Abstract
Background
We reviewed systematically the results of genetic studies investigating associations between putative susceptibility genes for attention-deficit hyperactivity disorder (ADHD) and neuropsychological traits relevant for this disorder.
Methods
We identified papers for review through the PubMed database.
Results
Twenty-nine studies examined 10 genes (DRD4, DAT1, COMT, DBH, MAOA, DRD5, ADRA2A, GRIN2A, BDNF and TPH2) in relation to neuropsychological traits relevant for ADHD. For DRD4, the continuous performance test (CPT) and derived tasks were the most used tests. Association of high reaction time variability with the 7-repeat allele absence appears to be the most consistent result and seems to be specific to ADHD. Speed of processing, set-shifting and cognitive impulsiveness were less frequently investigated but seem to be altered in the 7-repeat allele carriers. No effect of genotype was found on response inhibition (the stop and go/no-go tasks). For DAT1, 4 studies provide conflicting results in relation to omission and commission errors from CPT and derived tasks. High reaction time variability seems to be the most replicated cognitive marker associated with the 10-repeat homozygosity. The other genes have attracted fewer studies, and the reported findings need to be replicated.
Limitations
Although we aimed to perform a formal meta-analysis, this was not possible because the number of studies using the same neurocognitive endophenotypes was limited. We referred only minimally to the various theoretical frameworks in this field of research; more detail would have been beyond the scope of our systematic review. Finally, sample sizes in most of the studies we reviewed were small. Thus, some negative findings could be attributed to a lack of statistical power, and positive results should be considered preliminary until they are replicated in extended samples.
Conclusion
Several methodological issues, including measurement errors, developmental changes in cognitive abilities, sex, psychostimulant effects and presence of comorbid conditions, represent confounding factors and may explain conflicting results.
Abstract
Contexte
Nous avons revu systématiquement des résultats des études génétiques ayant examiné l'association entre des gènes de susceptibilité au trouble hyperactivité/déficit de l'attention (THADA) et les marqueurs neuropsychologiques les plus incriminés dans ce trouble.
Méthodes
Nous avons recueilli des articles analysés dans cette revue par le moyen d'interrogation de la base de données PubMed.
Résultats
Vingt-neuf études ont examiné 10 gènes (DRD4, DAT1, COMT, DBH, MAOA, DRD5, ADRA2A, GRIN2A, BDNF et TPH2) en association aux traits neuropsychologiques impliqués dans le THADA. Pour DRD4, le « continuous performance test » (CPT) et les tâches dérivées représentent les tâches les plus utilizées. L'association d'une grande variabilité des temps de réaction avec l'absence de l'allèle « 7-repeat » apparaît comme le résultat le plus solide et semble être spécifique au THADA. La vitesse de traitement, la flexibilité et l'impulsivité cognitives ont été moins fréquemment étudiées mais semblent être perturbées chez les porteurs de l'allèle « 7-repeat ». Il n'existe pas d'effet du génotype sur la capacité d'inhibition (tâches stop et go/no-go). Pour DAT1, 4 études rapportent des résultats discordants concernant les erreurs d'omission et de commission au CPT et tâches dérivées. Une grande variabilité des temps de réaction semble être le marqueur cognitif le plus répliqué en association à l'homozygosité de l'allèle « 10-repeat ». Les autres gènes ont fait l'objet de moins d'études dont les résultats nécessitent des réplications.
Limites
Une méta-analyse n'a pas pu être réalisée vu le faible nobre d'études utilisant le même endophénotype cognitif. Nous nous sommes référés au minimum aux différentes approches théoriques dans ce champ de recherche car les aborder en détail aurait dépassé l'objectif de cette étude d'examen critique. Enfin, les échantillons étudiés sont de petite taille. De ce fait, quelques résultats négatifs pourraient être attribués au manque de puissance statistique et les associations positives devraient être considérées comme préliminaires jusqu'à leur réplication dans des échantillons plus grands.
Conclusion
Plusieurs problèmes méthodologiques incluant les erreurs de mesure, l'effet du développement sur les performances cognitives, le sexe, les effets des traitements psychostimulants et la présence de comorbidités sont relevés. Ils représentent des facteurs confondants et peuvent contribuer à la discordance des résultats.
Introduction
Attention-deficit hyperactivity disorder (ADHD) is a highly prevalent, childhood-onset disorder characterized by age-inappropriate levels of inattention, hyperactivity and impulsivity.1 The disorder often persists into adulthood with deleterious effects on educational and social outcomes. Twin, family and adoption studies have suggested that there is a strong genetic contribution to ADHD, with a mean heritability estimate of 76%.2 However, it has been difficult to implicate any specific gene in ADHD beyond reasonable doubt. The difficulties encountered in identifying genetic variants increasing the risk for ADHD could be at least in part owing to the heterogeneity and complexity of this clinical syndrome.3,4 Consequently, it has been proposed that the use of intermediate phenotypes (or endophenotypes) relevant to ADHD are likely to be more informative than the DSM-IV1 diagnoses, allowing for increased detection of genetic effects.5–7
The most popular model of ADHD is that the essential impairment underlying the clinical symptoms is a deficit in response inhibition that impairs the capacity of the individual to withhold a prepotent response when engaged in a task.8 Further research has led to the observation that other deficits in executive function are associated with ADHD; a meta-analysis of 83 studies has shown that children with ADHD demonstrate substantial impairment on measures of working memory, planning and organization, set shifting, processing speed, inattention and impulsivity in addition to deficits in response inhibition (effect sizes between 0.43 and 0.69).9 Several of these intermediate phenotypes have been examined in genetic association studies.
Alternatively, many features of ADHD are described as related to problems with regulating allocation of energy and effort.10 This “cognitive– energetic” model defines state regulation as the allocation of extra effort to sustain performance in the presence of stressors such as high presentation rates of stimuli. Although activation normally increases with event rates, long interevent intervals engender a suboptimal hypoactivation in people with ADHD, who then are unable to summon the necessary effort to adjust appropriately to the demands of the situation. Reaction time variability, one of the most replicated deficits in ADHD across a variety of tasks, is thought to be one of the best indices of such state regulation difficulties and has been equally examined as an intermediate phenotype.11
In addition to these purely cognitive models, it has also been proposed that ADHD may result from emotional deregulation in the form of delay aversion.12 According to this model, in children with ADHD waiting is associated with a negative emotional tone that can be appreciated using tasks that require them to wait longer to maximize gain. However, to our knowledge, no genetic studies have been designed to test this model. As a relatively large body of research has now been published, it may be informative to explore whether this approach investigating endophenotype and quantitative traits has produced more consistent findings compared with the classical approach centred on the categorical syndrome of ADHD and its subcategories. The main objective of our study was to systematically review and discuss the results of studies investigating neuropsychological and genetic correlations in ADHD.
Methods
We identified papers for inclusion in this review by searching journal abstracts available online through the National Library of Medicine's PubMed database using a number of keyword searches. Each search combined 1 key word referring to 1 endophenotype (e.g., “neuropsychological,” “cognitive,” “attention,” “executive,” “memory,” “inhibition,” “IQ,” “reaction time,” “state regulation,” “endophenotype”), a name of a gene that has been previously associated with ADHD (e.g., “DRD4” or “dopamine [DA] receptor 4”) and the term “ADHD.” We completed the search with a systematic screening of the references of the identified and relevant papers. We limited our search to English-language papers. Minimum selection criteria for these genetic association studies were the use of clinically established diagnoses and the inclusion of children and/or adolescent participants. Box 1 summarizes the different neuropsychological tasks used in these studies and provides a succinct description of the reported scores. We mentioned the available data on the heritability and occurrence in unaffected relatives of the intermediate traits used for genetic association.
Box 1.
For each study, we calculated the effect size (Cohen d) in the direction of the deficit (high risk worse than low risk) and the percentage of variance in the endophenotype explained by the genotype variance using the following formula: percentage of variance = [(nlow risk + nhigh risk) / (nlow risk × nhigh risk)] + [d2 / 2(nlow risk + nhigh risk)],28 where nlow risk and nhigh risk represent the number of people in the low-and high-risk groups, respectively.
Results
We included in our review 29 studies examining 10 genes (DRD4, DAT1, COMT, DBH, MAOA, DRD5, ADRA2A, GRIN2A, BDNF and TPH2) in relation to neuropsychological traits relevant for ADHD.
