Abstract
Cognitive changes in addicts and in animals exposed to addictive drugs have been extensively investigated over the past decades. One advantage of studying addiction using cognitive paradigms is that neural processing in addicts or drug-exposed animals can be compared to that in normal subjects. Tests of cognitive flexibility that measure the ability to change responding to a previously rewarded or punished stimulus are of potential interest in the study of addiction, because addiction can itself be viewed as an inability to change responding to stimuli previously associated with drug reward. One such test is reversal learning, which is impaired in cocaine addicts and animals that have chronically self-administered or been exposed to cocaine. A circuit including orbitofrontal cortex, basolateral amygdala and striatum subserves reversal learning. In rats that have been previously exposed to cocaine, neurons in these regions show selective and distinctive changes in how they encode information during reversal learning. These changes suggest that in these rats, orbitofrontal cortex loses the ability to signal expected outcomes, and basolateral amygdala becomes inflexible in its encoding of cue significance. These changes could explain cocaine-induced impairments to cognitive flexibility and may have theoretical importance in addiction.
Keywords: addiction, cocaine, orbitofrontal cortex, basolateral amygdala, reversal, associative learning
The notion that addiction is a “brain disease” – that the brain of an addict has entered a pathological state that is fundamentally different from that of a non-addict – was famously promoted by Dr. Alan Leshner, former director of NIDA, in a Science article in the 1990’s (Leshner, 1997). Much important research since that time has been driven by this conception, and results have borne it out to such a degree that it has become a truism in the scientific community. One of the implications of this idea is that an addict may learn and process information differently than a non-addict. Such changes to cognitive function would be expected to be long-lasting, and could in theory enhance ongoing vulnerability to relapse and compulsive drug-taking. Thus they could provide an important underlying contribution to the behavioral patterns that define addiction.
The hypothesis that cognitive dysfunctions in addicts form an important part of the disease was first prominently raised around the time of Dr. Leshner’s seminal paper. Several important reviews suggested that deficits in response inhibition or in decision-making, particularly in psychostimulant users, were mediated by disruptions to frontal cortical regions, and that such deficits could contribute to some aspects of addiction (Jentsch and Taylor, 1999; Robbins and Everitt, 1999; Rogers and Robbins, 2001). However, because most of the supporting evidence at that time had been gathered in human addicts, it was impossible to conclude from these correlational studies whether such deficits were drug-induced or whether they indicated a pre-existing trait in those vulnerable to addiction.
Subsequent animal studies, to be reviewed below, have established that cognitive dysfunctions are induced by chronic exposure to addictive drugs, particularly to psychostimulants. Like the studies in human addicts, these studies have used cognitive tasks that have also been used to study brain function in normal humans or animals. This approach has the advantage that it allows abnormalities in the brains of addicts or drug-exposed animals to stand out against a background of what is known about normal brain function, and thereby their relationship to behavior to be better understood. Data that is derived from this approach complements that derived from more traditional models of addiction, in which animals are trained to self-administer drugs under various schedules and conditions. It is important to note, however, that cognitive tests normally measure responding based on stimuli associated with natural rewards rather than on drug rewards, and that therefore the extent to which abnormalities in addicts and drug-exposed animals measured in such paradigms underlie actual drug-seeking and –taking behaviour remains an open question (Schoenbaum and Shaham, 2008).
Paradigms designed to probe cognitive flexibility are of particular interest in the study of addiction. Cognitive flexibility is a broad concept that refers to the ability to adapt one’s cognitive representations, and hence behavior, to changing conditions. In particular, it includes the ability to change encoding and responding to stimuli that have previously predicted the availability of reward or punishment. On the face of it, addiction involves a disruption in this ability; that is, addicts have difficulty in changing the drug-seeking and –taking responses that are triggered by stimuli formerly associated with drug reward. Indeed, human cocaine and alcohol addicts are impaired on gambling tasks and reversal learning tasks that probe cognitive flexibility, and monkeys that have been passively exposed to cocaine also show deficits in reversal learning when tested later, in the absence of drug (Bechara et al., 2002; Ersche et al., 2008; Fillmore and Rush, 2006; Grant et al., 2000; Jentsch et al., 2002; Rogers et al., 1999; Rogers and Robbins, 2001). In our lab, rats with previous experience either with self-administration of cocaine or with passive cocaine injections, are abnormally slow to learn reversals, even though they learn initial contingencies at a normal rate (Calu et al., 2007; Schoenbaum et al., 2004). Other paradigms, such as those involving reinforcer devaluation, also demonstrate that experience with psychostimulants disrupts the ability to change responding to reward-associated cues when conditions have changed (Nelson and Killcross, 2006; Schoenbaum and Setlow, 2005).
What, then, is the neural basis for cocaine-induced disruptions to these forms of cognitive flexibility? The areas of the brain involved in cognitive flexibility include many of the same areas that have been found to exhibit long-lasting structural and functional changes in addiction (Robinson and Kolb, 2004; Volkow and Fowler, 2000). This fact accords with the recent conception of addiction as an associative learning disorder that co-opts brain areas and circuits normally involved in learning about natural rewards (Di Chiara, 1999; Hyman and Malenka, 2001; Kelley, 2004; Robbins and Everitt, 1999). These areas include subcortical regions, such as basolateral amygdala (ABL) and striatum, and prefrontal cortical regions such as orbitofrontal cortex (OFC). We have found that each of these regions exhibits changed encoding of cues and/or cue-outcome associations during reversal learning in rats chronically exposed to cocaine. Below, these changes, and their relationships to behavior, will be reviewed. In keeping with the strategy for studying cognition in addicts outlined above, this review will first discuss what is known about the neural basis of cognitive flexibility in several key brain areas, before detailing the changes that occur after chronic cocaine exposure.
Cognitive Flexibility in Normal Animals
Long-standing evidence has implicated the OFC in cognitive flexibility. Thus, OFC damage in many species causes severe reversal deficits while preserving a normal ability to learn initial contingencies (Bohn et al., 2003; Brown and McAlonan, 2003; Chudasama and Robbins, 2003; Dias et al., 1996; Izquierdo et al., 2004; Jones and Mishkin, 1972; Kim and Ragozzino, 2005; Meunier et al., 1997; Rolls et al., 1994; Schoenbaum et al., 2002; Schoenbaum et al., 2003a; Teitelbaum, 1964). In order to understand the role that OFC plays in cognitive flexibility, it will be helpful to discuss some of the hypotheses that have been advanced to explain it. One straightforward explanation is that OFC normally acts to inhibit prepotent responding that has become inappropriate after reversal (Jones and Mishkin, 1972; Mishkin, 1964). Animals lacking OFC would therefore respond perseveratively after reversal simply because they cannot inhibit the response that was well learned before reversal. However, even within go, no-go reversal-learning paradigms, OFC-lesioned animals demonstrate an intact ability to withhold responding during initial learning. For example, in our paradigm, OFC-lesioned rats retain the normal ability to inhibit responding to a new odor cue that predicts delivery of a bitter quinine solution. Only after the initial meaning of a cue is changed to its opposite is the OFC-dependent learning deficit revealed. Furthermore, OFC-lesioned animals are able to learn to inhibit responding in other settings, including simple extinction of responding and inhibition of the prepotent tendency to select a greater quantity of food (Chudasama et al., 2006; Gallagher et al., 1999; Izquierdo et al., 2004; Pickens et al., 2003). Thus, the role of OFC in cognitive flexibility is more specific than simply conferring an ability to inhibit responding per se.
