Abstract
Background
There is an interest in investigating the relation between emotional memory impairments in schizophrenia and specific symptom dimensions. We explored potential links between emotional memory and social anhedonia severity in patients with schizophrenia and in healthy individuals.
Methods
Twenty-nine patients with schizophrenia and 27 matched healthy individuals completed the Chapman Revised Social Anhedonia Scale and then performed an emotional face recognition memory task involving happy, sad and neutral face expressions. We calculated emotional memory performance using 2 independent measures: the discrimination accuracy index Pr and the response bias Br. We also measured valence ratings of the face stimuli. We performed correlation analyses using the inter-individual variability in social anhedonia severity and the individual score obtained for each memory performance variable and for each face valence rating condition.
Results
Patients with schizophrenia reported higher levels of social anhedonia compared with healthy individuals. They also showed lower recognition accuracy for faces compared with healthy participants. We found no significant correlation between social anhedonia severity and any of the memory performance variables for both patients with schizophrenia and healthy individuals. Regarding potential links between social anhedonia severity and face valence ratings, we found that individuals with elevated social anhedonia had a tendency to rate the face stimuli as more negative.
Limitations
Our negative finding may be partly explained by a lack of statistical power owing to our small patient sample. In addition, our patient sample had unusually high estimated IQ scores, which highlights potential issues regarding the generalization of our findings. Finally, we used a yes–no recognition memory task with a very short retention interval delay.
Conclusion
Our results suggest that social anhedonia is not directly linked to emotional memory deficits and biases and does not interfere with the modulatory effect of positively valenced emotion on memory.
Abstract
Contexte
L'étude du lien entre les atteintes de la mémoire émotionnelle dans la schizophrénie et certaines dimensions spécifiques des symptômes suscite l'intérêt. Nous avons exploré les liens potentiels entre la mémoire émotionnelle et la gravité de l'anhédonie sociale chez des patients schizophrènes et des témoins en bonne santé.
Méthodes
Nous avons administré l'échelle révisée d'anhédonie sociale de Chapman à 29 patients atteints de schizophrénie et à 27 témoins jumelés, avant de leur proposer une tâche mémorielle de reconnaissance de visages arborant des expressions heureuses, tristes et neutres. Nous avons calculé la performance de la mémoire émotionnelle à l'aide de 2 paramètres indépendants : l'indice Pr de précision de la discrimination et l'indice Br de biais de la réponse. Nous avons aussi mesuré les cotes de valence correspondant aux stimuli faciaux. Nous avons effectué des analyses de corrélation à partir de la variabilité interindividuelle de la gravité de l'anhédonie sociale et du score individuel obtenu pour chaque variable du rendement mémoriel et pour chaque score de valence faciale.
Résultats
Les patients atteints de schizophrénie ont présenté des taux plus élevés d'anhédonie sociale que les témoins en bonne santé. Ils ont aussi moins bien reconnu les visages, comparativement aux témoins. Nous n'avons noté aucune corrélation significative entre la gravité de l'anhédonie sociale et les diverses variables du rendement mémoriel, tant chez les patients atteints de schizophrénie que chez les témoins en bonne santé. Quant aux liens potentiels entre la gravité de l'anhédonie sociale et les cotes de valence émotionnelle, nous avons noté que les participants présentant une importante anhédonie sociale avaient tendance à donner une interprétation plus sombre des expressions faciales.
Limites
Ce résultat négatif peut en partie s'expliquer par le manque de puissance statistique inhérent à la petite taille de notre échantillon. De plus, les patients de notre échantillon présentaient des QI inhabituellement élevés, ce qui peut empêcher la généralisation de nos résultats. Finalement, notre tâche mémorielle demandait que l'on réponde par oui ou par non et l'intervalle de rétention était très bref.
Conclusion
Nos résultats donnent à penser que l'anhédonie sociale n'est directement liée ni aux déficits ni aux biais de la mémoire émotionnelle et n'interfère pas avec l'effet modulateur d'une émotion à valence positive sur la mémoire.
Introduction
In recent years, the candidate symptom approach in schizophrenia has led to a growing interest in social anhedonia. Social anhedonia is defined as a reduced capacity to experience pleasure in social situations, and multiple studies have reported elevated levels in people with schizophrenia.1–3 Social anhedonia among people with schizophrenia is a stable condition across time and clinical status and is relatively independent of symptoms such as depression and psychosis.1 Moreover, elevated social anhedonia in healthy individuals has been associated with an increased vulnerability to the development of schizophrenia.4–6 Further, it has been proposed that anhedonia is an enduring trait in which the severity varies based on a wide range of individual differences.7
It was recently hypothesized that the severity of anhedonia measured by self-report questionnaires among patients with schizophrenia may be moderated by episodic memory deficits.8 Indeed, self-report anhedonia questionnaires, such as the Chapman scales,9 require patients to reflect on hedonic memories to estimate the intensity of their emotional experiences. Recalling the subjective experience of pleasure associated with past hedonic experiences could be quite difficult for some patients in light of the episodic memory deficits that characterize schizophrenia.10,11 Thus, the congruence between the measurement of anhedonia through self-report questionnaires and the genuine reduction of the hedonic capacity could be compromised by these memory deficits. This possible relation between anhedonia and episodic memory has been examined in patients with schizophrenia8,12 and in healthy individuals.13 Burbridge and Barch12 examined whether performance of a standard nonemotional episodic memory task moderated the relation between anhedonia questionnaire ratings and the ratings of affective eliciting stimuli. Their results showed that better memory performance was not associated with a stronger relation between anhedonia ratings and subjective emotional experience of positive stimuli. In the experimental protocol by Horan and colleagues,8 patients had to first rate their emotional responses to pleasant foods and film clips and then complete a surprise memory recall task about their emotions after a 4-hour delay. Results showed no significant difference between patients and controls in the delayed recall of the pleasant emotions. They concluded that self-reported anhedonia is not secondary to deficiencies in encoding and short-term retention for pleasurable experiences. However, as acknowledged by the authors, their memory task might not have been sensitive enough to variations in anhedonia, as performance was quite high owing to the small number of evocative stimuli and to the use of a short delay between encoding and recalling the hedonic experiences. Finally, in 2006, Mathews and Barch13 showed that higher levels of physical and social anhedonia in healthy individuals did not influence the memory performance for emotional words.