Dopamine receptor 4 (DRD4)
The DRD4 gene is located on chromosome 11p15.5, having a highly polymorphic variable number tandem repeat (2, 3, 4, 5 or 7 copies of a 48-bp repeat sequence) within the coding region (exon 3). In the general population, the most prevalent alleles are the 4- (~67%), 7- (~12%) and 2- (~10%) repeat alleles.29 The frequencies of these alleles vary widely in human populations.30 In addition to coding for 1 of the 5 DA receptors, the DRD4 gene has been widely investigated in ADHD because D4 receptors are mainly expressed in brain regions such as the anterior cingulate cortex that are known to be important for attention and inhibition. Moreover, the 7-repeat allele was reported to be associated with blunted response to DA in Chinese hamster ovary cell lines expressing D4 receptor variants.31 A recent meta-analysis of case–control and family-based studies in European populations showed a significant preferential transmission of the 7-repeat allele from parents to children affected with ADHD (odds ratio [OR] = 1.34, 95% confidence interval [CI] 1.23–1.45).30 The 5-repeat allele was also associated with an increased risk (OR = 1.68, 95% CI 1.17–2.41). Conversely, the 4-repeat allele showed a reduced association in both case–control and family-based studies (OR = 0.9, 95% CI 0.84–0.97), suggesting that this allele likely confers a protective effect.
Contrary to expectation, 4 studies reported that the 7-repeat risk allele was associated with relatively better attention than the short-repeat alleles. Swanson and colleagues32 were the first to report that the subgroup of participants with at least the 7-repeat allele did not display neuropsychological deficits. In this study, 3 tasks were used to probe 3 anatomic networks implicated in attention. The battery consisted of a colour-word task based on conflict resolution (probing anterior network), a cued-detection task requiring shifting and maintenance of attention (probing postero-parietal network and frontal brain regions) and a go-change task (tapping alerting network but also anterior network). Additionally, the 7-absent subgroup showed longer reaction times and greater standard deviations. The authors invoked the heterogeneity of ADHD with the possibility that in participants without the 7-repeat allele ADHD developed from other genetic and nongenetic risk factors. They argued also that the 7-repeat allele may have been associated with behavioural features rather than cognitive deficits. Subsequently, Manor and colleagues,33 using the test of variables of attention, reported that children with ADHD carrying the 7-repeat allele exhibited better commission and omission scores and lower reaction time variability compared with those carrying the shortest allele (the 2-repeat allele). Moreover, a “dose effect” across the different alleles was found with a trend for an inverse correlation between number of repeats and impaired performance. In a recent study, Bellgrove and colleagues34 reported that carriers of the 7-repeat allele performed significantly better than noncarriers in terms of commission errors and had less reaction time variability on the sustained attention to response task. Furthermore, the probands with the 7-repeat allele did not differ from a control group with respect to their sustained attention to response task performances. In line with these case–control results, in family-based analyses better performance on the sustained attention to response task tended to predict biased transmission of the 7-repeat allele from heterozygous parents. In a more recent study from the same group,35 spectral analysis of reaction time variability and genotyping of control children supported the hypothesis that the association of this attentional pattern with the absence of the 7-repeat allele was somewhat specific to ADHD.
Intriguingly, 3 other studies reported opposite results. Langley and colleagues36 compared cognitive performances of 2 groups of medication-naive children with ADHD. The group with the 7-repeat allele appeared to show greater impulsiveness (faster and less accurate responses in the matched familiar figures test and faster reaction times in the stop task) that those without this allele. However, children with and without the 7-repeat allele did not differ in response inhibition (stop and go/no-go tasks) or continuous performance test (CPT) measures. In a study by Waldman,7 performances on the trail making test (part A: processing speed; part B: set-shifting ability) were examined as an endophenotype in a sample of children with ADHD and their siblings. Performances in the 2 parts of the trail making test were associated with DRD4 genotypes. Participants carrying 2 copies of the 7-repeat allele exhibited longer response times, independently of diagnostic status. For part A, an additive effect of the 7-repeat allele was suggested, whereas a recessive effect was found for this allele in part B. Moreover, with the use of logistic regression analyses, part A response time seemed to mediate part of the effects of DRD4 on ADHD status, whereas part B response time tended to moderate these effects. Recently, Kieling and colleagues37 reported that probands with 1 or more 7-repeat alleles showed more commission errors on the CPT, whereas 4-repeat homozygosity was associated with reduced commission and omission errors.
Finally, 1 study did not identify any differences in cognitive performance in relation to DRD4 genotype. Barkley and colleagues,38 in a longitudinal study, reported no differences between ADHD adolescents with and without the 7-repeat allele on the matched familiar figures test, the Gordon diagnostic system and the Wisconsin card sorting test.
Apart from the variable number tandem repeat polymorphism, Bellgrove and colleagues34 examined the association of 2 additional polymorphisms located within the promoter region of the DRD4 gene to sustained attention to response task scores in children with ADHD. Probands' homozygosity for the A allele at –521 single nucleotide polymorphism (SNP) was associated with greater reaction time variability. Moreover, family-based analysis showed that higher errors in a composite score of commission and omission errors predicts transmission of the A allele from heterozygous parents to affected children. For the –616 SNP, there was no effect on sustained attention to response task scores.
In summary, with regard to the 7-repeat allele, the CPT and derived tasks (sustained attention to response task, test of variables of attention and Gordon diagnostic system) were the most used cognitive tests. Association of high reaction time variability with the 7-repeat allele absence appears to be the most consistent result and seems to be specific to ADHD. Speed of processing (trail making test part A), set shifting (trail making test part B) and cognitive impulsiveness (matched familiar figures test) were less frequently investigated but seem to be altered in the 7-repeat allele carriers. No effect of genotype was found on response inhibition (the stop and go/no-go tasks) (Table 1).
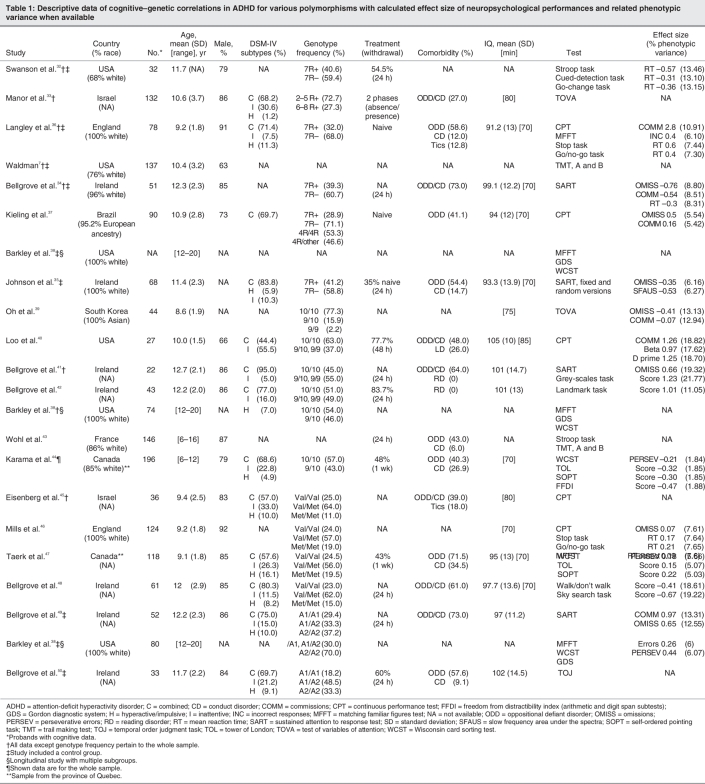
Table 1
Dopamine transporter (DAT1)
The DA transporter (DAT1) gene harbours a 40-bp repeat sequence variable number tandem repeat polymorphism located in the 3′-untranslated region.51 The most common alleles are the 10- (480-bp, 71.9%) and 9-repeat (440-bp, 23.4%) alleles,52 although these frequencies are variable from one population to the next.53 Homozygosity for the 10-repeat allele was reported to be associated with higher DA transporter protein in the striatum,54,55 a region where it is heavily expressed56 and where it serves as the primary means of DA reuptake.57 Several studies have suggested a relation between ADHD and the DAT1 variable number tandem repeat, the 10-repeat allele being the risk allele; however, a roughly equal number of studies have failed to detect such a relation.4 In a review by Faraone and colleagues,3 the pooled OR associated with the 10-repeat allele in family-based association studies was estimated to be 1.13 (95% CI 1.03–1.24).
