Abstract
Prior work suggests that active experience impacts infants’ understanding of simple actions. The present studies compared the impact of active and observational experience on infants’ ability to identify the goal of a novel tool use event. Infants received active practice and training using a cane to retrieve an out-of-reach toy, or matched observational experience, prior to taking part in a habituation paradigm that assessed infants’ ability to identify the goal of another person’s tool use acts. Active training alone facilitated 10-month-old infants’ ability to identify the goal of the tool use event. Active experience using tools may enable infants to build motor representations of tool use events that subsequently guide action perception and support action understanding.
Introduction
The ability to use tools to achieve otherwise unobtainable goals is a hallmark of advanced intelligence (Parker & Gibson, 1977). Although the exact nature of tool use representations is currently a matter of intense debate (see Hauser & Santos, in press), mature tool use representations encompass many elements. Adults understand that tools possess key properties (Hauser & Santos, in press) that support particular patterns of usage (Boronat et al., 2005; Johnson-Frey, Newman-Norlund & Grafton, 2005; Kan et al., 2006) toward functions that they were designed to perform (Bloom, 1996; Kelemen, 1999; Matan & Carey, 2001). Recent studies indicate that many of these aspects of tool use understanding are present by early childhood (Berger, Adolph & Lobo, 2005; Brown, 1990; Casler & Keleman, 2005; 2006; Chen & Siegler, 2000; Connolly & Dalgleish, 1989; Defeyter & German, 2003).
A critical aspect of tool use, and a necessary precursor for building more sophisticated tool use representations, is the ability to recognize that objects can be used as a means to an end. Even before children become adept users of the tools of our culture they are able to use one object to act on and obtain a desired object: by 6 to 7 months of age infants can be trained to use an intermediary object as a means toward a desired end (Munakata, McClelland, Johnson & Siegler, 1997), and over the next six months of life infants’ behavior on means-end tasks becomes increasingly systematic and spontaneous (e.g., Bates, Carlson-Luden & Bretherton, 1980; Uzgiris & Hunt, 1975; Piaget, 1953; Willatts, 1990, 1999).
These developments in infants’ means-end action parallel changes in infants’ perception of others’ means-end acts. Sommerville and Woodward (2005a) habituated 10- and 12-month-old infants to a sequence in which an actor pulled one of the two cloths, each supporting a different out-of-reach toy, in order to obtain her target toy. The locations of the toys were reversed following habituation and on test trials infants saw the actor grasp a new cloth, supporting the same toy she had acted toward previously, or grasp the same cloth that she had initially, supporting a new toy. Twelve-month-old infants looked longer to the new toy trials than the new cloth trials, suggesting that they construed the actor’s actions on the cloth as directed toward the toy. Ten-month-old infants’ habituation performance was related to their ability to solve a similar cloth-pulling task in their own actions. Infants who produced predominantly planful solutions to solve the action task (based on how frequently they intentionally used the cloth as a means to get the toy) looked longer to the new toy than new cloth events, whereas infants who infrequently used planful strategies to solve the action task showed the reverse pattern of looking. These findings suggest a transition between 10 and 12 months of age in infants’ ability to view others’ actions on intermediary objects as directed toward goal objects, and provide evidence that this emerging ability is linked to infants’ skill at solving similar sequences in their own actions.
A subsequent study investigated whether action experience exerts a causal impact on action perception. Previous research indicates that infants can encode the goal of a direct reach and grasp by 5 to 6 months of age (Woodward, 1998), an age by which infants are adept at executing smooth and efficient goal-directed reaches (Bertenthal & Clifton, 1998). Sommerville, Woodward and Needham (2005) provided pre-reaching infants with a reaching intervention, which facilitated infants ability to apprehend objects, either prior to or following a habituation paradigm that assessed their ability to recognize the goal-directed nature of a simple reach and grasp event. Infants who received the reaching intervention before the habituation paradigm encoded the goal of the reach and grasp events, whereas infants who received the tasks in the reserve order did not.
The aforementioned findings suggest that providing infants with action experience impacts their action perception. However, these findings raise the question of whether active experience uniquely impacts infants’ action perception. Action experience may impact infants’ action perception by increasing infants’ observational experience with certain goal-directed actions. Infants might learn about salient action effects (Hofer, Hauf & Aschersleben, 2005), behavioral regularities that signal goal attainment (Baldwin, Baird, Saylor & Clark, 2001), object affordances (Gibson & Pick, 2000), and other people’s emotional reactions to goal attainment (Brand, Baldwin & Ashburn, 2002) by observing their own or others’ actions.
Alternately, active experience may play a unique or privileged role in infants’ action perception. Active experience appears to preferentially influence tool use behavior before the end of the first year of life. Although toddlers readily learn to use tools from watching others (Barrett, Davis & Needham, 2007; Brown, 1990; Casler & Keleman, 2005; 2006; Chen & Siegler, 2000; Meltzoff, 2007), 9- to 12-month-old infants demonstrate no improvements (Goubet et al., 2006; Provasi, Dublon & Bloch, 2001; Sommerville & Woodward, 2005a), or only marginal improvements (Abravenel & Gingold, 1985; Nielsen, 2006; Provasi et al., 2001) following observations of tool use or means end acts. Moreover, infants’ self experience has been hypothesized to play a formative role in their understanding of goals and intentions, broadly construed. According to Meltzoff (2002b; 2005; 2007) infants understand others’ behavior by analogy to the self: when infants see another individual act they reflect on the internal states that typically accompany the witnessed behavior when it is self performed.