Although recent findings suggest that self-reported anhedonia among patients with schizophrenia is not influenced by episodic memory deficits, there is still a need for a more precise investigation of potential links between anhedonia and emotional memory. First, it is uncertain whether the severity of a specific type of anhedonia modulates the memory performance for a congruent type of emotional stimuli. This suggests that self-reported social anhedonia among patients with schizophrenia may be moderated by memory performance for social emotional information only. Second, it is still unclear whether social anhedonia is directly associated with the failure of positive emotional valence to enhance memory among patients with schizophrenia.14 Finally, it may be important to examine whether or not self-reported social anhedonia is linked to specific memory biases. Psychiatric symptoms can bias cognitive and affective information processing by altering the way such information is processed, organized and represented. Brébion and colleagues15 examined the relation between memory biases and both positive and negative symptoms in 40 patients with schizophrenia. They found that hallucination scores correlated with an increased bias toward false recognition of nonpresented words (familiarity bias), whereas anhedonia was significantly correlated with a more conservative bias, that is, with fewer false recognitions of nonpresented words (novelty bias). As a whole, emotional memory biases and deficits associated with social anhedonia still need to be better understood.
The primary goal of our study was to determine whether or not social anhedonia measured in people with schizophrenia and in healthy individuals would be associated with variations in emotional memory performance and/or biases during an emotional face recognition memory task. We hypothesized that the severity of social anhedonia among patients with schizophrenia would be significantly and negatively correlated with the recognition memory accuracy for happy faces and with the magnitude of the enhancement of recognition memory accuracy by positively valenced information. We also expected that elevated social anhedonia among patients with schizophrenia would be associated with a novelty detection bias for happy faces.
Methods
To investigate these associations, we used an emotional face recognition memory task validated by our research group.16,17 We used faces depicting different emotional expressions because these are powerful social stimuli that convey crucial information used in social interactions; such facial expressions have been extensively employed to examine emotional processing in both nonclinical18,19 and psychiatric populations.20–23 One important advantage for using facial expressions as emotional stimuli is that they are not confounded by the overlap between stimulus emotionality and complexity or the unusualness that is often encountered with words or complex pictures.24–26 We specifically chose sad and happy expressions because these are basic emotions that are encountered in everyday life and are easily decoded, even among psychiatric patients.21,22 In previous studies, our group used a similar version of the emotional face recognition memory task and demonstrated that both happy and sad expressions significantly modulate recognition memory performance and biases for face stimuli in healthy individuals.17,27
Participants
We screened all participants to exclude those with a past or current neurologic condition (including head trauma with loss of consciousness) that could affect cognition; current drug or alcohol abuse (i.e., DSM-IV28 criteria not met for abuse for at least 3 months); and family history of hereditary neurologic disorders. We obtained written informed consent from all participants after providing them with a complete description of the study. The institutional review board of the Douglas Mental Health University Institute approved our protocol.
We recruited outpatients with schizophrenia from various clinics of the Douglas Institute. Patients' treating psychiatrists diagnosed schizophrenia according to the DSM-IV criteria. A trained research assistant independently confirmed the diagnosis using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I).29 We assessed positive and negative symptoms with the Scale for the Assessment of Positive Symptoms30 and the Scale for the Assessment of Negative Symptoms,31 respectively. We measured overall function using the Global Assessment Scale.32
We recruited healthy individuals through advertisements in local newspapers, and we examined them according to the nonpatient edition of the SCID-I to rule out any current or past Axis I psychiatric disorders. We excluded healthy individuals with a family history (first-degree relative) of schizophrenia spectrum disorders. We selected a group of healthy individuals who were representative of the normal population; we did not assess Axis II disorders and other personality traits. Efforts were made to match healthy individuals and patients with schizophrenia based on sex, age and education.
Procedure
We conducted the study at the Douglas Mental Health University Institute. Participants first completed the social anhedonia scale, and we subsequently assessed them using the Rey Auditory Verbal Learning Test,33 a standardized verbal memory measure to estimate nonemotional verbal episodic memory performance. We estimated full-scale IQ using the Wechsler Abbreviated Scale of Intelligence34 to ensure that any possible group differences in terms of episodic memory performance could not be attributed to a substantial disparity in intellectual ability. Three patients and 1 healthy individual refused to be assessed with the Rey Auditory Verbal Learning Test, and 3 healthy individuals refused to be assessed with the Wechsler Abbreviated Scale of Intelligence. Finally, participants performed the experimental emotional face recognition memory test in our laboratory on a personal computer using E-Prime software (Psychology Software Tools).