Loo and colleagues40 reported that children carrying 2 copies of the 10-repeat allele exhibit higher commission errors, impulsive responses (β score) and reaction time variability compared with carriers of the 9-repeat allele. Bellgrove and colleagues,41 who used a CPT–like task (the sustained attention to response task), found higher reaction time variability in children who were homozygous for the 10-repeat allele. However, in this study, the 2 groups did not differ in omission and commission errors.
In contrast, Oh and colleagues39 reported fewer omission errors in the test of variables of attention in patients with 2 copies of the 10-repeat allele compared with carriers of 1 copy. No significant differences were observed between the 2 groups with regard to commission errors, reaction times or reaction time variability. Recently, Barkley and colleagues38 reported that 2 groups of adolescents with ADHD (homozygous or heterozygous for the 10-repeat allele), did not differ in performance on the Gordon diagnostic system, a continuous performance–like test. Additionally, it was found that controls who were heterozygous for the 10-repeat allele made more omission errors compared with homozygous controls.
Spatial attentional asymmetry has been described in children with ADHD.58–60 Bellgrove and colleagues,42 using the grey-scales task, reported that probands with 2 copies of the 10-repeat allele showed an attenuated spatial asymmetry, whereas heterozygous children showed the typical leftward attentional asymmetry. In a second study with an extended sample, they reported that left-sided inattention (measured by the landmark task) was associated with the 10-repeat allele.41 The authors also reported that the landmark asymmetry index predicted biased transmission of the 10-repeat allele from parents to affected children.
Finally, some indices of executive functions (Wisconsin card sorting test, trail making test and Stroop test) appear not to be modulated by the DAT1 variable number tandem repeat polymorphism,38,43 whereas better performance on other indices such as the tower of London, the self-order pointing task and the Weschler intelligence scale for children–III arithmetic and digit span subtests was reported to be associated to the 10/10 genotype compared with the 9/10 genotype.44 Measures of cognitive impulsiveness (matched familiar figures test) seem not to be modulated by the DAT1 variable number tandem repeat polymorphism.38
In summary, 4 studies provide conflicting results in relation to omission and commission errors from CPT and derived tasks (sustained attention to response task, test of variables of attention and Gordon diagnostic system). Interestingly, high reaction time variability seems to be the most replicated cognitive marker associated with 10-repeat homozygosity. Interesting results regarding spatial attentional asymmetry and some executive tasks have been reported but need replication (Table 1).
Catechol-O-methyltransferase (COMT)
The human COMT gene has been localized to chromosomal region 22q11.1-q11.2. Studies focused on a functional SNP in exon 4 (472G/A) that leads to an amino acid substitution (valine → methionine) at position 108 or 158 of the coding sequence of the soluble and the membrane-bound COMT, respectively.61 Homozygosity for methionine leads to a 3- to 4-fold reduction in COMT activity compared with homozygosity for valine.61 Given that DAT1 may play a reduced role in the control of synaptic DA within the prefrontal cortex (PFC),62–65 it has been suggested that the variation of COMT activity may modulate largely synaptic availability of DA in the PFC. A recent meta-analysis concluded that there is a small but significant relation between Val158Met genotype (Val as risk allele) and executive function (measured by Wisconsin card sorting test) in healthy individuals (d = 0.29, 95% CI 0.02–0.55). However, pooled studies of participants with schizophrenia were not significant (d = –0.07, 95% CI –0.4 to 0.26).66
In ADHD, 2 studies examined the association between this polymorphism and performance on a set of cognitive tasks known to tap into the PFC. The first study examined the Val158Met polymorphism in 124 children and used the matched familiar figures test, the CPT and the stop and go/no-go tasks.46 The second study genotyped the Val108/158Met polymorphism in 118 children and obtained cognitive scores from the Wisconsin card sorting test, the tower of London and the self-order pointing task evaluating a range of executive functions including working memory, planning and set shifting.47 Both studies suggested that in children with ADHD there were no effects of COMT polymorphism on neurocognitive function, especially executive function. A third study conducted by Bellgrove and colleagues48 analyzed sustained attention capacity (estimated from performance on 2 subtests of the “test of everyday attention for children”) in relation to COMT genotype. Unexpectedly, impairment in sustained attention was found to be pronounced in children carrying at least 1 copy of the Met allele. No distortion in the transmission of COMT gene variants from parents to affected children was found. Given that performances on tasks mediated by the PFC can be impaired by both hypo-and hyper-dopaminergic states,67 it was hypothesized that the slower clearance of DA associated with the Met allele of the COMT gene may be disadvantageous to cognition in ADHD.48 Finally, a family-based study conducted by Eisenberg and colleagues45 showed a trend for an association when transmission of the COMT Val allele was examined in probands who scored better than the 50th percentile on the CPT commission errors score. Only participants with the Met/Met genotype had markedly fewer commission errors, whereas no significant differences were observed between Val/Val and Val/Met genotypes. Moreover, the association was significant when transmission of the COMT Val allele was examined in probands selected on the basis of clinical severity (score on the Conners teaching rating hyperactivity scale better than the 50th percentile) or when probands with inattentive type (DSM-IV criteria) were excluded (Table 1).
Dopamine β-hydroxylase (DBH)
The DBH gene located on chromosome 9q3468 encodes an enzyme that catalyzes the conversion of DA into norepinephrine and is particularly expressed in the PFC.69 A –1021 C/T polymorphism was reported to be responsible for up to 50% of the variation of plasma DBH activity.70 However, it is another polymorphism in intron 5 (the 5′ TaqI polymorphism) that has been often tested in clinical samples of patients with ADHD with consistent findings. In fact, a meta-analysis by Faraone and colleagues3 suggested a significant association between ADHD and the 5′ TaqI polymorphism. When the family-based studies are pooled, the OR is estimated to be 1.33 (95% CI 1.11–1.59).
Anomalies in the temporal allocation of visual attention have been reported in ADHD.71,72 Using a temporal order judgment task, Bellgrove and colleagues49 reported that children with ADHD were impaired in allocating attention to visual targets that appeared in close temporal proximity (50 ms) compared with controls. The ADHD probands who were homozygous for the A2 allele exhibited poorer performances on this task than noncarriers of this allele. Employing a logistic regression extension of the transmission disequilibrium test, the authors also found that performance on this task predicted distorted transmission of A2 alleles from parents to affected children. In a second study, children possessing 2 copies of the A2 allele had significantly more commission and omission errors and greater reaction time variability (as assessed by the sustained attention to response task) than those who did not carry this allele.50
Barkley and colleagues38 reported neuropsychological correlates of the DBH TaqI polymorphism in a large group of adolescents with ADHD and a matched control group. Comparisons showed that genotype (2 copies of A2 v. 1 or 0 copies) had no effect on any measures in the control group. In the ADHD group, participants who were homozygous for the A2 allele made more errors on the Wisconsin card sorting test (problem-solving) and the matched familiar figures test (cognitive impulsiveness).
Apart form the TaqI polymorphism, 1 study examined the association between the –1021 C/T polymorphism and executive function, as reflected by a composite measure (CPT and Wisconsin card sorting test) in children and adolescents with ADHD.73 The CC genotype was associated with a diminished performance (Table 1).
Monoamine oxidase A (MAOA)
Several studies have suggested a relation between ADHD and alleles of the MAOA gene.4 The 30-bp variable number tandem repeat 1.2 kb polymorphism was studied in relation to cognitive performance in ADHD. This polymorphism has alleles with 2, 3, 3.5, 4 and 5 repeats.74 The 2- and 3-repeat alleles have been associated with low transcriptional efficiency of the gene and with impulsivity and aggressive behaviour.75,76 Manor and colleagues74 examined the role of this polymorphism in the test of variables of attention in a population of 112 children with ADHD. They found that participants carrying the long MAOA alleles (4- and 5-repeat) made more commission errors than those without the alleles. This association was markedly attenuated after administration of methylphenidate. More recently, 7 SNPs in a region spanning 31 kb from intron 5 to the 3′UTR were reported to be significantly associated with ADHD, but were independent from IQ level.77
Dopamine receptor 5 (DRD5)
The DRD5 gene consists of a single exon on chromosome 4. Two recent meta-analyses confirmed that a polymorphic microsatellite without a known functional significance confered a small but significant risk for ADHD.78,79 An association was observed between the 148-bp repeat allele and 4 variables of the test of variables of attention (commission errors, omission errors, reaction times and reaction time variability).80 However, the authors reported these findings with caution and recommended independent replication.
