Abstract
Since the announcement of the STEP trial results in the past months, we have heard many sober pronouncements on the possibility of an HIV vaccine. On the other hand, optimistic quotations have been liberally used, from Shakespeare's Henry V's "Once more unto the breach, dear friends" to Winston Churchill's definition of success as "going from one failure to another with no loss of enthusiasm". I will forgo optimistic quotations for the phrase "Sang Froid", which translates literally from the French as "cold blood"; what it really means is to avoid panic when things look bad, to step back and coolly evaluate the situation. This is not to counsel easy optimism or to fly in face of the facts, but I believe that while the situation is serious, it is not desperate.
I should stipulate at the outset that I am neither an immunologist nor an expert in HIV, but someone who has spent his life in vaccine development. What I will try to do is to provide a point of view from that experience.
There is no doubt that the results of STEP were disappointing: not only did the vaccine fail to control viral load, but may have adversely affected susceptibility to infection. But HIV is not the only vaccine to experience difficulties; what lessons can we glean from prior vaccine development?
Lessons from vaccinology
First, look at an uncomplicated example: the rubella vaccine. This is a live attenuated virus that was isolated in WI-38 fetal fibroblasts during the 1962/63 rubella pandemic and attenuated by low temperature passage in those same cells [1]. By selection of clones replicating at low temperature, we obtained a virus that consistently multiplied in seronegative humans and that evoked both humoral and mucosal immune responses that blocked superinfection [2]. Why was it successful in giving immunity? Of course, the answer is this: neutralizing antibodies to rubella present in the serum and on the mucosa are correlates of protection in preventing both nasopharyngeal implantation and subsequent viremia [3].
However, things are not always that easy. Take the paramyxoviruses measles and respiratory syncytial virus (RSV) as examples. Live measles virus has been a great success in eliminating the disease, but in the early days there was also a licensed killed measles vaccine. Unfortunately, when vaccinated children were exposed to wild measles they suffered an atypical disease that included severe pulmonary, hepatic and dermatologic manifestations. Similarly, a formalin inactivated RSV vaccine was tested in infants, many of whom developed severe respiratory disease after subsequent natural infection with the virus [4].
The pathogenetic features of these adverse reactions were similar [Table 1]. In both cases, the antibodies elicited had either disappeared or were non-protective because directed against the wrong protein, the T cell response was Th2 biased and contributed to the pathology, and replication of wild virus was enhanced [5-8]. Although I will not argue that this type of reaction could also explain the putative enhanced acquisition of HIV in the STEP and Phambili trials, it at least illustrates the idea that in the absence of functional antibodies, cellular immunity of the wrong type can enhance, rather than diminish susceptibility.
Table 1.
Severe reactions to Inactivated Measles and RSV Vaccines
| Following exposure, vaccinees had exaggerated disease in lungs. |
|---|
| Pathology included immune complex deposition and high replication in the lungs. |
| Vaccines elicited non-protective, low avidity, waning antibodies. |
| Vaccines elicited strong CD4+ proliferation with a Th2 cytokine response, including IL-13 |
| Caused cessation of use of both vaccines |
Another type of misadventure happened with the first licensed rotavirus vaccine. This was an orally administered mixture of a simian rotavirus and reassortants of human and simian rotaviruses in which the simian virus contributed 10 of the 11 double-stranded RNA segments. Although protective, it caused intussusception (intestinal invagination) in approximately one in 10,000 vaccinees [9]. This happened because the supposedly attenuated simian vector retained pathogenicity for the infant intestine, causing diarrhea and fever [10]. This problem was solved in my former laboratory by substituting a bovine rotavirus as vector, and in another lab, by classical attenuation of a human rotavirus [11,12]. Neither of the new vaccines causes intussusception [13,14]. The point is that the choice of a supposedly attenuated vector is a key issue, and that the wrong choice of vector brings safety problems.
Another lesson from vaccinology is that correlates of immunity may be complex, and antibody and cellular immunity are often collaborative. This point can be illustrated with reference to cytomegalovirus (CMV) [15,16]. As in HIV, superinfection may occur in previously infected individuals, but the course of secondary infection is much less pathogenic than in non-immune subjects. This is particularly important when infection occurs in pregnancy, as the fetal outcomes after primary or secondary infection are quite different.
Antibody against CMV alone may protect against primary infection, but if infection occurs, cellular immunity is critical in controlling it. In addition, challenge dose is an important variable, and can overcome moderate levels of immunity, a fact that may apply to HIV. This was shown by challenge studies in which seronegative volunteers could be infected with 10 PFU of a low-passage CMV, whereas naturally seropositive volunteers were protected against 100 PFU, but could also be infected if the dose was raised to 1000 PFU [17].
Nevertheless, two vaccines in development have shown moderate ability to prevent or modify CMV infection. One is based on a live attenuated virus, and one on a glycoprotein that induces neutralizing antibody [17,18]. Thus, the fact that superinfection has been demonstrated in some already HIV-infected people does not necessarily rule out a role for immunity in controlling disease after infection [19,20].
Another example is immunity to smallpox after vaccinia, about which one can say that antibody is key: high titers give sterile immunity. However, as antibody wanes, infection may occur, which CD8+ T cells must control. Antibody lasts forever after vaccination and CD4+ T cells last almost forever, but CD8+ T cells disappear after about 20 years. Thus, although complete protection is temporary, protection against severe disease is permanent [21].
The last agent I would like to discuss before turning to HIV is hepatitis C. There are many similarities between the two agents, including the rapid development of geographical variation, with a 30% nucleotide difference between hepatitis C genotypes [22]. Although hepatitis C is a flavivirus, it shares a number of properties with HIV, as shown in Table 2.
Table 2.
Similarities between Hepatitis C and HIV
| HCV | HIV | |
|---|---|---|
| Envelope and Core Ags | + | + |
| Glycosylated envelope protein | + | + |
| Envelope is neut. target, but hypervariable | + | + |
| Chronic viremia | + | + |
| Escape mutation | + | + |
| Geographical genetic variation | + | + |
| PD-1 Upregulated | + | + |
| High titer neutralizing Ab protects | + | + |
| Strong cell responses against multiple epitopes necessary for control of viremia | + | + |
| CD4+ cells needed to sustain CD8+ T cells | + | + |
| Tcm cells needed for long-term control | + | + |
Interestingly, patients who resolve acute hepatitis C infections have higher levels of neutralizing antibodies early in infection than do those who go on to chronic infection (Figure 1). As in the case of HIV, antibodies do not help when they develop late in chronic infection [23]. The target of neutralizing antibodies is the E2 envelope protein, and as in HIV, escape occurs [24,25].
Figure 1.
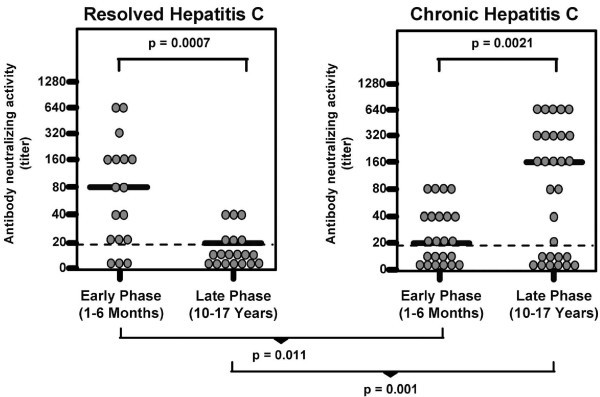
Neutralizing antibodies in patients with resolved or chronic hepatitis C.
However, it appears that late in the infection, induction or reconstitution of cellular immune responses also correlates with recovery from chronic hepatitis C viremia [26-30]. Those cellular responses are mediated by both CD4+ and CD8+ T cells directed against non-structural as well as structural proteins; to be effective, those responses must be strong, highly avid, and directed against multiple epitopes [31-34]. Although a crucial difference between the two viruses is the lack of integration by hepatitis C in contrast to HIV, I think it is instructive to see that a chronic infection can be counteracted by standard immune responses [32].
