Abstract
The treatment of schizophrenia for the last half century has been with dopamine (DA) D2 receptor blockers, implicating a hyperdopamine basis for psychosis. However, a 2007 report found that the glutamate agonist LY404039 was effective in schizophrenia, suggesting a hypoglutamate state for the illness. Although phencyclidine psychosis also supports a hypoglutamate cause, assessing the basic and clinical findings shows that phencyclidine has DA D2 agonist actions as well. Accurate Dreiding models of phencyclidine and the LY glutamate agonists precisely fit the known tetrahedral model of the D2 receptor that accommodates all DA agonists. A further view is that metabotropic glutamate agonists also exert D2 agonism, and their antipsychotic doses (about 100 mg/d) are predicted by their dissociation constants (about 20 nM) for D2. Hence, the clinical antipsychotic action of a glutamate agonist may depend on its ability to interfere with DA neurotransmission by its DA partial agonism.
Abstract
Depuis un demi-siècle, on traite la schizophrénie au moyen de bloqueurs des récepteurs dopaminergiques D2 sur la base des fondements hyperdopaminergiques de la psychose. Or, un rapport de 2007 a révélé que l'agoniste du glutamate LY404039 était efficace dans la schizophrénie, évoquant le rôle possible d'un déficit en glutamate dans la maladie. Bien que la psychose liée à la phencyclidine étaye également le rôle étiologique du déficit en glutamate, l'analyse des fondements et des signes cliniques révèle que la phencyclidine exerce également des actions dopaminergiques D2 agonistes. Des modèles de Dreiding précis de la phencyclidine et des agonistes du glutamate LY correspondent étroitement au modèle tétraédrique connu du récepteur D2, qui concorde avec tous les agonistes dopaminergiques. Selon une autre hypothèse, les agonistes métabotropiques du glutamate exercent aussi un agonisme D2 et leurs doses antipsychotiques (environ 100 mg/j) sont estimées en fonction de leurs constantes de dissociation (environ 20 nM) pour le D2. Ainsi, l'action antipsychotique clinique de l'agoniste du glutamate pourrait dépendre de sa capacité d'interférer avec la neurotransmission de la dopamine par l'entremise de son action dopaminergique agoniste partielle.
Introduction
The biological basis for psychotic signs and symptoms in schizophrenia is not known. Although abnormalities in several neurotransmitters have been found in the brains of patients with schizophrenia, much attention has focused on the roles of dopamine (DA) and glutamate neurotransmission underlying the disease. The purpose of this commentary is not to argue whether a DA or glutamate mechanism dominates the biology of psychosis, but rather to see to what extent DA mechanisms may underly glutamate drug action to develop a possible parsimony of antipsychotic drug action. In other words, this commentary is only one view, and its possible controversial nature is to explore and question the state of the art.
A main cornerstone for the DA basis of schizophrenia1,2 is the fact that antipsychotics alleviate psychotic signs and symptoms by inhibiting the action of DA on DA D2 receptors in accordance with the established relation between clinical doses and the antipsychotic affinities for D2 receptors. This correlation includes both the traditional antipsychotics, the recent so-called atypical antipsychotics, as well as the new partial DA agonists aripiprazole and bifeprunox.
At the same time, however, it has long been proposed that an underactivity of glutamate receptors may contribute to psychosis on the basis that phencyclidine, which blocks N-methyl-D-aspartate (NMDA) receptors, is a psychotogen. Thus, the hypoglutamate theory of schizophrenia advocates the stimulation of glutamate receptors to alleviate psychosis.3–5 Because a trial of the metabotropic glutamate receptor agonist LY404039 was clinically effective in treating schizophrenia,6 the present commentary examines the pharmacological selectivity of phencyclidine and the LY agonist congeners.
Pharmacological evidence for a hypoglutamate basis of psychosis
Phencyclidine action
Although there are DA agonist and antagonist drugs that are highly selective for DA D2 receptors, glutamate antagonists are not especially selective for glutamate receptors, which makes it difficult to test the possible glutamate basis for schizophrenia. Despite the nonselective actions of phencyclidine and the related compound ketamine, these glutamate antagonists have been extensively used to examine the possible hypoglutamate basis of schizophrenia.7,8
At the same time, however, there are many studies indicating that phencyclidine affects both the DA system and the glutamate system, influencing biology and behaviour. Specifically, phencyclidine activates presynaptic DA neurons because it causes ipsilateral turning in rats with hemilesioned nigral neurons and inhibits nigral neuron firing, both effects that are blocked by the DA D2 receptor-blocking action of haloperidol.9–11
These presynaptic actions of phencyclidine may be consistent with its possible action on presynaptic DA receptors of the high-affinity type (D2High), which are presumed to exist on DA nigral cell terminals and on prolactin-secreting cells in the anterior pituitary gland in addition to the known post-synaptic location of D2High receptors.