Adrenergic receptor 2 (ADRA2A)
Initial studies did not identify an association between ADHD and the MspI polymorphism of the ADRA2A gene.81–84 However, a study by Park and colleagues85 found a significant association between ADHD and 2 SNPs, one in the promoter and another in the 3′ untranslated region. Waldman and colleagues86 investigated a set of executive measures in relation to polymorphisms of the ADRA2A gene in the sample of ADHD children studied by Park and colleagues.85 The promoter region polymorphism (MspI) was found to be associated and linked with performances on tower of London and trail making tests and with reaction time variability on the stop signal task, whereas the 3′ untranslated region SNP (DraI) was associated with trail making time scores.
Glutamate receptor, ionotropic, N-methyl-D-aspartate (GRIN2A)
The GRIN2A gene is located in the16p13 locus that was linked to ADHD.87 A family-based study reported a significant association between ADHD and a GRIN2A polymorphism (Grin2a–5).88 In contrast to these findings, a family-based study did not identify any evidence supporting the association of 4 polymorphisms (including Grin2a–5, all without known functional consequence) and ADHD.89 Equally, no evidence for association between any of these GRIN2A polymorphisms and cognitive phenotypes of inhibitory control (stop task), verbal short-term memory (forward digit span) and verbal working memory (backward digit span) were observed.
Trypotphan hydroxylase 2 (TPH2)
The TPH2 gene encodes the rate-limiting enzyme in the synthesis of serotonin in humans and was shown to be associated with completed suicide90 and major depression.91 Four studies have shown an association between multiple SNPs in this gene and ADHD.92–95 Recently, a significant association was observed between total errors of omission in the test of variables of attention and 2 SNPs (rs1386488, rs6582072).95 Similarly, a significant association was observed between rs1386488 and another SNP (rs1386497) and total reaction time scores as well as total reaction time variability scores. These intronic SNPs are part of 8 markers found to have strong linkage disequilibrium with each other and that compose a single haplotype block associated with ADHD in the tested sample. A second study tested IQ level and 4 SNPs previously reported to be associated with ADHD, but analyses were negative.77
Brain-derived neurotropic factor (BDNF)
The BDNF gene was suggested to play an important role in the pathophysiology of several psychiatric disorders. Particularly, the Val66Met polymorphism was implicated in hippocampal function, as reflected by reports of associations with structural and functional measures.96 In children with ADHD, although an initial study reported an association of this polymorphism with a preferential transmission of the valine allele,97 5 studies failed to replicate this finding.98–102 Verbal short-term memory and working memory, as evaluated by the digit span test, were also examined and yielded a negative finding.99
Gene–gene interactions
Additive or interactive effects of 2 or more genes on neuropsychological traits pertinent to ADHD have been reported, especially for polymorphisms of the DRD4 (a copy of the 7-repeat allele as a risk genotype) and DAT1 (homozygosity for the 10-repeat allele as a risk genotype) genes. In fact, it was speculated that the combination of these 2 risk genotypes may lead to an extreme hypodopaminergic state correlated with poor cognitive function. This assumption was evidenced by findings from a longitudinal epidemiologic investigation of 2 independent birth cohorts in which children presenting with ADHD symptoms and carrying both dopaminergic risk genotypes scored an average of 8.2 IQ points lower than children with no risk genotypes.103 However, 2 association studies implicating children with clinically diagnosed ADHD failed to replicate such influence of DAT1 and DRD4 polymorphisms on IQ performance.77,104
Discussion
Although associations between polymorphisms of different candidate genes and ADHD have previously been reported, the functional consequences of allelic variation within these genes remain uncertain. It has been suggested that many of these genes may contribute to neuropsychological deficits observed in children with ADHD. The approach of linking a genetic risk factor to an alternative or intermediate neuropsychological phenotype has led to a growing interest in the last few years. Since 1999, about 30 studies mostly examining 4 candidate genes (DAT1, DRD4, COMT and DBH) in relation to the neurocognitive phenotypes relevant for ADHD have been published.
Limitations
Our review had some limitations. Although we aimed to perform a formal meta-analysis, this was not possible because the number of studies using the same neurocognitive endophenotypes was limited. This field of research has multiple approaches that can be attributed to various neuropsychological parameters relevant in ADHD. We referred only minimally to the various theoretical frameworks in this field of research; more detail would have been beyond the scope of our systematic review. To qualify as endophenotypes, neuropsychological deficits in patients with ADHD have to meet a number of criteria, including heritability, independence from fluctuation in behavioural manifestations of the disorder over time, cosegregation with the illness within families and higher occurrence in nonaffected family members than in the general population.6 Although the approach of using endophenotypes to improve genetic studies has been widely publicized in the recent psychiatric genetic literature, several potential limitations have to be considered when applying this approach. One of the major problems is that using endophenotypes without evidence of familiality can lead to an overanalysis of the data and findings that do not make biological sense.105 Furthermore, measurement errors and/or the presence of confounding factors may limit the capacity of reaching reliable findings. Measurement errors in behavioural research may arise from important within-subject variation in performance. This problem may be particularly crucial in ADHD because patients with this disorder are characterized by important reaction time variability of neuropsychological performance.5 Thus, it is advisable to determine the test–retest reliability of the proposed neuropsychological tasks used in genetic studies of ADHD.106 Alternatively, this increased variability is considered by some authors to be the most consistent neuropsychological abnormality in ADHD across many reaction time–based tasks and might reflect a unitary construct.107 Under the assumption of high test–retest variability is an intrinsic characteristic of ADHD, this trait could be targeted by genetic studies, although more genetic epidemiological studies are needed to confirm its genetic underpinnings.
Furthermore, confounding factors may participate in variation of the intermediate phenotype through nongenetic factors. For example, it has been shown that maternal smoking during pregnancy is a risk factor for both ADHD and executive dysfunctions in offspring.108,109 Failure to take these confounding factors into account may result in spurious findings and prevent replicating results from one study to another.
Another important issue in this field of research is that different aspects of human cognition mature at different ages.110 Given that examined populations vary widely in age, it is possible that this variable explains part of the discrepancies between studies. Davidson and colleagues111 reported that performance in cognitive flexibility task progresses developmentally and does not reach adult levels in 13-year-old children. Moreover, tasks sensitive to executive function at a young age may become too simple and automated to reflect executive processes in older individuals.112 Furthermore, these dynamic changes in cognitive process over time may be supported by changes in monoamine metabolism, as suggested by experimental data from animal and human studies.113,114 For example, in rats, COMT activity is correlated with age.115,116 Thus, it is possible that the role of COMT in the catabolism of DA is developmentally regulated, with children relying less on this catabolic pathway than adults. Conversely, it has been reported that DAT1 density is inversely correlated with age.117 It is therefore possible that DA metabolism relies more on DAT1 than on COMT activity in children compared with adults. These developmentally dynamic changes in the activity/expression of key proteins involved in monoamine metabolism, compounded with their different brain distributions and differential involvement in multiple functions (e.g., tonic and phasic DA regulation) highlight the importance of a study sample with a narrow age range.
Another factor that needs to be taken into consideration is sex. It is very well established that the clinical expression of ADHD differs between males and females.118 Although differences in neuropsychological profiles between males and females with ADHD have not been widely studied, it possible that the difference in symptoms is at least in part secondary to different neuropsychological abnormalities. Furthermore, it has been shown in animal models that the behavioural consequences of gene defects are expressed differently between males and females.119 For example, it was shown that the frequencies of the Val/Met alleles are different between males and females in an Israeli population.45 Also, Qian and colleagues120 showed that the association/ linkage between COMT alleles and ADHD may depend on sex. Assuming that these differential allelic effects are true, it is evident that the results of studies may vary widely because of the proportion of males and females included in each study.