Innate immunity
So what can be said about immune protection against HIV? With regard to innate immune responses, we know that they are clearly valuable [35,36], both immediately after infection and as adjuvants to adaptive immune responses. The question is: do they have memory? A method of maintaining elevated innate immune responses after immunization, particularly NK cells or intracellular APOBEC3G, could be valuable, although contrariwise after HIV infection, it has been reported that an HIV-induced ligand is responsible for CD4+ T cell destruction by NK cells [37].
On the other hand, a recent report [38] shows that the gene Apobec3 encodes Rfv3, which enhances neutralizing antibody responses against lentiviruses. This opens a new avenue of research to counteract its antagonist, the Vif element of HIV. In addition, soluble CD40 ligand has been shown recently to enhance HIV-specific memory T cell responses [39,40].
Antibodies
Clearly, everybody would like to know how to induce a neutralizing response that covers primary isolates from all of the clades. A recent list of approaches is shown in Table 3[41]. To this list may be added: the recent studies attempting to mimic the b12-like antibodies produced by some infected individuals [42]; studies using alloantigens like hsp70 as part of an immunization regimen that apparently evokes a wider breadth of neutralization [43]; and the use of AAV as a vector to carry an antibody-producing gene into the cells of a vaccinee [44].
Table 3.
Novel approaches to the design of envelope immunogens
| Mimic native trimer on virion surface |
|---|
| Redirect immune responses to conserved conformational epitopes |
| Add disulfides or other amino acids to stabilize conformational epitopes |
| Bind envelope to CD4 or CD4-mimetic peptide |
| Remove carbohydrate residue or entire carbohydrate side chains |
| Redirect responses away from variable epitopes |
| Remove one or more variable loops |
| Add carbohydrate side chains to hide |
| variable regions |
However, short of the ideal of broad neutralization with a single antigen, it is not beyond our abilities to produce multivalent vaccines. Because of multiple serotypes or subtypes, numerous licensed vaccines are actually multivalent, including those for Human Papilloma, Influenza, Meningococcal, Pneumococcal, Polio, and Rotavirus. Moreover, every year we change the valences of influenza vaccines to match the evolution of the virus. Although this is not an ideal scenario, most years it works well; on condition that surveillance is good, and that there are regional manufacturers, it is practical to make different vaccines for different areas.
Thus, although antibodies to conserve epitopes are highly desirable, antibodies to gp120 loops that mutate and are regional in distribution may require continuous updating and regionalization of vaccine antigens (as for flu), as well as the inclusion of multiple gp 120s. Even with breakthroughs in finding conserved epitopes, I doubt that we can escape totally from having to make multivalent or regional HIV vaccines [45]. Indeed, recent reports suggest that multivalent HIV envelopes do give broad neutralizing responses [46-49].
Does antibody protect against HIV infection? Clearly, non-human primate studies using HIVIG or monoclonal antibodies strongly support an affirmative answer [50-53]. In addition, the burden of evidence is in favor of a protective ability of maternal neutralizing antibodies in prevention of HIV transmission to the newborn [54]. Moreover, it has been reported that neutralizing antibodies develop rapidly and in high titer after HIV-2 infection, which could explain the much slower disease progression in HIV-2 patients [55-57].
How much antibody is needed for protection? A number of estimates have been made, and these are summarized in Table 4[50,52,58-61]. In addition, although superinfection is a fact in the presence of low levels of homologous neutralizing antibodies, there are data suggesting high levels are protective [62]. So if high levels of antibody are necessary for protection, in line with the need for multiple hits to neutralize the virion, and as HIV spreads from the site of implantation within several days, effector B cells must be in the circulation and producing antibody at the time of exposure [63,64]. Thus, booster doses of an AIDS vaccine will be necessary to maintain protective levels of antibody.
Table 4.
Estimates of titers of neutralizing antibodies required for sterile protection against HIV.
| Investigator | Titer | SN End Point |
Species | Remarks |
|---|---|---|---|---|
| Trkola et al [58] | 1/200 | 70% | Human | Acute infection |
| Parren et al [60] | 1/400 | 90% | Macaques | SHIV Challenge |
| Nishimura et al. [61] | 1/38 | 100% | Macaques | SHIV Challenge |
| Mascola et al [50,52] | 1/50, 1/29–1/88 | 90% | Macaques | SHIV Challenge |
| Trkola et al [59] | 1/400 | 90% | Human | Rebound after HAART |
Indeed, booster doses are commonly needed for vaccines, even for some that are highly efficacious. They are almost always needed for inactivated vaccines, e.g. tetanus, diphtheria, and polysaccharide conjugates (exceptions are hepatitis A and hepatitis B), and are often needed for live vaccines, e.g. measles, mumps, and smallpox (exceptions are rubella and OPV). This may be an inconvenient truth, but the use of adjuvants might help prolong immunity.
The new adjuvants now available in vaccinology are legion, and they increase breadth as well as height of antibody responses. They include oil-in-water and water-in-oil emulsions, saponins, liposomes, lipopolysaccharides, cytokines, cationic polymers for DNA plasmids, mast cell activiators and numerous toll-like receptor (TLR) agonists. A recent report showed that an oil-in-water emulsion containing monophosphoryl lipid A and QS-21 substantially increased the number of primary isolates that could be neutralized in vitro by rabbit antisera [45,65]. Much more work is needed in this area [66-68].
Cellular immunity
I think it is safe to say that cytotoxicity mediated by CD8+ T cells can for a time suppress HIV viral load, even if it can not prevent acquisition of infection [69-79]. Clinical data correlating CTL responses with control of viral load and macaque studies by many labs have made that point clearly. Two examples are illustrative: in Figure 2, CD8+ T cells were clearly associated with low viral loads after challenge with SHIV [70]; and in a study of a DNA prime/adenovirus 5 boost, CTL against gag alone reduced viral load after SIV challenge (Figure 3). Moreover, among many other factors, elite HIV controllers, long-term non-progressors, and multiply exposed sex workers all have evidence of potent CD8+ T cells in the blood and in the mucosa [80-82], as well as innate immune factors [83].
Figure 2.
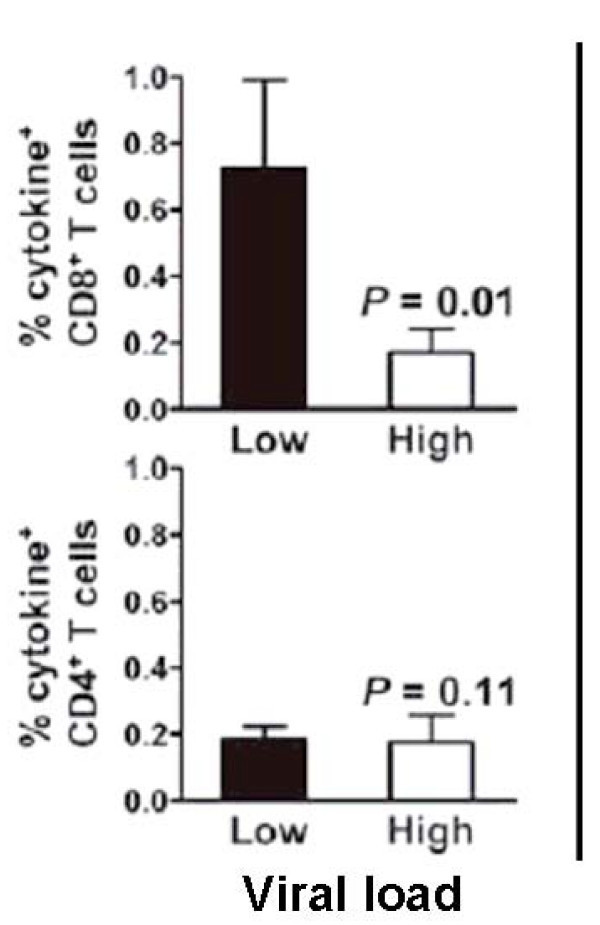
Control of High SHIV Viral Load by CD8+ Cells After Vaccination.