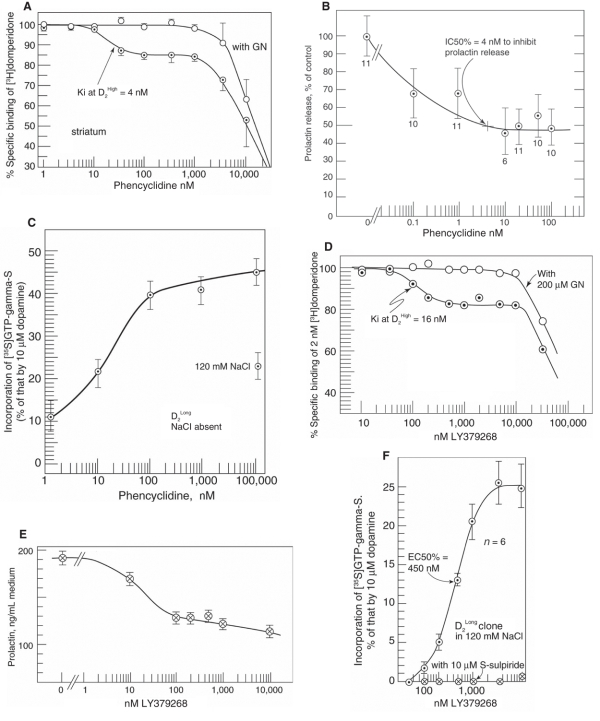
Although many experiments show that phencyclidine is an antagonist of ionotropic glutamate receptors, phencyclidine also has a DA-like partial agonist action on presynaptic or postsynaptic D2High receptors. For example, phencyclidine recognizes the functional DA D2High receptor, which is converted to its low-affinity state by guanylylimidodiphosphate (Fig. 1A),12,13 directly inhibits the release of prolactin in rat anterior pituitary cells in primary culture (Fig. 1B),14 and stimulates the incorporation of [35S]GTP-γ-S into human cloned DA D2 receptors (Fig. 1C).13
Fig. 1: Dopamine (DA)–like agonist actions of phencyclidine and LY379268. (A) Phencyclidine recognized the high-affinity state of the D2 receptor, D2High, as labelled by [3H]domperidone. The presence of 200 μM guanylylimidodiphosphate abolished this high-affinity component, indicating the agonist nature of this phencyclidine-recognized state (reproduced with permission from Seeman P, Guan H-C. Phencyclidine and glutamate agonist LY379268 stimulate DA D2High receptors: D2 basis for schizophrenia. Synapse 2008;62:819-28. ©2008 Wiley-Liss, Inc.13). (B) Phencyclidine inhibited the release of prolactin from anterior pituitary cells in culture (reproduced with permission from Seeman P, Lasaga M. Dopamine agonist action of phenyclidine. Synapse 2005;58:275-7. ©2005 Wiley-Liss, Inc.14). (C) Phencyclidine stimulated the incorporation of [35S]GTP-γ-S in human cloned DA D2Long receptors (in CHO cells) in the absence of NaCl (top line), as well as in the presence of 120 mM NaCl which was about 25% of that compared with the absence of NaCl (30 min incubation) (modified, with permission13). (D) The glutamate agonist LY379268 recognized the high-affinity state of the D2 receptor, D2High, as labelled by [3H]domperidone. The presence of 200 μM guanylylimidodiphosphate abolished this high-affinity component, indicating the agonist nature of this drug-recognized state (with permission from Seeman P, Caruso C, Lasaga M. Dopamine partial agonist actions of the glutamate receptor agonists LY354740 and LY379268. Synapse 2008;62:154-8. ©2008 Wiley-Liss, Inc.16). (E) LY379268 directly inhibited the secretion of prolactin from anterior pituitary cells in culture (with permission16). (F) LY379268 stimulated the incorporation of [35S]GTP-γ-S into DA D2Long receptors, an effect selectively blocked by 10 μM of the D2 antagonist S-sulpiride (with permission16).
The DA agonist nature of the 3 glutamate antagonists, phencyclidine, ketamine and dizocilpine (MK 801), is indicated by their ability to inhibit the binding of [3H]domperidone to D2 receptors in rat striatal tissue in the D2High concentration range with dissociation constants of 4 nM, 8 nM and 24 nM, respectively.13 For all 3 compounds, the presence of 200 μM guanylylimidodiphosphate removed the high-affinity phase of the competition of the compound with [3H]domperidone, indicating that phencyclidine, ketamine and dizocilpine would have agonist action at the high-affinity state of D2.