Measurement of social anhedonia
We used the Chapman Revised Social Anhedonia Scale35 to measure trait anhedonia severity. The Chapman anhedonia questionnaires9,35 are by far the most common anhedonia scales used to study the trait-like dimension of anhedonia.1,5,36–40 The Social Anhedonia Scale is a 40-item self-rated questionnaire with true or false answers on items that describe various common social situations. Some examples of the items presented are “I have always enjoyed looking at photographs of friends” and “I have often found it hard to resist talking to a good friend, even when I have other things to do.” The total score ranges from 0 to 40 (the lower the score, the less severe the anhedonia). In both patients with schizophrenia and healthy individuals, internal reliability for the Social Anhedonia Scale is consistently good with the α coefficient typically exceeding 0.80.41
Emotional face recognition memory task
We selected photographs of individuals depicting sad, happy or neutral facial expressions from 4 databases: Karolinska Directed Emotional Faces,42 the Psychological Image Collection at Stirling (http://pics.psych.stir.ac.uk), the Computer Vision Laboratory (http://lrv.fri.uni-lj.si/facedb.html) and the Purdue Robot Vision Lab AR (http://rvl1.ecn.purdue.edu/_aleix/aleix_face_DB.html) data sets, which our group has used as part of 2 functional magnetic resonance imaging (fMRI) emotional memory studies.16,17 We modified all images using Adobe Photoshop 7.0 (Adobe Systems) to achieve uniform face size, contrast and resolution and to convert them to grey-scale. In addition, we removed hair from the images so that the sex discrimination task was based only on the facial features. Overall, we used 168 stimuli, each corresponding to a different individual. We divided stimuli into 2 subsets, each with 28 sad, happy and neutral faces (half of the faces were male). The emotional face recognition memory task consisted of 2 phases: encoding and recognition.
During the encoding phase, 84 faces taken from one subset (counterbalanced across subjects) were presented twice, in a pseudorandom order. We instructed participants to make a sex judgment by clicking predetermined “male” and “female” buttons and to remember the stimuli. We chose this incidental task during the encoding phase to limit group differences in emotion discrimination and identification. Previous studies have shown that people with schizophrenia have difficulties with the explicit evaluation of emotional faces.43,44 Hence, using an incidental task that can be performed equally well by both groups likely minimizes this difference.
We assessed the recognition phase immediately after the encoding phase. During the recognition phase, the same stimuli were presented with an equal number of new faces. We asked participants to judge whether each face had been previously presented by clicking on predetermined “old” and “new” buttons. The stimulus time presentation was 2.5 seconds with an intertrial of 1.5 seconds. After the recognition phase, we asked participants to rate all the faces in terms of valence. To achieve a more precise and idiosyncratic rating from participants, each face was accompanied with a continuous line with the label “very negative” at the left end of the line and the label “very positive” at the right end of the line. We informed participants that the middle of the line was associated with neutrality. Using a mouse, participants moved an arrow on this line and clicked the left button once the arrow was well positioned on the line according to the emotional valence of the face. This procedure indicated their personal emotional valence rating for each face presented. The continuous line was in fact an ordinal scale ranging from “1” (very negative) to “323” (very positive).
Because of the nature of face stimuli, it is possible to objectively determine the degree of physical similarity among stimuli within and between emotional categories. This latter point is critical when investigating the potential expression-induced modulation of memory performance, although it has rarely been taken into account in previous research. Specifically, the degree of similarity is determined among the faces belonging to each emotional category with the eigenface method on the basis of a principal component analysis technique.45 This eigenface method has been used for our face stimuli, and the results of this analysis are published elsewhere.17,27 Briefly, the eigenface analysis has shown that any modulation of memory performance by emotion was unlikely to be caused by differences in the degree of similarity among faces for the 3 expressions.
We calculated memory performance according to the Two-High Threshold Model46 by means of the discrimination accuracy index Pr (Pr = H – FA) and the response bias Br (Br = FA / [1-(H – FA)]), where H and FA represent proportions of hits and false alarms, respectively. The former provides an unbiased estimate of the accuracy in the response to old and new items, where higher values correspond to better (more accurate) memory. The response bias, in contrast, is an index of the overall tendency to respond “old” or “new” regardless of accuracy. In this case, positive values indicate a tendency to say “old” (i.e., familiarity or liberal bias), whereas the negative side of the scale represents a novelty or conservative bias (that is, a propensity to say “new”). Importantly, the Pr and the Br measures are independent.46 We also calculated an index of the modulatory effect of positive emotion on the discrimination accuracy by subtracting the accuracy index Pr for neutral faces from the accuracy index Pr for happy faces. The result reflects an index of the positive modulation (i.e., Pollyanna effect), that is, the increased discrimination accuracy for happy faces compared with neutral faces.
Statistical analysis
For each group separately, we verified normality of distribution for the demographic variables and measures on the Rey Auditory Verbal Learning Test, Wechsler Abbreviated Scale of Intelligence, Social Anhedonia Scale and the emotional face memory task for each face expression condition (accuracy Pr, response bias Br, gender identification task at the encoding phase, reaction time at the recognition phase and face valence rating). We did not consider normality correction to be necessary for any of these variables. We removed 2 patients from the analyses: 1 owing to an extreme score (34) on the Social Anhedonia Scale (Grubb test for outliers: G statistic for suspected outlier score = 3.18; critical value for n = 29: 2.73) and 1 owing to technical problems during the emotional face memory task. We compared demographic, cognitive and clinical data between groups using Student t tests, except for the sex variable, for which we used a χ2 test. We explored potential relations among severity of social anhedonia, face valence ratings and memory performance measures using parametric correlation analyses. We correlated the individual scores measured with the Social Anhedonia Scale with the subjective ratings of the happy, sad and neutral faces and with the individual scores for the Pr-happy, Br-happy, positive modulation index, Pr-sad, Br-sad, Pr-neutral and Br-neutral performance measures.
