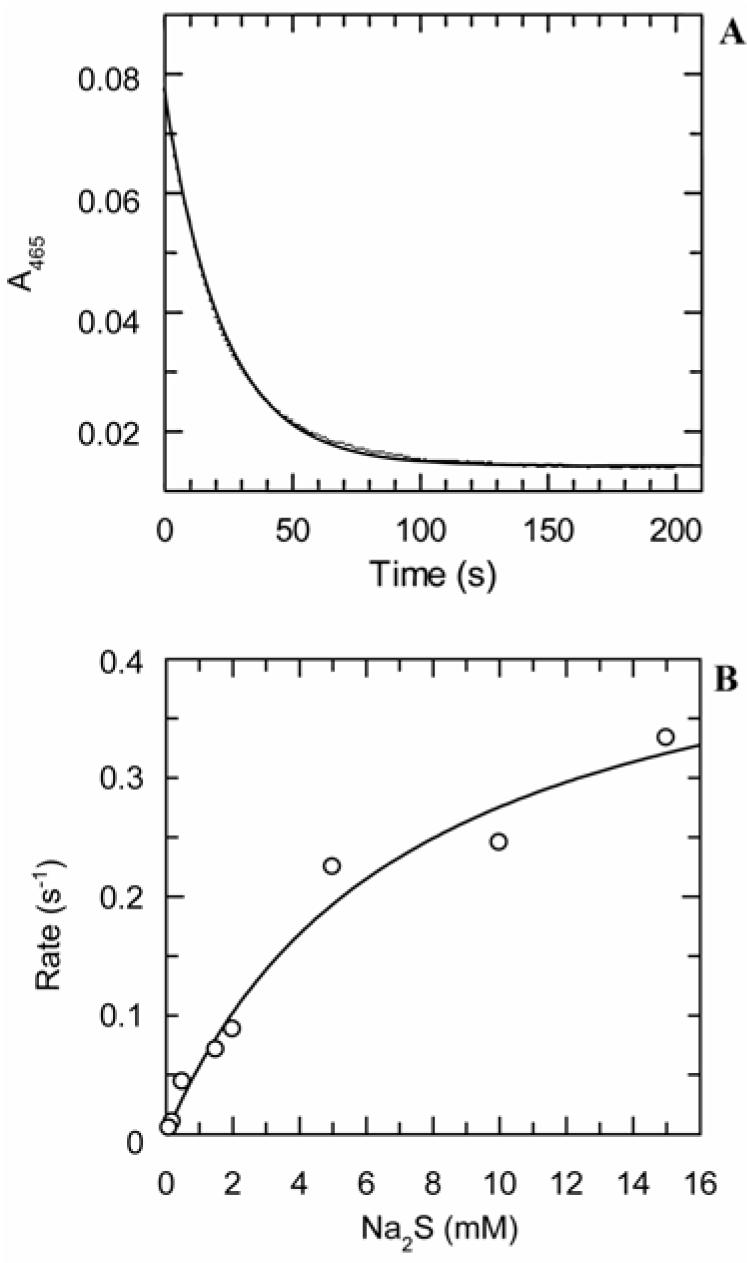
Kinetics of quenching of the α-aminoacrylate intermediate by bisulfide. The CysM/O-phospho-L-serine α-aminoacrylate intermediate was pre-formed by mixing CysM with a stoichiometric quantity of O-phospho-L-serine in 50 mM Tris-HCl, pH 8.0 at room temperature (∼22 °C). The solution containing the intermediate was then mixed with a solution of sodium sulfide in the same buffer, resulting in reaction of the α-aminoacrylate intermediate. The final solution contained the reducing agent tris(2-carboxyethyl)phosphane (TCEP) at a concentration of 2 mM. (A) Representative trace with exponential fit showing decay of absorbance at 465 nm after rapid mixing of the pre-formed CysM/O-phospho-L-serine α-aminoacrylate intermediate (11 μM) with sodium sulfide (0.5 mM). (B) Plot of the first order rates of quenching extracted from the exponential fits at various bisulfide concentrations (0.1 – 15 mM), fit to a hyperbolic function, which describes a rapid equilibrium binding model. The Kd for bisulfide was found to be 7 ± 2 mM and the first order rate constant for the carbon-sulfur bond-forming addition reaction was 0.48 ± 0.07 s-1.