Although phencyclidine, ketamine and dizocilpine stimulate the incorporation of [35S]-GTP-γ-S into D2Long receptors, different laboratories can get differing results because this stimulation is markedly reduced by NaCl (Fig. 1C), especially above 120 mM, with D2Short being much more sensitive than D2Long to the inhibition by NaCl.13 For example, Odagaki and Toyoshima12 used 60-minute incubation and 100–150 mM NaCl in their assay, both conditions of which resulted in a complete loss of stimulation of [35S]GTP binding by phencyclidine on both D2Short and D2Long.13
Are metabotropic glutamate agonists selective for glutamate receptors?
The metabotropic glutamate receptor agonists LY379268 and LY354740 are agonists for metabotropic glutamate-2 and -3 receptors.15 These compounds, however, are also DA partial agonists on DA D2High receptors (Fig. 1D and Fig. 1F), interfere with the action of DA in vitro and inhibit the release of prolactin from rat isolated anterior pituitary cells in culture, as noted above (Fig. 1E).16
More recently, one of these LY glutamate agonists, namely LY404039 (in the form of an oral prodrug, LY2140023), has been used to treat schizophrenia.6 However, because LY404039 is essentially identical to LY379268, it appears reasonable to consider that LY404039 also has a DA partial agonist action, would interfere with DA neurotransmission and would, therefore, be clinically effective in treating schizophrenia.
Because LY404039 is not comercially available, it is difficult to study or confirm the alleged molecular actions of this particular compound, or even whether LY404039 has any partial AD agonist–like effects. Therefore, at this time the pharmacological selectivity of LY404039 remains to be confirmed.
Therefore, to investigate the neurochemical basis of schizophrenia, it is essential to consider whether glutamate group II agonists similar to LY379268 are selective. Woolley and colleagues17 and Fell and colleagues18 reported that phencyclidine evoked hyperactive behaviour in mGlu2 and mGlu3 receptor knockout mice and that the glutamate agonists LY379268 and LY404039 reduced the phencyclidine effect in mGlu3 receptor knockout mice but not in mGlu2 receptor knockout mice. Those 2 reports concluded that the target for the glutamate agonists was the mGlu2 receptor.
However, it should be noted that mGlu2 receptor knockout mice are supersensitive to DA, as indicated by their enhanced response to cocaine;19 that the functional D2High sites are markedly elevated in the striata of mGlu2 receptor knockout mice; and that D2 receptors in the striatum are 67-fold more DA sensitive to agonist stimulation in mGlu2 receptor knockout tissue (but only 4-fold more sensitive in the mGlu3 knockouts) compared with wild-type mouse striatal tissue.20
Therefore, considering that phencyclidine has a partial DA agonist action (Fig. 1), it is reasonable to expect a higher dose of the LY drug to inhibit the phencyclidine-induced ambulation of the mGlu2 receptor knockout mouse compared with the mGlu3 receptor knockout mouse. In other words, the phencyclidine dose–response curve would be right-shifted by the LY drugs in the mGlu2 receptor knockout mice but not in the mGlu3 receptor knockout mice.
Although LY379268 is an agonist at metabotropic glutamate receptors with dissociation constants of 15 nM,15 this compound also has similar dissociation constants of 20 to 30 nM at D2High receptors (Fig. 1). These data indicate that LY379268 would bind in vivo to both sets of receptors.