We performed statistical tests using SPSS for Windows (version 15.0, SPSS Inc.). We performed Student t tests and 2-factor mixed design analyses of variance (ANOVA), with group as a between-subject factor and emotional face expression (neutral, sad or happy faces) as a within-subject factor, to compare patients with schizophrenia and healthy individuals for the different task variables. We performed paired t tests to test whether a specific condition bias (Br-sad for instance) was significantly different from the baseline “0” (i.e., no novelty or familiarity bias). For each group separately, we also used the parametric Pearson r coefficient to correlate severity of social anhedonia with the emotional memory task variables. A straight Bonferroni correction across all correlations would have been too conservative given the number of a priori hypotheses. However, to provide additional false-positive protection, we considered correlations to be significant only if they had a p < 0.01.
Results
Participants
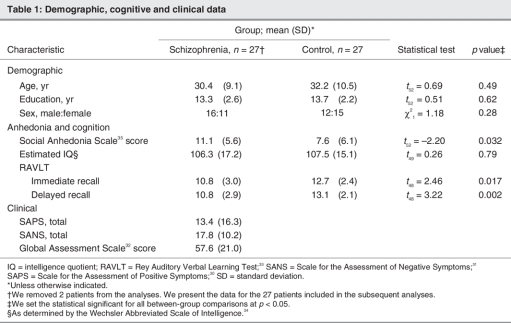
We included in our study participants (29 with schizophrenia and 27 healthy individuals) aged 20–56 years with an estimated IQ of at least 75. At the time of assessment, all patients had been clinically stable for at least 4 weeks and had been on a fixed medication regimen for at least 6 weeks. All but 3 patients were taking antipsychotic medication. Twenty-three were taking second-generation antipsychotics (8 olanzapine, 9 risperidone, 3 quetiapine and 3 clozapine) and 3 were taking conventional antipsychotics (1 loxapine and 2 zuclopenthixol) — no patients were taking a combination of both. The mean dose of medication was equivalent to 313 mg/day of chlorpromazine (standard deviation [SD] 340 mg/d),47 and medication was not discontinued for the purposes of the study. None of the patients had a concurrent mood disorder at the time of the study, although 7 patients were receiving concomitant antidepressant medication (1 venlafaxine, 2 citalopram, 1 mirtazapine, 1 paroxetine, 1 fluoxetine and 1 trazodone), and 2 patients were taking a mood stabilizing medication (valproic acid). No patients were taking benzodiazepines. Table 1 provides demographic, cognitive and clinical data for all participants.
Table 1
Anhedonia and cognition
Table 1 reports the mean scores on the Social Anhedonia Scale for each group. Patients with schizophrenia reported significantly higher levels of social anhedonia compared with healthy individuals. They also showed a significant impairment compared with the healthy individuals for both immediate and delayed recalls on the standardized measure of nonemotional verbal episodic memory (Rey Auditory Verbal Learning Test). This verbal memory deficit was not explained by a difference in global intellectual ability, as suggested by the absence of a significant group difference in estimated IQ.
Valence ratings of the faces
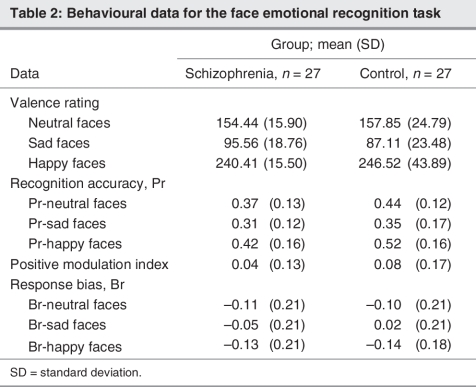
We used a 2-factor mixed design ANOVA to test whether patients with schizophrenia and healthy individuals differed for the face valence ratings. The analysis showed no significant main effect of group (F1,52 = 0.02, p = 0.90) and no significant group by face expression interaction (F2,104 = 0.98, p = 0.38), indicating that patients with schizophrenia and healthy individuals rated the faces comparably. A significant main effect of face expression (F2,104 = 380.48, p < 0.001) showed that the 3 face expression conditions were rated differently in terms of valence (pairwise comparisons: sad < neutral < happy). Table 2 shows the valence rating data.
Table 2
Emotional face memory task
Sex identification task: encoding phase
A nonsignificant main effect of group (F1,52 = 0.49, p = 0.49) and nonsignificant group by face expression interaction (F2,104 = 0.21, p = 0.80) suggest that patients with schizophrenia and healthy participants were not significantly different regarding their capacity to properly identify the sex of each face during the encoding phase (% accuracy for both groups combined: neutral faces 98%, sad faces 95% and happy faces 95%). Their was a significant main effect of face expression (F2,104 = 13.82, p < 0.001), suggesting that both patients and healthy individuals were more accurate in the sex identification of neutral faces compared with sad and happy faces.
Reaction time: recognition phase
As shown by the nonsignificant main effect of group (F1,52 = 0.03, p = 0.87) and the nonsignificant group by face expression interaction (F2,104 = 0.47, p = 0.63), there was no difference between patients with schizophrenia and healthy participants regarding reaction time during the recognition phase. Furthermore, the emotional face expressions did not significantly modulate reaction time (main effect of face expression: F2,104 = 0.77, p = 0.47).
Discrimination index Pr and positive modulation index
Patients with schizophrenia showed an overall lower recognition accuracy for faces (Pr) compared with healthy individuals (Table 2), as revealed by a significant main effect of group (F1,52 = 4.66, p = 0.036). Moreover, a significant main effect of face expression (F2,104 = 23.68, p < 0.001) indicated that face expressions modulated recognition accuracy (pairwise comparisons: Pr-sad < Pr-neutral < Pr-happy). Thus, faces depicting a sad expression were recognized less well compared with both neutral and happy faces. On the other hand, participants had a better memory for the happy faces compared with both neutral and sad conditions. The absence of a significant group by face expression interaction (F2,104 = 1.39, p = 0.25) suggests that the sad and happy face expressions had a similar modulatory effect on recognition accuracy for both groups.