Another factor that might be important to take into consideration while interpreting these studies is pharmacological treatment. Indeed, in most studies, children with ADHD were receiving long-term stimulant medications that were withdrawn for at least 24 hours. However, animal studies indicate that withdrawal of chronic stimulant treatment may lead to decreased DA neuronal firing.121 Few studies reviewed here comprised medication-naive participants,36,37,73 whereas some others included a subgroup of medication-naive children without showing comparisons to receiving stimulant medication.32,35,40,42,44,47,50 Interestingly, in 2 studies with test sessions before and after stimulant administration, the genetic–cognitive correlations disappeared15 or were markedly attenuated39 after the administration of methylphenidate (0.3 mg/kg). This observation could exemplify the neutralization of a small genetic effect on cognition by a large dopaminergic tone induced by treatment.
Some disorders frequently associated with ADHD might interfere with the measurement of neuropsychological performances. For example, reading disability and ADHD co-occur in about 15%–40% of patients,122 and it is possible that the 2 conditions interact to shape neuropsychological performances of affected individuals. Thus, controlling for such learning disabilities might also be a critical issue.
Correlations between polymorphisms of the candidate genes and neuropsychological measures were performed frequently following a case–control association design in which the behavioural phenotype was serving as the dependent variable and the genotypes (or the number of high-risk alleles) for the candidate gene as a categorical explanatory variable. Results drawn from this type of analysis can suffer from bias owing to the possibility of population stratification. An interesting alternative, when parent/offspring trios are available, is the use of quantitative extensions of the transmission disequilibrium test (general test123 and logistic regression124), which might help to determine whether cognitive measures could predict distorted parental transmission of high-versus low-risk alleles to ADHD probands. However, including a control group is highly recommended. In fact, ensuring sensitivity and specificity of the neuropsychological measures to ADHD is an important issue that appears to establish differences between the clinical and control groups.
Finally, sample sizes of most of the studies we reviewed were small. Recent reviews of the association between the COMT Val/Met polymorphism and executive function suggest that the effect of this polymorphism may be very modest and that very large sample sizes (> 1000 participants) are needed to reliably detect such an effect.125 Thus, some negative findings could be attributed to a lack of statistical power, and positive results should be considered preliminary until they are replicated in extended samples.
Meta-analyses of candidate genes for susceptibility to ADHD yielded ORs ranging from 1.13 to 1.44, which represent small genetic effects.3 It was suggested that for an additive genetic model, the proportion of phenotypic variance explained by the associated genes is about 3.2%.126 Under the assumption that endophenotypes are less complex than the clinical syndrome of ADHD and that they have more tractable genetic underpinnings, it may be expected that the variance in endophenotypes that is explained by variation in candidate genes should be higher than 3.2%. In our review, whenever possible, we provided the effect sizes and the percentage of variance in endophenotypes explained by a number of candidate genes. The latter varied from 5% to 19%. Although it may be concluded that these proportions of variances in endophenotypes explained by candidate genes are higher than the proportions of variances in ADHD, this conclusion may be too premature given that studies assessing the same gene and the same endophenotype were scant and prevented any reliable estimation of the effect sizes, which are often overestimated in studies first published. Statistical aspects aside, approaches associating intermediate phenotypes to genetic variants are undeniably valuable in bridging the gap between genes and the clinical symptoms of ADHD.
Conclusion
Despite the promises raised by the use of endophenotypes in the genetic research in ADHD, the findings reported until recently have been poorly replicated. Methodological issues related to the neuropsychological phenotype of ADHD, measurement errors, developmental variation of cognition, sex effect, action of stimulant treatment and the presence of comorbid conditions represent potential sources of confusion. Other factors such as population stratification and small effect sizes that are common to genetic association studies contribute to the problem. Until much larger studies with optimal control of confounding factors are conducted, usefulness of neuropsychological endophenotypes in ADHD cannot be truly assessed.
Footnotes
Contributors: Drs. Kebir, Tabbane and Joober designed the study. Dr. Kebir acquired the data, which all authors analyzed. Drs. Kebir and Joober wrote and reviewed the article, which Drs. Tabbane and Sengupta also reviewed. All authors provided approval for publication.
Competing interests: None declared for Drs. Kebir, Tabbane and Sengupta. Dr. Joober is a paid consultant with Janssen Canada and Pfizer Canada and has received educational grants from Janssen Canada and AstraZeneca Canada.
Correspondence to: Dr. R. Joober, Douglas Mental Health University Institute and McGill University, 6875 boul. Lasalle, Montréal QC H4H 1R3; fax 514 888-4064; ridha.joober@douglas.mcgill.ca
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 1994.
- 2.Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet 2005;366:237-48. [DOI] [PubMed]
- 3.Faraone SV, Perlis RH, Doyle AE, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry 2005;57:1313-23. [DOI] [PubMed]
- 4.Waldman ID, Gizer IR. The genetics of attention deficit hyperactivity disorder. Clin Psychol Rev 2006;26:396-432. [DOI] [PubMed]
- 5.Castellanos FX, Tannock R. Neuroscience of attention-deficit/ hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci 2002;3:617-28. [DOI] [PubMed]
- 6.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry 2003;160:636-45. [DOI] [PubMed]
- 7.Waldman ID. Statistical approaches to complex phenotypes: evaluating neuropsychological endophenotypes for attention-deficit/hyperactivity disorder. Biol Psychiatry 2005;57:1347-56. [DOI] [PubMed]
- 8.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 1997;121:65-94. [DOI] [PubMed]
- 9.Willcutt EG, Doyle AE, Nigg JT, et al. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry 2005;57:1336-46. [DOI] [PubMed]
- 10.Sergeant JA. Modeling attention-deficit/hyperactivity disorder: a critical appraisal of the cognitive-energetic model. Biol Psychiatry 2005;57:1248-55. [DOI] [PubMed]
- 11.Andreou P, Neale BM, Chen WAI, et al. Reaction time performance in ADHD: improvement under fast-incentive condition and familial effects. Psychol Med 2007;37:1703-15. [DOI] [PMC free article] [PubMed]
- 12.Sonuga-Barke EJS. Causal models of attention-deficit/ hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol Psychiatry 2005;57:1231-8. [DOI] [PubMed]
- 13.Holmes J, Hever T, Hewitt L, et al. A pilot twin study of psychological measures of attention deficit hyperactivity disorder. Behav Genet 2002;32:389-95. [DOI] [PubMed]
- 14.Myles-Worsley M, Coon H. Genetic and developmental factors in spontaneous selective attention: a study of normal twins. Psychiatry Res 1997;71:163-74. [DOI] [PubMed]
- 15.Murphy KR, Barkley RA. Parents of children with attention-deficit/hyperactivity disorder: psychological and attentional impairment. Am J Orthopsychiatry 1996;66:93-102. [DOI] [PubMed]
- 16.Asarnow RF, Nuechterlein KH, Subotnik KL, et al. Neurocognitive impairments in nonpsychotic parents of children with schizophrenia and attention-deficit/hyperactivity disorder: the University of California, Los Angeles Family Study. Arch Gen Psychiatry 2002;59:1053-60. [DOI] [PubMed]
- 17.Doyle AE, Willcutt EG, Seidman LJ, et al. Attention-deficit/hyperactivity disorder endophenotypes. Biol Psychiatry 2005;57:1324-35. [DOI] [PubMed]
- 18.Groot AS, de Sonneville LM, Stins JF, et al. Familial influences on sustained attention and inhibition in preschoolers. J Child Psychol Psychiatry 2004;45:306-14. [DOI] [PubMed]
- 19.Nigg JT, Blaskey LG, Stawicki JA, et al. Evaluating the endophenotype model of ADHD neuropsychological deficit: results for parents and siblings of children with ADHD combined and inattentive subtypes. J Abnorm Psychol 2004;113:614-25. [DOI] [PubMed]
- 20.Schachar RJ, Chen S, Logan GD, et al. Evidence for an error monitoring deficit in attention deficit hyperactivity disorder. J Abnorm Child Psychol 2004;32:285-93. [DOI] [PubMed]
- 21.Kuntsi J, Stevenson J. Psychological mechanisms in hyperactivity: II. The role of genetic factors. J Child Psychol Psychiatry 2001;42:211-9. [PubMed]
- 22.Pennington BF, Bennetto L, McAleer O, et al. Executive functions and working memory: theoretical and measurement issues. In: Lyon GR, Krasnegor NA, editors. Attention, memory and executive function. Baltimore (MD): Paul H. Brooks; 1996. p. 327-48.