Quenching of the α-aminoacrylate intermediate by CysO-COSH
Addition by rapid mixing of a solution of CysO-COSH to a pre-formed solution of the α-aminoacrylate under single turnover conditions also caused a decay in the absorbance at 465 nm. This decay could be fit to a single exponential function to give the observed rate for decay at the various concentrations of CysO-COSH examined. The rate of reaction of CysO-COSH with the CysM-bound aminoacrylate intermediate could not be saturated at the highest available CysO-COSH concentration (250 μM), which approached the solubility limit for the protein. We therefore restricted our analysis to the reaction of CysO-COSH in the 0–125 μM concentration range. A comparison of the specificity of CysM for the nucleophilic substrates, which was the aim of the present study, is readily obtained from such an analysis. The data describing the dependence of the rate of reaction of CysO-COSH in this concentration range with the CysM-bound aminoacrylate intermediate fit well to a line with a slope of 88 ± 6 mM-1 s-1. This value can be interpreted as an estimate of the second order rate constant for the reaction (Figure 5). The y-intercept of this line had a value of 0.61 ± 0.43 s-1.
Figure 5.
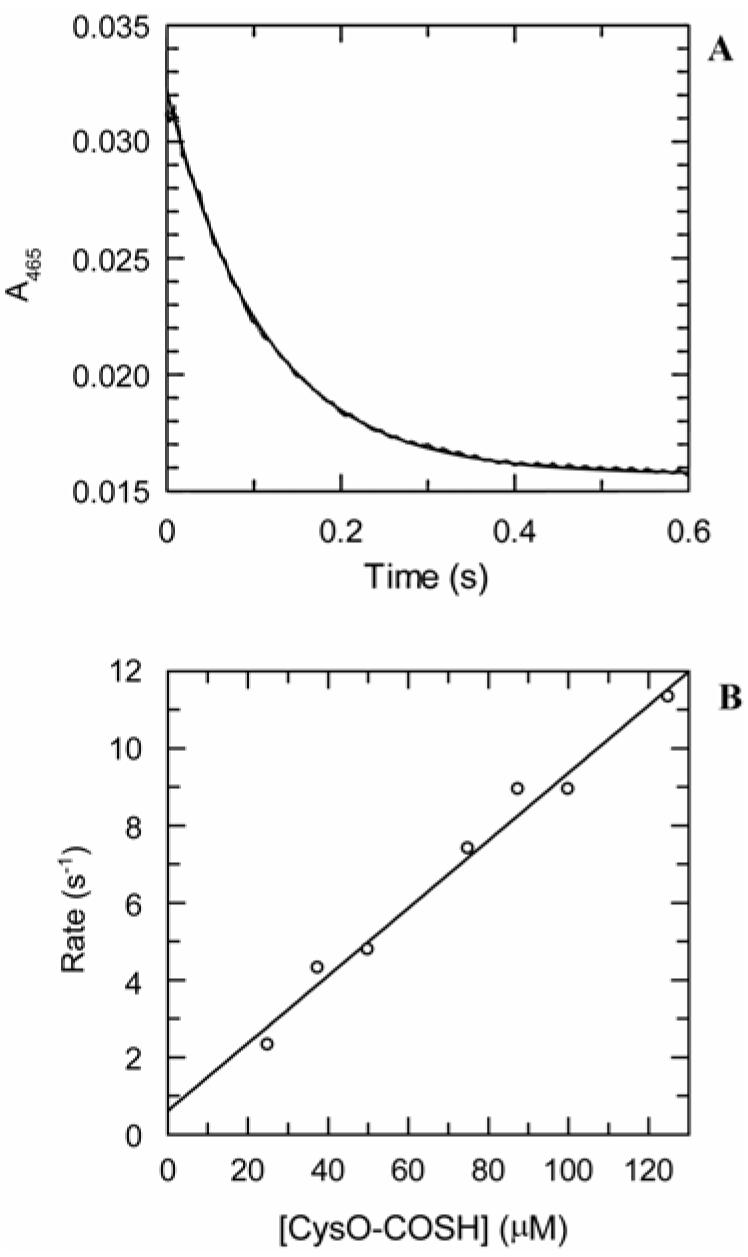
Kinetics of quenching of the α-aminoacrylate intermediate by CysO-COSH. The CysM/O-phospho-L-serine α-aminoacrylate intermediate was pre-formed by mixing CysM (14 μM) with O-phospho-L-serine (11 μM) in 50 mM Tris-HCl, pH 8.0 at room temperature (∼22 °C). This solution was then rapidly mixed with a solution containing CysO-COSH in the same buffer. (A) Representative trace with exponential fit showing decay of absorbance at 465 nm after rapid mixing of the CysM/O-phospho-L-serine α-aminoacrylate intermediate with CysO-COSH (0.05 mM) in 50 mM Tris-HCl at pH 8 and at room temperature. (B) Plot of the first-order rates of decay at the various CysO-COSH concentrations with a linear fit. The slope of this line is a measure of the specificity of the enzyme for this nucleophilic substrate and was found to be 88 ± 6 mM-1 s-1.
DISCUSSION
Kinetic scheme for CysM