These dissociation constants at D2High for LY379268 and its closely related congeners (including LY35474016) would predict antipsychotic clinical daily doses of about 100 mg/d, as illustrated in Figure 2,21 in agreement with that used by Patil and colleagues.6,22
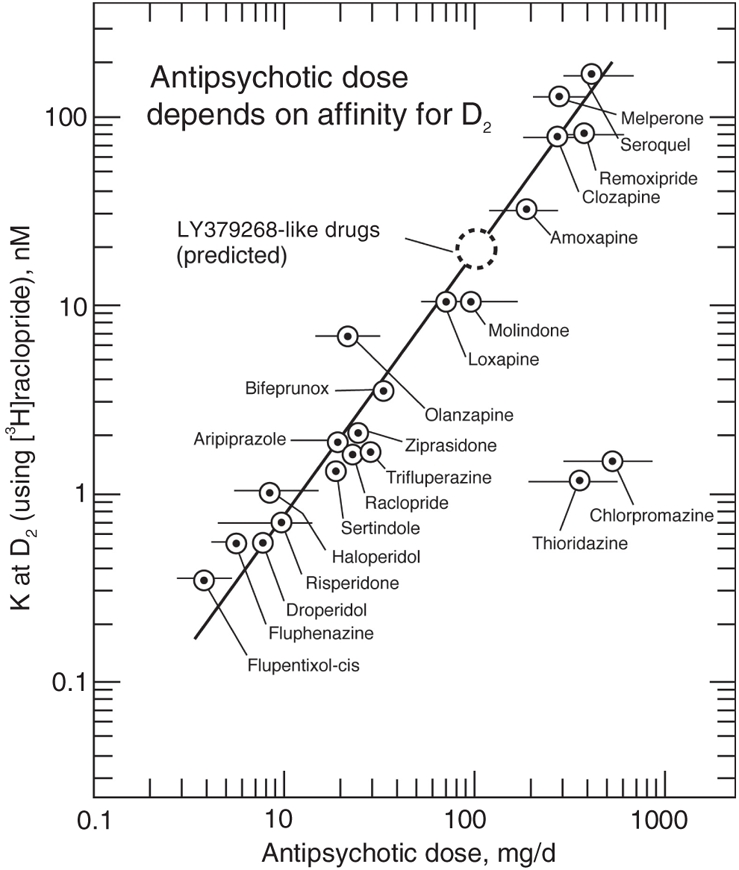
Fig. 2: The clinical doses of antipsychotic drugs correlate with their potencies (expressed as dissociation constants, Ki values) at the human cloned dopamine (DA) D2Long receptor. The doses of chlorpromazine and thioridazine appear disproportionately high, because at least 98% of these molecules bind to plasma proteins; when corrected for such plasma protein binding, the free concentrations of all the antipsychotics correlate even more closely to the drug concentrations (in the plasma water) that block DA D2 receptors. Note that the DA partial agonists, aripiprazole and bifeprunox, also fit the correlation when using human cloned D2Long receptors and [3H]raclopride. The glutamate receptor agonists LY354740 and LY379268 have DA partial agonist dissociation constants, KiHigh, of 20–40 nM at the functional DA D2High receptor, data predicting that compounds of this class would have an antipsychotic action at clinical doses of the order of 100 mg/d6 (modified, with permission from Seeman P. Dopamine and schizophrenia. Scholarpedia 2007;2:3634. Revision #3726921).
Phencyclidine and LY379268 attachment to D2High receptors in vivo
Because the DA D2 receptor is functional in its high-affinity state,13 D2High, and because low concentrations of (+)PHNO or radioactive (+)PHNO label D2High receptors,23 it is possible to test the direct binding of phencyclidine and LY379268 to DA D2High receptors by the inhibition of [3H](+)PHNO binding to striatum in vivo, but measured ex vivo.
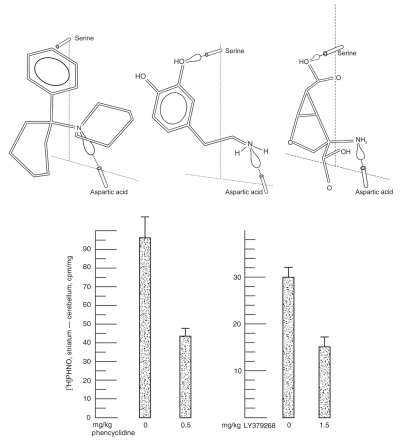
The binding of phencyclidine to D2High receptors in vivo has been directly confirmed by the intravenous injection of phencyclidine, which inhibited the binding of [3H]PHNO to the striatum, as measured ex vivo. An example of such results is shown in Figure 3, where 0.5 mg/kg phencyclidine inhibited 52% of the control binding of [3H](+)PHNO to the striatum ex vivo.13 Similar experiments with LY379268 found that 1.5 mg/kg inhibited the binding of [3H](+)PHNO by 50% (Fig. 313).
Fig. 3: (Top) Proposed molecular conformations of dopamine (DA; centre), phencyclidine (left), and LY379268 (right), using Dreiding molecular models to fit onto the tetrahedral model of the D2 receptor.24 The lone pair of electrons is shown as a balloon that hydrogen bonds with the serine and aspartate amino acid residues of the receptor protein. Although phencyclidine does not have the hydrogen-bonding hydroxyl group, it nevertheless recognizes the D2High state with a dissociation constant of 3–4 nM. The more potent phenyl-meta-OH-phencyclidine would fit perfectly. (Bottom) Inhibition of [3H](+)PHNO binding to DA D2High receptors ex vivo. The intravenous injection of phencyclidine or LY379268 inhibited binding of the injected [3H](+)PHNO. The absolute amount of specific binding (i.e., striatum–cerebellum) varied between different batches of rats because the average weight of the rats on the left was 250 g, whereas that on the right was 375 g (modified, with permission from Seeman P, Guan H-C. Phencyclidine and glutamate agonist LY379268 stimulate dopamine D2High receptors: D2 basis for schizophrenia. Synapse 2008;62:819-28. ©2008 Wiley-Liss, Inc.13).