We used a Student t test to directly compare patients and healthy individuals for the positive modulation index. Healthy individuals were not significantly different from patients with schizophrenia (t52 = 0.99, p = 0.33) regarding the enhancement of memory for the happy expression compared with the neutral expression.
Response biases
Patients with schizophrenia and healthy individuals did not significantly differ in their overall tendency to respond “old” (familiarity bias) or “new” (novelty bias) (main effect of group: F1,52 = 0.16, p = 0.68). In addition, there was no significant group by face expression interaction (F2,104 = 2.02, p = 0.14). We observed a significant main effect of face expression (F2,104 = 14.75, p < 0.001), indicating an overall modulatory effect of emotional face expression on response bias (both groups combined: Br-neutral –0.10, Br-sad –0.02, Br-happy –0.13). Both groups showed a significant novelty bias for happy faces (Br-happy v. baseline “0”; patients: t26 = –3.08, p = 0.005; healthy individuals: t26 = –4.17, p < 0.001) and neutral faces (Br-neutral v. baseline “0”; patients: t26 = –2.64, p = 0.014; healthy individuals: t26 = –2.45, p = 0.021). However, there was no significant familiarity or novelty bias for sad faces for either patients with schizophrenia or healthy individuals (Br-sad v. baseline “0”; patients: t26 = –1.23, p = 0.23; healthy individuals: t26 = 0.43, p = 0.67) (Table 2).
Social anhedonia severity, face valence ratings and emotional memory variables
For each group separately, we performed correlation analyses using the individual scores on the Social Anhedonia Scale and the individual scores obtained at each memory performance measure (Pr-happy, Br-happy, positive modulation index, Pr-sad, Br-sad, Pr-neutral and Br-neutral) and at each face valence rating condition.
Patients with schizophrenia
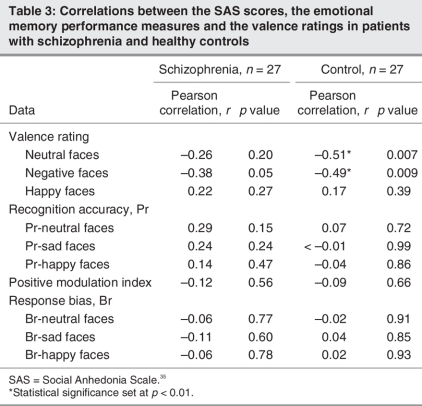
No happy, sad and neutral face valence ratings were significantly correlated with severity of social anhedonia (p < 0.01 required for significance). Regarding memory performance per se, there was no significant correlation between any of the memory performance variables and severity of social anhedonia (Table 3).
Table 3
Healthy individuals
There was no significant correlation between happy face valence ratings and severity of social anhedonia. Conversely, scores on the Social Anhedonia Scale were significantly and negatively correlated with ratings of sad faces, suggesting that healthy individuals with high levels of social anhedonia rated the sad faces more negatively. In addition, we also observed a significant correlation between neutral face valence ratings and scores on the Social Anhedonia Scale, suggesting that healthy individuals with elevated social anhedonia perceived neutral stimuli more negatively. Finally, we observed no significant correlation between any of the memory performance variables and severity of social anhedonia (Table 3).
Discussion
Social anhedonia is a prevalent symptom among patients with schizophrenia that can interfere with the initiation and maintenance of social interactions. In this exploratory study, we examined potential links between the severity of this symptom and emotional memory. We used an emotional face recognition memory task to assess 2 different populations on both their accuracy in recognizing emotional faces (Pr) and their response bias (Br) during the recognition. Among our main hypotheses, we predicted that social anhedonia in schizophrenia would have a detrimental effect on recognition memory accuracy for happy faces and on the enhancement of memory for positively valenced information. We also expected social anhedonia to be associated with a novelty bias for happy faces. Contrary to our hypotheses, the memory performance measures did not significantly correlate with severity of social anhedonia in patients with schizophrenia or healthy individuals.
Severity of social anhedonia and face valence ratings
We explored whether social anhedonia influences face valence ratings. We found that healthy individuals with elevated social anhedonia rated the sad faces more negatively. We observed a similar nonsignificant trend among patients with schizophrenia. Social anhedonia could be linked to an altered evaluation of negatively valenced information, thus individuals with elevated social anhedonia may perceive more negativity from social information. This assumption is supported by the presence of a significant negative correlation found among healthy individuals between severity of social anhedonia and neutral face valence ratings. This significant correlation suggests that healthy individuals with elevated social anhedonia perceive more negativity even in neutrally valenced social information. In both his original48 and revised49 theories, Paul Meehl pointed out that anhedonia could contribute to or be a consequence of what he described as the aversive drift (i.e., the tendency to take on a burdensome, threatening, gloomy, negative emotional charge). The fact that participants with elevated social anhedonia rated the neutral and sad faces as more negative could reflect this aversive drift. However, variations in social anhedonia did not influence happy face valence ratings in either group.