Furthermore, a variety of evidence indicates that observation and production share a common computational code and neural architecture (“mirror neuron system”) both in adults (e.g., Grafton et al., 1996; Grèzes and Decety, 2001; Hamzei et al., 2003; Prinz, 1997) and in children and infants (Falyck-Ytter, Gredeback & von Hofsten, 2006; Fecteau et al., 2004; Longo & Bertenthal, 2006; Lepage & Théoret, 2007). This system may support action perception and understanding (Jacobs & Jeannerod, 2004; Sommerville & Decety, 2006; Wilson & Knoblich, 2005) by enabling a covert simulation of an observer’s own motor plans during observation (e.g., Jeannerod, 2001). Although shared neural resources for action and perception ultimately result in a bidirectional influence of action on perception and perception on action, motor activation during action observation has been hypothesized to arise as a result of correlated visual-motor experience during action production and practice (Heyes, Bird, Johnson & Haggard, 2005) and the degree of mirror neuron system activation during observation is mediated by motor expertise and experience (but not observational experience; Calvo-Merino et al., 2004, 2006; Järveläinen et al., 2004). Thus, action expertise and experience may be critical to the establishment and operation of this system.
The present study directly investigated the impact of active versus observational experience on infants’ understanding of a novel tool use event: using a cane to retrieve an out-of-reach toy. At 10 months of age infants rarely spontaneously use the cane as a tool, but their ability to do so improves as a function of training and practice (Sommerville, Feldman & Dillon, 2008). If active experience plays a privileged role in infants’ action understanding, then infants should demonstrate greater understanding of the goal-directed nature of the tool use sequence following active training than following observation. In contrast, if infants first identify actors’ goals in tool use events from observation, then infants receiving observational experience should outperform infants receiving active experience. Finally, it is possible that active and observational experience exert equivalent effects on infants’ tool use understanding, in which case infants should benefit equally from active and observational experience.
Method
Participants
Fifty-one 10-month-old infants participated in the experiment. Infants ranged in age from 9 months, 15 days to 10 months, 19 days (mean age = 9 months, 24 days). Twenty-five infants were female. The infants were all full term (at least 37 weeks gestation) and from a large city in the United States. Participants were recruited through a database maintained by a large university in the Pacific Northwest. Thirty-five infants were identified as White, Non-hispanic, 5 as mixed ethnicities, 1 as African American, 1 as Asian/Pacific Islander and 9 unreported. Eleven additional infants began the procedure but were not included in the final sample because they became fussy during the task and did not complete the procedure (n = 4), there was an experimental error (n = 3), because they refused to watch test events (n = 3) or due to parental interference (n = 1). Seventeen infants took part in the baseline condition, 18 infants took part in the training condition, and 16 infants took part in the observation condition.
Procedure
Infants either completed only the habituation paradigm (baseline condition), the active training task followed by the habituation paradigm (active training condition) or the observation session followed by the habituation paradigm (observation condition). Testing of infants in the observation condition occurred once the baseline and active training conditions were completed because the observation session was designed to closely parallel the training session. The conditions did not vary in terms of average age or gender composition, ns.
Active training task
Infants sat in a high chair in front of a testing table. During the task infants used a cane to retrieve a series of out-of-reach toys. Infants acted on the red- and green-striped canes (dimensions) that were subsequently featured in the habituation paradigm. The orientation of the canes mirrored the orientation featured in the habituation paradigm.
Infants received two pre- and two post-test trials in which the experimenter placed the toy (either a yellow rubber duck or the purple hippopotamus; both featured in the subsequent habituation paradigm) out of the infants’ reach in the crook of the cane and infants were encouraged to get the toy. Pre- and post-test trials ended after a) infants solved the task, b) infants acted on the cane without attending to the toy for 10 seconds, or c) 30 seconds elapsed. No assistance was given to the infants on these trials, and infants were not rewarded for obtaining the toy.
During training trials, the experimenter used a variety of methods designed to enhance infants’ ability to use the cane to retrieve a variety of bath toys. These included tapping on the toy, tapping on the cane, helping infants to pull the cane, modeling cane-pulling, using red- and green-striped canes that moved in tracks to enable infants to more readily pull the canes and praising infants after they obtained the toy (the experimenter displayed a mild positive expression and said “Good job”). Infants proceeded to testing after a) they had received a minimum of 4 opportunities to act on the cane to get the toy and b) they used the cane to get the out-of-reach toy on at least 3 consecutive trials. Infants received up to 9 training trials (mean = 6.2). The training session lasted an average of 6 minutes and 35 second (range: 4 minutes and 24 seconds to 8 minutes and 47 seconds).
Observation session
The observation session was designed to parallel the training condition. The condition was designed as a “third-party” observational experience, thus infants sat directly facing the experimenter but several feet away, and neither experimenter interacted with infants during the observation session. Infants watched a series of 10 cane-pulling trials (matched to the number of cane-pulling trials that training infants completed) that were modeled after those produced by the most successful infants from the training condition. Because it would have been difficult for the actor to accurately reproduce the exact actions of the infants in the training condition, the actor solved the problem planfully on every trial. This ensured that, if anything, the quality of problem-solving attempts that infants saw exceeded those of the attempts that infants in the training condition performed. An on-line observer verified that infants watched each of the ten trials; if infants failed to do so they did not proceed to the habituation paradigm.
The pre-test and post-test trials were administered in an identical manner to those of the active training condition. During training trials experimenter used the same training cues that were used most frequently by the experimenter in the training condition: infants saw the experimenter model pulling the cane once (following the pre-test trials) and the experimenter tapped on the toy twice during each trial. After solving each trial the “infant” experimenter maintained a neutral facial expression similar to that produced by infants in the active training condition and played with the toy for the same average duration as infants in the active training condition (approximately 5 seconds). The adult experimenter praised the actor after each training trial, to a level that matched the training condition. The length of the training session was matched to the training session (mean = 6 minutes and 23 seconds; range = 6 minutes and 30 seconds to 8 minutes and 10 seconds; p > .6).