Mode of glutamate agonist attachment to the D2 receptor
The competition between a DA agonist and [3H]domperidone for DA D2 receptors generally reveals a biphasic pattern, as illustrated in Figure 1A and Figure 1D. Using [3H]domperidone, the high-affinity phase is generally sharply demarcated from the low-affinity concentration phase for all DA agonists. This distinct biphasic pattern for DA agonists does not occur when using [3H]spiperone or [3H]raclopride, which is why it is essential to use [3H]domperidone when searching for agonist competition at D2High receptors.
What are the molecular requirements and molecular conformations of glutamate agonist compounds that would facilitate their attachment to the DA D2 receptor? Although the answer is not known, the data show that all compounds that inhibit the binding of [3H]domperidone at D2High have DA agonist activity. For example, DA agonists need a hydroxyl group to form a hydrogen bond with the serine residue on the D2 receptor, as illustrated in the known tetrahedral pharmacophore model24 in Figure 3. The absence of this hydroxyl group prevents DA agonist action and prevents recognition of the D2High state.
This generalization also holds for aminotetralins, benzo[f]quinolines and apomorphine, the latter having hydroxyl groups at the 10 and 11 positions, as compared with aporphine (sic), which has no hydroxyl groups and is not an agonist and does not recognize D2High.
Phencyclidine readily recognizes D2High by its inhibition of [3H]domperidone with a KiHigh value of about 4 nM, as shown in Figure 1A. However, because phencyclidine does not have any hydroxyl or other hydrogen-bonding substituent, how would phencyclidine attach to the D2 receptor? The phencyclidine conformation in Figure 3 suggests one possible molecular fit, with the phenyl group hydrophobically associating with hydrophobic amino acids in the D2 protein. Although this molecular configuration is speculative, it has recently been confirmed in our laboratory (unpublished data) that meta-hydroxy-phencyclidine, which is twice as potent as phencyclidine,25 is more potent than phencyclidine at D2High; meta-hydroxy-phencyclidine readily fits the tetrahedral model shown in Figure 3. In addition, the glutamate agonists LY379268 and LY404039 comfortably fit the tetrahedral model of the D2 receptor, as shown in Figure 3.
Although there may be a DA component of action by the LY compounds, it is difficult to speculate at present how much each of the behavioural actions can be attributed to a DA component or to a glutamate component, especially when LY404039 is not made available.
Neurochemical basis for schizophrenia: glutamate or DA?
The published data show that phencyclidine and ketamine have low Ki values at D2High, lower than those for phencyclidine or ketamine at the NMDA receptor (as labelled by [3H]MK801). This general finding suggests that the in vivo action of phencyclidine and ketamine may include a DA agonist component at D2 receptors, and may contribute to the psychotic component of phencyclidine or ketamine clinical action.
In fact, at plasma concentrations of phencyclidine between 25 ng/mL and 75 ng/mL (the latter corresponding to about 200 nM), the clinical profile of phencyclidine action has many components, including hyperactivity, psychotic behaviour, nystagmus and lethargy. Phencyclidine concentrations higher than 100 ng/mL are associated with coma, toxicity and hyper-reflexia, all of which may lead to death. It is the higher concentrations of phencyclidine (above 200 nM) that appear to be related to these toxic clinical actions of phencyclidine, and these may arise in connection with phencyclidine action at the NMDA ionotropic receptors.
As for the metabotropic agonist LY379268, its KiHigh values of 5–30 nM at D2High are similar to its Ki values of 14 nM and 15 nM at the mGluR2 receptor (cloned mGlu2 and rat brain membranes, respectively). It is likely, therefore, that the action of LY379268 in vivo26 has both glutamate and DA components of action. This is supported by the data in Figure 3, which indicate that LY379268 has a significant affinity in vivo for D2High. In fact, the data in Figure 3 indicate that 1.5 mg/kg LY379268 inhibits 50% of the in vivo binding of [3H](+)PHNO to D2High, similar to the dose of LY379268 for inhibition of phencyclidine-induced ambulation.26
The D2-stimulating actions of LY379268 and its associated inhibition of the stimulating action of DA on D2 in vitro (Fig. 3) appears to be similar to the DA-inhibiting action for the partial agonist aripiprazole, an effective antipsychotic. This similarity may warrant referring to LY379268 as a DA partial agonist.