The minimal kinetic scheme for CysM (Scheme 3) can be derived from the full pathway (Scheme 2) by its collapse due to the kinetic silence of the geminal diamine intermediates (3 and 7) and the aldimine intermediate 4. There was neither a lag in formation of the observable α-aminoacrylate intermediate, nor a dependence of the amplitude for aminoacrylate formation on the substrate concentration, suggesting that the forward partitioning of all intermediates up to and including the imine 4 must be, respectively, rapid and favorable. Indeed in the case of the cysteine synthase OASS-A from Salmonella typhimurium LT-2, the CysM-bound PLP-OAS aldimine intermediate (equivalent to structure 4) could only be observed on a low millisecond timescale and a precise rate for its formation was not reported (22). The kinetic pathway of CysM thus resembles that reported for the O-acetylserine sulfhydrylase enzymes with respect to the “elimination” half-reaction which generates the aminoacrylate intermediate. However, in the second CysM half-reaction a hitherto unexplored mechanistic variation presents itself in the form of CysO-COSH, which acts as a sulfide equivalent. Additionally, this half-reaction of CysM offered a more detailed insight than had been previously available in any system into the kinetics of sulfur transfer mediated by CysO-like proteins. The biosynthetic enzymes for thiamin (23) and quinolobactin (12) are experimentally much less tractable with regard to making detailed kinetic measurements on the sulfur transfer reaction.
Scheme 3.
Minimal kinetic pathway and determined rate constants for CysM
Formation of the α-aminoacrylate intermediate
Mixing of CysM with an excess of O-acetyl-L-serine or O-phospho-L-serine (Figure 1A and 1B, respectively) produces initially a red shift of the absorbance at 412 nm due to the enzyme-PLP internal aldimine (1). This is consistent with the data observed for reaction of the previously-studied O-acetylserine sulfhydrylase A enzyme from Salmonella typhimurium with OAS, and is attributed to the transient formation of the enzyme-PLP external aldimine (4), which then rapidly converts to the aminoacrylate intermediate. The lack of an isosbestic point in the data obtained with CysM contrasts with the situation for the reaction of OAS with the B-isozyme from S. typhimurium (24).