Habituation paradigm
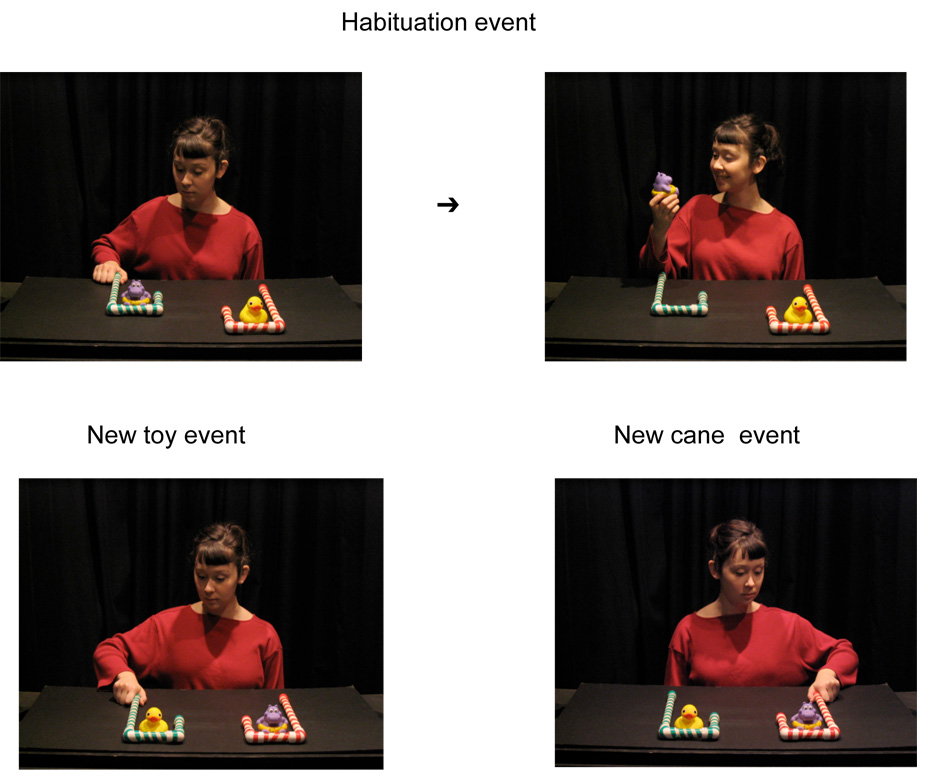
The habituation paradigm was modeled after Sommerville and Woodward (2005b; see Figure 1). Infants saw an actor (who was unaware of the condition the infant was assigned to) sitting behind a stage that supported the red and green-striped canes, each surrounding a different toy (the duck and hippopotamus). On habituation trials the actor reached toward and grasped the stalk of one of the canes, pulled it towards her, grasped the toy sitting in the crook of the cane and picked up the toy. Recording of infants' looking began once the actor had picked up the toy and ended when infants looked away from the event for two consecutive seconds. At the end of each trial, the screen was raised and then lowered to begin the next trial. The habituation phase ended after infants' looking time, totaled across three consecutive trials, fell to half of its initial level. Thus, infants received a minimum of six habituation trials. If the habituation criterion was not met after 14 trials, the habituation phase was ended and test trials began.
Figure 1.
The cane-pulling habituation paradigm.
Prior to the test phase the screen was raised and the locations of the toys were switched; the canes stayed in place. On test trials the actor grasped one of the canes and then remained stationary. Infants' looking was recorded from the time that the actor grasped the stalk of the cane until the infant looked away from the event for two consecutive seconds. On new toy trials, the actor grasped the same cane she had grasped during habituation trials, which now surrounded a different toy. On new cane trials, the actor grasped the other cane, which now surrounded the toy that had been grasped during habituation trials. Infants received two trials of each type in alternation for a total of four test trials. The location of the toys on habituation trials, the side to which the actor first reached during habituation trials and the test event shown first were counterbalanced across infants.
Coding and reliability
Training performance
We were most interested in infants’ performance on the pre- and post-test trials. To ensures that trials in which infants solved the task inadvertently were not counted toward their success we coded whether infants solved the cane-pulling task in an apparently planful manner. Trials were scored as planful if infants looked at the toy, pulled the cane while maintaining attention to the toy and quickly and immediately grasped the toy (within three seconds; cf. Sommerville & Woodward, 2005).
In addition, we coded the training trials for frequency of interventions on the toy by the experimenter, the experimenter’s interventions on the cane, the frequency of use of the sliding cane, the extent to which the experimenter assisted the infant in pulling the cane, the frequency of modeling attempts and the experimenter’s overall use of praise during the task to determine whether any of these variables accounted for infants’ improvement from pre- to post-test trials.
Reliability coding was completed for 25% of the sample for the pre- and post-test trials. The primary and secondary coder agreed on the classification of 94 % of pre and post-trials.
Habituation paradigm
Infants’ looking time was calculated online by an observer who watched infants on a video monitor using a computer program to calculate looking times and habituation criteria (Pinto, 1994). The observer was unaware of the trial type that infants were viewing and was only able to see infants on the monitor. A secondary observer, unaware of infants’ condition or the trial type infants saw first, coded infants’ eye gaze from videotape. Trials on which the primary and secondary observer identified the same look away as ending the trial were counted as agreements. The primary and secondary coder agreed upon 92% of trials for the baseline condition and 99% of trials for the training condition and 98% of trials for the observation condition. Disagreements were randomly distributed, χ2 (df = 1) = 1.0, p >.9.