- 23.Seidman LJ, Biederman J, Monuteaux MC, et al. Neuropsychological functioning in nonreferred siblings of children with attention deficit/hyperactivity disorder. J Abnorm Psychol 2000;109:252-65. [DOI] [PubMed]
- 24.Swan GE, Carmelli D. Evidence for genetic mediation of executive control: a study of aging male twins. J Gerontol B Psychol Sci Soc Sci 2002;57:P133-43. [DOI] [PubMed]
- 25.Ando J, Ono Y, Wright MJ. Genetic structure of spatial and verbal working memory. Behav Genet 2001;31:615-24. [DOI] [PubMed]
- 26.Stins JF, van Baal GC, Polderman TJ, et al. Heritability of Stroop and flanker performance in 12-year old children. BMC Neurosci 2004;5:49. [DOI] [PMC free article] [PubMed]
- 27.Fan J, Wu Y, Fossella JA, et al. Assessing the heritability of attentional networks. BMC Neurosci 2001;2:14. [DOI] [PMC free article] [PubMed]
- 28.Cooper H, Hedges L. The handbook of research synthesis. New York (NY): Russel Sage Foundation; 1994.
- 29.Petronis A, Van Tol HH, Lichter JB, et al. The D4 dopamine receptor gene maps on 11p proximal to HRAS. Genomics 1993;18:161-3. [DOI] [PubMed]
- 30.Li D, Sham PC, Owen MJ, et al. Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD). Hum Mol Genet 2006;15:2276-84. [DOI] [PubMed]
- 31.Asghari V, Sanyal S, Buchwaldt S, et al. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J Neurochem 1995;65:1157-65. [DOI] [PubMed]
- 32.Swanson J, Oosterlaan J, Murias M, et al. Attention deficit/ hyperactivity disorder children with a 7-repeat allele of the dopamine receptor D4 gene have extreme behavior but normal performance on critical neuropsychological tests of attention. Proc Natl Acad Sci U S A 2000;97:4754-9. [DOI] [PMC free article] [PubMed]
- 33.Manor I, Tyano S, Eisenberg J, et al. The short DRD4 repeats confer risk to attention deficit hyperactivity disorder in a family-based design and impair performance on a continuous performance test (TOVA). Mol Psychiatry 2002;7:790-4. [DOI] [PubMed]
- 34.Bellgrove MA, Hawi Z, Lowe N, et al. DRD4 gene variants and sustained attention in attention deficit hyperactivity disorder (ADHD): effects of associated alleles at the VNTR and –521 SNP. Am J Med Genet B Neuropsychiatr Genet 2005;136B:81-6. [DOI] [PubMed]
- 35.Johnson KA, Kelly SP, Robertson IH. Absence of the 7-repeat variant of the DRD4 VNTR is associated with drifting sustained attention in children with ADHD but not in controls. Am J Med Genet B Neuropsychiatr Genet. In press. [DOI] [PubMed]
- 36.Langley K, Marshall L, van der Bree M, et al. Association of the dopamine D4 receptor gene 7-repeat allele with neuropsychological test performance of children with ADHD. Am J Psychiatry 2004;161:133-8. [DOI] [PubMed]
- 37.Kieling C, Roman T, Doyle AE, et al. Association between DRD4 gene and performance of children with ADHD in a test of sustained attention. Biol Psychiatry 2006;60:1163-5. [DOI] [PubMed]
- 38.Barkley RA, Smith KM, Fisher M, et al. An examination of the behavioral and neuropsychological correlates of three ADHD candidate gene polymorphisms (DRD47+, DBH Taq1 A2, and DAT1 40 bp VNTR) in hyperactive and normal children followed to adulthood. Am J Med Genet B Neuropsychiatr Genet 2006;141B:487-98. [DOI] [PMC free article] [PubMed]
- 39.Oh KS, Shin DW, Oh GT, et al. Dopamine transporter genotype influences the attention deficit in Korean boys with ADHD. Yonsei Med J 2003;44:787-92. [DOI] [PubMed]
- 40.Loo SK, Specter E, Smolen A, et al. Functional effects of the DAT1 polymorphism on EEG measures in ADHD. J Am Acad Child Adolesc Psychiatry 2003;42:986-93. [DOI] [PubMed]
- 41.Bellgrove MA, Hawi Z, Kirley A, et al. Dissecting the attention deficit hyperactivity disorder (ADHD) phenotype: sustained attention, response variability and spatial attentional asymmetries in relation to dopamine transporter (DAT1) genotype. Neuropsychologia 2005;43:1847-57. [DOI] [PubMed]
- 42.Bellgrove MA, Hawi Z, Kirley A, et al. Association between dopamine transporter (DAT1) genotype, left-side inattention, and an enhanced response to methylphenidate in attention-deficit hyperactivity disorder. Neuropsychopharmacology 2005;30:2290-7. [DOI] [PubMed]
- 43.Wohl M, Boni C, Asch M, et al. Lack of association of the dopamine transporter gene in a French ADHD sample. Am J Med Genet B Neuropsychiatr Genet 2008;147B:1509-10. [DOI] [PubMed]
- 44.Karama S, Grizenko N, Sonuga-Barke EJS, et al. Dopamine transporter 3′UTR VNTR genotype is a marker of performance on executive function tasks in children with ADHD. BMC Psychiatry 2008;8:45. [DOI] [PMC free article] [PubMed]
- 45.Eisenberg J, Mei-Tal G, Steinberg A, et al. Haplotype relative risk study of catechol-O-methyltransferase (COMT) and attention deficit hyperactivity disorder (ADHD): association of the high-enzyme activity Val allele with ADHD impulsive-hyperactive phenotype. Am J Med Genet 1999;88:497-502. [PubMed]
- 46.Mills S, Langley K, Van den Bree M, et al. No evidence of association between catechol-O-methyltransferase (COMT) Val158Met genotype and performance on neuropsychological tasks in children with ADHD: a case-control study. BMC Psychiatry 2004;4:15. [DOI] [PMC free article] [PubMed]
- 47.Taerk E, Grizenko N, Ben Amor L, et al. Catechol-O-methyltransferase (COMT) Val108/158Met polymorphism does not modulate executive function in children with ADHD. BMC Med Genet 2004;5:30. [DOI] [PMC free article] [PubMed]
- 48.Bellgrove MA, Domschke K, Hawi Z, et al. The methionine allele of the COMT polymorphism impairs prefrontal cognition in children and adolescents with ADHD. Exp Brain Res 2005;163:352-60. [DOI] [PubMed]
- 49.Bellgrove MA, Mattingley JB, Hawi Z, et al. Impaired temporal resolution of visual attention and dopamine beta hydroxylase genotype in attention-deficit/hyperactivity disorder. Biol Psychiatry 2006;60:1039-45. [DOI] [PubMed]
- 50.Bellgrove MA, Hawi Z, Gill M, et al. The cognitive genetics of attention deficit hyperactivity disorder (ADHD): sustained attention as a candidate phenotype. Cortex 2006;42:838-45. [DOI] [PubMed]
- 51.Vandenbergh DJ, Persico AM, Hawkins AL, et al. Human dopamine transporter gene maps to chromosome 5p15.3 and displays a VNTR. Genomics 1992;14:1104-6. [DOI] [PubMed]
- 52.Doucette-Stamm LA, Blakely DJ, Tian J, et al. Population genetic study of the human dopamine transporter gene (DAT1). Genet Epidemiol 1995;12:303-8. [DOI] [PubMed]
- 53.Mitchell RJ, Howlett S, Earl L, et al. Distribution of the 3′ VNTR polymorphism in the human dopamine transporter gene in world populations. Hum Biol 2000;72:295-304. [PubMed]
- 54.Heinz A, Goldman D, Jones DW, et al. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology 2000;22:133-9. [DOI] [PubMed]
- 55.Cheon KA, Ryu YH, Kim JW, et al. The homozygosity for 10-repeat allele at dopamine transporter gene and dopamine transporter density in Korean children with attention deficit hyperactivity disorder: relating to treatment response to methylphenidate. Eur Neuropsychopharmacol 2005;15:95-101. [DOI] [PubMed]
- 56.Krause KH, Dresel SH, Krause J, et al. The dopamine transporter and neuroimaging in attention deficit hyperactivity disorder. Neurosci Biobehav Rev 2003;27:605-13. [DOI] [PubMed]