Therefore, the DA partial agonist action of LY379268, and its interference with endogenous DA action,16 is compatible with the antipsychotic clinical effect of its closely related congener LY404039. The DA agonist-like properties of these compounds, however, are best examined not by using [3H]raclopride or [3H]spiperone,6 which do not readily reveal the D2High state, but by competition with [3H]domperidone.
Animal models of schizophrenia
Considering that schizophrenia is a uniquely human disease of thought disorder, there is no appropriate animal model for this illness. Nevertheless, many animal models have been proposed on the basis of unusual behaviours produced by various experimental treatments. The consistent finding in these animal models is that the proportion of DA D2High receptors in the striatum is elevated by 100%–300% above normal. These models include long-term administration of amphetamine, methamphetamine, phencyclidine, cocaine, caffeine and cannabis, hippocampal and cerebral cortex lesions, rats socially isolated since birth, and gene knockouts of D4 receptors, adrenoceptors, GABA-β-1 receptors, trace amine receptors, metabotropic glutamate-2 and -3 receptors, and many other neural pathway proteins.27
Conclusion
First, the stimulation of DA D2High receptors by phencyclidine and ketamine supports the DA component to the psychotic symptoms of schizophrenia, given that these drugs induce similar symptoms resembling the disorder. Their D2High potencies are equal to or more potent than their antagonist actions on glutamate NMDA receptors. Second, because phencyclidine has a D2 agonist action and haloperidol is relatively selective in blocking DA D2 receptors, it is important to note that haloperidol blocks the clinical psychotic actions of phencyclidine in individuals without schizophrenia who had recently ingested phencyclidine.28–31 Although it is often said that high doses of potent full D2 antagonists fail to block many of the actions of phencyclidine and ketamine, Ögren and Goldstein32 have unambiguously shown that haloperidol and remoxipride (which is highly D2-selective) blocked the motor stimulation elicited by phencyclidine or by dizocilpine without eliciting catalepsy. For example, they state that “doses of remoxipride (10 μg/kg and 40 μg/kg) almost completely blocked (80%–100% inhibition) the effects of both the 2 mg/kg and 3 mg/kg doses of phencyclidine,” without any sign of catalepsy. Third, although it is often said that haloperidol fails to attenuate ketamine or phencyclidine effects in humans, it is a clinical fact that haloperidol, which is reasonably selective for D2, reduces or blocks the clinical psychotic action of ketamine,33 consistent with a D2 agonist component of ketamine action. Moreover, haloperidol, chlorpromazine and pimozide, all D2 antagonists, were clinically effective in treating phencyclidine psychosis. As Giannini and colleagues30,31,33 stated, these results further support the role of the D2 receptor in phencyclidine psychosis and suggest that D2 blockers such as haloperidol or pimozide be employed as the treatment of choice in phencyclidine psychosis (see also Green and colleagues34). Although Lahti and colleagues8 found that ketamine given to patients with schizophrenia who were prescribed haloperidol worsened psychotic symptoms, they suggested that the pre-existing level of psychosis (average of 9 years in their study) may have sensitized the clinical action of ketamine. Finally, the nausea caused by ketamine in 15%–25% of individuals35,36 may stem from its D2 agonist action.
In conclusion, as noted by Jentsch and Roth4, “it is important to point out that phencyclidine cannot be argued to support a single ‚transmitter hypothesis' of schizophrenia.” In fact, the combined phencyclidine actions of DA agonism and NMDA antagonism may model the different components of thought disorder and cognitive impairment in schizophrenia, as indicated by Krystal and colleagues.7
As noted previously, mice with mGlu2 receptor knockouts are supersensitive to DA13,37 with their striata showing an increase of 150% in the proportion of D2High states. This behavioural and biochemical DA supersensitivity is similar to that found in the many published animal models of schizophrenia.27 Moreover, the behavioural DA supersensitivity in the animal models matches that found in about 75% of individuals with schizophrenia.27 In other words, whether the cause of schizophrenia is a glutamate gene mutation or any one of a vast number of other possible mutations,38 the clinical signs and symptoms arise from hyperactive DA neurotransmission, the target of treatment.