Figure 3.
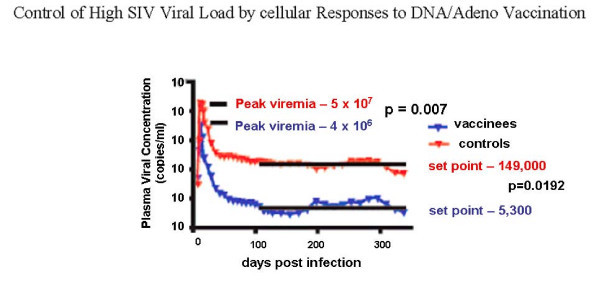
Control of High SIV Viral Load by cellular Responses to DNA/Adeno Vaccination.
However, there are issues of quantity. Recently, CTL responses were measured after two conventional live vaccines, smallpox and yellow fever [84]. In terms of percent CD8+ T cells specific to the vaccine, smallpox vaccine induced about 10% vaccinia-specific cells producing IFN-gamma, whereas yellow fever vaccine induced more than 2% yellow fever-specific cells. Compare those figures to the data from the STEP study in which only 0.5 to 1% of CD8+ T cells were specific to HIV (J McElrath, personal communication, 2008). Thus, it is legitimate to ask whether paucity of response played a role in the STEP failure.
Numerous factors influence the quality of CTL response, some of which are listed in Table 5. Many groups have demonstrated the importance of polyfunctionality, as defined by cytokine and chemokine secretion, in the control of HIV viral load [85-89] Other aspects of function that are suggested to be important include: CTL avidity [90,91]; number of epitopes seen by the CD8+ T cells [90,92], which is a reason for exploring the use of consensus and mosaic sequences to induce responses to more epitopes [86,91,93,94]; presence of polyfunctional CD8s in the rectal mucosa; preservation of Th17 cells [95]; and persistence of both CD4+ and CD8+ central memory T cells [92,96]. As it is likely that semen of HIV-transmitting patients will have both cell-free and cell-associated virus, it appears necessary that the CD8+ T cells be capable of killing infected cells in the inoculum [97].
Table 5.
Cellular immune responses to HIV that could be improved
| Quantity of specific CD8+ cells |
|---|
| Polyfunctionality of CD8+ cells |
| Avidity of CD8+ cells |
| Number of epitopes seen |
| Intestinal homing of CD8+ cells |
| Th17/Tregs balance |
| Increased CD4+ central memory cells |
| Increased CD8+ central memory cells |
My goal here is not to exhaustively examine all of the important T cell responses, but rather to say that there are numerous leads with regard to improving cellular immune responses to an HIV vaccine, and that the failure of the first trial of this idea says only that the responses induced were inadequate to simulate those induced during natural infection that appear to control HIV temporarily.
Mucosal immunity
It has become a cliché to say that vaccines can not provide sterile immunity. In my view, this is a canard. As indicated in Table 6, if the pathogenic agent is injected into the blood stream, as in arbovirus infection, or acts by a toxin, as in tetanus, sterile immunity is undoubted. In addition, if the agent implants first on the mucosa, as in many infections, sterile immunity is achievable on condition that mucosal immunity is sufficient to abort that replication. Examples of this principle include resistance to measles and rubella after vaccination with live viruses that induce both serum and mucosal antibody [21,98], and live or killed influenza vaccines, after which induction of either serum or mucosal antibody can completely prevent infection [99].
Table 6.
Do vaccines elicit "sterile" immunity?
| Yes | Depends Mucosal Presence of Antibody |
|---|---|
| Diphtheria | Polio |
| Hepatitis A | Hib |
| Hepatitis B | Influenza |
| Lyme | Measles |
| Rabies | Pertussis |
| Tetanus | Rubella |
| Yellow Fever | Varicella |
Mucosal immunity is as complex as systemic immunity [100-104]. Secretory IgA may neutralize on the surface or block transcytosis [105]. Second, the CTL population in the intestine is numerous and can kill HIV-infected cells, which is important in view of the evidence that preservation of intestinal memory CD4+ T cells contributes to a good prognosis for the subsequent course of HIV infection [106,107]. Third, serum IgG can diffuse onto mucosal surfaces, particularly in the respiratory tract. The latter fact probably accounts for the reduction of pharyngeal carriage of encapsulated bacteria by conjugated polysaccharide vaccines, and the reduction of virus titer in the pharynx and stool of IPV vaccinees [21,102].
I alluded to the live, orally administered rotavirus vaccine previously, and there is another lesson to be learned from the rotavirus story: rotavirus diarrhea is caused by replication of the virus in intestinal cells. There are three important proteins of the virus: two of these, vp4 and vp7, induce neutralizing antibodies, whereas the third, vp6, induces non-neutralizing antibodies and cellular immune responses.
With regard to the correlates of immunity, efficacy studies show that type-specific neutralizing antibodies are an important factor in protection. However, studies of natural immunity show that non-neutralizing as well as neutralizing antibodies to vp6 also correlate with protection [108,109]. Moreover, studies in animals demonstrate that CTL in the intestinal lining against vp6 also have a role [110]. Finally, measurement of serum IgA antibodies provide a surrogate of protection by the vaccine [111], indicating that secretory IgA in the intestinal mucosa plays a major role in that protection [112].
Thus, mucosal immunity collaborates with other functions to control rotavirus, a theme reflected in studies of macaques and of Kenyan sex workers (Figure 4), in whom systemic T cell proliferation and neutralizing antibodies at the level of the genital and intestinal tracts were synergistic in protection against HIV [113,114].
Figure 4.
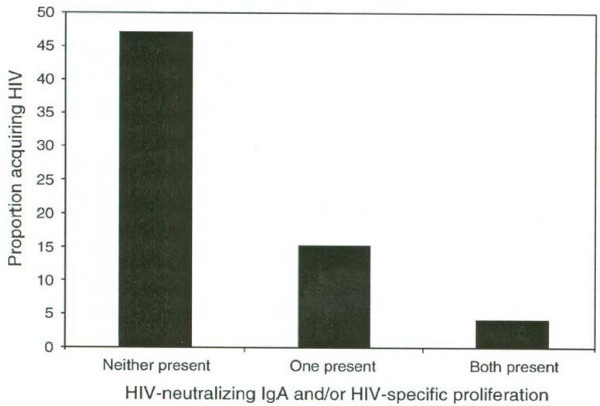
Acquisition of HIV by Kenyan Sex Workers Prevented by Genital IgA and Systemic T Cell Proliferation. Obviously, there are many problems to solve in attempting mucosal immunization. One approach is to mix routes of administration, for example priming with oral vaccination and following with parenteral boost. Moreover, it is not impossible to consider mixed intranasal and intrarectal administration to immunize both the genital and gastrointestinal tract. Aerosol administration of HPV vaccine has been reported to induce IgA secreting cells in the genital tract [115], and there is recent work suggesting that sublingual administration of antigens may be a way around compartmentalization of mucosal immunity [116] (see table 7).
Obviously, there are many problems to solve in attempting mucosal immunization. One approach is to mix routes of administration, for example priming with oral vaccination and following with parenteral boost. Moreover, it is not impossible to consider mixed intranasal and intrarectal administration to immunize both the genital and gastrointestinal tract. Aerosol administration of HPV vaccine has been reported to induce IgA secreting cells in the genital tract [115], and there is recent work suggesting that sublingual administration of antigens may be a way around compartmentalization of mucosal immunity [116] (see table 7).