The kinetics of formation of the aminoacrylate may be described in terms of a rapid equilibrium binding model (Scheme 2) where the rate of dissociation of the ES complex, k-1, is much greater than the rate of the subsequent, kinetically significant, chemical step, i.e. the β-elimination reaction (k2). This model has been used previously to describe the formation of the aminoacrylate intermediate from OAS, catalyzed by O-acetylserine sulfhydrylase enzymes from Salmonella typhimurium and Escherichia coli, and predicts a hyperbolic dependence of the observed rate constant for chemistry on the substrate concentration, with saturation of the rate of the chemical step at concentrations of substrate which saturate the ES complex. In this model, an aminoacrylate intermediate at a CysM active site under conditions of zero substrate concentration may only undergo reverse reaction. Hence, the y-intercept of such a hyperbola corresponds to the back-rate for the chemical reaction (k-2). Equation (2) also defines the equilibrium constant K1 for substrate association, which is the reciprocal of the dissociation constant (Kd) The quotient K1[S]/(K1[S] + 1) is a fraction that ranges from 0 to 1, reflecting the formation of the ES complex. The rates of formation of the aminoacrylate intermediate in a single turnover from both O-acetyl- and O-phospho-L-serine were found to exhibit hyperbolic dependence on the substrate concentrations, consistent with this model. Both substrates have comparable dissociation constants (Kd) of 6 mM (OPS) and 5 mM (OAS). The Michaelis constants (Km) for these amino acids with respect to enzymes which utilize them as substrates appear to be, broadly speaking, in the mid-micromolar to low millimolar range (25, 26). The relatively high dissociation constants for the amino acid substrates are similar to that determined for OAS in studies on the O-acetylserine sulfhydrylase A enzyme from Salmonella typhimurium. However, the rates of the elimination reaction to form the aminoacrylate intermediate were found to vary substantially between O-acetyl- and O-phospho-L-serine. The rate of the β-elimination reaction of acetic acid to give the aminoacrylate intermediate (0.025 s-1) is 850-fold slower than that of the elimination of, formally, [HPO4]2- (17 s-1), suggesting the presence of stabilizing interactions at the CysM active site which facilitate such a rate enhancement.
In the case of OAS, the apparent second order rate constant for formation of the aminoacrylate was found to be 0.005 mM-1 s-1, compared with 2.8 mM-1 s-1 for O-phospho-L-serine. This 560-fold specificity suggests that O-phospho-L-serine and not O-acetyl-L-serine is the physiologically relevant substrate for CysM. Given the similarity of the dissociation constants for both substrates, the ultimate origin of this specificity appears to be the chemical reactivity of OPS at the CysM active site.
In order to further evaluate the plausibility of OPS as a substrate for CysM, we investigated the CysM-catalyzed reaction of cysteine. It would seem logical that physiologically-relevant substrates for cysteine synthases must greatly out-compete the intrinsic cysteine synthase-catalyzed reactivity of cysteine itself. Cysteine reacts with biphasic kinetics in the presence of CysM as it both forms and quenches the aminoacrylate intermediate. In the case of OASS-A from Salmonella typhimurium, the quenching reaction has been reported to form a thioether product (27). The time course of reaction of cysteine with CysM is thus described by a double exponential function with the first and second phases resulting from the formation and quenching of the aminoacrylate, respectively (Figure 3). The formation of the aminoacrylate from cysteine also exhibits rapid-equilibrium binding kinetics, with the apparent second order rate constant (0.0044 mM-1 s-1) suggesting that L-cysteine itself is an equally-good substrate for CysM as OAS. Such an observation points strongly to OPS as a more likely substrate for CysM than OAS. This contrasts with the situation for the previously-studied OASS-B from Salmonella typhimurium, which does not utilize OPS as a substrate (28).
Quenching of the α-aminoacrylate intermediate
The reaction of the CysM-bound α-aminoacrylate intermediate in the carbon-sulfur bond-forming conjugate addition constitutes the second half-reaction characteristic of cysteine synthases. In all previously-studied systems bisulfide is the sulfur donor. CysM offers a hitherto unexplored mechanistic variation on the existing paradigm in the form of CysO-COSH, the thiocarboxylated sulfide carrier protein which acts as a sulfide source for this second half reaction. Additionally, the CysM system allowed kinetic characterization of the sulfide transfer event mediated by CysO-COSH. This characterization has not been possible for any of the other sulfide carrier proteins. To characterize this second half-reaction of CysM we examined the reaction of the pre-formed aminoacrylate intermediate with both CysO-COSH and bisulfide.