The relation between anhedonia ratings and the evaluation or experience of emotional stimuli has been investigated in recent studies,8,12–14 yielding mixed results. Consistent with our findings, Burbridge and Barch12 did not find any significant correlation between social and physical Chapman anhedonia ratings and self-reported experience of multiple types of positive stimuli in either patients with schizophrenia or controls. On the other hand, Mathews and Barch13 reported that in healthy individuals both physical and social anhedonia are associated with less positive valence ratings of positive words. Similarly, Herbener and colleagues14 observed the same association using positive scenes in their healthy sample but not in their schizophrenia sample. The authors suggested a dissociation between immediate reports of positive emotional responses and estimation of delayed hedonic experiences among patients with schizophrenia. In general, it is logical to expect that a reduced capacity to feel pleasure will significantly impact the immediate self-report of emotional response to positive stimuli. However, equivocal results in the literature suggest that interfering variables, such as cognitive deficits, may alter the link between self-reported anhedonia and immediate report of hedonic experiences among patients with schizophrenia. It should be noted that we did not require our participants to rate their own pleasure during the observation of the faces; we asked them to evaluate the extent of which a facial expression looked happy, sad or neutral. Happy expressions are intense social stimuli that serve a communicatory function and are automatically and quickly processed by highly specialized neural systems.50 Thus it is possible that variations in the capacity to experience pleasure with others are not directly associated with the capacity to consciously and accurately evaluate the emotional valence of positively valenced social stimuli.
Social anhedonia and emotional face recognition memory
There have been a limited number of studies that have directly investigated the link between anhedonia and episodic memory performance.8,12,13 These studies were mostly driven by the “anhedonia paradox” usually found in schizophrenia. Indeed, there is a contradiction between the high levels of anhedonia usually reported by patients with schizophrenia through questionnaires and clinical interviews and the immediate report of normal levels of positive emotions during exposure to hedonic stimuli in a laboratory. Results from these recent studies do not support the hypothesis that memory deficits among patients with schizophrenia explain the anhedonia paradox. Consistent with these studies and contrary to our hypotheses, our results also suggest that interindividual variability in social anhedonia is not linked to the memory performance and bias for emotional social stimuli. It is important to continue the investigation of potential links between cognitive/memory deficits and severity of social anhedonia for several reasons. First, elevated social anhedonia in healthy individuals increases the likelihood of psychiatric illnesses such as schizophrenia developing. The reduced capacity to feel pleasure in social situations does not by itself explain the vulnerability; thus a better characterization of the cognitive and psychological features that accompany social anhedonia in healthy individuals is needed. Second, it has been proposed that anhedonia characterizes subgroups of patients with schizophrenia.9,51,52 Linking anhedonia to specific cognitive deficits such as emotional memory impairments could therefore contribute to the cognitive characterization of more homogeneous subgroups of patients. Moreover, the possible link between anhedonia and emotional memory was of particular interest in schizophrenia given the anhedonia paradox. Several key questions remain regarding the nature of anhedonia in schizophrenia. The anhedonia paradox raises 2 important questions.
First, what do self-report anhedonia questionnaires measure among patients with schizophrenia? Researchers have recently proposed that neurocognitive deficits could interfere with the measurement of a genuine capacity to feel pleasure among patients with schizophrenia using self-report questionnaires. Recent data suggest that episodic memory impairments do not explain the anhedonia paradox. An alternative target could be related to the anticipation of pleasure. Indeed, Gard and colleagues53 recently proposed that patients with schizophrenia experience disturbances in the experience of pleasure related to future activities. They found that patients with schizophrenia predicted that future events would be less pleasurable and reported less pleasure in anticipation of future positive events. In addition, ratings of anhedonia on the Scale for the Assessment of Negative Symptoms were significantly correlated with the patients' reports of anticipatory pleasure but not with consummatory pleasure (i.e., genuine rewarding feeling of pleasure in the presence of a positive stimulus/activity). Anticipation of an upcoming pleasure is likely influenced by several factors, including current mood, memory of past hedonic experiences and personality traits. Pleasure anticipation impairments among patients with schizophrenia may originate from an abnormal integration of these factors.
Second, is there a genuine reduction in the capacity to feel pleasure among patients with schizophrenia? Do people with schizophrenia always feel comparable levels of pleasure compared with healthy individuals when directly engaged in an enjoyable activity? This is quite unintuitive given the apparent flat affect and lack of interest that clinicians continuously observe among these individuals. One could propose that people with schizophrenia “manage” to feel normal levels of pleasure in simple hedonic situations (smoking a cigarette, viewing beautiful pictures, eating cookies) involving more basic positive emotions. Since these simple hedonic situations/ stimuli are often used in experimental paradigms, this would explain the absence of a significant difference between patients and controls for the subjective pleasure felt during these experiments (consummatory pleasure is apparently preserved). On the other hand, it is possible that people with schizophrenia will struggle in their capacity to genuinely feel pleasure when confronted with more complex hedonic situations that involve more complex positive emotions such as pride, accomplishment or complicity. These positive emotions are more rarely experimentally tested but are, nonetheless, just as important and are often involved in multiple real-life situations. Anhedonia is usually divided into 2 dimensions: social anhedonia and physical anhedonia. It seems that social anhedonia, as measured by self-report questionnaires, refers to a greater extent of complex positive emotions than physical anhedonia. One wonders if an experimental paradigm distinguishing simple basic pleasures (mostly sensory-based pleasures) from more complex ones (e.g., pride, accomplishment) could demonstrate that consummatory pleasure is indeed impaired among patients with schizophrenia, but only for hedonic stimuli/situations involving complex positive emotions.
Limitations
Our results should be interpreted with some caution considering some limitations of our protocol. First, even though the size of our patient sample (n = 27) was equivalent to that of similar recent studies,8,12,14 it was still a limited number of participants for a correlational approach. It is possible that our negative finding was partly explained by a lack of statistical power.