Table 7.
Some newer strategies for an HIV vaccine
| Replicating vectors, e.g. Adenoviruses 4 and 7, CMV, Sendai, VSV, alphavirus-VSV |
|---|
| DNA plasmids with electroporation |
| Non-parenteral routes of administration: intranasal, rectal, sublingual |
| DNA/NYVAC prime boost regimen |
| Gene-driven HIV antibody |
| Anti-phospholipid antibodies |
| Live, attenuated HIV, e.g. Δnef, Δnef/vpr, ΔGY |
| (Canarypox/gp prime-boost 120 trial still ongoing in Thailand) |
The future
Of course, we must look at new vectors [117-122]. Replicating adenovirus vectors boosted by viral proteins have given promising results in prevention of SIV infection in macaques [123-125]. An interesting observation made in those studies and in other studies in macaques is the protection afforded by non-neutralizing antibodies through their action on infected cells by mechanisms such as ADCC [126-128]. This echoes the theme mentioned in relation to rotavirus.
Cytomegalovirus is under test as a replicating vector, as are measles, Sendai viruses and VSV [129,130]. DNA plasmids are enjoying a renaissance thanks to the concomitant use of electroporation and new adjuvants [49,131,132]. In addition, the European Consortium, has reported polyfunctional T cell responses in humans after a DNA-NYVAC vaccinia regimen [133]. Non-parenteral routes of administration of non-replicating vectors are also being explored [134]. Transfer of the gene for a neutralizing antibody via an adeno-associated virus vector to vaccinees is another intriguing approach [44]. Homology of anti-phospholipid antibodies and HIV epitopes is being explored [135]. And some of our hearts still belong to live attenuated HIV [136-138], although this is a contentious area owing to safety concerns. One should also keep in mind that the canarypox prime, gp120 boost trial in Thailand has survived analyses for the futility of efficacy and will be reported this year, and that the prime-boost concept using a DNA prime and Ad5 boost, which gives enhanced immune responses in comparison with Ad5 alone, remains to be tested in the clinic [139].
In summary, I believe that an effective HIV vaccine will need to stimulate neutralizing antibodies, as well as CD4+ and CD8+ cellular responses in the blood and on the mucosa. This is hardly a novel conclusion, and it is a tall order, but the biology of the virus and the history of vaccinology tell us, respectively, that those responses are necessary and that they have been feasible to induce for previous vaccines.
At the beginning of this article, I disdained the use of optimistic or pessimistic quotations to justify opinions about the future of HIV vaccine development. I have tried to be realistic in my own assessment of the situation and I will close with one quotation, because it is definitely realistic, as everyone who has ever worked in a laboratory knows. It comes from Emile Roux, the associate of Pasteur and a brilliant scientist in his own right. He said, "Science appears calm and triumphant when it is completed; but science in the process of being done is only contradiction and torment, hope and disappointment." Let us not give up, for as Roux would agree, the goal is worth it.
Competing interests
The author is a paid consultant to Sanofi Pasteur, Merck, and other vaccine manufacturers.
Acknowledgements
This article is based on a keynote address delivered at the AIDS Vaccine Conference in Cape Town, South Africa, on 13 October 2008.
References
- Plotkin SA, Farquhar JD, Katz M, Buser F. Attenuation of RA 27-3 rubella virus in WI-38 human diploid cells. Am J Dis Child. 1969;12(2):178–185. doi: 10.1001/archpedi.1969.02100040180004. [DOI] [PubMed] [Google Scholar]
- Plotkin SA, Farquhar JD, Ogra PL. Immunologic properties of RA27/3 rubella virus vaccine. Journal of American Medical Association. 1973;12:585–590. doi: 10.1001/jama.225.6.585. [DOI] [PubMed] [Google Scholar]
- Fogel A, Gerichter CB, Barnea B, Handsher R, Heeger E. Response to experimental challenge in persons immunized with different rubella vaccines. J Pediatr. 1978;12(1):26–29. doi: 10.1016/s0022-3476(78)80064-x. [DOI] [PubMed] [Google Scholar]
- Polack FP. Atypical measles and enhanced respiratory syncytial virus disease (ERD) made simple. Pediatr Res. 2007;12(1):111–115. doi: 10.1203/PDR.0b013e3180686ce0. [DOI] [PubMed] [Google Scholar]
- Polack FP, Hoffman SJ, Moss WJ, Griffin DE. Altered synthesis of interleukin-12 and type 1 and type 2 cytokinesin rhesus macaques during measles and atypical measles. J Infect Dis. 2002;12(1):13–19. doi: 10.1086/338009. [DOI] [PubMed] [Google Scholar]
- Polack FP, Hoffman SJ, Crujeiras G, Griffin DE. A role for nonprotective complement-fixing antibodies with low avidity for measles virus in atypical measles. Nat Med. 2003;12(9):1209–1213. doi: 10.1038/nm918. [DOI] [PubMed] [Google Scholar]
- De Swart RL, Kuiken T, Timmerman HH, van AG, Hoogen BG Van Den, Vos HW. et al. Immunization of macaques with formalin-inactivated respiratory syncytial virus (RSV) induces interleukin-13-associated hypersensitivity to subsequent RSV infection. J Virol. 2002;12(22):11561–11569. doi: 10.1128/JVI.76.22.11561-11569.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregoning JS, Yamaguchi Y, Harker J, Wang B, Openshaw PJ. The role of T cells in the enhancement of respiratory syncytial virus infection severity during adult reinfection of neonatally sensitized mice. J Virol. 2008;12(8):4115–4124. doi: 10.1128/JVI.02313-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intussusception among recipients of rotavirus vaccine – United States, 1998–1999. MMWR Morb Mortal Wkly Rep. 1999;12(27):577–581. [PubMed] [Google Scholar]
- Joensuu J, Koskenniemi E, Vesikari T. Symptoms associated with rhesus-human reassortant rotavirus vaccine in infants. Pediatr Infect Dis J. 1998;12(4):334–340. doi: 10.1097/00006454-199804000-00013. [DOI] [PubMed] [Google Scholar]
- Clark HF, Offit PA, Plotkin SA, Heaton PM. The new pentavalent rotavirus vaccine composed of bovine (strain WC3) – human rotavirus reassortants. Pediatr Infect Dis J. 2006;12(7):577–583. doi: 10.1097/01.inf.0000220283.58039.b6. [DOI] [PubMed] [Google Scholar]
- Bernstein DI, Sander DS, Smith VE, Schiff GM, Ward RL. Protection from rotavirus reinfections: two-year prospective study. J Infect Dis. 1991;12:277–283. doi: 10.1093/infdis/164.2.277. [DOI] [PubMed] [Google Scholar]
- Vesikari T, Matson DO, Dennehy P, Van DP, Santosham M, Rodriguez Z. et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;12(1):23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC. et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;12(1):11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin Infect Dis. 2004;12(2):233–239. doi: 10.1086/421999. [DOI] [PubMed] [Google Scholar]
- Plotkin SA. Is there a formula for an effective CMV vaccine? J Clin Virol. 2002;12(Suppl 2):S13–S21. doi: 10.1016/s1386-6532(02)00093-8. [DOI] [PubMed] [Google Scholar]
- Plotkin SA, Starr SE, Friedman HM, Gonczol E, Weibel RE. Protective effects of Towne cytomegalovirus vaccine against low-passage cytomegalovirus administered as a challenge. J Infect Dis. 1989;12(5):860–865. doi: 10.1093/infdis/159.5.860. [DOI] [PubMed] [Google Scholar]
- Pass R, Zhang C, Simpson T, Cytomegalovirus (CMV) envelope glycoprotein B (gB) vaccine in young women. [Abstract] Infectious Diseases Society of America, (late-breaker), San Diego, CA, October 4–7, 2007. 2007.