The aminoacrylate intermediate could be quenched by bisulfide to give L-cysteine. The dependence of the observed rate for quenching on the concentration of bisulfide was hyperbolic, also consistent with a rapid-equilibrium binding model. From the hyperbolic fit to the data, the rate constant k5 for quenching of the aminoacrylate by bisulfide was found to be 0.48 s-1, with a Kd for bisulfide of 7 mM. This value suggests that appreciable binding of bisulfide to CysM should occur only at a bisulfide concentration generally regarded as toxic to M. tuberculosis (29). In contrast, the rate of quenching of the aminoacrylate in the presence of CysO-COSH depended linearly on the CysO-COSH concentration in the 0–125 μM range. The rate of quenching of the aminoacrylate by CysO-COSH could not be fully saturated at a CysO-COSH concentration of 250 μM, which approaches the solubility limit of the protein. The kinetic difference between CysO-COSH and bisulfide as nucleophiles provides an insight into the physical processes involved with the two sulfur sources. The quenching of the aminoacrylate by CysO-COSH requires association of the two proteins, likely followed by conformational changes in one or both proteins to place the Gly-Gly-SH C-terminal sulfur atom of CysO-COSH in a position optimal for β-addition to the aminoacrylate intermediate (5). It is not surprising that these steps should be slower than the subsequent chemical reaction. The slope of the line describing the concentration dependence of the quenching rate (88 mM-1 s-1) may thus be regarded as an estimate of the second order rate constant k4 for binding of CysO-COSH to CysM. This is three orders of magnitude greater than the value of k5/Kd for bisulfide, computed for that substrate to be 0.07 mM-1 s-1, and which is an estimate of the specificity of the enzyme for this substrate. Comparison of these values strongly suggests that CysO-COSH is the nucleophilic substrate in vivo. The CysM active site with the aminoacrylate intermediate present cannot be readily accessible to the bulk solvent in the absence of CysO, as demonstrated by the longevity of the aminoacrylate even under conditions of exposure to nucleophiles such as cysteine, and its slow rate of reaction with bisulfide. In the case of OASS-A from Salmonella typhimurium, the rate of the β-addition reaction is thought to be diffusion-limited when bisulfide is the nucleophilic substrate (30). Therefore it is likely that binding of CysO causes a conformational change in CysM which facilitates the nucleophilic addition.
The y-intercept of the linear fit describing the concentration-dependence of the rate of quenching of the OPS-derived CysM-aminoacrylate intermediate by CysO-COSH is nonzero and has a value of 0.61 ± 0.43 s-1, which is likely to estimate the rate of the rate-limiting step for back-reaction of the S-CysO-Cys external aldimine (6), i.e. re-formation of the aminoacrylate and CysO-COSH.
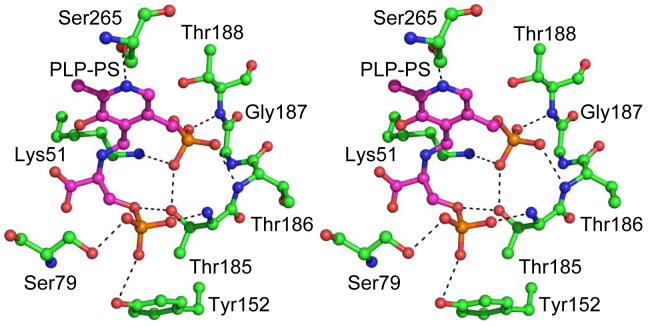
Structural analysis of O-phospho-L-serine bound to CysO/CysM
The structure of CysO/CysM has recently been solved (S. Ealick in press). Using this structure, a model for the O-phospho-L-serine/PLP imine at the active site of CysM was constructed (Figure 6). In this model, the phosphate forms H-bonds with the OH of Ser79 the NH of Thr185 and potentially with the OH of Tyr152. There are no nearby positively charged residues or other candidates for phosphate binding residues. The predicted binding is consistent with the proposal that O-phospho-L-serine is the physiological substrate for CysM.
Figure 6.
Stereoview of the active site model of the O-phospho-L-serine-PLP imine at the active site of CysM. The O-phospho-L-serine-PLP imine is indicated with cyan carbon atoms.
