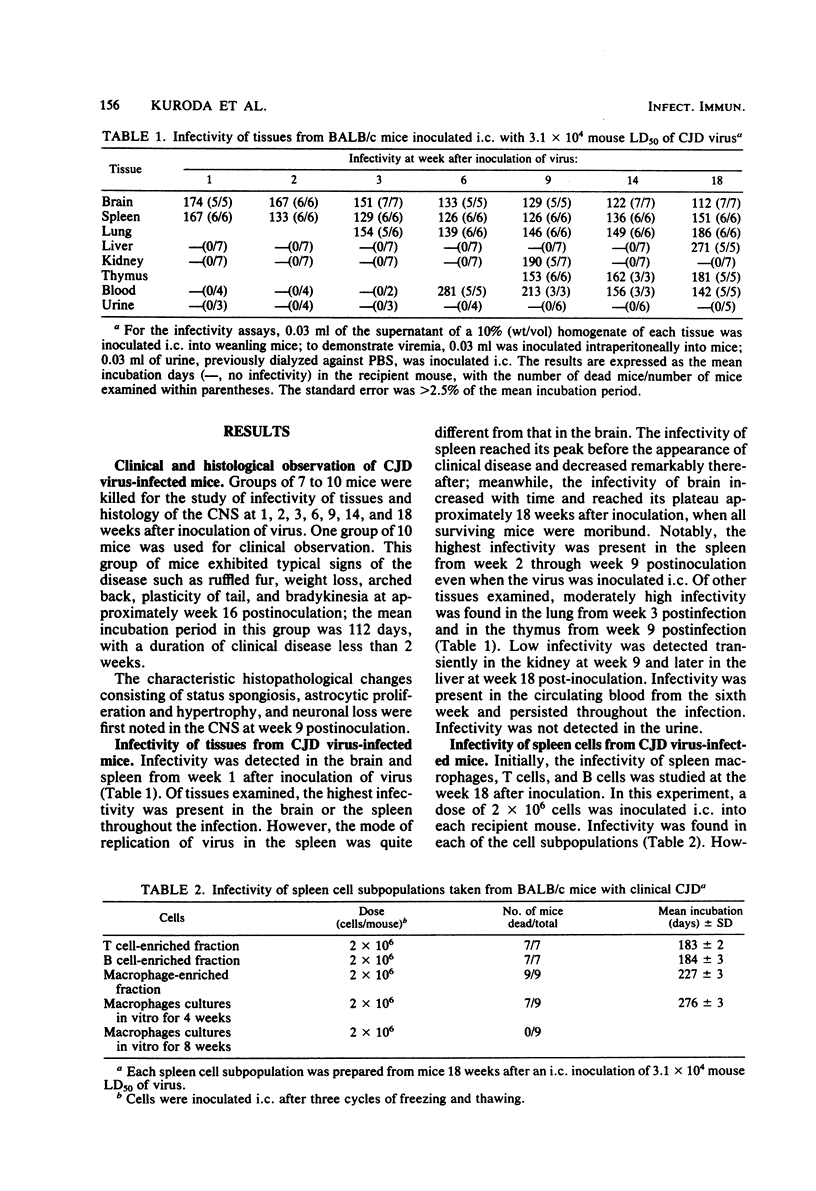
Abstract
The mode of replication of the "unconventional virus" of Creutzfeldt-Jakob disease was studied in BALB/c mice infected intracerebrally. Virus was detected in the brain, spleen, lung, thymus, liver, kidney, and blood, but not in urine, at various time intervals after inoculation. The highest infectivity was present in the spleen from the second through the ninth weeks postinfection. Density gradient separation of spleen cells with colloidal silica (Percoll) revealed that the highest concentration of virus was present in blastoid cells from lower-density (1.05 to 1.07 g/ml) fractions. These results suggest that blastoid cells play an important role as the initial replication site of virus in the pathogenesis of Creutzfeldt-Jakob disease in mice.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asher D. M., Gibbs C. J., Jr, Gajdusek D. C. Pathogenesis of subacute spongiform encephalopathies. Ann Clin Lab Sci. 1976 Jan-Feb;6(1):84–103. [PubMed] [Google Scholar]
- Brown P., Green E. M., Gajdusek D. C. Effect of different gradient solutions on the buoyant density of scrapie infectivity. Proc Soc Exp Biol Med. 1978 Sep;158(4):513–516. doi: 10.3181/00379727-158-40236. [DOI] [PubMed] [Google Scholar]
- Clarke M. C., Haig D. A. Presence of the transmissible agent of scrapie in the serum of affected mice and rats. Vet Rec. 1967 Apr 22;80(16):504–504. doi: 10.1136/vr.80.16.504. [DOI] [PubMed] [Google Scholar]
- Dickinson A. G., Fraser H., McConnell I., Outram G. W. Mitogenic stimulation of the host enhances susceptibility to scrapie. Nature. 1978 Mar 2;272(5648):54–55. doi: 10.1038/272054a0. [DOI] [PubMed] [Google Scholar]
- Edelman R., Wheelock E. F. Vesicular stomatitis virus replication in human leukocyte cultures: enhancement by phytohemagglutinin. Science. 1966 Nov 25;154(3752):1053–1055. doi: 10.1126/science.154.3752.1053. [DOI] [PubMed] [Google Scholar]
- Eklund C. M., Kennedy R. C., Hadlow W. J. Pathogenesis of scrapie virus infection in the mouse. J Infect Dis. 1967 Feb;117(1):15–22. doi: 10.1093/infdis/117.1.15. [DOI] [PubMed] [Google Scholar]
- Fraser H., Dickinson A. G. Pathogenesis of scrapie in the mouse: the role of the spleen. Nature. 1970 May 2;226(5244):462–463. doi: 10.1038/226462a0. [DOI] [PubMed] [Google Scholar]
- Fraser H., Dickinson A. G. Studies of the lymphoreticular system in the pathogenesis of scrapie: the role of spleen and thymus. J Comp Pathol. 1978 Oct;88(4):563–573. doi: 10.1016/0021-9975(78)90010-5. [DOI] [PubMed] [Google Scholar]
- Gajdusek D. C. Unconventional viruses and the origin and disappearance of kuru. Science. 1977 Sep 2;197(4307):943–960. doi: 10.1126/science.142303. [DOI] [PubMed] [Google Scholar]
- Garfin D. E., Stites D. P., Perlman J. D., Cochran S. P., Prusiner S. B. Mitogen stimulation of splenocytes from mice infected with scrapie agent. J Infect Dis. 1978 Sep;138(3):396–400. doi: 10.1093/infdis/138.3.396. [DOI] [PubMed] [Google Scholar]
- Garfin D. E., Stites D. P., Zitnik L. A., Prusiner S. B. Suppression of polyclonal B cell activation in scrapie-infected C3H/HeJ mice. J Immunol. 1978 Jun;120(6):1986–1990. [PubMed] [Google Scholar]
- Gmelig-Meyling F., Waldmann T. A. Separation of human blood monocytes and lymphocytes on a continuous Percoll gradient. J Immunol Methods. 1980;33(1):1–9. doi: 10.1016/0022-1759(80)90077-0. [DOI] [PubMed] [Google Scholar]