Second, our patient sample had unusually high estimated IQ scores, which highlights potential issues regarding the generalization of our findings. All patients recruited for this study also participated in an independent fMRI study. Patients with schizophrenia who accept to participate in our fMRI studies are usually highly functional and interested in medical research. To prevent potential problems during the scanning session, we usually avoid recruiting patients who are not clinically stable, not functional or who show a total absence of motivation for participating in the study. This could partly explain why patients recruited in this study showed such a high mean estimated IQ. Yet, it is possible that our findings only apply to a subgroup of highly functional patients with elevated IQ.
Finally, we used a yes–no recognition memory task with a very short retention interval delay (i.e., a few minutes). One could suggest that greater modulation of recognition memory by emotion could have been observed with a task involving a longer retention interval delay. However, previous studies, including several from our group, have shown that a simple yes–no recognition memory task using an incidental encoding task can nonetheless produce a significant enhancement of memory for emotional stimuli.16,17,54 Based on these studies, we felt our task was appropriate to answer our research questions.
Conclusion
Our results suggest that social anhedonia is not directly linked to emotional memory deficits and biases and does not interfere with the modulatory effect of positively-valenced emotion on memory.
Acknowledgments
This study was supported by an operating grant from the Canadian Institutes of Health Research (#53280). Dr. Lepage is supported by a salary award from the Fonds de recherche en santé du Québec. Dr. Harvey was supported by a doctoral studentship from the Fonds de recherche en santé du Québec. We thank Drs. M. Brodeur and M. Pelletier for assistance with clinical assessments.
Footnotes
Contributors: Drs. Harvey, Sergerie, Armony and Lepage designed the study. Dr. Harvey acquired the data and analyzed it together with Mr. Bodnar. Dr. Harvey wrote the article; Mr. Bodnar and Drs. Sergerie, Armony and Lepage reviewed it. All authors gave final approval for publication.
Competing interests: None declared.
Correspondence to: Dr. M. Lepage, Douglas Mental Health University Institute, Frank B. Common Pavilion, 6875 LaSalle Blvd., Montréal QC H4H 1R3; martin.lepage@douglas.mcgill.ca
References
- 1.Blanchard JJ, Horan WP, Brown SA. Diagnostic differences in social anhedonia: a longitudinal study of schizophrenia and major depressive disorder. J Abnorm Psychol 2001;110:363-71. [DOI] [PubMed]
- 2.Blanchard JJ, Mueser KT, Bellack AS. Anhedonia, positive and negative affect, and social functioning in schizophrenia. Schizophr Bull 1998;24:413-24. [DOI] [PubMed]
- 3.Katsanis J, Iacono WG, Beiser M. Anhedonia and perceptual aberration in first-episode psychotic patients and their relatives. J Abnorm Psychol 1990;99:202-6. [DOI] [PubMed]
- 4.Chapman LJ, Chapman JP, Kwapil TR, et al. Putatively psychosis-prone subjects 10 years later. J Abnorm Psychol 1994;103:171-83. [DOI] [PubMed]
- 5.Gooding DC, Tallent KA, Matts CW. Clinical status of at-risk individuals 5 years later: further validation of the psychometric high-risk strategy. J Abnorm Psychol 2005;114:170-5. [DOI] [PubMed]
- 6.Mason O, Startup M, Halpin S, et al. Risk factors for transition to first episode psychosis among individuals with ‚at-risk mental states'. Schizophr Res 2004;71:227-37. [DOI] [PubMed]
- 7.Meehl PE. Primary and secondary hypohedonia. J Abnorm Psychol 2001; 110:188-93. [DOI] [PubMed]
- 8.Horan WP, Green MF, Kring AM, et al. Does anhedonia in schizophrenia reflect faulty memory for subjectively experienced emotions? J Abnorm Psychol 2006;115:496-508. [DOI] [PubMed]
- 9.Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J Abnorm Psychol 1976;85:374-82. [DOI] [PubMed]
- 10.Aleman A, Hijman R, de Haan EH, et al. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry 1999;156:1358-66. [DOI] [PubMed]
- 11.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 1998;12:426-45. [DOI] [PubMed]
- 12.Burbridge JA, Barch DM. Anhedonia and the experience of emotion in individuals with schizophrenia. J Abnorm Psychol 2007;116:30-42. [DOI] [PubMed]
- 13.Mathews JR, Barch DM. Episodic memory for emotional and non-emotional words in individuals with anhedonia. Psychiatry Res 2006;143:121-33. [DOI] [PubMed]
- 14.Herbener ES, Rosen C, Khine T, et al. Failure of positive but not negative emotional valence to enhance memory in schizophrenia. J Abnorm Psychol 2007;116:43-55. [DOI] [PubMed]
- 15.Brébion G, David AS, Jones H, et al. Hallucinations, negative symptoms, and response bias in a verbal recognition task in schizophrenia. Neuropsychology 2005;19:612-7. [DOI] [PubMed]
- 16.Sergerie K, Lepage M, Armony JL. A process-specific functional dissociation of the amygdala in emotional memory. J Cogn Neurosci 2006;18:1359-67. [DOI] [PubMed]
- 17.Sergerie K, Lepage M, Armony JL. Influence of emotional expression on memory recognition bias: a functional magnetic resonance imaging study. Biol Psychiatry 2007;62:1126-33. [DOI] [PubMed]
- 18.Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biol Psychiatry 2002;51:59-67. [DOI] [PubMed]
- 19.Posamentier MT, Abdi H. Processing faces and facial expressions. Neuropsychol Rev 2003;13:113-43. [DOI] [PubMed]
- 20.Kohler CG, Bilker W, Hagendoorn M, et al. Emotion recognition deficit in schizophrenia: association with symptomatology and cognition. Biol Psychiatry 2000;48:127-36. [DOI] [PubMed]
- 21.Kohler CG, Turner TH, Bilker WB, et al. Facial emotion recognition in schizophrenia: intensity effects and error pattern. Am J Psychiatry 2003;160:1768-74. [DOI] [PubMed]
- 22.Leppanen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr Opin Psychiatry 2006;19:34-9. [DOI] [PubMed]
- 23.Mandal MK, Pandey R, Prasad AB. Facial expressions of emotions and schizophrenia: a review. Schizophr Bull 1998;24:399-412. [DOI] [PubMed]
- 24.Adolphs R, Denburg NL, Tranel D. The amygdala's role in long-term declarative memory for gist and detail. Behav Neurosci 2001;115:983-92. [DOI] [PubMed]
- 25.Ochsner KN. Are affective events richly recollected or simply familiar? The experience and process of recognizing feelings past. J Exp Psychol Gen 2000;129:242-61. [DOI] [PubMed]
- 26.Talmi D, Moscovitch M. Can semantic relatedness explain the enhancement of memory for emotional words? Mem Cognit 2004;32:742-51. [DOI] [PubMed]
- 27.Lepage M, Sergerie K, Pelletier M, et al. Episodic memory bias and the symptoms of schizophrenia. Can J Psychiatry 2007;52:702-9. [DOI] [PubMed]
- 28.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 1994.