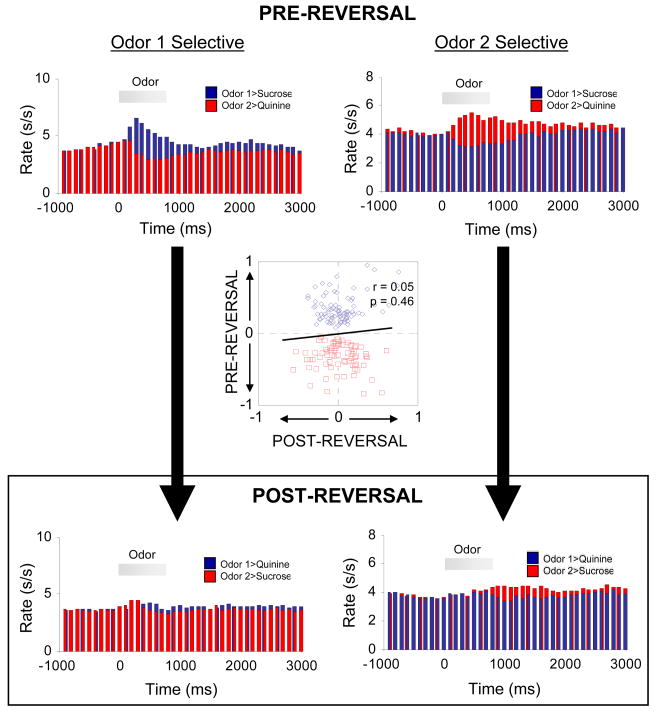
A second account of the role of OFC in reversal learning is that it drives behavior according to associative information representing the significance of a particular cue (Rolls et al., 1996; Thorpe et al., 1983). Neuronal firing representing cue significance has been found in OFC, but of course it has been found in other areas of the brain also (Paton et al., 2006; Saddoris et al., 2005; Schoenbaum et al., 1999; Setlow et al., 2003; Stalnaker et al., 2007b). What makes OFC special under this account, and the reason that it would be required for reversal learning, is its ability to rapidly change its firing of cue significance when contingencies change. However, recent evidence argues against this account. While OFC does contain some neurons that reverse firing when the significance of cues is reversed (Critchley and Rolls, 1996; Rolls et al., 1996; Schoenbaum et al., 1999), the population of cue-selective OFC neurons, taken as a whole, does not do so. In fact, as shown in Figure 2, OFC neurons generally fail to track cue significance across reversal. This situation contrasts with that in other brain regions, such as ABL, in which associative encoding does tend to track cue significance across reversal (Paton et al., 2006; Saddoris et al., 2005; Schoenbaum et al., 1999; Stalnaker et al., 2007b). Furthermore, we have recently found that the rapidity of reversal learning is inversely related to the flexibility of cue-selective encoding in OFC (Stalnaker et al., 2006). All of this evidence argues against the idea that OFC is directly driving behavior in reversal learning, according to its encoding of cue significance.
Figure 2.
Cue-selective neurons in OFC do not generally reverse their selectivity when contingencies are reversed. Population response of neurons in OFC identified as cue-selective during learning. Average activity per neuron is shown, synchronized to odor onset, before and after reversal. The population response fails to reverse cue-selectivity. Inset scatterplot compares the cue-selectivity indices before (X-axis) and after reversal (Y-axis) for all the cue-selective neurons used to construct the population histograms. Blue and red symbols show data for “Odor 1 Selective” neurons and “Odor 2 Selective” neurons, respectively. The cue-selectivity indices show no correlation. Cue-selectivity index was defined as (frO1 − frO2)/(frO1+frO2), where fr = firing rate during cue-sampling, O1 = odor cue that predicted sucrose before reversal; O2 = odor cue that predicted quinine before reversal. Data adapted from Stalnaker et al, European Journal of Neuroscience, 2006.
An alternative explanation for the importance of OFC to reversal learning, and to cognitive flexibility more generally, lies in its ability to signal the value of an expected outcome, rather than simply to drive behavior based on the associative history of a particular cue. Neuronal recordings and functional imaging in OFC, in many species and in many paradigms, have demonstrated that activity there tracks with and anticipates the value of rewarding or punishing outcomes (Blair et al., 2006; Dolan, 2007; Feierstein et al., 2006; Furuyashiki et al., 2008; Gottfried et al., 2003; Hikosaka and Watanabe, 2004; O’Doherty et al., 2002; Roberts, 2006; Roesch and Olson, 2004, 2005; Roesch et al., 2006; Schoenbaum et al., 1998; Schoenbaum et al., 2003b; Tremblay and Schultz, 1999). Furthermore, an intact OFC is necessary for animals to respond according to the current value of an anticipated outcome in Pavlovian reinforcer devaluation paradigms (Gallagher et al., 1999; Izquierdo et al., 2004; Pickens et al., 2003). In reversal learning, the encoding of expected outcomes could contribute to flexible behavior by allowing the recognition, after reversal, that the values of outcomes do not match those predicted by the preceding cues. Thus, without a signal from OFC indicating the value of expected outcomes, it would be more difficult to recognize that conditions have changed and that new learning and/or behavior is necessary. An OFC-dependent recognition of changed conditions could thus drive reversals by facilitating learning of the new contingencies in other areas of the brain, which themselves could influence behavior directly. Under this hypothesis, OFC-lesioned animals would perseverate because they continue to encode the old contingencies, or fail to encode the new ones, in other associative learning areas.