- Blish CA, Blay WM, Haigwood NL, Overbaugh J. Transmission of HIV-1 in the face of neutralizing antibodies. Curr HIV Res. 2007;12(6):578–587. doi: 10.2174/157016207782418461. [DOI] [PubMed] [Google Scholar]
- Piantadosi A, Ngayo MO, Chohan B, Overbaugh J. Examination of a Second Region of the HIV Type 1 Genome Reveals Additional Cases of Superinfection. AIDS Res Hum Retroviruses. 2008;12(9):1221. doi: 10.1089/aid.2008.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;12(3):401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- Simmonds P. Genetic diversity and evolution of hepatitis C virus – 15 years on. J Gen Virol. 2004;12(Pt 11):3173–3188. doi: 10.1099/vir.0.80401-0. [DOI] [PubMed] [Google Scholar]
- Pestka JM, Zeisel MB, Blaser E, Schurmann P, Bartosch B, Cosset FL. et al. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc Natl Acad Sci USA. 2007;12(14):6025–6030. doi: 10.1073/pnas.0607026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton M, Abrignani S. Prospects for a vaccine against the hepatitis C virus. Nature. 2005;12(7053):961–966. doi: 10.1038/nature04081. [DOI] [PubMed] [Google Scholar]
- Von Hahn T, Yoon JC, Alter H, Rice CM, Rehermann B, Balfe P. et al. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology. 2007;12(2):667–678. doi: 10.1053/j.gastro.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Elmowalid GA, Qiao M, Jeong SH, Borg BB, Baumert TF, Sapp RK. et al. Immunization with hepatitis C virus-like particles results in control of hepatitis C virus infection in chimpanzees. Proc Natl Acad Sci USA. 2007;12(20):8427–8432. doi: 10.1073/pnas.0702162104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;12(10):1395–1406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimme R, Neumann-Haefelin C, Boettler T, Blum HE. Adaptive immune responses to hepatitis C virus: from viral immunobiology to a vaccine. Biol Chem. 2008;12(5):457–67. doi: 10.1515/bc.2008.061. [DOI] [PubMed] [Google Scholar]
- Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P. et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;12(9):1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett SE, Guerra B, Brasky K, Miskovsky E, Houghton M, Klimpel GR. et al. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology. 2001;12(6):1479–1487. doi: 10.1053/jhep.2001.24371. [DOI] [PubMed] [Google Scholar]
- Shoukry NH, Grakoui A, Houghton M, Chien DY, Ghrayeb J, Reimann KA. et al. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;12(12):1645–1655. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerly D, Heckerman D, Allen TM, Chisholm JV III, Faircloth K, Linde CH. et al. Increased cytotoxic T-lymphocyte epitope variant cross-recognition and functional avidity are associated with hepatitis C virus clearance. J Virol. 2008;12(6):3147–3153. doi: 10.1128/JVI.02252-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland GT, El-Kamary SS, Klenerman P, Nicosia A. Hepatitis C vaccine: supply and demand. Lancet Infect Dis. 2008;12(6):379–386. doi: 10.1016/S1473-3099(08)70126-9. [DOI] [PubMed] [Google Scholar]
- Lin Y, Kwon T, Polo J, Zhu YF, Coates S, Crawford K. et al. Induction of broad CD4+ and CD8+ T-cell responses and cross-neutralizing antibodies against hepatitis C virus by vaccination with Th1-adjuvanted polypeptides followed by defective alphaviral particles expressing envelope glycoproteins gpE1 and gpE2 and nonstructural proteins 3, 4, and 5. J Virol. 2008;12(15):7492–7503. doi: 10.1128/JVI.02743-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner T, Wang Y, Pido-Lopez J, Whittall T, Bergmeier LA, Babaahmady K. The emerging role of innate immunity in protection against HIV-1 infection. Vaccine. 2008;12(24):2997–3001. doi: 10.1016/j.vaccine.2007.11.060. [DOI] [PubMed] [Google Scholar]
- Pido-Lopez J, Whittall T, Wang Y, Bergmeier LA, Babaahmady K, Singh M. et al. Stimulation of cell surface CCR5 and CD40 molecules by their ligands or by HSP70 up-regulates APOBEC3G expression in CD4(+) T cells and dendritic cells. J Immunol. 2007;12(3):1671–1679. doi: 10.4049/jimmunol.178.3.1671. [DOI] [PubMed] [Google Scholar]
- Vieillard V, Le GR, Dausset J, Debre P. A vaccine strategy against AIDS: an HIV gp41 peptide immunization prevents NKp44L expression and CD4+ T cell depletion in SHIV-infected macaques. Proc Natl Acad Sci USA. 2008;12(6):2100–2104. doi: 10.1073/pnas.0711629105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago ML, Montano M, Benitez R, Messer RJ, Yonemoto W, Chesebro B. et al. Apobec3 encodes Rfv3, a gene influencing neutralizing antibody control of retrovirus infection. Science. 2008;12(5894):1343–1346. doi: 10.1126/science.1161121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miconnet I, Pantaleo G. A soluble hexameric form of CD40 ligand activates human dendritic cells and augments memory T cell response. Vaccine. 2008;12(32):4006–4014. doi: 10.1016/j.vaccine.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Liu J, Yu Q, Stone GW, Yue FY, Ngai N, Jones RB. et al. CD40L expressed from the canarypox vector, ALVAC, can boost immunogenicity of HIV-1 canarypox vaccine in mice and enhance the in vitro expansion of viral specific CD8+ T cell memory responses from HIV-1-infected and HIV-1-uninfected individuals. Vaccine. 2008;12(32):4062–4072. doi: 10.1016/j.vaccine.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MI, Fauci AS. An HIV vaccine – evolving concepts. N Engl J Med. 2007;12(20):2073–2081. doi: 10.1056/NEJMra066267. [DOI] [PubMed] [Google Scholar]
- Burioni R, Mancini N, De MD, Clementi N, Perotti M, Nitti G. et al. Anti-HIV-1 response elicited in rabbits by anti-idiotype monoclonal antibodies mimicking the CD4-binding site. PLoS ONE. 2008;12(10):e3423. doi: 10.1371/journal.pone.0003423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaahmady K, Bergmeier LA, Lehner T. Combining human antisera to human leukocyte antigens, HIVgp120 and 70 kDa heat shock protein results in broadly neutralizing activity to HIV-1. AIDS. 2008;12(11):1267–1276. doi: 10.1097/QAD.0b013e328304b3a6. [DOI] [PubMed] [Google Scholar]
- Lewis AD, Chen R, Montefiori DC, Johnson PR, Clark KR. Generation of neutralizing activity against human immunodeficiency virus type 1 in serum by antibody gene transfer. J Virol. 2002;12(17):8769–8775. doi: 10.1128/JVI.76.17.8769-8775.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson Hedestam GB, Fouchier RA, Phogat S, Burton DR, Sodroski J, Wyatt RT. The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus. Nat Rev Microbiol. 2008;12(2):143–155. doi: 10.1038/nrmicro1819. [DOI] [PubMed] [Google Scholar]
- Graham BS, Koup RA, Roederer M, Bailer RT, Enama ME, Moodie Z. et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 DNA candidate vaccine. J Infect Dis. 2006;12(12):1650–1660. doi: 10.1086/509259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MS, Xu L, Beaudry K, Martin KL, Beddall MH, Miura A. et al. Multiclade human immunodeficiency virus type 1 envelope immunogens elicit broad cellular and humoral immunity in rhesus monkeys. J Virol. 2005;12(5):2956–2963. doi: 10.1128/JVI.79.5.2956-2963.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Pal R, Mascola JR, Chou TH, Mboudjeka I, Shen S. et al. Polyvalent HIV-1 Env vaccine formulations delivered by the DNA priming plus protein boosting approach are effective in generating neutralizing antibodies against primary human immunodeficiency virus type 1 isolates from subtypes A, B, C, D and E. Virology. 2006;12(1):34–47. doi: 10.1016/j.virol.2006.02.032. [DOI] [PubMed] [Google Scholar]