Results
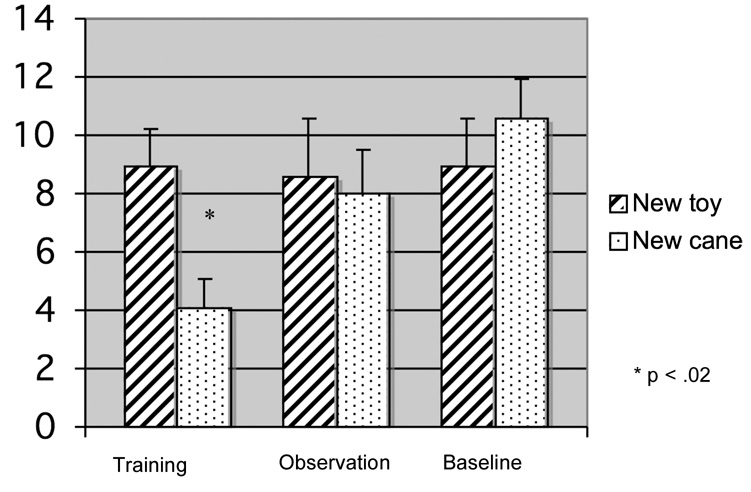
Our main question of interest was whether infants’ looking times to the test events varied as a function of condition. Because within-condition analyses revealed that observation infants’ test preference was mediated by the test event shown first, we performed a between-subjects’ analysis on infants’ looking on the first test trial. Infants’ looking times were entered into an ANOVA with test trial type (new toy versus new cane event) and condition (baseline vs. observation vs. training) as the between-subjects variables1. This analysis revealed a main effect of condition, F(2,45)=3.3, p<.05, which was qualified by a condition by test trial type interaction, F(2,45) = 3.9, p<.03; hp2= .15. Planned comparisons revealed that only training infants looked longer to the new toy event than to the new cane event, t(16) = 2.2, p<.02; hp2 = .33, suggesting that these infants attended more heavily to a change in the actor’s goal versus a change in the cane that the actor on (see Figure 2).
Figure 2.
Mean looking times (standard errors) on the first test trial as a function of trial type and condition.
To investigate whether observational experience exerted any impact on infants’ attention to the test events, we examined infants’ recovery from habituation to the test events. Infants in each condition differed systematically in their response to the test events: baseline infants responded only to the new cane event, t(16) = 2.3, p<.04; hp2 = .25, observation infants recovered to both the new cane, t(15) = 3.6, p<.002; hp2 = .46, and new toy events, t(15) = 4.7, p<.001; hp2 = .60, and training infants recovered attention selectively to the new toy event, t(17) = 2.2, p<.04; hp2 = .232,3. Taken together with the above findings, these results indicate that only training infants selectively encoded the goal of the cane-pulling sequence.
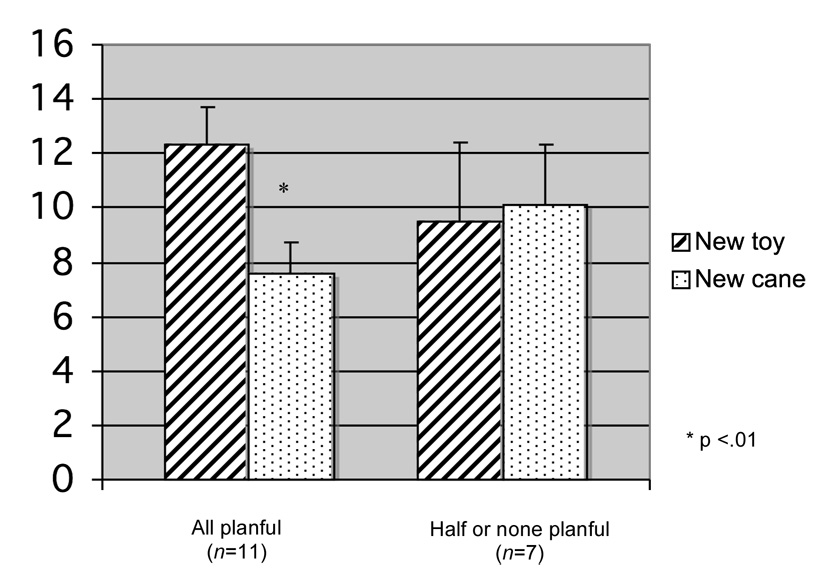
We next focused on the impact of individual differences in active training performance on infants’ subsequent interpretations of the cane-pulling event. Training infants significantly improved from pre-test, M = .30, SE = .08, to post-test trials, M = .70, SE = .10; t(16) = 2.9, p < .01; hp2= .404. Eleven infants performed planful strategies on both post-test trials and seven infants performed planful strategies on half (n=4) or no post-test trials (n=3). To investigate whether this variability was related to infants’ test event preference, we entered infants’ looking times into an ANOVA with trial type (new cane vs. new toy) as the within-subjects variable and training performance (all planful vs. half or fewer planful) and test event shown first (new toy vs. new cane) as the between-subjects variables. This analysis revealed a marginal effect of trial type, F(1,14) = 3.4, p<.09, which was qualified by a test trial type by post-test performance interaction, F(1,14) = 4.5, p<.05; hp2 = .27, and no other significant effects or interactions (ns). Infants who demonstrated planful strategies on all post-test trials looked significantly longer on new toy trials than on new cane trials, t(10) = 3.5, p<.01; hp2 = .55. Infants’ looking times to the test events as a function post-test performance are depicted in Figure 35.
Figure 3.