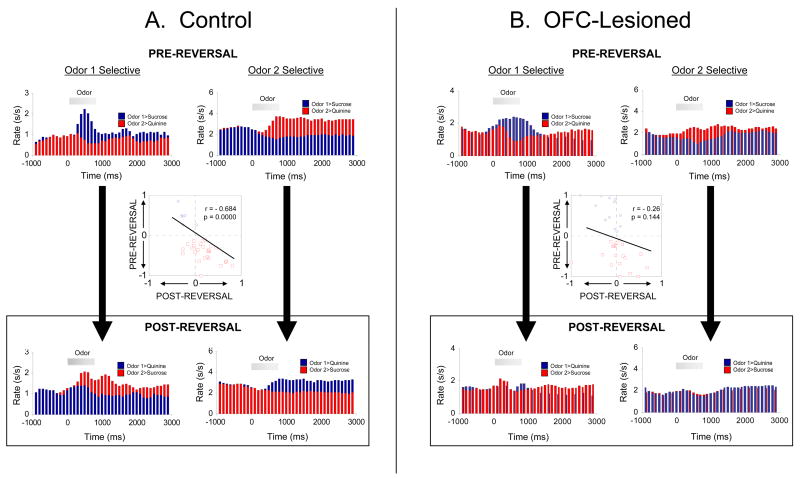
As a test of the hypothesis that OFC facilitates changes in associative encoding in other brain regions, we recorded neural activity in ABL in rats with ipsilateral neurotoxic lesions of OFC, as they learned and reversed 2-odor discrimination problems (Saddoris et al., 2005). In each problem, one odor predicted the availability of sucrose solution, and the other odor predicted the availability of aversive quinine solution. After reversal, the opposite contingencies were in effect. Figure 3 shows the effects of reversal on the firing of cue-selective ABL neurons in these rats, on which the influence of OFC would have been greatly diminished. The inflexibility of associative encoding of cue significance is evident in the population histograms showing that the populations that developed cue selectivity during initial learning failed to show selectivity after reversal, and in the scatterplot which shows that there was no correlation between cue-selectivity before and after reversal. Thus, as predicted, the removal of OFC input created encoding in ABL that failed to track the cue significance when contingencies changed.
Figure 3.
Flexibility of associative encoding in ABL depends on input from OFC. Population response of cue-selective neurons in ABL in control rats (A), or in rats with ipsilateral lesions of OFC (B). Average activity per neuron is shown, synchronized to odor onset, before and after reversal. Unlike the populations recorded in intact rats, the population response recorded in OFC-lesioned rats does not reverse cue-selectivity. Inset scatterplot compares the cue-selectivity indices before (X-axis) and after reversal (Y-axis) for all the cue-selective neurons used to construct the population histograms. Blue and red symbols show data for “Odor 1 Selective” neurons and “Odor 2 Selective” neurons, respectively. Again, in contrast to the inverse correlation in intact rats, the cue-selectivity indices in OFC-lesioned rats showed no correlation, indicating that ABL neurons in OFC-lesioned rats had lost their tendency to reverse. Data adapted from Stalnaker et al., Nature Neuroscience, 2007 and Saddoris et al, Neuron, 2005.
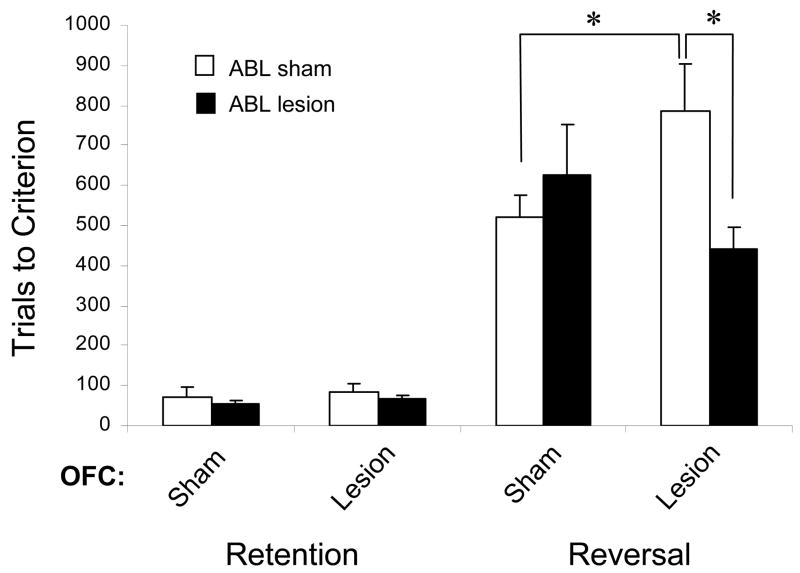
But is inflexible encoding in ABL actually retarding reversal, thereby causing the OFC-dependent reversal deficit? To test this question, we created bilateral lesions of ABL in rats with bilateral OFC lesions and then trained them on a series of reversal learning problems (Stalnaker et al., 2007a). If the proximal cause of the deficits arising from OFC damage is perseverative encoding in ABL, then removal of this encoding by lesions would remove the deficit. If, on the other hand, OFC drives reversal of behavior directly, then creating the additional lesions in ABL would have no effect on the original deficit or might even make the deficit worse. As shown in Figure 4, the results matched the former prediction: rats with lesions of only OFC were impaired on learning reversals, while rats with lesions of both areas performed as well as controls. Bilateral lesions of ABL by themselves did not have any significant effect either on initial learning or on reversal learning. These results of these two studies taken together suggest that encoding in ABL becomes inflexible and therefore interferes with normal reversal learning when input from OFC is eliminated. This is consistent with the idea that OFC normally facilitates flexible encoding in ABL, and perhaps in other situations in other brain regions, through its signalling of outcome expectancies.
Figure 4.
Reversal impairments caused by OFC lesions are abolished by lesions of ABL. Bars show the average number of trials (+SEM) required to retain and reverse a 2-odor discrimination problem for controls, rats with OFC lesions, rats with OFC lesions combined with bilateral ABL lesions, or rats with ABL lesions alone. As expected, OFC lesions impaired reversal learning. This impairment was abolished by pre-training lesions of ABL. ABL lesions alone had no effect. *, p < 0.05. Data adapted from Stalnaker et al, Neuron, 2007.
Of course, ABL is not itself necessary for normal reversal learning, and, in the absence of OFC, even hinders the rate of reversal learning. What then is the role of ABL in behavioral flexibility? The answer to this question likely depends on the type of information required for flexible responding. ABL seems to be particularly critical when flexible responding is based upon knowledge of the associations between cues and outcomes. Many recording studies reveal that ABL neurons respond to cues that have been associated with negative or positive outcomes (Belova et al., 2004; Cain and Bindra, 1972; Fuster and Uyeda, 1971; Maren, 2000; Muramoto et al., 1993; Nishijo et al., 1988; Quirk et al., 1995; Saddoris et al., 2005; Sanghera et al., 1979; Schoenbaum et al., 1999; Toyomitsu et al., 2002). Evidence suggests that this information is encoded earlier in ABL than in OFC, and that ABL is necessary for the significance of cues to be learned and encoded in other regions, such as OFC (Schoenbaum et al., 1999; Schoenbaum et al., 2003b). For example, in Pavlovian reinforcer devaluation paradigms, ABL is necessary for learning the association between a cue and an outcome in order to enable later adjustments in responding to that cue when the value of the outcome changes (Hatfield et al., 1996; Pickens et al., 2003). Therefore, cognitive flexibility may often depend on ABL when the behavior is fundamentally dependent on acquisition of cue-outcome associations. Importantly, this is not the case in reversals, because there are likely many different types of associative information utilized in a reversal. Thus no one type of information is necessary; instead the rate of reversal learning more likely reflects the type of involved information that is most resistant to change – the bottleneck. The results of the experiment reviewed above suggest that outcome-related information in ABL becomes this bottleneck in OFC-lesioned rats.