- HORSTMANN D. M. Poliomyelitis virus in blood of orally infected monkeys and chimpanzees. Proc Soc Exp Biol Med. 1952 Mar;79(3):417–419. doi: 10.3181/00379727-79-19398. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kleinman L. F., Kibrick S., Ennis F., Polgar P. Herpes simplex virus replication in human lymphocyte cultures stimulated with phytomitogens and anti-lymphocyte globulin. Proc Soc Exp Biol Med. 1972 Dec;141(3):1095–1099. doi: 10.3181/00379727-141-36941. [DOI] [PubMed] [Google Scholar]
- Lavelle G. C., Sturman L., Hadlow W. J. Isolation from mouse spleen of cell populations with high specific infectivity for scrapie virus. Infect Immun. 1972 Mar;5(3):319–323. doi: 10.1128/iai.5.3.319-323.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis E. E., Gorgacs E. J., Manuelidis L. Viremia in experimental Creutzfeldt-Jakob disease. Science. 1978 Jun 2;200(4345):1069–1071. doi: 10.1126/science.349691. [DOI] [PubMed] [Google Scholar]
- Marsh R. F., Miller J. M., Hanson R. P. Transmissible mink encephalopathy: studies on the peripheral lymphocyte. Infect Immun. 1973 Mar;7(3):352–355. doi: 10.1128/iai.7.3.352-355.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauel J., Defendi V. Regulation of DNA synthesis in mouse macrophages. I. Sources, action and purification of the macrophage growing factor (MGF). Exp Cell Res. 1971 Mar;65(1):33–42. doi: 10.1016/s0014-4827(71)80047-2. [DOI] [PubMed] [Google Scholar]
- Outram G. W., Dickinson A. G., Fraser H. Reduced susceptibility to scrapie in mice after steroid administration. Nature. 1974 Jun 28;249(460):855–856. doi: 10.1038/249855a0. [DOI] [PubMed] [Google Scholar]
- Outram G. W. The pathogenesis of scrapie in mice. Front Biol. 1976;44:325–357. [PubMed] [Google Scholar]
- PATTISON I. H., MILLSON G. C. Distribution of the scrapie agent in the tissues of experimentally inoculated goats. J Comp Pathol. 1962 Jul;72:233–244. doi: 10.1016/s0368-1742(62)80026-5. [DOI] [PubMed] [Google Scholar]
- Paul P. S., Mengeling W. L., Brown T. T., Jr Replication of porcine parvovirus in peripheral blood lymphocytes, monocytes, and peritoneal macrophages. Infect Immun. 1979 Sep;25(3):1003–1007. doi: 10.1128/iai.25.3.1003-1007.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siakotos A. N., Gajdusek D. C., Gibbs C. J., Jr, Traub R. D., Bucana C. Partial purification of the scrapie agent from mouse brain by pressure disruption and zonal centrifugation in sucrose-sodium chloride gradients. Virology. 1976 Mar;70(1):230–237. doi: 10.1016/0042-6822(76)90261-0. [DOI] [PubMed] [Google Scholar]
- Tateishi J., Ohta M., Koga M., Sato Y., Kuroiwa Y. Transmission of chronic spongiform encephalopathy with kuru plaques from humans to small rodents. Ann Neurol. 1979 Jun;5(6):581–584. doi: 10.1002/ana.410050616. [DOI] [PubMed] [Google Scholar]
- Tateishi J., Sato Y., Koga M., Doi H., Ohta M. Experimental transmission of human subacute spongiform encephalopathy to small rodents. I. Clinical and histological observations. Acta Neuropathol. 1980;51(2):127–134. doi: 10.1007/BF00690454. [DOI] [PubMed] [Google Scholar]
- Willems F. T., Melnick J. L., Rawls W. E. Replication of poliovirus in phytohemagglutinin-stimulated human lymphocytes. J Virol. 1969 May;3(5):451–457. doi: 10.1128/jvi.3.5.451-457.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