Role of CysM and CysO-COSH
Formation of the CysO-COSH thiocarboxylate represents a considerable energy investment for M. tuberculosis, with the direct involvement of at least two nucleoside triphosphate molecules required to produce O-phospho-L-serine and activate CysO for formation of the C-terminal thiocarboxylate. The underlying reasons justifying this investment are not clear, but it is possible that the thiocarboxylate moiety represents a stable, oxidation-resistant and relatively non-toxic source of sulfide from which cysteine can be produced in the highly oxidizing environment of the macrophage. Inhibitors of CysM may thus have potential as anti mycobacterial chemotherapeutic agents.
CONCLUSIONS
A new cysteine biosynthetic pathway, in Mycobacterium tuberculosis, involving a sulfide carrier protein (CysO) and a cysteine synthase (CysM) has recently been described. Here we report the kinetic characterization of this system and determine that O-phospho-L-serine rather than O-acetyl-L-serine is the cosubstrate. The chemical mechanism of CysM involves formation of an α-aminoacrylate intermediate consistent with its assignment as a member of the PLP-dependent β-replacement family of enzymes. O-acetyl-L-serine has a comparable Kd to that of O-phospho-L-serine, but the elimination of acetic acid is substantially slower than that of [HPO4]2-, resulting in a much smaller apparent second order rate constant for formation of the aminoacrylate intermediate. This appears to be analogous to the situation described for the hyperthermophilic archaeon Aeropyrum pernix K1, which has been shown to possess a cysteine synthase enzyme which selectively catalyzes the O-phosphoserine sulfhydrylation reaction (6). Our data show that the thiocarboxylated sulfur carrier protein (CysO-COSH) is likely to serve as the endogenous nucleophilic substrate in M. tuberculosis, having an apparent second order rate constant for quenching of the aminoacrylate intermediate that is more than 1200 times faster than bisulfide, the nucleophilic substrate assigned for homologous bacterial cysteine synthases. The underlying reasons for involvement of a sulfur carrier protein in this pathway remain unclear. One possibility is that the oxidation-resistance of the thiocarboxylate moiety may be a important key factor in protecting sulfide from oxidation in the macrophage. This is the first kinetic characterization of sulfur transfer from a sulfide carrier protein.
ACKNOWLEDGEMENTS
We thank Dr. Jeremiah Hanes for helpful discussions relating to experimental design and data analysis.
Abbreviations
- OAS
O-acetyl-L-serine
- OASS
O-acetylserine sulfhydrylase
- OPS
O-phospho-L-serine
- EDTA
ethylenediamine tetraacetic acid
- TCEP
tris(2-carboxyethyl)phosphine
- PLP
pyridoxal-5′-phosphate
- Tris
tris(hydroxymethyl)aminomethane
Footnotes
This work was supported by National Institutes of Health Grants DK44083 (to T.P.B.) and DK67081 (to S.E.E.). C.T.J. was the recipient of an NIH Chemistry/Biology Interface Traineeship.