- Wang S, Kennedy JS, West K, Montefiori DC, Coley S, Lawrence J. et al. Cross-subtype antibody and cellular immune responses induced by a polyvalent DNA prime-protein boost HIV-1 vaccine in healthy human volunteers. Vaccine. 2008;12(31):3947–3957. doi: 10.1016/j.vaccine.2007.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola JR, Lewis MG, Stiegler G, Harris D, VanCott TC, Hayes D. et al. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6 PD by passive transfer of neutralizing antibodies. J Virol. 1999;12(5):4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE. et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;12(2):207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- Mascola JR. Passive transfer studies to elucidate the role of antibody-mediated protection against HIV-1. Vaccine. 2002;12(15):1922–1925. doi: 10.1016/s0264-410x(02)00068-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kawada M, Takeda A, Igarashi H, Matano T. Post-infection immunodeficiency virus control by neutralizing antibodies. PLoS ONE. 2007;12(6):e540. doi: 10.1371/journal.pone.0000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barin F, Jourdain G, Brunet S, Ngo-Giang-Huong N, Weerawatgoompa S, Karnchanamayul W. et al. Revisiting the role of neutralizing antibodies in mother-to-child transmission of HIV-1. J Infect Dis. 2006;12(11):1504–1511. doi: 10.1086/503778. [DOI] [PubMed] [Google Scholar]
- Bjorling E, Scarlatti G, von GA, Albert J, Biberfeld G, Chiodi F. et al. Autologous neutralizing antibodies prevail in HIV-2 but not in HIV-1 infection. Virology. 1993;12(1):528–530. doi: 10.1006/viro.1993.1160. [DOI] [PubMed] [Google Scholar]
- Shi Y, Brandin E, Vincic E, Jansson M, Blaxhult A, Gyllensten K, Evolution of human immunodeficiency virus type 2 coreceptor usage, autologous neutralization, envelope sequence and glycosylation. J Gen Virol. pp. 3385–3396. [DOI] [PubMed]
- Leligdowicz A, Rowland-Jones S. Tenets of protection from progression to AIDS: lessons from the immune responses to HIV-2 infection. Expert Rev Vaccines. 2008;12(3):319–331. doi: 10.1586/14760584.7.3.319. [DOI] [PubMed] [Google Scholar]
- Trkola A, Kuster H, Rusert P, von WV, Leemann C, Weber R. et al. In vivo efficacy of human immunodeficiency virus neutralizing antibodies: estimates for protective titers. J Virol. 2008;12(3):1591–1599. doi: 10.1128/JVI.01792-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trkola A, Kuster H, Rusert P, Joos B, Fischer M, Leemann C. et al. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med. 2005;12(6):615–622. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C. et al. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 2001;12(17):8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y, Igarashi T, Haigwood N, Sadjadpour R, Plishka RJ, Buckler-White A. et al. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. J Virol. 2002;12(5):2123–2130. doi: 10.1128/jvi.76.5.2123-2130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Strain MC, Frost SD, Pillai SK, Wong JK, Wrin T. et al. Lack of neutralizing antibody response to HIV-1 predisposes to superinfection. Virology. 2006;12(1):1–5. doi: 10.1016/j.virol.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG. et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci USA. 2008;12(21):7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CJ, Li Q, Abel K, Kim EY, Ma ZM, Wietgrefe S. et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;12(14):9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang PF, Cham F, Dong M, Choudhary A, Bouma P, Zhang Z. et al. Extensively cross-reactive anti-HIV-1 neutralizing antibodies induced by gp140 immunization. Proc Natl Acad Sci USA. 2007;12(24):10193–10198. doi: 10.1073/pnas.0608635104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG, Schweighardt B, Wrin T, Galovich J, Hoh R, Sinclair E. et al. Neutralizing antibody responses against autologous and heterologous viruses in acute versus chronic human immunodeficiency virus (HIV) infection: evidence for a constraint on the ability of HIV to completely evade neutralizing antibody responses. J Virol. 2006;12(12):6155–6164. doi: 10.1128/JVI.00093-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattentau Q. Correlates of antibody-mediated protection against HIV infection. Current Opinion in HIV and AIDS. 2008;12:368–374. doi: 10.1097/COH.0b013e3282f9ae79. [DOI] [PubMed] [Google Scholar]
- Brown BK, Wieczorek L, Sanders-Buell E, Rosa BA, Robb ML, Birx DL. et al. Cross-clade neutralization patterns among HIV-1 strains from the six major clades of the pandemic evaluated and compared in two different models. Virology. 2008;12(2):529–538. doi: 10.1016/j.virol.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Kuroda MJ, Schmitz JE, Charini WA, Nickerson CE, Lifton MA, Lord CI. et al. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J Immunol. 1999;12(9):5127–5133. [PubMed] [Google Scholar]
- Wilson NA, Reed J, Napoe GS, Piaskowski S, Szymanski A, Furlott J. et al. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J Virol. 2006;12(12):5875–5885. doi: 10.1128/JVI.00171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;12(3):406–416. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Hess C, Altfeld M, Thomas SY, Addo MM, Rosenberg ES, Allen TM. et al. HIV-1 specific CD8+ T cells with an effector phenotype and control of viral replication. Lancet. 2004;12(9412):863–866. doi: 10.1016/S0140-6736(04)15735-8. [DOI] [PubMed] [Google Scholar]
- Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA. et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;12(5403):857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;12(9):6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacha JB, Chung C, Rakasz EG, Spencer SP, Jonas AK, Bean AT. et al. Gag-specific CD8+ T lymphocytes recognize infected cells before AIDS-virus integration and viral protein expression. J Immunol. 2007;12(5):2746–2754. doi: 10.4049/jimmunol.178.5.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifson JD, Rossio JL, Piatak M Jr, Parks T, Li L, Kiser R. et al. Role of CD8(+) lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J Virol. 2001;12(21):10187–10199. doi: 10.1128/JVI.75.21.10187-10199.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J. et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;12(12):4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereyra F, Addo MM, Kaufmann DE, Liu Y, Miura T, Rathod A. et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis. 2008;12(4):563–571. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- Saez-Cirion A, Lacabaratz C, Lambotte O, Versmisse P, Urrutia A, Boufassa F. et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci USA. 2007;12(16):6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentini L, Fenizia C, Naddeo V, Clerici M. Not just sheer luck! Immune correlates of protection against HIV-1 infection. Vaccine. 2008;12(24):3002–3007. doi: 10.1016/j.vaccine.2007.11.062. [DOI] [PubMed] [Google Scholar]
- Potter SJ, Lacabaratz C, Lambotte O, Perez-Patrigeon S, Vingert B, Sinet M. et al. Preserved central memory and activated effector memory CD4+ T-cell subsets in human immunodeficiency virus controllers: an ANRS EP36 study. J Virol. 2007;12(24):13904–13915. doi: 10.1128/JVI.01401-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase AJ, Yang HC, Zhang H, Blankson JN, Siliciano RF. Preservation of FoxP3+ regulatory T cells in the peripheral blood of human immunodeficiency virus type 1-infected elite suppressors correlates with low CD4+ T-cell activation. J Virol. 2008;12(17):8307–8315. doi: 10.1128/JVI.00520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni PS, Butera ST, Duerr AC. Resistance to HIV-1 infection: lessons learned from studies of highly exposed persistently seronegative (HEPS) individuals. AIDS Rev. 2003;12(2):87–103. [PubMed] [Google Scholar]