The fact that rats with both OFC and ABL lesions can still learn reversals at a normal rate demonstrates that other brain regions can support reversal learning, at least under some conditions. One possibility would the striatum. There is considerable evidence that dorsolateral striatum, in particular, is involved in encoding stimulus-response (S-R) associations, or habits (Jog et al., 1999; Schmitzer-Torbert and Redish, 2004; Yin and Knowlton, 2006; Yin et al., 2004), which could support reversal learning. In addition, lesions of some regions of the striatum can impair reversal learning (Ferry et al., 2000; Ragozzino et al., 2002), and neurons in both the dorsal and ventral striatum show cue-selective activity, some of which reverses its selectivity during reversal learning (Setlow et al., 2003; Takahashi et al., 2007).
Information Processing in Orbitofrontal Cortex in Addiction
Many lines of evidence suggest that the structure and function of OFC is disrupted in users of addictive drugs, both in response to drug-associated cues and in the context of cognitive paradigms (Jentsch and Taylor, 1999; Schoenbaum et al., 2006; Schoenbaum and Shaham, 2008; Volkow and Fowler, 2000). Imaging studies in cocaine (Volkow et al., 1991) and methamphetamine (Volkow et al., 2001) users reveal altered metabolism in the OFC and, in cocaine users, abnormal neuronal activation in response to drug-associated cues (Volkow and Fowler, 2000). In addition, human psychostimulant or poly-drug addicts and animals exposed to psychostimulants display cognitive deficits that resemble those seen after OFC damage (Bechara et al., 2001; Coffey et al., 2003; Ersche et al., 2008; Fillmore and Rush, 2006; Grant et al., 2000; Jentsch et al., 2002; Roesch et al., 2007; Rogers et al., 1999; Schoenbaum et al., 2004; Schoenbaum and Setlow, 2005).
As discussed above, an important contribution of OFC to cognitive flexibility may arise from its ability to signal the value of expected outcomes. The question therefore arises as to what extent the altered function of OFC in addicts reflects a specific disruption of this signalling, as opposed to a more general hypofunction. To examine this question, we exposed rats to the same regimen of cocaine exposure that we have found to disrupt reversal learning, and then implanted recording electrodes in OFC. After recovery, we compared neural correlates in OFC as rats learned and reversed a series of 2-odor discrimination problems in the absence of cocaine (Stalnaker et al., 2006). Many aspects of neural activity were similar between cocaine-treated rats and saline-treated controls. For example, the distribution of baseline firing rates did not differ, nor did the percentage of cue-selective neurons that developed during initial learning, and nor did the percentage of outcome-selective neurons that developed during initial learning. Thus, at a gross level, the OFC continued to function normally after cocaine exposure.
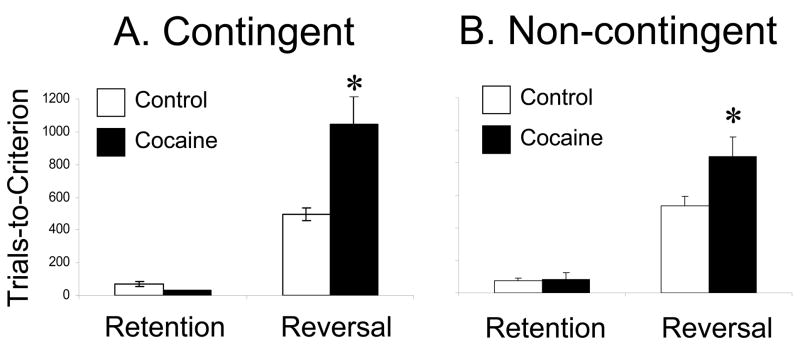
However, we discovered two marked differences between the two groups. In saline-treated rats, many outcome-selective neurons became activated during cue sampling, thereby signalling the predicted outcome as the rat sampled an odor. Sucrose-selective neurons would tend to become activated by the odor that predicted sucrose, and quinine-selective neurons would tend to become activated by the odor that predicted quinine. Cocaine-treated rats, in stark contrast, failed to develop cue-selectivity that signalled the predicted outcome. Instead, cue-selective activity in this population was just as likely to signal the unpredicted outcome as the predicted outcome. We quantified this result by analyzing the distribution of cue-selectivity indices for outcome selective neurons in each group. As shown in Figure 5, analysis of these distributions showed that in saline-treated rats, the distributions were skewed towards the appropriate cue for the outcome, while in cocaine-treated rats, the distributions were evenly distributed across both cues. Thus chronic cocaine exposure caused OFC neurons to fail to accurately signal the expected outcome during odor sampling in this task.
Figure 5.
Cue-selectivity indices for neurons that developed outcome-expectant firing during the pre-criterion block, firing differentially after the rat’s response, in anticipation of either sucrose or quinine delivery. On the top row are shown the populations that developed quinine-expectant firing, and on the bottom row are shown the populations that developed sucrose-expectant firing. Red or blue bars represent neurons that were significantly selective for one or the other of the two odors. In both quinine-expectant and sucrose-expectant populations, neurons in control rats were more likely to develop cue-selectivity to the cue that predicted their preferred outcome. Thus the distribution for quinine-expectant neurons is skewed to the left, and that in sucrose-expectant neurons in skewed to the right. In contrast, in both populations in cocaine-treated rats, neurons were equally likely to develop cue-selectivity to either cue. Thus, the distributions are symmetrically distributed around zero. Cue-selectivity indices were calculated from activity during odor sampling, using the same formula as in Figure 2. Data adapted from Stalnaker et al., European Journal of Neuroscience, 2006.
The second marked difference caused by cocaine exposure was a difference in the degree to which cue-selective neural activity reversed after contingencies were reversed. Consistent with the results of previous experiments, control rats showed a moderate level of plasticity, with about 25% of cue selective neurons reversing their cue-selectivity across reversal. In contrast, plasticity was actually greater in cocaine-treated rats, with about 34% of cue-selective neurons reversing their cue-selectivity. However, plasticity in cocaine-treated rats showed an abnormal relationship to performance. As mentioned earlier, in control rats, plasticity in OFC was inversely proportional to reversal performance: the worse the performance, the more likely cue-selective neurons were to reverse their selectivity. This provides evidence that OFC cue-selectivity is not directly driving performance; instead, it seems to be responding to feedback about performance. This was not true in the cocaine-treated rats; in this population, rats that performed poorly on the reversals showed no evidence of reversal of encoding in OFC. These results suggest that that cocaine-treated rats have lost the normal feedback mechanisms that operate when outcomes do not match expectations, as happens when rats are slow to reverse their performance in response to reversed contingencies.