- 29.Spitzer RL, Williams JBW, Gibbon M, et al. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry 1992;49:624-9. [DOI] [PubMed]
- 30.Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS). Iowa City (IA): University of Iowa; 1984.
- 31.Andreasen NC. Modified Scale for the Assessment of Negative Symptoms (SANS). Iowa City (IA): University of Iowa; 1984.
- 32.Endicott J, Spitzer RL, Fleiss JL, et al. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 1976;33:766-71. [DOI] [PubMed]
- 33.Rey A. L'examaen clinique en psychologie. Paris: Presses Universitaires de France; 1964.
- 34.Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio (TX): Psychological Corporation; 1999.
- 35.Eckblad M, Chapman LJ, Chapman JP, et al. The revised social anhedonia scale. Unpublished test. Madison (WI): University of Wisconsin; 1982.
- 36.Camisa KM, Bockbrader MA, Lysaker P, et al. Personality traits in schizophrenia and related personality disorders. Psychiatry Res 2005;133:23-33. [DOI] [PubMed]
- 37.Collins LM, Blanchard JJ, Biondo KM. Behavioral signs of schizoidia and schizotypy in social anhedonics. Schizophr Res 2005;78:309-22. [DOI] [PubMed]
- 38.Dubal S, Jouvent R. Time-on-task effect in trait anhedonia. Eur Psychiatry 2004;19:285-91. [DOI] [PubMed]
- 39.Harvey PO, Pruessner J, Czechowska Y, et al. Individual differences in trait anhedonia: a structural and functional magnetic resonance imaging study in non-clinical subjects. Mol Psychiatry 2007;12:767-75. [DOI] [PubMed]
- 40.Kontaxakis VP, Kollias CT, Havaki-Kontaxaki BJ, et al. Physical anhedonia in the acute phase of schizophrenia. Ann Gen Psychiatry 2006;5:1. [DOI] [PMC free article] [PubMed]
- 41.Horan WP, Kring AM, Blanchard JJ. Anhedonia in schizophrenia: a review of assessment strategies. Schizophr Bull 2006;32:259-73. [DOI] [PMC free article] [PubMed]
- 42.Lundqvist D, Flykt A, Öhman A. The Karolinska Directed Emotional Faces [CD-ROM]. Stockholm (Sweden): Department of Clinical Neuroscience, Psychology section, Karolinska Institutet; 1998.
- 43.Bigelow NO, Paradiso S, Adolphs R, et al. Perception of socially relevant stimuli in schizophrenia. Schizophr Res 2006;83:257-67. [DOI] [PubMed]
- 44.Leppänen JM, Niehaus DJ, Koen L, et al. Emotional face processing deficit in schizophrenia: a replication study in a South African Xhosa population. Schizophr Res 2006;84:323-30. [DOI] [PubMed]
- 45.Turk M, Pentland A. Eigenfaces for recognition. J Cogn Neurosci 1991;3:71-86. [DOI] [PubMed]
- 46.Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen 1988;117:34-50. [DOI] [PubMed]
- 47.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 2003;64:663-7. [DOI] [PubMed]
- 48.Meehl PE. Schizotaxia, schizotypia, schizophrenia. Am Psychol 1962;17:827-38.
- 49.Meehl PE. Toward an integrated theory of schizotaxia, schizotypy and schizophrenia. J Personal Disord 1990;4:1-99.
- 50.Adolphs R. Neural systems for recognizing emotion. Curr Opin Neurobiol 2002;12:169-77. [DOI] [PubMed]
- 51.Kirkpatrick B, Buchanan RW. Anhedonia and the deficit syndrome of schizophrenia. Psychiatry Res 1990;31:25-30. [DOI] [PubMed]
- 52.Schürhoff F, Szöke A, Bellivier F, et al. Anhedonia in schizophrenia: A distinct familial subtype? Schizophr Res 2003;61:59-66. [DOI] [PubMed]
- 53.Gard DE, Kring AM, Gard MG, et al. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res 2007;93:253-60. [DOI] [PMC free article] [PubMed]
- 54.Buchanan TW. Retrieval of emotional memories. Psychol Bull 2007;133:761-79. [DOI] [PMC free article] [PubMed]