- Miller JD, Most RG van der, Akondy RS, Glidewell JT, Albott S, Masopust D. et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;12(5):710–722. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Turk G, Gherardi MM, Laufer N, Saracco M, Luzzi R, Cox JH. et al. Magnitude, breadth, and functional profile of T-cell responses during human immunodeficiency virus primary infection with B and BF viral variants. J Virol. 2008;12(6):2853–2866. doi: 10.1128/JVI.02260-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchfield JW, Lemongello D, Walker DH, Garcia JC, Asmuth DM, Pollard RB. et al. Multifunctional human immunodeficiency virus (HIV) gag-specific CD8+ T-cell responses in rectal mucosa and peripheral blood mononuclear cells during chronic HIV type 1 infection. J Virol. 2007;12(11):5460–5471. doi: 10.1128/JVI.02535-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada M, Igarashi H, Takeda A, Tsukamoto T, Yamamoto H, Dohki S. et al. Involvement of multiple epitope-specific cytotoxic T-lymphocyte responses in vaccine-based control of simian immunodeficiency virus replication in rhesus macaques. J Virol. 2006;12(4):1949–1958. doi: 10.1128/JVI.80.4.1949-1958.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Santra S, Schmitz JE, Roederer M, Letvin NL. Magnitude and quality of vaccine-elicited T-cell responses in the control of immunodeficiency virus replication in rhesus monkeys. J Virol. 2008;12(17):8812–8819. doi: 10.1128/JVI.00204-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daucher M, Price DA, Brenchley JM, Lamoreaux L, Metcalf JA, Rehm C. et al. Virological outcome after structured interruption of antiretroviral therapy for human immunodeficiency virus infection is associated with the functional profile of virus-specific CD8+ T cells. J Virol. 2008;12(8):4102–4114. doi: 10.1128/JVI.02212-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MS, Ng HL, Dagarag M, Ali A, Yang OO. Epitope-dependent avidity thresholds for cytotoxic T-lymphocyte clearance of virus-infected cells. J Virol. 2007;12(10):4973–4980. doi: 10.1128/JVI.02362-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyakov IM, Isakov D, Zhu Q, Dzutsev A, Berzofsky JA. A novel functional CTL avidity/activity compartmentalization to the site of mucosal immunization contributes to protection of macaques against simian/human immunodeficiency viral depletion of mucosal CD4+ T cells. J Immunol. 2007;12(11):7211–7221. doi: 10.4049/jimmunol.178.11.7211. [DOI] [PubMed] [Google Scholar]
- Kawada M, Tsukamoto T, Yamamoto H, Takeda A, Igarashi H, Watkins DI. et al. Long-term control of simian immunodeficiency virus replication with central memory CD4+ T-cell preservation after nonsterile protection by a cytotoxic T-lymphocyte-based vaccine. J Virol. 2007;12(10):5202–5211. doi: 10.1128/JVI.02881-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W, Perkins S, Theiler J, Bhattacharya T, Yusim K, Funkhouser R. et al. Polyvalent vaccines for optimal coverage of potential T-cell epitopes in global HIV-1 variants. Nat Med. 2007;12(1):100–106. doi: 10.1038/nm1461. [DOI] [PubMed] [Google Scholar]
- Santra S, Korber BT, Muldoon M, Barouch DH, Nabel GJ, Gao F. et al. A centralized gene-based HIV-1 vaccine elicits broad cross-clade cellular immune responses in rhesus monkeys. Proc Natl Acad Sci USA. 2008;12(30):10489–10494. doi: 10.1073/pnas.0803352105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, Differential Th17 CD4 T cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008. [DOI] [PMC free article] [PubMed]
- Sun Y, Schmitz JE, Buzby AP, Barker BR, Rao SS, Xu L. et al. Virus-specific cellular immune correlates of survival in vaccinated monkeys after simian immunodeficiency virus challenge. J Virol. 2006;12(22):10950–10956. doi: 10.1128/JVI.01458-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero E, Bona R, Muratori C, Federico M. Virological consequences of early events following cell-cell contact between human immunodeficiency virus type 1-infected and uninfected CD4+ cells. J Virol. 2008;12(16):7773–7789. doi: 10.1128/JVI.00695-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RT, Markowitz LE, Albrecht P, Stewart JA, Mofenson LM, Preblud SR. et al. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;12(5):1036–1042. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- Belshe RB, Gruber WC, Mendelman PM, Mehta HB, Mahmood K, Reisinger K. et al. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J Infect Dis. 2000;12(3):1133–1137. doi: 10.1086/315323. [DOI] [PubMed] [Google Scholar]
- Haynes BF, Shattock RJ. Critical issues in mucosal immunity for HIV-1 vaccine development. J Allergy Clin Immunol. 2008;12(1):3–9. doi: 10.1016/j.jaci.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard MP, Bansal GP, Pedroza-Martins L, Dodet B, Mehra V, Schito M. et al. Mucosal immunity and HIV/AIDS vaccines: Report of an International Workshop, 28–30 October 2007. Vaccine. 2008;12(32):3969–3977. doi: 10.1016/j.vaccine.2008.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvet JP, Fischetti VA. Diversity of antibody-mediated immunity at the mucosal barrier. Infect Immun. 1999;12(6):2687–2691. doi: 10.1128/iai.67.6.2687-2691.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattock RJ, Haynes BF, Pulendran B, Flores J, Esparza J. Improving defences at the portal of HIV entry: mucosal and innate immunity. PLoS Med. 2008;12(4):e81. doi: 10.1371/journal.pmed.0050081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;12(30):5467–5484. doi: 10.1016/j.vaccine.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Mestecky J, Jackson S, Moldoveanu Z, Nesbit LR, Kulhavy R, Prince SJ. et al. Paucity of antigen-specific IgA responses in sera and external secretions of HIV-type 1-infected individuals. AIDS Res Hum Retroviruses. 2004;12(9):972–988. doi: 10.1089/aid.2004.20.972. [DOI] [PubMed] [Google Scholar]
- Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL. et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;12(5362):427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- Ling B, Veazey RS, Hart M, Lackner AA, Kuroda M, Pahar B. et al. Early restoration of mucosal CD4 memory CCR5 T cells in the gut of SIV-infected rhesus predicts long term non-progression. AIDS. 2007;12(18):2377–2385. doi: 10.1097/QAD.0b013e3282f08b32. [DOI] [PubMed] [Google Scholar]
- Kallewaard NL, McKinney BA, Gu Y, Chen A, Prasad BV, Crowe JE Jr. Functional maturation of the human antibody response to rotavirus. J Immunol. 2008;12(6):3980–3989. doi: 10.4049/jimmunol.180.6.3980. [DOI] [PubMed] [Google Scholar]
- Garaicoechea L, Olichon A, Marcoppido G, Wigdorovitz A, Mozgovoj M, Saif L. et al. Llama-derived single-chain antibody fragments directed to rotavirus VP6 protein possess broad neutralizing activity in vitro and confer protection against diarrhea in mice. J Virol. 2008;12(19):9753–9764. doi: 10.1128/JVI.00436-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Wen K, Azevedo MS, Gonzalez AM, Zhang W, Saif LJ. Virus-specific intestinal IFN-gamma producing T cell responses induced by human rotavirus infection and vaccines are correlated with protection against rotavirus diarrhea in gnotobiotic pigs. Vaccine. 2008;12(26):3322–3331. doi: 10.1016/j.vaccine.2008.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark HF, Offit PA, Vidor E. In: Vaccines. Plotkin S, Orenstein WA, Offit PA, editor. Saunders-Elsevier; 2008. Rotavirus Vaccines; pp. 715–734. [Google Scholar]