Finally, although emphasis in the present commentary is on the targeting of the D2 receptor by glutamate compounds, it is important to note that, because the striatal DA system is influenced by the prefrontal cortex and hippocampus, DA alterations and enhanced release in the striatum could be secondary to glutamate disruptions in the prefrontal cortex and hippocampus.39
Acknowledgments
This work was supported by the Ontario Mental Health Foundation, a Canadian Institutes of Health Research grant MOP57927, the Dr. Karolina Jus estate, the Medland family, the O'Rorke family, the Rockert family, the Essel Foundation, Constance E. Lieber and Stephen Lieber.
Footnotes
Competing interests: None declared.
Correspondence to: Dr. P. Seeman, Department of Pharmacology, Medical Science Bldg., Rm. 4344, University of Toronto, Toronto ON M5S 1A8; fax 416 971-2445; philip.seeman@utoronto.ca
References
- 1.Van Rossum JM. The significance of dopamine-receptor blockade for the action of neuroleptic drugs. In: Brill H, Cole JO, Deniker P, et al., editors. Neuro-Psycho-Pharmacology: Proceedings of the Fifth International Congress of the Collegium Internationale Neuro-Psycho-Pharmacologicum, 1966 March 28–31; Washington. Amsterdam: Excerpta Medica Foundation; 1967. p. 321-9.
- 2.Baumeister AA, Francis JL. Historical development of the dopamine hypothesis of schizophrenia. J Hist Neurosci 2002;11:265-77. [DOI] [PubMed]
- 3.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 1991;148:1301-8. [DOI] [PubMed]
- 4.Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 1999;20:201-25. [DOI] [PubMed]
- 5.Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry 2001;158:1367-77. [DOI] [PubMed]
- 6.Patil ST, Zhang L, Martenyi F, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med 2007;13:1102-7. [DOI] [PubMed]
- 7.Krystal JH, Perry EB Jr, Gueorguieva R, et al. Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: implications for glutamatergic and dopaminergic model psychoses and cognitive function. Arch Gen Psychiatry 2005;62:985-94. [DOI] [PubMed]
- 8.Lahti AC, Koffel B, LaPorte D, et al. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 1995;13:9-19. [DOI] [PubMed]
- 9.Fessler RG, Sturgeon RD, Meltzer HY. Phencyclidine-induced ipsilateral rotation in rats with unilateral 6-hydroxydopamine-induced lesions of the substantia nigra. Life Sci 1979;24:1281-8. [DOI] [PubMed]
- 10.Sturgeon RD, Fessler RG, London SF, et al. A comparison of the effects of neuroleptics on phencyclidine-induced behaviors in the rat. Eur J Pharmacol 1981;76:37-53. [DOI] [PubMed]
- 11.Freeman AS, Bunney BS. The effects of phencyclidine and N-allylnormetazocine on midbrain dopamine neuronal activity. Eur J Pharmacol 1984;104:287-93. [DOI] [PubMed]
- 12.Odagaki Y, Toyoshima R. Dopamine D2 receptor-mediated G protein activation assessed by agonist-stimulated [35S]guanosine 5′-O-(γ-thiotriphosphate) binding in rat striatal membranes. Prog Neuropsychopharmacol Biol Psychiatry 2006;30:1304-12. [DOI] [PubMed]
- 13.Seeman P, Guan HC. Phencyclidine and glutamate agonist LY379268 stimulate dopamine D2High receptors: D2 basis for schizophrenia. Synapse 2008;62:819-28. [DOI] [PubMed]
- 14.Seeman P, Lasaga M. Dopamine agonist action of phencyclidine. Synapse 2005;58:275-7. [DOI] [PubMed]
- 15.Schoepp DD, Jane DE, Monn JA. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology 1999;38:1431-76. [DOI] [PubMed]
- 16.Seeman P, Caruso C, Lasaga M. Dopamine partial agonist actions of the glutamate receptor agonists LY 354,740 and LY 379,268. Synapse 2008;62:154-8. [DOI] [PubMed]
- 17.Woolley ML, Pemberton DJ, Bate S, et al. The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity. Psychopharmacology (Berl) 2008;196:431-40. [DOI] [PubMed]
- 18.Fell MJ, Johnson BG, Svensson KA, et al. Evidence for the role of metabotropic glutamate (mGlu)2 not mGlu3 receptors in the preclinical antipsychotic pharmacology of the mGlu2/3 receptor agonist (-)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY404039). J Pharmacol Exp Ther 2008;326:209-17. [DOI] [PubMed]
- 19.Morishima Y, Miyakawa T, Furuyashiki T, et al. Enhanced cocaine responsiveness and impaired motor coordination in metabotropic glutamate receptor subtype 2 knockout mice. Proc Natl Acad Sci U S A 2005;102:4170-5. [DOI] [PMC free article] [PubMed]
- 20.Seeman P, Battaglia G, Corti C, et al. Glutamate Receptor mGlu2 and mGlu3 knockout striata are dopamine supersensitive, with elevated D2High receptors and marked supersensitivity to the domapine agonist (+)PHNO. Synapse 2009;63:247-51. [DOI] [PubMed]
- 21.Seeman P. Dopamine and schizophrenia. Scholarpedia 2007;2:3634. Revision #37269. Available: www.scholarpedia.org /article /Dopamine_and_schizophrenia (accessed 2008 Oct 1).