REFERENCES
- 1.Scientific Working Group Report on Tuberculosis. World Health Organization; Geneva: 2006. [Google Scholar]
- 2.Kredich NM, Tomkins GM. The enzymic synthesis of L-cysteine in Escherichia coli and Salmonella typhimurium. J. Biol. Chem. 1966;241:4955–4965. [PubMed] [Google Scholar]
- 3.Johnson CM, Roderick SL, Cook PF. The serine acetyltransferase reaction: acetyl transfer from an acylpantothenyl donor to an alcohol. Arch. Biochem. Biophys. 2007;433:85–95. doi: 10.1016/j.abb.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Tai C-H, Cook PF. Pyridoxal-5′-phosphate-dependent α,β-elimination reactions: Mechanism of O-acetylserine sulfhydrylase. Acc. Chem. Res. 2001;34:49–59. doi: 10.1021/ar990169l. [DOI] [PubMed] [Google Scholar]
- 5.Mino K, Ishikawa K. Characterization of a novel thermostable O-acetylserine sulfhydrylase from Aeropyrum pernix K1. J. Bacteriol. 2003;185:2277–2284. doi: 10.1128/JB.185.7.2277-2284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mino K, Ishikawa K. A novel O-phospho-L-serine sulfhydrylation reaction catalyzed by O-acetylserine sulfhydrylase from Aeropyrum pernix K1. FEBS Lett. 2003;551:133–138. doi: 10.1016/s0014-5793(03)00913-x. [DOI] [PubMed] [Google Scholar]
- 7.Wheeler PR, Coldham NG, Keating L, Gordon SV, Wooff EE, Parish T, Hewinson RG. Functional demonstration of reverse transsulfuration in the Mycobacterium tuberculosis complex reveals that methionine is the preferred sulfur source for pathogenic mycobacteria. J. Biol. Chem. 2005;280:8069–8078. doi: 10.1074/jbc.M412540200. [DOI] [PubMed] [Google Scholar]
- 8.Sauerwald A, Zhu W, Major T, Roy H, Palioura S, Jahn D, Whitman WB, Yates JR, 3rd, Ibba M, Söll D. RNA-dependent cysteine biosynthesis in Archaea. Science. 2005;307:1969–1972. doi: 10.1126/science.1108329. [DOI] [PubMed] [Google Scholar]
- 9.Burns KE, Baumgart S, Dorrestein PC, Zhai H, McLafferty FW, Begley TP. Reconstitution of a new cysteine biosynthetic pathway in Mycobacterium tuberculosis. J. Am. Chem. Soc. 2005;127:11602–11603. doi: 10.1021/ja053476x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park J-H, Dorrestein PC, Zhai H, Kinsland C, McLafferty FW, Begley TP. Biosynthesis of the thiazole moeity of thiamin pyrophosphate (vitamin B1) Biochemistry. 2003;42:12430–12438. doi: 10.1021/bi034902z. [DOI] [PubMed] [Google Scholar]
- 11.Rudolph MJ, Wuebbens MM, Rajagopalan KV, Schindelin H. Crystal structure of molybdopterin synthase and its evolutionary relationship to ubiquitin activation. Nat. Struct. Biol. 2001;8:42–46. doi: 10.1038/83034. [DOI] [PubMed] [Google Scholar]
- 12.Godert AM. Dissertation. Cornell University; 2006. Investigating the Biosynthesis of Thio-Quinolobactin and the Development of a Proteomics Probe for Thiamin Utilizing Enzymes. [Google Scholar]
- 13.Matthijs S, Baysse C, Koedam N, Tehrani KA, Verheyden L, Budzikiewicz H, Schäfer M, Hoorelbeke B, Meyer J-M, De Greve H, Cornelis P. The Pseudomonas siderophore quinolobactin is synthesized from xanthurenic acid, an intermediate of the kynurenine pathway. Mol. Microbiol. 2004;52:371–374. doi: 10.1111/j.1365-2958.2004.03999.x. [DOI] [PubMed] [Google Scholar]
- 14.Hershko A, Ciechanover A. The ubiquitin system. Ann. Rev. Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 15.Mueller EG. Trafficking in persulfides: delivering sulfur in biosynthetic pathways. Nat. Chem. Biol. 2006;2:185–194. doi: 10.1038/nchembio779. [DOI] [PubMed] [Google Scholar]
- 16.Lehmann C, Begley TP, Ealick SE. Structure of the Escherichia coli ThiS—ThiF complex, a key component of the sulfur transfer system in thiamin biosynthesis. Biochemistry. 2006;45:11–19. doi: 10.1021/bi051502y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao T, Cohen RE. A cryptic protease couples deubiquitination and degradation by the proteasome. Nature. 2002;419:403–407. doi: 10.1038/nature01071. [DOI] [PubMed] [Google Scholar]