Mean looking times (standard errors) to the test trials as a function of post-test performance (training condition).
Discussion
The current findings support the hypothesis that active training impacts infants’ understanding of another person’s tool use actions over and above observational experience. Training infants selectively encoded the goal of the cane-pulling sequence, and their ability to do so was related to the extent to which they could successfully use the cane to get the toy independently on post-test trials. Matched observational experience was not sufficient to lead to selective encoding of the goal of the sequence. However, following observational experience infants recovered attention to any change to the structure of the sequence, suggesting that observational experience may exert weaker effects on infants’ action perception than active experience and/or that the impact of observational experience on action understanding may vary as a function of age or developmental level. Future work can distinguish between these alternatives by testing infants across a variety of ages and by investigating whether infants can identify the goal of the tool use event when presented with a greater amount of observational experience.
Are there alternate interpretations of our findings? The observation and training conditions differed from one another not only in terms of whether the infants acted or not, but also in terms of how successful the witnessed tool use attempts were: infants in the training condition experienced both failure and success at using the tool, whereas observation infants witnessed only tool use success. In a recent study Want and Harris (2001) found that 3-year-olds, but not 2-year-olds, benefited from witnessing another person’s tool use success and failure (above only witnessing tool use success). Thus, is unlikely that exposure to tool use failures would enhance infants’ tool use understanding. Nevertheless, future work should directly address this possibility by manipulating the extent of the actor’s success during the observation task.
The impact of tool use experience on tool use perception is consistent with recent claims that a motor resonance mechanism is present and operative in infancy and may underlie action understanding (Bertenthal & Longo, 2007; Falck-Ytter et al., 2006; Lepage & Théoret, 2007). Several authors have recently claimed that the mirror neuron system plays an important role in action anticipation (e.g. Sommerville & Decety, 2006; Wilson & Knoblich, 2005). In monkeys mirror neurons discharge in response to fully visible goal-directed actions, but also when monkeys are presented with only the beginning of a goal-directed action toward a hidden object (Umiltà et al., 2001). Moreover, motor representations are prospective: current actions reflecting impending action goals and intentions (e.g., Claxton, Keen & McCarty, 2003; Flanagan & Johansson, 2003; Johnson-Frey, McCarty & Keen, 2004). In the current study, activation of motor representations during action observation may have supported anticipation of the actor’s upcoming actions, leading to enhanced attention to the change in the actor’s goal. Our results may also have implications for the developmental trajectory of the mirror neuron system (see Bertenthal & Longo, 2007; Kilner & Blakemore, 2007; Lepage & Théoret, 2007 for various perspectives on this issue). In the present study, infants’ ability to identify the goal of the tool use sequence relied on their own competence at performing the sequence; in adults, the extent of mirror neuron system activation is mediated by the observer’s own motor history (Calvo-Merino et al., 2004; 2006; Cross et al., 2006; Järveläinen et al., 2004). These findings suggest that the mirror neuron system likely undergoes significant developments within and beyond infancy, based in part on the observer’s growing action experience and abilities.
Finally, self-produced experience is likely only one means by which infants come to understand the actions of others. Past research indicates that infants capitalize on a range of cues within the ongoing stream of action, such as salient action effects (Hauf et al., 2004), behavioral regularities (Baldwin et al., 2001), equifinality of action (Kiraly et al., 2003), efficiency of action (Csibra et al., 1999; Gergely, Nadasdy, Csibra & Biro), self-propelled movement (Baron-Cohen, 1995; Premack & Premack, 1997) and contingency cues (Shimuzu & Johnson, 2004) to identify goal-directed action and agents. In addition, emerging evidence suggests that infants draw on causal knowledge (Sommerville & Woodward, 2005a; Woodward & Sommerville, 2000), prior information about an actor’s action tendencies (Sommerville & Crane, 2008) and language utterances (Sommerville, Crane & Braun, 2008) to make sense of others’ behavior. Thus, it is likely that infants capitalize on a variety of mechanisms and information sources to support their action understanding. A future task for developmental researchers will be to identify how these information sources and mechanisms are integrated in the development of action perception and interpretation.
Acknowledgments
A University of Washington Royalty Research Fellowship and a National Institute of Child Health and Development Grant (1RO3HD053616) to the first author supported this work. We would like to thank all of the parents and infants who participated in this research. We would also like to thank Allyss Dillon and the students and staff at the Early Childhood Cognition Lab who helped with data collection and coding. We are grateful to three anonymous reviewers for comments on an earlier version of this manuscript.
Footnotes
Preliminary analyses revealed no main effects of toy on the infants’ right or first reach on infants’ looking times to the test events, ps>.3. Therefore, in subsequent analyses we collapsed across these variables. Two infants (baseline condition n = 1, observation condition n = 1) were excluded from analyses because they were outliers (more than 2 standard deviations above the mean on total looking to the test events)
Prior familiarization with the toys and tools, rather than observation of tool use acts per se, may have facilitated observation infants’ ability to process elements of the initial tool use event, in turn accounting for increased sensitivity to test event changes. However, infants in the observation and baseline conditions habituated in the same number of trials (observation: M = 7.3, SE = .6; baseline: M = 7.7, SE = .4; ns), suggesting that familiarity with the toys and tools did not speed infants’ processing of the tool use event. Future work can directly distinguish between these two alternatives by testing infants in a condition in which they are familiarized with the toys and tools independently prior to taking part in the habituation paradigm.