These results provide a potential explanation for the behavioral deficits of the cocaine-treated rats. They suggest that the failure of OFC of cocaine-treated rats to signal expected outcomes at the time of cue sampling leads to a lack of feedback regarding the predictive relationships of cues to outcomes. This lack of feedback could lead to slower reversal performance, as well as to abnormal plasticity in cue-selectivity in OFC. In addition, a similar drug-induced neural deficit in human drug users could lead to difficulty in changing drug-seeking behavior in response to negative feedback – that is, to the mismatch between expectations of reward triggered by drug-associated cues on the one hand, and the actual negative outcomes that occur after drug use on the other.
Effects of Cocaine Exposure on Information Processing in Basolateral Amygdala and Striatum
As described above, the flexibility of encoding of cue significance in ABL depends on the integrity of OFC. Damage to OFC causes abnormally inflexible encoding in ABL, and the resulting miscoding of the old associations during a reversal seems to be the proximal cause of the reversal impairment caused by OFC lesions. Based on this evidence, one might expect that encoding of cue significance in ABL might also be inflexible after drug exposure in addicts and models of addiction. Indeed, evidence suggests that ABL becomes persistently and abnormally responsive to drug-associated cues in addiction (Bonson et al., 2002; Carelli et al., 2003; Childress et al., 1999; Ciccocioppo et al., 2001; Kilts et al., 2001). For instance, imaging studies frequently reveal activations of amygdala during exposure to drug-associated cues that elicit craving in addicts (Bonson et al., 2002; Childress et al., 1999; Kilts et al., 2001). In animal models, lesions or pharmacological manipulations of ABL, including those that block memory reconsolidation, block cue-induced relapse, suggesting that memories stored in amygdala may mediate relapse (Lee et al., 2005; Lee et al., 2006) (Fuchs et al., 2006; Kruzich and See, 2001; See et al., 2003). These memories are remarkably resistant to change, persisting long into abstinence (Ciccocioppo et al., 2004; Lu et al., 2004), through extinction (Weiss et al., 2001), and remaining unaltered even in the face of adverse outcomes or cues that represent adverse outcomes (Deroche-Gamonet et al., 2004; Vanderschuren and Everitt, 2004). The persistence and apparent invulnerability to change of these memories has been argued to contribute to the lack of control that characterizes decision-making in addiction (Vanderschuren and Everitt, 2005; Weiss, 2005).
These findings raise the question as to whether the persistence of these memories, which seem to be encoded in ABL, is specific to drug-associated cues, or whether there exists a more general persistence in the encoding of cue significance in ABL in drug users. To test this question, we performed a similar experiment to the one described above for OFC, except that we recorded in ABL (Stalnaker et al., 2007b). As we have reported previously in control rats (Saddoris et al., 2005; Schoenbaum et al., 1999), ABL neurons in both groups rapidly developed selective firing to each of the odor cues as the rats learned their predictive meaning. In controls, these cue-selective populations tended to reverse their cue-selectivity after reversal, such that they tracked the outcome predicted by the cue rather than the sensory features of the cue itself. In contrast, in cocaine-treated rats, cue-selective neurons failed to reverse their cue-selectivity after reversal. This pattern of activity was not related to the identity of the cue, because it was not generally present at the beginning of recording sessions. Rather, it developed with initial learning, and then failed to change in response to the change in contingencies after reversal. The contrast between the flexibility in cue-selectivity in controls and the inflexibility in cocaine-treated rats is illustrated by the population histograms and scatter plots shown in Figure 6.
Figure 6.
Previous cocaine treatment causes inflexible encoding of cue significance across reversal. Shown are population histograms before and after reversal for all neurons recorded in ABL that were significantly selective for the sucrose-predictive cue (odor 1 selective) or the quinine-predictive cue (odor 2 selective) during the post-criterion pre-reversal trial block. In saline-treated rats, neurons in both populations reversed their cue-selectivity across reversal. In contrast, in cocaine-treated rats, neurons that developed selectivity to the sucrose-predictive cue during learning remained selective for the same cue after reversal, even though it now predicted quinine. Neurons in cocaine-treated rats that developed selectivity to the quinine-predictive cue during learning failed to reverse their selectivity after reversal, instead showing a phasic response to both cues. Insets show a quantitative analysis of the flexibility of these populations across reversal. Neurons in saline-treated rats showed a negative correlation between their pre-reversal vs. post-reversal cue-selectivity indices; neurons in cocaine-treated rats showed a positive correlation between the two. Data adapted from Stalnaker et al., Nature Neuroscience, 2007.
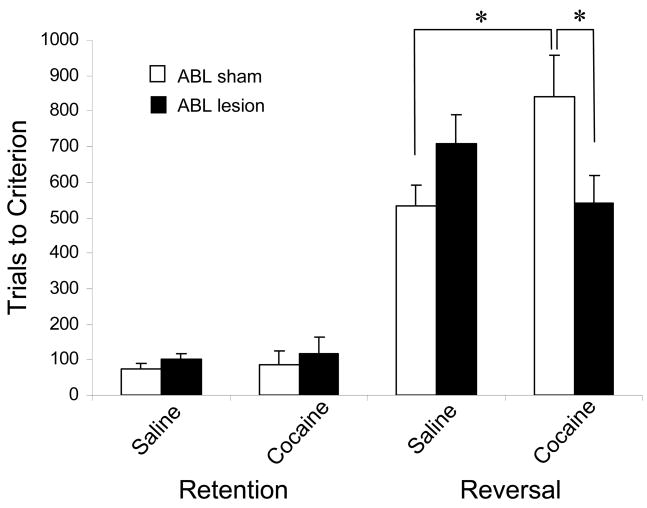
Of course, the inflexibility of cue-selectivity in ABL in cocaine-treated rats is similar to that observed after lesions of OFC. This is consistent with the hypothesis that cocaine-induced disruptions to OFC outcome-expectancy signals might cause the inflexibility in ABL, thereby causing inflexible behavior like that seen after OFC lesions. To test whether inflexible encoding in ABL in cocaine-treated rats might be causing inflexible behavior, we performed another experiment in which we first exposed rats to a regimen of cocaine injections, and then made bilateral lesions to ABL (Stalnaker et al., 2007b), and finally tested rats on reversal learning, again in the absence of cocaine. As discussed above, bilateral lesions of ABL eliminated OFC-dependent reversal impairments. Remarkably, as illustrated in Figure 7, we found the same effect in cocaine-treated rats: those with bilateral ABL lesions performed just as well as controls, while those with sham lesions of ABL performed significantly worse. Thus, just as in OFC-lesioned rats, encoding in ABL of cocaine-exposed rats seemed to be interfering with the ability to learn reversals quickly. These results are consistent with the hypothesis that rigid associative encoding in ABL after reversal is the proximal cause of the cocaine-induced reversal impairment. Furthermore, it is possible that a similar mechanism could contribute to the persistence of encoding of drug-associated cues in ABL that seems to play a central role in relapse.
Figure 7.