- 57.Garris PA, Wightman RM. Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: an in vivo voltammetric study. J Neurosci 1994;14:442-50. [DOI] [PMC free article] [PubMed]
- 58.Carter CS, Krener P, Chaderjian M, et al. Asymmetrical visual-spatial attentional performance in ADHD: evidence for a right hemispheric deficit. Biol Psychiatry 1995;37:789-97. [DOI] [PubMed]
- 59.Nigg JT, Swanson JM, Hinshaw SP. Covert visual spatial attention in boys with attention deficit hyperactivity disorder: lateral effects, methylphenidate response, and results for parents. Neuropsychologia 1997;35:165-76. [DOI] [PubMed]
- 60.McDonald S, Bennett KM, Chambers H, et al. Covert orienting and focusing of attention in children with attention deficit hyperactivity disorder. Neuropsychologia 1999;37:345-56. [DOI] [PubMed]
- 61.Lachman HM, Papolos DF, Saito T, et al. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 1996;6:243-50. [DOI] [PubMed]
- 62.Sesack SR, Hawrylak VA, Guido MA, et al. Cellular and subcellular localization of the dopamine transporter in rat cortex. Adv Pharmacol 1998;42:171-4. [DOI] [PubMed]
- 63.Dougherty DD, Bonab AA, Spencer TJ, et al. Dopamine transporter density in patients with attention deficit hyperactivity disorder. Lancet 1999;354:2132-3. [DOI] [PubMed]
- 64.Lewis DA, Melchitzky DS, Sesack SR, et al. Dopamine transporter immunoreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. J Comp Neurol 2001;432:119-36. [DOI] [PubMed]
- 65.Moron JA, Brockington A, Wise RA, et al. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci 2002;22:389-95. [DOI] [PMC free article] [PubMed]
- 66.Barnett JH, Jones PB, Robbins TW, et al. Effects of the catechol-O-methyltransferase Val158Met polymorphism on executive function: a meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Mol Psychiatry 2007;12:502-9. [DOI] [PubMed]
- 67.Arnsten AFT. Catecholamine modulation of prefrontal cortical cognitive function. Trends Cogn Sci 1998;2:436-47. [DOI] [PubMed]
- 68.Craig SP, Buckle VJ, Lamouroux A, et al. Localization of the human dopamine beta hydroxylase (DBH) gene to chromosome 9q34. Cytogenet Cell Genet 1988;48:48-50. [DOI] [PubMed]
- 69.Gaspar P, Berger B, Febvret A, et al. Catecholamine innervation of the human cerebral cortex as revealed by comparative immunohistochemistry of tyrosine hydroxylase and dopamine-beta-hydroxylase. J Comp Neurol 1989;279:249-71. [DOI] [PubMed]
- 70.Zabetian CP, Anderson GM, Buxbaum SG, et al. A quantitative-trait analysis of human plasma-dopamine beta-hydroxylase activity: evidence for a major functional polymorphism at the DBH locus. Am J Hum Genet 2001;68:515-22. [DOI] [PMC free article] [PubMed]
- 71.Hollingsworth DE, McAuliffe SP, Knowlton BJ. Temporal allocation of visual attention in adult attention deficit hyperactivity disorder. J Cogn Neurosci 2001;13:298-305. [DOI] [PubMed]
- 72.Li CS, Lin WH, Chang HL, et al. A psychophysical measure of attention deficit in children with attention-deficit/hyperactivity disorder. J Abnorm Psychol 2004;113:228-36. [DOI] [PubMed]
- 73.Kieling C, Genro JP, Hutz MH, et al. The -1021 C/T DBH polymorphism is associated with neuropsychological performance among children and adolescents with ADHD. Am J Med Genet B Neuropsychiatr Genet 2008;147B:485-90. [DOI] [PubMed]
- 74.Manor I, Tyano S, Mel E, et al. Family-based and association studies of monoamine oxidase A and attention deficit hyperactivity disorder (ADHD): preferential transmission of the long promoter-region repeat and its association with impaired performance on a continuous performance test (TOVA). Mol Psychiatry 2002;7:626-32. [DOI] [PubMed]
- 75.Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet 1998;103:273-9. [DOI] [PubMed]
- 76.Manuck SB, Flory JD, Ferrell RE, et al. A regulatory polymorphism of the monoamine oxidase-A gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsivity. Psychiatry Res 2000;95:9-23. [DOI] [PubMed]
- 77.Sonuga-Barke EJ, Brookes KJ, Buitelaar J, et al. Intelligence in DSM-IV combined type attention-deficit/hyperactivity disorder is not predicted by either dopamine receptor/transporter genes or other previously identified risk alleles for attention-deficit/ hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet 2008;147:316-9. [DOI] [PubMed]
- 78.Maher BS, Marazita ML, Ferrell RE, et al. Dopamine system genes and attention deficit hyperactivity disorder: a meta-analysis. Psychiatr Genet 2002;12:207-15. [DOI] [PubMed]
- 79.Lowe N, Kirley A, Hawi Z, et al. Joint analysis of DRD5 marker concludes association with ADHD confined to the predominantly inattentive and combined subtypes. Am J Hum Genet 2004;74:348-56. [DOI] [PMC free article] [PubMed]
- 80.Manor I, Corbex M, Eisenberg J, et al. Association of the dopamine D5 receptor with attention deficit hyperactivity disorder (ADHD) and scores on a continuous performance test (TOVA). Am J Med Genet B Neuropsychiatr Genet 2004;127B:73-7. [DOI] [PubMed]
- 81.Comings DE, Gade-Andavolu R, Gonzalez N, et al. Additive effect of three noradrenergic genes (ADRA2a, ADRA2C, DBH) on attention-deficit hyperactivity disorder and learning disabilities in Tourette syndrome subjects. Clin Genet 1999;55:160-72. [DOI] [PubMed]
- 82.Xu C, Schachar R, Tannok W, et al. Linkage study of the alpha2A adrenergic receptor in attention-deficit hyperactivity disorder families. Am J Med Genet 2001;105:159-62. [PubMed]
- 83.Comings DE, Gonzalez NS, Cheng Li SC, et al. A “line item” approach to the identification of genes involved in polygenic behavioral disorders: the adrenergic alpha2A (ADRA2A) gene. Am J Med Genet B Neuropsychiatr Genet 2003;118B:110-4. [DOI] [PubMed]
- 84.Roman T, Schmitz M, Polanczyk GV, et al. Further evidence for the association between attention deficit/hyperactivity disorder and the dopamine-beta-hydroxylase gene. Am J Med Genet 2002;114:154-8. [DOI] [PubMed]
- 85.Park L, Nigg JT, Waldman ID, et al. Association and linkage of alpha-2A adrenergic receptor gene polymorphisms with childhood ADHD. Mol Psychiatry 2005;10:572-80. [DOI] [PubMed]
- 86.Waldman ID, Nigg JT, Gizer IR, et al. The adrenergic receptor α-2A gene (ADRA2A) and neuropsychological executive functions as putative endophenotype for childhood ADHD. Cogn Affect Behav Neurosci 2006;6:18-30. [DOI] [PubMed]
- 87.Smalley SL, Kustanovich V, Minassian SL, et al. Genetic linkage of attention-deficit/hyperactivity disorder on chromosome 16p13, in a region implicated in autism. Am J Hum Genet 2002;71:959-63. [DOI] [PMC free article] [PubMed]
- 88.Turic D, Langley K, Mills S, et al. Follow-up of genetic linkage findings on chromosome 16p13: evidence of association of N-methyl-D-aspartate glutamate receptor 2A gene polymorphism with ADHD. Mol Psychiatry 2004;9:169-73. [DOI] [PubMed]
- 89.Adams J, Crosbie J, Ickowicz A, et al. Glutamate receptor, ionotropic, N-methyl D-aspartate 2A (GRIN2A) gene as a positional candidate for attention-deficit/hyperactivity disorder in the 16p13 region. Mol Psychiatry 2004;9:494-9. [DOI] [PubMed]
- 90.Zill P, Büttner A, Eisenmenger W, et al. Single nucleotide polymorphism and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene in suicide victims. Biol Psychiatry 2004;56:581-6. [DOI] [PubMed]