To further determine whether the observational session exerted a subtle impact on infants’ habituation performance we coded infants’ “micro look-aways” (looks away from the observation session trials that lasted 1 second or less) produced by each infant on each trial from videotape as an index of attentional quality. We categorized infants as high quality observers, M = .2; range = 0.0 to .3, or low quality observers, M = .6; range = .4 to .8, on the basis of a median split and subsequently entered infants’ looking times to the test events into an ANOVA with test trial type (new toy vs. new cane) as the within-subjects variable and observation quality (high vs. low) and test event shown first (new toy vs. new cane) as the between-subjects variables. This analysis revealed an interaction between test trial type and observation quality, F(1,12)=5.4, p<.04; hp2 = .31. High quality observers showed a non-significant preference for the new event whereas low quality observers showed a non-significant preference for the new cane event.
Pre-test trials were not recorded for one infant in the training condition. To assess whether the training methods that the experimenter used exerted an impact on infants’ cane-pulling performance we correlated the number of each of the different training attempts with infants’ post-test scores. These analyses revealed that infants’ post-test performance was unrelated to the specific training attempts used by the experimenter, all ps > .2.
To ensure that the impact of training on infants’ habituation performance did not result from mere familiarity with the canes, we coded the amount of time infants spent touching and looking at the canes during the training session, and investigated the relation between this measure of cane familiarity and the extent of infants’ new toy preference. We found no relation between infants’ overall attention to and contact with the cane and their habituation performance, ns.
References
- Abravanel E, Gingold H. Learning via observation during the second year of life. Developmental Psychology. 1985;21:614–623. [Google Scholar]
- Baldwin DA, Baird JA, Saylor MM, Clark MA. Infants parse dynamic action. Child Development. 2001;72:708–717. doi: 10.1111/1467-8624.00310. [DOI] [PubMed] [Google Scholar]
- Barrett TM, Davis EF, Needham A. Learning about tools in infancy. Developmental Psychology. 2007;43:352–368. doi: 10.1037/0012-1649.43.2.352. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Mindblindness: An essay on autism and theory of mind. Cambridge, MA: The MIT Press; 1995. [Google Scholar]
- Bates E, Carlson-Luden V, Bretherton I. Perceptual aspects of tool using in infancy. Infant Behavior and Development. 1980;3:127–140. [Google Scholar]
- Berger SA, Adolph KE, Lobo SA. Out of the toolbox: Toddlers differentiate wobbly and wooden handrails. Child Development. 2005;76:1294–1307. doi: 10.1111/j.1467-8624.2005.00851.x. [DOI] [PubMed] [Google Scholar]
- Bertenthal B, Clifton RK. Perception and action. In: Damon W, Kuhn D, Siegler R, editors. Handbook of Child Psychology. Cognition, perception and language. Vol. 2. New York: Wiley; 1998. pp. 51–102. [Google Scholar]
- Bloom P. Intention, history and artifact concepts. Cognition. 1996;60:1–29. doi: 10.1016/0010-0277(95)00699-0. [DOI] [PubMed] [Google Scholar]
- Boronat CB, Buxbaum LJ, Coslett HB, Tang K, Saffran EM, Kimberg DY, Detre JA. Distinctions between manipulation and function knowledge of objects: evidence from functional magnetic resonance imaging. Cognitive Brain Research. 2005;23:361–373. doi: 10.1016/j.cogbrainres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Brand RJ, Baldwin DA, Ashburn LA. Evidence for ‘motionese’: modifications in infant-directed action. Developmental Science. 2002;5:72–83. [Google Scholar]
- Brown AL. Domain-specific principles affect learning and transfer in children. Cognitive Science. 1990;14:107–133. [Google Scholar]
- Calvo-Merino B, Glaser DE, Grezes J, Passingham RE, Haggard P. Action observation and acquired motor skills: An fMRI study with expert dancers. Cerebral cortex. 2005 doi: 10.1093/cercor/bhi007. E-pub, Dec. 22, 2004. [DOI] [PubMed] [Google Scholar]
- Calvo-Merino B, Grèzes J, Glaser DE, Passingham RE, Haggard P. Seeing or doing? Influence of visual and motor familiarity in action observation. Current Biology. 2006;16:1905–1910. doi: 10.1016/j.cub.2006.07.065. [DOI] [PubMed] [Google Scholar]
- Casler K, Keleman D. Young children’s rapid learning about artifacts. Developmental Science. 2005;8:472–480. doi: 10.1111/j.1467-7687.2005.00438.x. [DOI] [PubMed] [Google Scholar]
- Casler K, Keleman D. Reasoning about artifacts at 24 months: The developing teleo-functional stance. Cognition. 2006 doi: 10.1016/j.cognition.2006.02.006. in press. [DOI] [PubMed] [Google Scholar]
- Csibra G, Gergely G, Biro S, Koos O, Brockbank M. Goal attribution without agency cues: The perception of “pure reason” in infancy. Cognition. 1999;72:237–267. doi: 10.1016/s0010-0277(99)00039-6. [DOI] [PubMed] [Google Scholar]
- Chen Z, Siegler RS. Across the great divide: Bridging the gap between understanding of toddlers' and older children's thinking. Monographs of the Society for Research in Child Development. 2000;65:1–108. [PubMed] [Google Scholar]
- Claxton LJ, Keen R, McCarty ME. Evidence of motor planning in infant reaching behavior. Psychological Science. 2003;14:354–356. doi: 10.1111/1467-9280.24421. [DOI] [PubMed] [Google Scholar]
- Connolly K, Dalgleish M. The emergence of a tool-using skill in infancy. Developmental Psychology. 1989;25:894–912. [Google Scholar]