- Azevedo MS, Yuan L, Iosef C, Chang KO, Kim Y, Nguyen TV. et al. Magnitude of serum and intestinal antibody responses induced by sequential replicating and nonreplicating rotavirus vaccines in gnotobiotic pigs and correlation with protection. Clin Diagn Lab Immunol. 2004;12(1):12–20. doi: 10.1128/CDLI.11.1.12-20.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattapallil JJ, Roederer M. Mucosa and vaccine-induced immune protection in nonhuman primates. Current Opinion in HIV and AIDS. 2008;12:387–392. doi: 10.1097/COH.0b013e3282f9ae66. [DOI] [PubMed] [Google Scholar]
- Hirbod T, Kaul R, Reichard C, Kimani J, Ngugi E, Bwayo JJ. et al. HIV-neutralizing immunoglobulin A and HIV-specific proliferation are independently associated with reduced HIV acquisition in Kenyan sex workers. AIDS. 2008;12(6):727–735. doi: 10.1097/QAD.0b013e3282f56b64. [DOI] [PubMed] [Google Scholar]
- Nardelli-Haefliger D, Lurati F, Wirthner D, Spertini F, Schiller JT, Lowy DR. et al. Immune responses induced by lower airway mucosal immunisation with a human papillomavirus type 16 virus-like particle vaccine. Vaccine. 2005;12(28):3634–3641. doi: 10.1016/j.vaccine.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Cuburu N, Kweon MN, Song JH, Hervouet C, Luci C, Sun JB. et al. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine. 2007;12(51):8598–8610. doi: 10.1016/j.vaccine.2007.09.073. [DOI] [PubMed] [Google Scholar]
- Vaine M, Wang S, Crooks ET, Jiang P, Montefiori DC, Binley J. et al. Improved induction of antibodies against key neutralizing epitopes by human immunodeficiency virus type 1 gp120 DNA prime-protein boost vaccination compared to gp120 protein-only vaccination. J Virol. 2008;12(15):7369–7378. doi: 10.1128/JVI.00562-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan CH, Nair N, Adams RJ, Zink MC, Lee EY, Polack FP. et al. Dose-dependent protection against or exacerbation of disease by a polylactide glycolide microparticle-adsorbed, alphavirus-based measles virus DNA vaccine in rhesus macaques. Clin Vaccine Immunol. 2008;12(4):697–706. doi: 10.1128/CVI.00045-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R, Kalyanaraman VS, Nair BC, Whitney S, Keen T, Hocker L. et al. Immunization of rhesus macaques with a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 vaccine elicits protective antibody response against simian human immunodeficiency virus of R5 phenotype. Virology. 2006;12(2):341–353. doi: 10.1016/j.virol.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Matano T, Kobayashi M, Igarashi H, Takeda A, Nakamura H, Kano M. et al. Cytotoxic T lymphocyte-based control of simian immunodeficiency virus replication in a preclinical AIDS vaccine trial. J Exp Med. 2004;12(12):1709–1718. doi: 10.1084/jem.20040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkevitch NV, Patterson LJ, Aldrich MK, Wu Y, Venzon D, Florese RH. et al. Durable protection of rhesus macaques immunized with a replicating adenovirus-SIV multigene prime/protein boost vaccine regimen against a second SIVmac251 rectal challenge: role of SIV-specific CD8+ T cell responses. Virology. 2006;12(1):83–98. doi: 10.1016/j.virol.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Buonaguro L, Devito C, Tornesello ML, Schroder U, Wahren B, Hinkula J. et al. DNA-VLP prime-boost intra-nasal immunization induces cellular and humoral anti-HIV-1 systemic and mucosal immunity with cross-clade neutralizing activity. Vaccine. 2007;12(32):5968–5977. doi: 10.1016/j.vaccine.2007.05.052. [DOI] [PubMed] [Google Scholar]
- Robert-Guroff M. Replicating and non-replicating viral vectors for vaccine development. Curr Opin Biotechnol. 2007;12(6):546–556. doi: 10.1016/j.copbio.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson LJ, Robert-Guroff M. Replicating adenovirus vector prime/protein boost strategies for HIV vaccine development. Expert Opin Biol Ther. 2008;12(9):1347–1363. doi: 10.1517/14712598.8.9.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson LJ, Beal J, Demberg T, Florese RH, Malkevich N, Venzon D. et al. Replicating adenovirus HIV/SIV recombinant priming alone or in combination with a gp140 protein boost results in significant control of viremia following a SHIV89.6P challenge in Mamu-A*01 negative rhesus macaques. Virology. 2008;12(2):322–337. doi: 10.1016/j.virol.2007.12.037. [DOI] [PubMed] [Google Scholar]
- Burton DR. Antibodies, viruses and vaccines. Nat Rev Immunol. 2002;12(9):706–713. doi: 10.1038/nri891. [DOI] [PubMed] [Google Scholar]
- Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM. et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;12(7158):101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- Weinhold KJ. Anti-HIV-1 ADCC: clinical and therapeutic implications. Biotechnol Ther. 1991;12(1–2):147–157. [PubMed] [Google Scholar]
- Koff WC, Parks CL, Berkhout B, Ackland J, Noble S, Gust ID. Replicating viral vectors as HIV vaccines Summary Report from IAVI Sponsored Satellite Symposium, International AIDS Society Conference, July 22, 2007. Biologicals. 2008. [DOI] [PubMed]
- Kawada M, Tsukamoto T, Yamamoto H, Iwamoto N, Kurihara K, Takeda A. et al. Gag-specific cytotoxic T-lymphocyte-based control of primary simian immunodeficiency virus replication in a vaccine trial. J Virol. 2008;12(20):10199–10206. doi: 10.1128/JVI.01103-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet. 2008;12(10):776–788. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S. Immunogenicity of DNA vaccines in humans: it takes two to tango. Hum Vaccin. 2008;12(6):449–452. doi: 10.4161/hv.4.6.6179. [DOI] [PubMed] [Google Scholar]
- Harari A, Bart PA, Stohr W, Tapia G, Garcia M, Medjitna-Rais E. et al. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J Exp Med. 2008;12(1):63–77. doi: 10.1084/jem.20071331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl-Hennig C, Kuate S, Franz M, Suh YS, Stoiber H, Sauermann U. et al. Atraumatic oral spray immunization with replication-deficient viral vector vaccines. J Virol. 2007;12(23):13180–13190. doi: 10.1128/JVI.01400-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam SM, McAdams M, Boren D, Rak M, Scearce RM, Gao F. et al. The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes. J Immunol. 2007;12(7):4424–4435. doi: 10.4049/jimmunol.178.7.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff WC, Johnson PR, Watkins DI, Burton DR, Lifson JD, Hasenkrug KJ. et al. HIV vaccine design: insights from live attenuated SIV vaccines. Nat Immunol. 2006;12(1):19–23. doi: 10.1038/ni1296. [DOI] [PubMed] [Google Scholar]
- Mansfield K, Lang SM, Gauduin MC, Sanford HB, Lifson JD, Johnson RP. et al. Vaccine protection by live, attenuated simian immunodeficiency virus in the absence of high-titer antibody responses and high-frequency cellular immune responses measurable in the periphery. J Virol. 2008;12(8):4135–4148. doi: 10.1128/JVI.00015-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan KM, Jordan AP, Hoxie JA. Effects of partial deletions within the human immunodeficiency virus type 1 V3 loop on coreceptor tropism and sensitivity to entry inhibitors. J Virol. 2008;12(2):664–673. doi: 10.1128/JVI.01793-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox KS, Clair JH, Prokop MT, Sykes KJ, Dubey SA, Shiver JW. et al. DNA gag/adenovirus type 5 (Ad5) gag and Ad5 gag/Ad5 gag vaccines induce distinct T-cell response profiles. J Virol. 2008;12(16):8161–8171. doi: 10.1128/JVI.00620-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