- 22.Seeman P. Glutamate agonists for schizophrenia stimulate dopamine D2High receptors. Schizophr Res 2008;99:373-4. [DOI] [PubMed]
- 23.Willeit M, Ginovart N, Graff A, et al. First human evidence of D-amphetamine induced displacement of a D2/3 agonist radioligand: a [11C]-(+)-PHNO positron emission tomography study. Neuropsychopharmacology 2008;33:279-89. [DOI] [PubMed]
- 24.Seeman P, Watanabe M, Grigoriadis D, et al. Dopamine D2 receptor binding sites for agonists. A tetrahedral model. Mol Pharmacol 1985;28:391-9. [PubMed]
- 25.Chaudieu I, Vignon J, Chichportiche M, et al. Role of the aromatic group in the inhibition of phencyclidine binding and dopamine uptake by PCP analogs. Pharmacol Biochem Behav 1989;32:699-705. [DOI] [PubMed]
- 26.Cartmell J, Monn JA, Schoepp DD. The metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus d-amphetamine motor behaviors in rats. J Pharmacol Exp Ther 1999;291:161-70. [PubMed]
- 27.Seeman P. All psychotic roads lead to increased dopamine D2High receptors: a perspective. Clin Schizophr Relat Psychoses 2008;1:351-355.
- 28.Castellani S, Giannini AJ, Adams PM. Physostigmine and haloperidol treatment of acute phencyclidine intoxication. Am J Psychiatry 1982;139:508-10. [DOI] [PubMed]
- 29.Castellani S, Giannini AJ, Boeringo J, et al. Phencyclidine intoxication: assessment of possible antidotes. J Toxicol Clin Toxicol 1982;19:313-9. [DOI] [PubMed]
- 30.Giannini AJ, Eighan MS, Loiselle RH, et al. Comparison of haloperidol and chlorpromazine in the treatment of phencyclidine psychosis. J Clin Pharmacol 1984;24:202-4. [DOI] [PubMed]
- 31.Giannini AJ, Nageotte C, Loiselle RH, et al. Comparison of chlorpromazine, haloperidol and pimozide in the treatment of phencyclidine psychosis: DA-2 receptor specificity. J Toxicol Clin Toxicol 1984-85;22:573-9. [DOI] [PubMed]
- 32.Ögren SO, Goldstein M. Phencyclidine-and dizocilpine-induced hyperlocomotion are differentially mediated. Neuropsychopharmacology 1994;11:167-77. [DOI] [PubMed]
- 33.Giannini AJ, Underwood NA, Condon M. Acute ketamine intoxication treated by haloperidol: a preliminary study. Am J Ther 2000;7:389-91. [DOI] [PubMed]
- 34.Green AI, Tohen MF, Hamer RM, et al. First episode schizophrenia-related psychosis and substance abuse disorders: acute response to olanzapine and haloperidol. Schizophr Res 2004;66:125-35. [DOI] [PubMed]
- 35.Dal D, Kose A, Honca M, et al. Efficacy of prophylactic ketamine in preventing postoperative shivering. Br J Anaesth 2005;95:189-92. [DOI] [PubMed]
- 36.Perry EB Jr, Cramer JA, Cho HS, et al. Psychiatric safety of ketamine in psychopharmacology research. Psychopharmacology (Berl) 2007;192:253-60. [DOI] [PubMed]
- 37.Corti C, Battaglia G, Molinaro G, et al. The use of knock-out mice unravels distinct roles for mGlu2 and mGlu3 metabotropic glutamate receptors in mechanisms of neurodegeneration/ neuroprotection. J Neurosci 2007;27:8297-308. [DOI] [PMC free article] [PubMed]
- 38.Walsh T, McClellan JM, McCarthy SE, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science 2008;320:539-43. [DOI] [PubMed]
- 39.Seamans JK, Gorelova N, Durstewitz D, et al. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci 2001;21:3628-38. [DOI] [PMC free article] [PubMed]