- Cross ES, Hamilton AFDC, Grafton ST. Building a motor simulation de novo: Observation of dance by dancers. Neuroimage. 2006;31:1257–1267. doi: 10.1016/j.neuroimage.2006.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Sommerville JA. Shared representations between self and others: A social cognitive neuroscience view. Trends in Cognitive Science. 2003;7:527–533. doi: 10.1016/j.tics.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Defeyter MA, German TP. Acquiring an understanding of design: evidence from children’s insight problem solving. Cognition. 2003;89:133–155. doi: 10.1016/s0010-0277(03)00098-2. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Carmant L, Tremblay C, Robert M, Bouthillier A, Theoret H. A motor resonance mechanism in children? Evidence for subdural electrodes in a 36-month-old child. Neuroreport. 2004;15:2625–2627. doi: 10.1097/00001756-200412030-00013. [DOI] [PubMed] [Google Scholar]
- Flack-Ytter T, Gredebäck G, von Hofsten C. Infants predict other people’s action goals. Nature Neuroscience. 2006;9:878–879. doi: 10.1038/nn1729. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Johansson RS. Action plans used in action observation. Nature. 2003;424:769–771. doi: 10.1038/nature01861. [DOI] [PubMed] [Google Scholar]
- Gergely G, Nadasdy Z, Csibra C, Biro S. Taking the intentional stance at 12 months of age. Cognition. 1995;56:165–193. doi: 10.1016/0010-0277(95)00661-h. [DOI] [PubMed] [Google Scholar]
- Gibson EJ, Pick AD. An ecological approach to perceptual learning and development. Oxford: Oxford University Press; 2000. [Google Scholar]
- Goubet N, Rochat P, Maire-Leblond C, Poss S. Learning from others in 9-18-month-old infants. Infant and Child Development. 2006;15:161–177. [Google Scholar]
- Grafton ST, Arbib MA, Fadiga L, Rizzolatti G. Localization of grasp representations in humans by positron emission tomography. Experimental Brain Research. 1996;112:103–111. doi: 10.1007/BF00227183. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Decety J. Functional anatomy of execution, mental simulation, observation, and verb generation of actions: A meta-analysis. Human Brain Mapping. 2001;12:1–19. doi: 10.1002/1097-0193(200101)12:1<1::AID-HBM10>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzei F, Rijntjes M, Dettmers C, Glauche V, Weiller C, Büchel C. The human action recognition system and its relationship to Broca’s area: an fMRI study. NeuroImage. 2003;19:637–644. doi: 10.1016/s1053-8119(03)00087-9. [DOI] [PubMed] [Google Scholar]
- Hauf P, Prinz W. The understanding of one’s own an other’s actions during infancy: “You-like-Me” or “Me-like-You”. Interaction studies: Social Behavior and Communication in Biological and Artificial Systems. 2005;6:429–445. [Google Scholar]
- Hauf P, Elsner B, Aschersleben G. The role of action effects in infants’ action control. Psychological Research. 2004;68:115–125. doi: 10.1007/s00426-003-0149-2. [DOI] [PubMed] [Google Scholar]
- Hauser MD, Santos LR. The evolutionary ancestry of our knowledge of tools: From percepts to concepts. To appear. In: Margolis E, Lawrence S, editors. The Creation of Mind. Oxford University Press; (in press) [Google Scholar]
- Heyes C, Bird G, Johnson H, Haggard P. Experience modulates automatic imitation. Cognitive Brain Research. 2005;22:233–240. doi: 10.1016/j.cogbrainres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Hofer T, Hauf P, Aschersleben G. Infants’ perception of goal-directed actions performed by a mechanical device. Infant Behavior & Development. 2005;28:466–480. [Google Scholar]
- Jacobs P, Jeannerod M. The motor theory of social cognition: A critique. Trends in Cognitive Sciences. 2004;9:21–25. doi: 10.1016/j.tics.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Järveläinen J, Schürmann M, Hari R. Activation of the human primary motor cortex during observation of tool use. Neuroimage. 2004;23:187–192. doi: 10.1016/j.neuroimage.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. Neural simulation of action: a unifying concept of motor cognition. NeuroImage. 2001;14:S103–S109. doi: 10.1006/nimg.2001.0832. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey SH, McCarty ME, Keen R. Reaching beyond spatial perception: Effects of intended future actions on visually guide prehension. Visual Cognition. 2004;11:371–399. [Google Scholar]
- Johnson-Frey SH, Newman-Norlund R, Grafton ST. A distributed left hemisphere network active during planning of everyday tool use skills. Cerebral Cortex. 2005;1:681–695. doi: 10.1093/cercor/bhh169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan IP, Kable JW, Van Scoyoc A, Chatterjee A, Thompson-Schill SL. Fractionating the left frontal response to tools: Dissociable effects of motor experience and lexical competition. Journal of Cognitive Neuroscience. 2006;18:267–277. doi: 10.1162/089892906775783723. [DOI] [PubMed] [Google Scholar]
- Kiraly I, Jovanovic B, Prinz W, Aschersleben G, Gergely G. The early origins of goal attribution in infancy. Consciousness and Cognition: An International Journal. 2003;12:752–769. doi: 10.1016/s1053-8100(03)00084-9. [DOI] [PubMed] [Google Scholar]
- Kelemen D. Function, goals and intention: children’s teleological reasoning about objects. Trends in Cognitive Sciences. 1999;3:461–468. doi: 10.1016/s1364-6613(99)01402-3. [DOI] [PubMed] [Google Scholar]
- Longo MR, Bertenthal BI. Common coding of observation and execution of action in 9-month-old infants. Infancy. 2006;10:43–59. doi: 10.1207/s15327078in1001_3. [DOI] [PubMed] [Google Scholar]