Reversal impairments caused by cocaine exposure are abolished by lesions of ABL. Bars show the average number of trials (+SEM) required to retain and reverse a 2-odor discrimination problem for cocaine or saline-treated rats with either sham or ABL lesions. As expected, previous cocaine exposure impaired reversal learning. This impairment was abolished by pre-training lesions of ABL. ABL lesions alone had no effect. *, p < 0.05. Data adapted from Stalnaker et al., Nature Neuroscience, 2007.
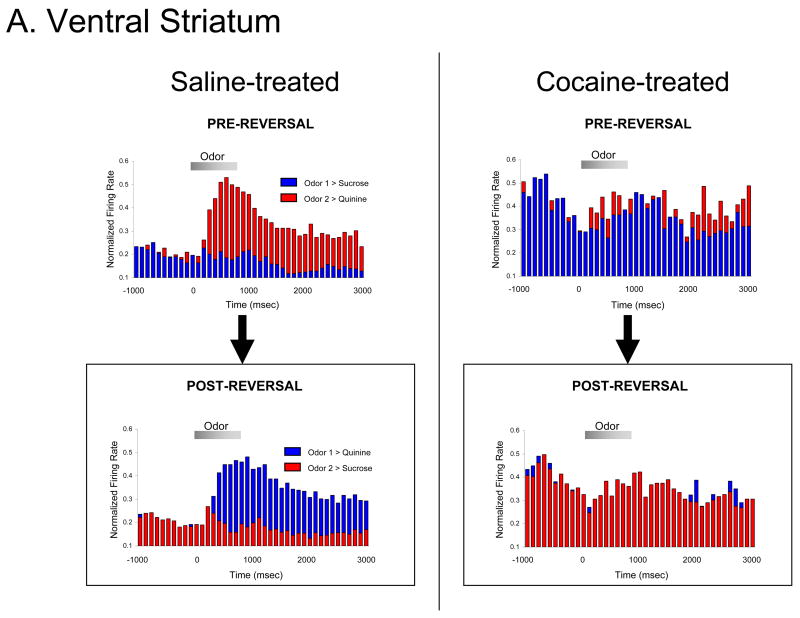
As described above, OFC and ABL are not the only brain regions that contribute to reversal learning, or that one might expect to show important changes after chronic cocaine exposure. Considerable theory and evidence suggests that the structure and function of both the dorsal and ventral striatum is altered in addiction, and that changes there could contribute to the compulsivity of drug-seeking in addiction (Belin and Everitt, 2008; Everitt and Robbins, 2005; Robinson and Berridge, 1993; Volkow et al., 1999; Volkow et al., 1997; Volkow et al., 2006; Wyvell and Berridge, 2000). One hypothesis is that chronic exposure to psychostimulants causes an increase in the efficacy of habitual, S-R associative encoding in dorsal striatum. Because behavior driven by S-R associations would be expected to be less flexible than associative encoding involving representations of outcomes, such a change might impair cognitive flexibility in addicts. We examined this question in the context of our reversal paradigm by recording neural activity in ventral and dorsal striatum of cocaine-exposed rats as they learned and reversed odor discrimination problems in the absence of cocaine (Takahashi et al., 2007). Interestingly, we found that neither region displayed the persistent and inflexible associative encoding that we observed in ABL after chronic cocaine. Encoding of cue significance in ventral striatum was marginally less flexible in cocaine-treated rats than in controls, but it was more remarkable for its absence. That is, while normal rats showed robust cue-selectivity in ventral striatum during acquisition and reversal of a series of odor discriminations, drug-treated rats showed very little, either before or after reversal. In dorsolateral striatum, cue-selectivity in cocaine-treated rats was similarly flexible to that in controls (Takahashi et al., 2007). This is illustrated for both regions in Figure 8. These results suggest that cognitive inflexibility after cocaine exposure may arise more from a degradation of outcome-related processing in OFC and ABL than from drug-induced changes to striatum.
Figure 8.
The effect of previous cocaine-treatment on cue selective populations in (A) ventral striatum and (B) dorsal striatum. Average normalized firing rates to each odor cue, synchronized to odor onset, are shown for cue-selective ventral striatum neurons and dorsal striatum neurons, in controls and in cocaine-treated rats. Cue-selectivity is absent from the population in ventral striatum after cocaine-treatment. Data adapted from Takahashi et al., Frontiers in Integrative Neuroscience, 2007.
Conclusions
The brain regions discussed in this review, particularly OFC, ABL and ventral striatum, are all closely interconnected in a circuit that is integrally involved in learning and guiding behavior based on positive and negative outcomes. Thus it is perhaps not surprising, in light of current theories of addiction, that all three regions show marked and persistent changes after cocaine exposure. What is remarkable, however, is that each of these regions shows selective and different changes in information processing that are of potential theoretical importance. After cocaine exposure, neurons in OFC show a very selective deficit in their ability to signal outcomes at the time of cue sampling. We have suggested that this change could lead to a deficit in recognizing the violations of expectations that would occur when conditions change and hence learning and behavior need to be updated. Such a deficit would be consistent with the rigid encoding of cue significance that occurs in ABL in these rats. It would also be consistent with the abnormal relationship between cue-selectivity in OFC and behavior that occurs after reversal in cocaine-treated rats. Finally, it could provide an explanation for both the cognitive inflexibility seen in addiction, and also, at least potentially, for the loss of control over behavior that constitutes the pathology of addiction. Going forward, it will be important to directly test the manner and degree to which these changes influence drug-seeking and –taking behavior. In addition, it would be desirable to test what kinds of manipulations might prevent these drug-induced alterations, and whether preventing them would have therapeutic value in the treatment of addiction. For the present, though, we would argue that the neural changes across these regions draw a more complete picture of addiction as a brain disease.
Figure 1.