- 91.Zill P, Baghai TC, Zwanzger P, et al. SNP and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene provide evidence for association with major depression. Mol Psychiatry 2004;9:1030-6. [DOI] [PubMed]
- 92.Sheehan K, Lowe N, Kirley A, et al. Tryptophan hydroxylase 2 (TPH2) gene variants associated with ADHD. Mol Psychiatry 2005;10:944-9. [DOI] [PubMed]
- 93.Walitza S, Renner TJ, Dempfle A, et al. Transmission disequilibrium of polymorphic variants in the tryptophan hydroxylase-2 gene in attention-deficit/hyperactivity disorder. Mol Psychiatry 2005;10:1126-32. [DOI] [PubMed]
- 94.Brookes K, Xu X, Chen W, et al. The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: association signals in DRD4, DAT1 and 16 other genes. Mol Psychiatry 2006;11:934-53. [DOI] [PubMed]
- 95.Manor I, Laiba E, Eisenberg J, et al. Association between trypotphan hydroxylase 2, performance on a continuance performance test and response to methylphenidate in ADHD participants. Am J Med Genet B Neuropsychiatr Genet 2008;47B:1501-8. [DOI] [PubMed]
- 96.Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 2003;112:257-69. [DOI] [PubMed]
- 97.Kent L, Green E, Hawi Z, et al. Association of the paternally transmitted copy of common Valine allele of the Val66Met polymorphism of the brain-derived neurotrophic factor (BDNF) gene with susceptibility to ADHD. Mol Psychiatry 2005;10:939-43. [DOI] [PubMed]
- 98.Friedel S, Horro FF, Wermter AK, et al. Mutation screen of the brain derived neurotrophic factor gene (BDNF): identification of several genetic variants and association studies in patients with obesity, eating disorders, and attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet 2005;132B:96-9. [DOI] [PubMed]
- 99.Lee J, Laurin N, Crosbie J, et al. Association study of the brain-derived neurotropic factor (BDNF) gene in attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet 2007;144B:976-81. [DOI] [PubMed]
- 100.Schimmelmann BG, Friedel S, Dempfle A, et al. No evidence for preferential transmission of common valine allele of the Val66Met polymorphism of the brain-derived neurotrophic factor gene (BDNF) in ADHD. J Neural Transm 2007;114:523-6. [DOI] [PubMed]
- 101.Xu X, Mill J, Zhou K, et al. Family-based association study between brain-derived neurotrophic factor gene polymorphisms and attention deficit hyperactivity disorder in UK and Taiwanese samples. Am J Med Genet B Neuropsychiatr Genet 2007;144B:83-6. [DOI] [PubMed]
- 102.Ribasés M, Hervás A, Ramos-Quiroga JA, et al. Association study of 10 genes encoding neurotrophic factors and their receptors in adult and child attention-deficit/hyperactivity disorder. Biol Psychiatry 2008;63:935-45. [DOI] [PubMed]
- 103.Mill J, Caspi A, Williams BS, et al. Prediction of heterogeneity in intelligence and adult prognosis by genetic polymorphisms in the dopamine system among children with attention-deficit/ hyperactivity disorder. Arch Gen Psychiatry 2006;63:462-9. [DOI] [PubMed]
- 104.Genro JP, Roman T, Zeni CP, et al. No association between dopaminergic polymorphisms and intelligence variability in attention-deficit/hyperactivity disorder. Mol Psychiatry 2006;11:1066-7. [DOI] [PubMed]
- 105.Szatmari P, Maziade M, Zwaigenbaum L, et al. Informative phenotypes for genetic studies of psychiatric disorders. Am J Med Genet B Neuropsychiatr Genet 2007;144B:581-8. [DOI] [PubMed]
- 106.Kuntsi J, Andreou P, Ma J, et al. Testing assumptions for endophenotype studies in ADHD: reliability and validity of tasks in a general population sample. BMC Psychiatry 2005;5:40. [DOI] [PMC free article] [PubMed]
- 107.Klein C, Wendling K, Huettner P, et al. Intra-subject variability in attention-deficit hyperactivity disorder. Biol Psychiatry 2006;60:1088-97. [DOI] [PubMed]
- 108.Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol 2003;25:427-36. [DOI] [PubMed]
- 109.Julvez J, Ribas-Fito N, Torrent M, et al. Maternal smoking habits and cognitive development of children at age 4 years in a population-based birth cohort. Int J Epidemiol 2007;36:825-32. [DOI] [PubMed]
- 110.Doyle AE, Faraone SV, Seidman LJ, et al. Are endophenotypes based on measures of executive functions useful for molecular genetic studies of ADHD. J Child Psychol Psychiatry 2005;46:774-803. [DOI] [PubMed]
- 111.Davidson MC, Amso D, Anderson LC, et al. Development of cognitive control and executive functions from 4 to 13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia 2006;44:2037-78. [DOI] [PMC free article] [PubMed]
- 112.Denckla MB. A theory and model of executive function: A neuropsychological perspective. In: Lyon GR, Krasnegor NA, editors. Attention, memory and executive function (ch. 15). Baltimore (MD): Paul H. Brooks; 1996. p. 263-78.
- 113.Carlsson A, Winblad B. Influence of age and time interval between death and autopsy on dopamine and 3-methoxytyramine levels in human basal ganglia. J Neural Transm 1976;38:271-6. [DOI] [PubMed]
- 114.Lee JJ, Chang CK, Liu IM, et al. Changes in endogenous monoamines in aged rats. Clin Exp Pharmacol Physiol 2001;28:285-9. [DOI] [PubMed]
- 115.Venero JL, Machado A, Cano J. Turnover of dopamine and serotonin and their metabolites in the striatum of aged rats. J Neurochem 1991;56:1940-8. [DOI] [PubMed]
- 116.Galva MD, Bondiolotti GP, Olasmaa M, et al. Effect of aging on lazabemide binding, monoamine oxidase activity and monoamine metabolites in human frontal cortex. J Neural Transm Gen Sect 1995;101:83-94. [DOI] [PubMed]
- 117.Meng SZ, Ozawa Y, Itoh M, et al. Developmental and age related changes of dopamine transporter, and dopamine D1 and D2 receptors in human basal ganglia. Brain Res 1999;843:136-44. [DOI] [PubMed]
- 118.Biederman J, Mick E, Faraone SV, et al. Influence of gender on attention deficit hyperactivity disorder in children referred to a psychiatric clinic. Am J Psychiatry 2002;159:36-42. [DOI] [PubMed]
- 119.Gogos JA, Morgan M, Luine V, et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A 1998;95:9991-6. [DOI] [PMC free article] [PubMed]
- 120.Qian Q, Wang Y, Zhou R, et al. Family-based and case-control association studies of catechol-O-methyltransferase in attention deficit hyperactivity disorder suggest genetic sexual dimorphism. Am J Med Genet B Neuropsychiatr Genet 2003;118:103-9. [DOI] [PubMed]
- 121.Brandon CL, Marinelli M, White FJ. Adolescent exposure to methylphenidate alters the activity of rat midbrain dopamine neurons. Biol Psychiatry 2003;54:1338-44. [DOI] [PubMed]
- 122.Willcutt EG, Pennington BF. Comorbidity of reading disability and attention-deficit/hyperactivity disorder: differences by gender and subtype. J Learn Disabil 2000;33:179-91. [DOI] [PubMed]
- 123.Abecasis GR, Cardon LR, Cookson WO. A general test of association for quantitative traits in nuclear families. Am J Hum Genet 2000;66:279-92. [DOI] [PMC free article] [PubMed]
- 124.Waldman ID, Robinson BF, Rowe DC. A logistic regression based extension of the TDT for continuous and categorical traits. Ann Hum Genet 1999;63:329-40. [DOI] [PubMed]
- 125.Flint J, Munafo MR. The endophenotype concept in psychiatric genetics. Psychol Med 2007;37:163-80. [DOI] [PMC free article] [PubMed]
- 126.Kuntsi J, Neale BM, Chen W, et al. The IMAGE project: methodological issues for the moleculargenetic analysis of ADHD. Behav Brain Funct 2006;2:27. [DOI] [PMC free article] [PubMed]