- 18.Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976;131:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 19.Cook PF, Hara S, Nalabolu SR, Schnackerz KD. pH Dependence of the absorbance and phosphorus-31 NMR spectra of O-acetylserine sulfhydrylase in the absence and presence of O-acetyl-L-serine. Biochemistry. 1992;31:2298–2303. doi: 10.1021/bi00123a013. [DOI] [PubMed] [Google Scholar]
- 20.Kinsland CL, Taylor SV, Kelleher NL, McLafferty FW, Begley TP. Overexpression of recombinant proteins with a C-terminal thiocarboxylate: Implications for protein semisynthesis and thiamin biosynthesis. Protein Science. 1998;7:1839–1842. doi: 10.1002/pro.5560070821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostrowski J, Kredich NM. Molecular characterization of the cysJIH promoters of Salmonella typhimurium and Escherichia coli: Regulation by cysB protein and N-acetyl-L-serine. J. Bacteriol. 1989;171(1):130–140. doi: 10.1128/jb.171.1.130-140.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woehl EU, Tai C–H, Dunn MF, Cook PF. Formation of the α-aminoacrylate intermediate limits the overall reaction catalyzed by O-acetylserine sulfhydrylase. Biochemistry. 1996;35:4776–4783. doi: 10.1021/bi952938o. [DOI] [PubMed] [Google Scholar]
- 23.Dorrestein PC, Zhai H, McLafferty FW, Begley TP. The biosynthesis of the thiazole phosphate moiety of thiamin: the sulfur transfer mediated by the sulfur carrier protein ThiS. Chem. Biol. 2004;11:1373–1381. doi: 10.1016/j.chembiol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Chattopadhyay A, Meier M, Ivaninskii S, Burkhard P, Speroni F, Campanini B, Bettati S, Mozzarelli A, Rabeh W, Li L, Cook PF. Structure, mechanism, and conformational dynamics of O-acetylserine sulfhydrylase from Salmonella typhimurium: comparison of A and B isozymes. Biochemistry. 2007;46:8315–8330. doi: 10.1021/bi602603c. [DOI] [PubMed] [Google Scholar]
- 25 (a).Ho C-L, Noji M, Saito K. Plastidic pathway of serine biosynthesis. Molecular cloning and expression of 3-phosphoserine phosphatase from Arabidopsis thaliana. J. Biol. Chem. 1999;274(16):11007–11012. doi: 10.1074/jbc.274.16.11007. [DOI] [PubMed] [Google Scholar]; (b) Singh SK, Yang K, Karthikeyan S, Huynh T, Zhang X, Phillips MA, Zhang H. The thrH gene product of Pseudomonas aeruginosa is a dual activity enzyme with a novel phosphoserine:homoserine phosphotransferase avtivity. J. Biol. Chem. 2004;279(13):13166–13173. doi: 10.1074/jbc.M311393200. [DOI] [PubMed] [Google Scholar]
- 26.Kredich NM, Becker MA. Cysteine biosynthesis: serine transacetylase and O-acetylserine sulfhydrylase (Salmonella typhimurium) Methods Enzym. 1971;17(B):459–470. [Google Scholar]
- 27.Flint DH, Tuminello JF, Miller TJ. Studies on the synthesis of the Fe-S cluster of dihydroxyacid dehydratase in Escherichia coli crude extract. J. Biol. Chem. 1996;271:16053–16067. doi: 10.1074/jbc.271.27.16053. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura T, Iwahashi H, Eguchi Y. Enzymatic proof for the identity of the S-sulfocysteine synthase and cysteine synthase B of Salmonella typhimurium. J. Bacteriol. 1984;158:1122–1127. doi: 10.1128/jb.158.3.1122-1127.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaksonen AH, Franzmann PD, Puhakka JA. Effects of hydraulic retention time and sulfide toxicity on ethanol and acetate oxidation in sulfate-reducing metal-precipitating fluidized-bed reactor. Biotechnol. Bioeng. 2004;86(3):332–343. doi: 10.1002/bit.20061. [DOI] [PubMed] [Google Scholar]
- 30.Rabeh WM, Alguindigue SS, Cook PF. Mechanism of the addition half of the O-acetylserine sulfhydrylase A reaction. Biochemistry. 2005;44:5541–5550. doi: 10.1021/bi047479i. [DOI] [PubMed] [Google Scholar]