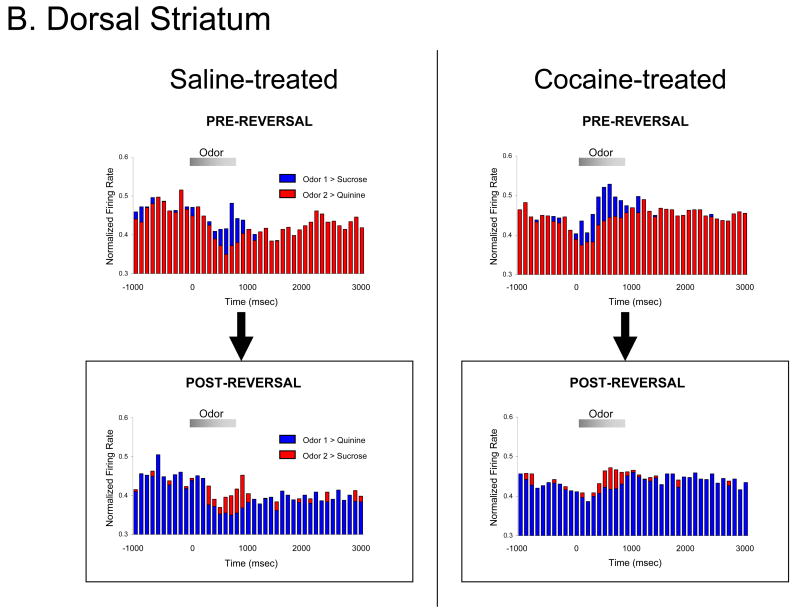
Previous experience with cocaine, via either self-administration training (A), or non-contingent injections (B), disrupts reversal learning. Self-administration training included 14 daily 3-hour sessions, with 0.75 mg/kg cocaine-HCl per infusion and an average of 24 infusions per day, and ended at least one month prior to behavioral testing on the go, no-go odor discrimination task described in the text. Non-contingent injection regimen consisted of 14 once-daily IP injections of 30 mg/kg cocaine-HCl, also ending at least one month prior to behavioral testing. In testing, rats first showed retention of a previously learned odor discrimination, and then acquired a reversal of that odor discrimination. Shown are average trials to criterion for two (A) or three (B) serial retention/reversals. Error bars indicate SEMs. *, p < 0.01, compared to controls. Data adapted from Calu et al., Learning & Memory 2007, and Stalnaker et al., Neuron, 2007.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40:1690–1705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Belova MA, Paton JJ, Salzman CD. Neural signals related to emotional learning and behavior in monkey amygdala. Society for Neuroscience Abstracts. 2004;30:84.84. [Google Scholar]
- Blair K, Marsh AA, Morton J, Vythilingam M, Jones M, Mondillo K, Pine DC, Drevets WC, Blair JR. Choosing the lesser of two evils, the better of two goods: specifying the roles of ventromedial prefrontal cortex and dorsal anterior cingulate in object choice. J Neurosci. 2006;26:11379–11386. doi: 10.1523/JNEUROSCI.1640-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn I, Giertler C, Hauber W. Orbital prefrontal cortex and guidance of instrumental behavior in rats under reversal conditions. Behavioral Brain Research. 2003;143:49–56. doi: 10.1016/s0166-4328(03)00008-1. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Brown VJ, McAlonan K. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behavioral Brain Research. 2003;146:97–130. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Cain DP, Bindra D. Responses of amygdala single units to odors in the rat. Experimental Neurology. 1972;35:98–110. doi: 10.1016/0014-4886(72)90062-3. [DOI] [PubMed] [Google Scholar]
- Calu DJ, Stalnaker TA, Franz TM, Singh T, Shaham Y, Schoenbaum G. Withdrawal from cocaine self-administration produces long-lasting deficits in orbitofrontal-dependent reversal learning in rats. Learn Mem. 2007;14:325–328. doi: 10.1101/lm.534807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Williams JG, Hollander JA. Basolateral amygdala neurons encode cocaine self-administration and cocaine-associated cues. Journal of Neuroscience. 2003;23:8204–8211. doi: 10.1523/JNEUROSCI.23-23-08204.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Kralik JD, Murray EA. Rhesus monkeys with orbital prefrontal cortex lesions can learn to inhibit prepotent responses in the reversed reward contingency task. Cerebral Cortex. 2006 doi: 10.1093/cercor/bhl025. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. Journal of Neuroscience. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F. Stimuli associated with a single cocaine experience elicit long-lasting cocaine-seeking. Nat Neurosci. 2004;7:495–496. doi: 10.1038/nn1219. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci U S A. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Experimental and Clinical Psychopharmacology. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Rolls ET. Olfactory neuronal responses in the primate orbitofrontal cortex: analysis in an olfactory discrimination task. Journal of Neurophysiology. 1996;75:1659–1672. doi: 10.1152/jn.1996.75.4.1659. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:951–953. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacol. 1999;375:13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Dolan RJ. The human amygdala and orbital prefrontal cortex in behavioural regulation. Philos Trans R Soc Lond B Biol Sci. 2007;362:787–799. doi: 10.1098/rstb.2007.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology (Berl) 2008;197:421–431. doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Feierstein CE, Quirk MC, Uchida N, Sosulski DL, Mainen ZF. Representation of spatial goals in rat orbitofrontal cortex. Neuron. 2006;51:495–507. doi: 10.1016/j.neuron.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Ferry AT, Lu XC, Price JL. Effects of excitotoxic lesions in the ventral striatopallidal--thalamocortical pathway on odor reversal learning: inability to extinguish an incorrect response. Exp Brain Res. 2000;131:320–335. doi: 10.1007/s002219900240. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Polydrug abusers display impaired discrimination-reversal learning in a model of behavioural control. J Psychopharmacol. 2006;20:24–32. doi: 10.1177/0269881105057000. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Feltenstein MW, See RE. The role of the basolateral amygdala in stimulus-reward memory and extinction memory consolidation and in subsequent conditioned cued reinstatement of cocaine seeking. Eur J Neurosci. 2006;23:2809–2813. doi: 10.1111/j.1460-9568.2006.04806.x. [DOI] [PubMed] [Google Scholar]
- Furuyashiki T, Holland PC, Gallagher M. Rat orbitofrontal cortex separately encodes response and outcome information during performance of goal-directed behavior. J Neurosci. 2008;28:5127–5138. doi: 10.1523/JNEUROSCI.0319-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM, Uyeda AA. Reactivity of limbic neurons of the monkey to appetitive and aversive signals. Electroencephalography and Clinical Neurophysiology. 1971;30:281–293. doi: 10.1016/0013-4694(71)90111-8. [DOI] [PubMed] [Google Scholar]
- Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. Journal of Neuroscience. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38:1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Han JS, Conley M, Gallagher M, Holland P. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka K, Watanabe M. Long- and short-range reward expectancy in the primate orbitofrontal cortex. European Journal of Neuroscience. 2004;19:1046–1054. doi: 10.1111/j.0953-816x.2004.03120.x. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nature Reviews Neuroscience. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Izquierdo AD, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. Journal of Neuroscience. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza R, 2nd, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science. 1999;286:1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- Jones B, Mishkin M. Limbic lesions and the problem of stimulus-reinforcement associations. Experimental Neurology. 1972;36:362–377. doi: 10.1016/0014-4886(72)90030-1. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kim J, Ragozzino KE. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiology of Learning and Memory. 2005;83:125–133. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruzich PJ, See RE. Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior. Journal of Neuroscience. 2001;21:RC155. doi: 10.1523/JNEUROSCI.21-14-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278:45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology. 2004;176:101–108. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- Maren S. Auditory fear conditioning increases CS-elicited spike firing in lateral amygdala neurons even after extensive overtraining. Eur J Neurosci. 2000;12:4047–4054. doi: 10.1046/j.1460-9568.2000.00281.x. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M. Effects of orbital frontal and anterior cingulate lesions on object and spatial memory in rhesus monkeys. Neuropsychologia. 1997;35:999–1015. doi: 10.1016/s0028-3932(97)00027-4. [DOI] [PubMed] [Google Scholar]