- Matan A, Carey S. Developmental changes within the core of artifact concepts. Cognition. 2001;78:1–26. doi: 10.1016/s0010-0277(00)00094-9. [DOI] [PubMed] [Google Scholar]
- McCarty ME, Clifton RK, Collard R. Problem-solving in infancy: The emergence of an action plan. Developmental Psychology. 1999;35:1091–1101. doi: 10.1037//0012-1649.35.4.1091. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN. Imitation as a mechanism of social cognition: Origins of empathy,theory of mind, and the representation of action. In: Goswami U, editor. Blackwell handbook of childhood cognitive development. Blackwell handbooks of developmental psychology. Malden, MA: Blackwell publishers; 2002b. pp. 6–25. [Google Scholar]
- Meltzoff AN. Imitation and other minds: The "Like Me" hypothesis. In: Hurley S, Chater N, editors. Perspectives on imitation: From cognitive neuroscience to social science. Cambridge: MIT Press; 2005. pp. 55–77. [Google Scholar]
- Meltzoff AN. The ‘Like Me’ framework for recognizing and becoming an intentional agent. Acta Psychologica. 2007 doi: 10.1016/j.actpsy.2006.09.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata Y, McClelland J, Johnson MH, Siegler RS. Rethinking infant knowledge: Toward an adaptive process account of successes and failures in object permanence tasks. Psychological Review. 1997;104:686–713. doi: 10.1037/0033-295x.104.4.686. [DOI] [PubMed] [Google Scholar]
- Nielsen M. Copying Actions and Copying Outcomes: Social Learning Through the Second Year. Developmental Psychology. 2006;42:555–565. doi: 10.1037/0012-1649.42.3.555. [DOI] [PubMed] [Google Scholar]
- Parker ST, Gibson KR. Object manipulation, tool use and sensorimotor intelligence in feeding adaptations in cebus monkeys and great apes. Journal of Human Evolution. 1977;6:623–641. [Google Scholar]
- Piaget J. The origins of intelligence in the child. London: Routledge & Kegan Paul; 1953. [Google Scholar]
- Premack D, Premack AJ. Infants attribute value + or − to the goal-directed actions of self-propelled objects. Journal of Cognitive Neuroscience. 1997;9:848–856. doi: 10.1162/jocn.1997.9.6.848. [DOI] [PubMed] [Google Scholar]
- Prinz W. Perception and action planning. European Journal of Cognitive Psychology. 1997;9:129–154. [Google Scholar]
- Provasi J, Dubon CD, Bloch H. Do 9- and 12-month-old infants learn means-end relations by observing. Infant Behavior and Development. 2001;24:195–213. [Google Scholar]
- Shimuzu YA, Johnson SC. Infants’ attribution of a goal to a morphologically unfamiliar agent. Developmental Science. 2004;7:425–430. doi: 10.1111/j.1467-7687.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- Sommerville JA, Decety J. Weaving the fabric of social interaction: Articulating developmental psychology and cognitive neuroscience in the domain of motor cognition. Psychonomic Bulletin and Review. 2006;13:179–200. doi: 10.3758/bf03193831. [DOI] [PubMed] [Google Scholar]
- Sommerville JA, Woodward AL. Pulling out the intentional structure of action: The relation between action processing and action production in infancy. Cognition. 2005a;95:1–30. doi: 10.1016/j.cognition.2003.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerville JA, Woodward AL. Infants’ sensitivity to the causal features of means-end sequences in action and perception. Infancy. 2005b;8:119–145. [Google Scholar]
- Sommerville JA, Crane CC. Infants use prior information about an actor’s goal to disambiguate an action sequence. 2008 Manuscript under review. [Google Scholar]
- Sommerville, Crane CC, Braun K. Once a frog-lover always a frog-lover?: Infants’ ability to transfer information about an actor’s goals across contexts. 2008 Manuscript under review. [Google Scholar]
- Sommerville JA, Feldman EN, Dillon AN. Infants’ ability to solve means-end sequences: Intertask relations and factors influencing performance. 2008 Manuscript in preparation. [Google Scholar]
- Sommerville JA, Woodward AL, Needham A. Action experience alters 3-month-old infants' perception of others' actions. Cognition. 2005;96:B1–B11. doi: 10.1016/j.cognition.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzgiris IC, Hunt JM. Oxford, England: University of Illinois Press; 1975. Assessment in infancy: Ordinal scales of psychological development. [Google Scholar]
- Umiltà MA, Kohler E, Gallese V, Fogassi L, Fadiga L, et al. “I know what you are doing”: A neurophysiological study. Neuron. 2001;32:91–101. doi: 10.1016/s0896-6273(01)00337-3. [DOI] [PubMed] [Google Scholar]
- Want SC, Harris PL. Learning from other people’s mistakes: causal understanding in learning to use a tool. Child Development. 2001;72:431–443. doi: 10.1111/1467-8624.00288. [DOI] [PubMed] [Google Scholar]
- Willatts P. Development of problem-solving strategies in infancy. In: Bjorklund DF, editor. Children's strategies: Contemporary views of cognitive development. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc.; 1990. pp. 23–66. [Google Scholar]
- Willatts P. Development of means-end behavior in young infants: Pulling a support to retrieve a distant object. Developmental Psychology. 1999;35:651–667. doi: 10.1037//0012-1649.35.3.651. [DOI] [PubMed] [Google Scholar]
- Wilson M, Knoblich G. The case for motor involvement in perceiving conspecifics. Psychological Bulletin. 2005;131:460–473. doi: 10.1037/0033-2909.131.3.460. [DOI] [PubMed] [Google Scholar]
- Woodward AL. Infants selectively encode the goal of an actor’s reach. Cognition. 1998;69:1–34. doi: 10.1016/s0010-0277(98)00058-4. [DOI] [PubMed] [Google Scholar]