- Mishkin M. Perseveration of central sets after frontal lesions in monkeys. In: Warren JM, Akert K, editors. The Frontal Granular Cortex and Behavior. McGraw-Hill; New York: 1964. pp. 219–241. [Google Scholar]
- Muramoto K, Ono T, Nishijo H, Fukuda M. Rat amygdaloid neuron responses during auditory discrimination. Neuroscience. 1993;52:621–636. doi: 10.1016/0306-4522(93)90411-8. [DOI] [PubMed] [Google Scholar]
- Nelson A, Killcross S. Amphetamine exposure enhances habit formation. Journal of Neuroscience. 2006;26:3805–3812. doi: 10.1523/JNEUROSCI.4305-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijo H, Ono T, Nishino H. Single neuron responses in alert monkey during complex sensory stimulation with affective significance. Journal of Neuroscience. 1988;8:3570–3583. doi: 10.1523/JNEUROSCI.08-10-03570.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Setlow B, Saddoris MP, Gallagher M, Holland PC, Schoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. Journal of Neuroscience. 2003;23:11078–11084. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Ragozzino KE, Mizumori SJ, Kesner RP. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behav Neurosci. 2002;116:105–115. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Drug addiction: bad habits add up. Nature. 1999;398:567–570. doi: 10.1038/19208. [DOI] [PubMed] [Google Scholar]
- Roberts AC. Primate orbitofrontal cortex and adaptive behaviour. Trends Cogn Sci. 2006;10:83–90. doi: 10.1016/j.tics.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004;304:307–310. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Olson CR. Neuronal activity in primate orbitofrontal cortex reflects the value of time. Journal of Neurophysiology. 2005;94:2457–2471. doi: 10.1152/jn.00373.2005. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Takahashi Y, Gugsa N, Bissonette GB, Schoenbaum G. Previous cocaine exposure makes rats hypersensitive to both delay and reward magnitude. Journal of Neuroscience. 2007;27:245–250. doi: 10.1523/JNEUROSCI.4080-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Taylor AR, Schoenbaum G. Encoding of time-discounted rewards in orbitofrontal cortex is independent of value representation. Neuron. 2006;51:509–520. doi: 10.1016/j.neuron.2006.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Curr Opin Neurobiol. 2001;11:250–257. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Critchley HD, Mason R, Wakeman EA. Orbitofrontal cortex neurons: role in olfactory and visual association learning. Journal of Neurophysiology. 1996;75:1970–1981. doi: 10.1152/jn.1996.75.5.1970. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. Journal of Neurology, Neurosurgery, and Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris MP, Gallagher M, Schoenbaum G. Rapid associative encoding in basolateral amygdala depends on connections with orbitofrontal cortex. Neuron. 2005;46:321–331. doi: 10.1016/j.neuron.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Sanghera G, Rolls ET, Roper-Hall A. Visual responses of neurons in the dorsolateral amygdala of the alert monkey. Experimental Neurology. 1979;63:610–626. doi: 10.1016/0014-4886(79)90175-4. [DOI] [PubMed] [Google Scholar]
- Schmitzer-Torbert N, Redish AD. Neuronal activity in the rodent dorsal striatum in sequential navigation: separation of spatial and reward responses on the multiple T task. J Neurophysiol. 2004;91:2259–2272. doi: 10.1152/jn.00687.2003. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nature Neuroscience. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J Neurosci. 1999;19:1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent S, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 2006;29:116–124. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci. 2004;19:1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B. Cocaine makes actions insensitive to outcomes but not extinction: implications for altered orbitofrontal-amygdalar function. Cereb Cortex. 2005;15:1162–1169. doi: 10.1093/cercor/bhh216. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learning and Memory. 2003a;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003b;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Shaham Y. The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biol Psychiatry. 2008;63:256–262. doi: 10.1016/j.biopsych.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, McLaughlin J, Fuchs RA. Muscarinic receptor antagonism in the basolateral amygdala blocks acquisition of cocaine-stimulus association in a model of relapse to cocaine-seeking behavior in rats. Neuroscience. 2003;117:477–483. doi: 10.1016/s0306-4522(02)00665-6. [DOI] [PubMed] [Google Scholar]
- Setlow B, Schoenbaum G, Gallagher M. Neural encoding in ventral striatum during olfactory discrimination learning. Neuron. 2003;38:625–636. doi: 10.1016/s0896-6273(03)00264-2. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Franz TM, Singh T, Schoenbaum G. Basolateral amygdala lesions abolish orbitofrontal-dependent reversal impairments. Neuron. 2007a;54:51–58. doi: 10.1016/j.neuron.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Roesch MR, Franz TM, Burke KA, Schoenbaum G. Abnormal associative encoding in orbitofrontal neurons in cocaine-experienced rats during decision-making. Eur J Neurosci. 2006;24:2643–2653. doi: 10.1111/j.1460-9568.2006.05128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker TA, Roesch MR, Franz TM, Calu DJ, Singh T, Schoenbaum G. Cocaine-induced decision-making deficits are mediated by miscoding in basolateral amygdala. Nat Neurosci. 2007b;10:949–951. doi: 10.1038/nn1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Roesch MR, Stalnaker TA, Schoenbaum G. Cocaine exposure shifts the balance of associative encoding from ventral to dorsolateral striatum. Frontiers in Integrative Neuroscience. 2007:1. doi: 10.3389/neuro.07.011.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum H. A comparison of effects of orbitofrontal and hippocampal lesions upon discrimination learning and reversal in the cat. Experimental Neurology. 1964;9:452–462. doi: 10.1016/0014-4886(64)90053-6. [DOI] [PubMed] [Google Scholar]
- Thorpe SJ, Rolls ET, Maddison S. The orbitofrontal cortex: neuronal activity in the behaving monkey. Experimental Brain Research. 1983;49:93–115. doi: 10.1007/BF00235545. [DOI] [PubMed] [Google Scholar]
- Toyomitsu Y, Nishijo H, Uwano T, Kuratsu J, Ono T. Neuronal responses of the rat amygdala during extinction and reassociation learning in elementary and configural associative tasks. European Journal of Neuroscience. 2002;15:753–768. doi: 10.1046/j.1460-9568.2002.01889.x. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Behavioral and neural mechanisms of compulsive drug seeking. Eur J Pharmacol. 2005;526:77–88. doi: 10.1016/j.ejphar.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, Alpert R, Hoff A. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry. 1991;148:621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Hitzemann R, Angrist B, Gatley SJ, Logan J, Ding YS, Pappas N. Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: implications in addiction. Am J Psychiatry. 1999;156:19–26. doi: 10.1176/ajp.156.1.19. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Weiss F, Martin-Fardon R, Ciccocioppo R, Kerr TM, Smith DL, Ben-Shahar O. Enduring resistance to extinction of cocaine-seeking behavior induced by drug-related cues. Neuropsychopharmacology. 2001;25:361–372. doi: 10.1016/S0893-133X(01)00238-X. [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci. 2000;20:8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]